Short abstract
Mitral annular calcification (MAC) involves calcium deposition in the fibrous annulus supporting the mitral valve (MV). When calcification extends onto the leaflets, valve opening can be restricted. The influence of MAC MV geometry on Doppler gradients is unknown. This study describes a novel methodology to rapid-prototype subject-specific MAC MVs. Replicated valves were used to assess the effects of distorted annular-leaflet geometry on Doppler-derived, transmitral gradients in comparison to direct pressure measurements and to determine if transmitral gradients vary according to measurement location. Three-dimensional echocardiography data sets were selected for two MAC MVs and one healthy MV. These MVs were segmented and rapid prototyped in their middiastolic configuration for in vitro testing. The effects of MV geometry, measurement modality, and measurement location on transmitral pressure gradient were assessed by Doppler and catheter at three locations along the MV's intercommissural axis. When comparing dimensions of the rapid-prototyped valves to the subject echocardiography data sets, mean relative errors ranged from 6.2% to 35%. For the evaluated MVs, Doppler pressure gradients exhibited good agreement with catheter-measured gradients at a variety of flow rates, though with slight systematic overestimation in the recreated MAC valves. For all of the tested MVs, measuring the transmitral pressure gradient at differing valve orifice positions had minimal impact on observed gradients. Upon the testing of additional normal and calcific MVs, these data may contribute to an improved clinical understanding of MAC-related mitral stenosis. Moreover, they provide the ability to statistically evaluate between measurement locations, flow rates, and valve geometries for Doppler-derived pressure gradients. Determining these end points will contribute to greater clinical understanding for the diagnosis MAC patients and understanding the use and application of Doppler echocardiography to estimate transmitral pressure gradients.
Keywords: mitral annular calcification, stenosis, Doppler echocardiography, experimentation
Introduction
Mitral annular calcification (MAC) is a condition involving calcium deposition in the fibrous annulus supporting the mitral valve. When calcification extends onto the valve leaflets, it can reduce valve opening. Contrary to stenosis caused by rheumatic disease, stenosis due to MAC creates an irregular valve orifice without commissural fusion [1,2]. MAC is frequently observed in the elderly and those with chronic kidney disease [3]. It is associated with hypertension, atherosclerosis, and concomitant aortic stenosis [4]. There is no effective medical therapy; when severe, it may require mitral valve (MV) replacement.
The indications for MV replacement rest on several diagnostic metrics, which include the transmitral pressure gradient, MV orifice area, and severity of calcification [4–6]. While Doppler-derived pressure gradients have been demonstrated to be in good agreement with transseptal catheter measurements in rheumatic disease, the influence of an irregular MAC MV leaflet geometry on Doppler gradients is unknown [7]. Moreover, it is unclear if the noncircular MAC MV orifice allows for greater error in Doppler measurement of transvalvular gradient [4].
One route towards directly assessing these clinical issues is through the use of in vitro mitral valve simulators. These simulators provide the unique advantage of isolating the effects of MV geometry on valvular function, which in humans can be difficult to clinically assess [7,8]. To this end, we describe the first use of a novel methodology to rapid-prototype subject-specific MAC MVs to assess the effect of their irregular geometry on Doppler-derived transmitral gradients in comparison to direct pressure measurements. We also compared pressure measurements at three transverse locations across each valve orifice, testing for the possible presence of localized variations in transvalvular gradient.
Methods
Subject Selection and Echocardiographic Acquisition.
This study was approved by the institutional review board at the Albert Einstein Medical Center (Philadelphia, PA). Three-dimensional and Doppler transesophageal echocardiography data sets were retrospectively selected from a series of subjects with MAC noted on clinically indicated studies. Data sets were excluded for poor image quality or inability to identify both the anterior and posterior leaflets. Two MAC subject MVs were selected based on these criteria and subsequently segmented for in vitro testing. To compare these valves to a control, a previously acquired data set from a healthy ovine animal was selected. While a healthy human data set was preferred, sheep have been demonstrated to be superb models of human cardiac and mitral valve anatomy, function, and left heart hemodynamics [9,10]. Sheep exhibit similar mitral valve annular areas, mitral leaflet size, and diastolic transmitral pressure gradients [11–13]. The healthy ovine data set consisted of an epicardial 3D echocardiography data set and Doppler-derived transmitral pressure measurements. The ovine animal included for this study received care in compliance with the protocols approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania in accordance with the guidelines for humane care [14].
Mitral Valve Segmentation and Rapid Prototyping.
A segmentation tool for real-time 3D echocardiography based upon user-controlled J0-splines was developed and implemented in matlab (The Mathworks, Inc., Natick, MA). This tool can segment any 4D Cartesian data set by scaling the data to 0.5-mm voxels and orienting the segmentation planes across the intercommissural axis (Fig. 1). The MV annulus and leaflets were segmented in 1.5-mm slices from commissure-to-commissure at middiastole. In each segmentation slice, the annulus was selected first, followed by the anterior and posterior leaflets. These steps were repeated for each slice in the 3D data set at middiastole.
Fig. 1.
Mitral valve segmentation methodology
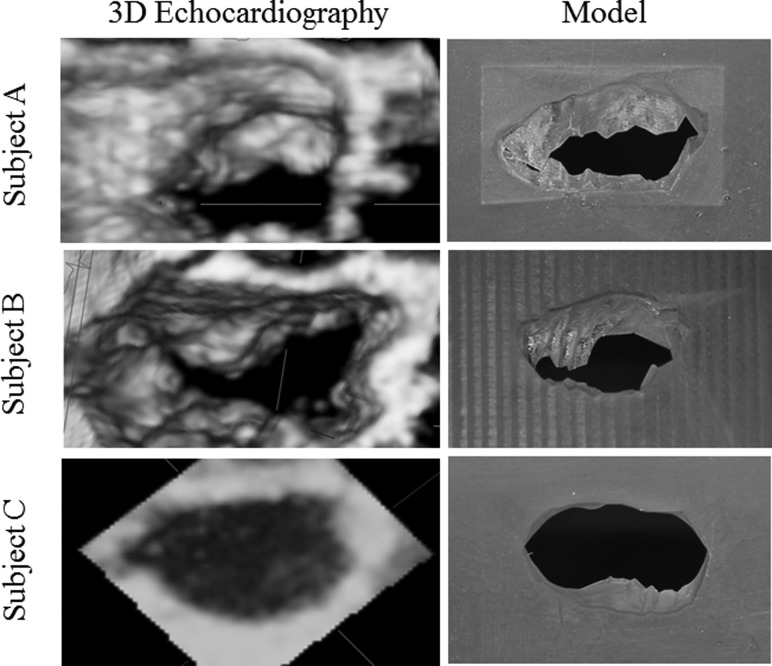
Following segmentation, the 3D coordinates of the MV leaflet geometry were exported into the solidworks 3D modeling software (SolidWorks Software, Waltham, MA). This program was used to mate the segmented MV geometry to a flat plate for mounting within the in vitro simulator. To mate the geometry with the plate, the virtual MV was given a 0.5-mm uniform thickness. After successful modeling, the segmented MV and mounting plate were rapid prototyped. Flexible MV models were considered for the present study, but significant challenges exist for fabricating patient-specific MVs with anisotropic properties and localized regions of stiffness to simulate annular-leaflet calcification. Due to these challenges, the MV models were fabricated to a resolution of ±0.1 mm from the rigid and water-resistant DSM Somos Proto Therm 12120 using stereolithography (Fineline Prototyping, Raleigh, NC). Images of the segmented mitral valves are displayed in Fig. 2.
Fig. 2.
Qualitative comparison of the patient 3D echocardiography mitral valves and the resultant segmented models; image pairs are shown from an en face atrial view
In Vitro Simulator.
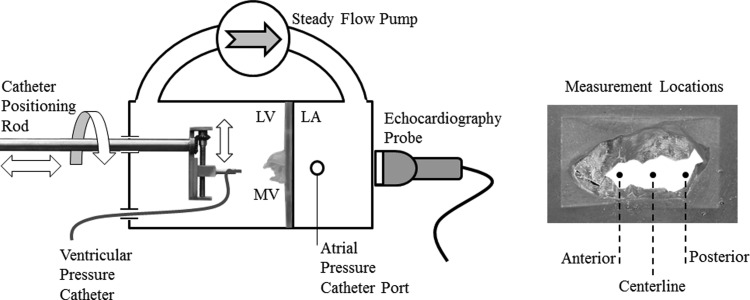
A steady flow loop was utilized to examine the effect of MV geometry and measurement modality on the transmitral pressure gradient (Fig. 3). This simulator consisted of a rigid atrium, rigid ventricle, steady flow pump, rigid tubing, a catheter positioning rod, and blood analog as the working fluid. The salt content in the working fluid (saline) was titrated to match the speed of sound in blood for the Doppler measurements. The rapid-prototyped MV models were mounted between the atrium and ventricle for experimentation. The steady flow pump (Little Giant Pump Co., 4E34N, Oklahoma City, OK) was able to achieve flow rates up to 35 l/min. Transmitral flow was measured using a calibrated electromagnetic flow probe (relative error ±5 ml/min) (Carolina Medical Electronics, FM501D, East Bend, NC) mounted proximal to the atrium.
Fig. 3.
Left experimental steady flow loop setup with the left atrium (LA), left ventricle (LV), and rapid-prototyped mitral valve (MV); right: en face view of the rapid-prototyped MV with the centerline, 1-cm anterior, and 1-cm posterior transmitral pressure measurement locations identified
The peak transmitral gradient was recorded by two modalities that included direct pressure catheter (Validyne DC-40, Northridge, CA) and Doppler measurement using a Phillips ie33 Matrix machine and X7-2 matrix array transducer (Phillips, Amsterdam, The Netherlands). The pressure catheter was calibrated to an accuracy of ±1 mmHg. The x7-2 probe exhibits the same operating characteristics, image capabilities, and performance as the transesophageal x7-2 t probe used clinically. The position of the left ventricular pressure catheter was controlled with a mechanically adjustable positioning rod (Fig. 3). Using this system, the pressure catheter could be positioned to a spatial accuracy of ±0.25 mm. Left atrial pressures were recorded using a static pressure tab just upstream of the valve orifice. Continuous-wave Doppler was used to calculate the transmitral pressure gradient for each transverse measurement location. Using this method, the pressure gradient is calculated from the peak transmitral fluid velocity using the modified Bernoulli equation [4].
Experimental Protocol and Data Analysis.
Each of the replicated MVs was tested to isolate the effect of valve geometry and location on the peak transmitral pressure gradient. Since the replicated MVs were fabricated to their middiastolic configurations, pulsatile transmitral flow could not be simulated. For these reasons, each valve was subjected to steady transmitral flow that was increased from 10 to 30 l/min in 5 l/min intervals. These flow rates were selected to represent the physiological range and extremes of peak middiastolic mitral flow rates observed clinically. At each flow rate, atrial and ventricular pressures were recorded by direct catheter and Doppler measurements at three locations along the intercommissural axis. These locations included 1 cm anterior from the valve centerline, centerline, and 1 cm posterior from the centerline. These measurements were repeated for each flow rate and location for six independent runs.
Transmitral flow and direct catheter pressure measurements were recorded for 15 seconds using a custom labview program (National Instruments, Austin, TX). All results are expressed as a mean ±1 standard deviation. All Doppler measurements were processed offline using the Philips qlab software (v.7.0; Philips Healthcare; Andover, MA). To compare the relative accuracy of the segmented models, qlab was used to determine the anterior-posterior and transverse annular diameters, posterior leaflet height, anterior leaflet height, and orifice area. This orifice area was determined by tracing the mitral orifice in a short-axis view that included the open commissures [4]. All measurements made on the rapid-prototyped MV models were completed in the solidworks 3D modeling software.
Results
One control and two MAC subject MVs were successfully segmented using the described methodology. Qualitative differences existed between the valves selected for in vitro testing. MAC subjects A and B demonstrated irregular, asymmetric orifice areas with restricted leaflet opening (Fig. 2). As expected, the healthy control valve (subject C) had a kidney-shaped orifice with anterior and posterior leaflets in the fully open position. To assess the relative accuracy of the segmentation, geometric valve measurements of the segmented models were compared to those measured by echocardiography in the subjects (Table 1). Results revealed the least relative error to be associated with the segmented orifice area, anterior leaflet height, and transverse annular diameter. The largest relative error involved the posterior leaflet height, particularly for the control ovine valve.
Table 1.
Comparison of mitral valve geometric measurements between echocardiography and the segmented mitral valve models
|
Subject A |
Subject B |
Subject C |
|||||
|---|---|---|---|---|---|---|---|
| Echo | Prototype | Echo | Prototype | Echo | Prototype | Mean relative error | |
| Orifice area (cm2) | 1.79 | 1.34 | 1.43 | 1.36 | 5.70 | 5.51 | 6.2% ± 3.7% |
| Anterior leaflet height (cm) | 1.90 | 1.98 | 1.70 | 1.89 | 1.90 | 1.78 | 7.2% ± 3.6% |
| Posterior leaflet height (cm) | 0.56 | 0.73 | 0.60 | 0.54 | 0.50 | 0.83 | 35% ± 28% |
| Anterior-posterior annular diameter (cm) | 1.90 | 1.86 | 1.93 | 1.60 | 2.73 | 2.37 | 10.8% ± 7.8% |
| Transverse annular diameter (cm) | 2.87 | 3.29 | 2.64 | 2.51 | 3.41 | 3.6 | 8.4% ± 5.4% |
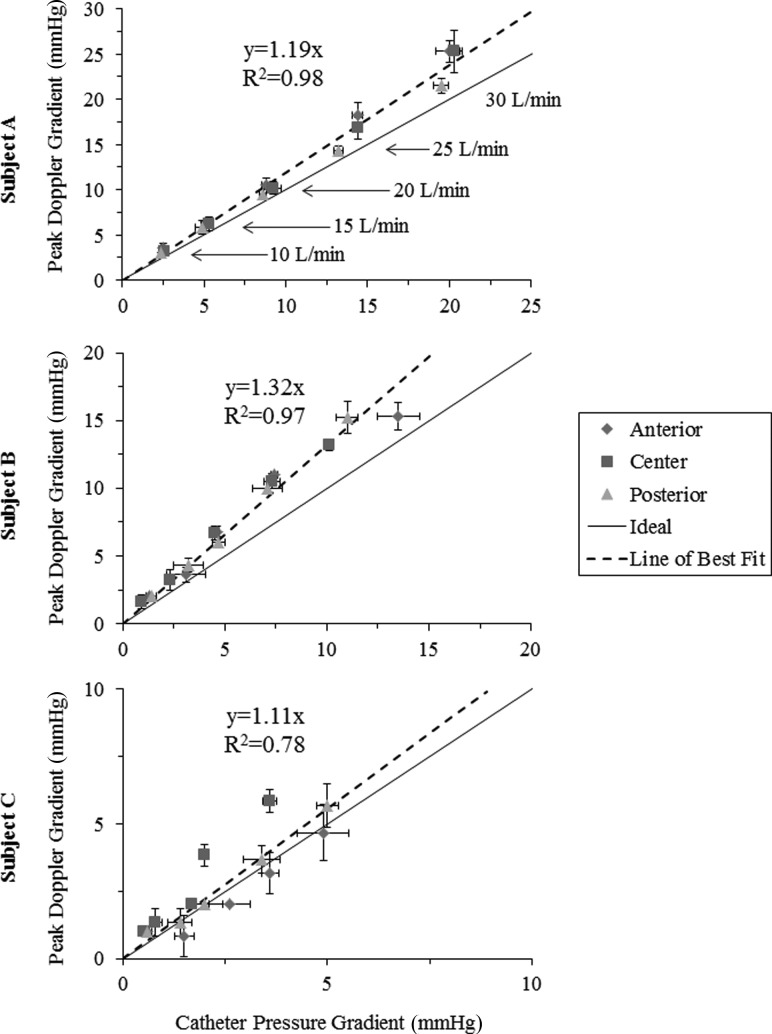
An ultimate goal of the developed methodology is to determine if Doppler echocardiography over- or underestimates the transmitral gradient in MAC patients. Direct transmitral pressure measurements were compared to those derived from Doppler echocardiography at each of the tested flow rates (Fig. 4). Data points close to the line of unity represent good agreement between peak Doppler and catheter measurements, whereas data points above the line indicate overestimation by Doppler. For increasing transmitral flow, Doppler-derived gradients increased linearly with direct pressure catheter measurements. Qualitative preliminary analyses indicate a slight overestimation of Doppler gradients in subjects A and B, with more agreement between Doppler and catheter gradients in the simulated healthy control valve (subject C).
Fig. 4.
Comparison of direct measurements and those derived from Doppler echocardiography (for flow rates of 10–30 l/min in 5 l/min intervals) based on data collected at the anterior commissure, centerline, and posterior commissure of each valve
A secondary goal of the developed methodology is to assess whether measurement location may contribute to variations in Doppler-derived pressure measurements. To evaluate, Doppler and direct pressure measurements were compared at three transverse locations across the valve orifice at increasing levels of mitral inflow (Figs. 3 and 4). It was hypothesized that localized pressure gradients might develop due to the abnormal valve geometries due to MAC. In these preliminary analyses, subject A and B demonstrated good qualitative agreement in the measured pressure gradient between the measurement locations. For the control valve (subject C), the Doppler-derived pressure drop across the valve centerline was larger than those measured by direct catheter at larger steady flow rates.
In addition to the goals of the developed methodology, the in vitro and in vivo Doppler-measured pressure gradients were compared to assess the ability of in vitro methodology to reproduce in vivo Doppler-measured pressure gradients. In the clinic, subject A exhibited a peak Doppler gradient of 9 mmHg (80 beats/min) at a flow rate of 17 l/min, while subject B exhibited a peak Doppler gradient of 9 mmHg (86 beats/min) at a flow rate of 13 l/min. By extrapolating the results of Fig. 4, in vitro simulation underestimated the in vivo Doppler gradient of subject A (7 mmHg versus 9 mmHg) and subject B (3 mmHg versus 9 mmHg). For subject C, the peak transmitral Doppler gradient observed in vivo was 5 mmHg (126 beats/min) at a flow rate of 37 l/min. Compared to the results herein, the in vitro gradient was extrapolated and found to overestimate the in vivo Doppler pressure gradient (8 mmHg versus 5 mmHg).
Discussion
MAC is prevalent in the general population and will become more common as the population ages [15,16]. MAC commonly produces small resting gradients across the MV, as measured by Doppler echocardiography [1]. Increases in heart rate and transvalvular flow, related to activity, increase the transvalvular gradient and could lead to symptoms. MAC is an increasing cause of mitral valve gradients, which progress over time and are occasionally severe enough to require valve replacement [1,2]. Doppler echocardiography has been validated as a noninvasive way to measure MV gradients in the case of rheumatic mitral stenosis [7]. However, in MAC-associated stenosis, such validation studies are lacking.
These results demonstrate a first step in the development of a methodology for assessing the functional significance of MAC valves and the use of Doppler echocardiography in their evaluation. Preliminary in vitro analyses demonstrate Doppler pressure gradients to have good agreement with catheter-measured gradients at a variety of flow rates, though with slight systematic overestimation. Differences in measured gradient at the anterior commissure, centerline, and posterior commissure of each valve were minor, and thus, for subjects A and B, measurement location had minimal impact on Doppler-derived pressure gradients. For subject B and C, larger standard deviations in Doppler and catheter pressure gradients were observed but were of a magnitude that is consistent with the measurement uncertainty in both modalities (±1 mmHg). For the healthy control valve, the centerline measurement exhibited larger gradients, which may be attributed to the likely more distinct parabolic flow profile through the valve's circular orifice. The uncertainties associated with direct catheter measurements may be improved in our ongoing studies with the use of computational fluid dynamics.
For our ongoing analyses, computational fluid dynamics will be used to simulate steady flow through the segmented patient-specific MV geometries. These trials will provide detailed pressure maps of the flow field for direct comparison to in vitro Doppler measurements. Computational fluid modeling may provide the ability to resolve interesting details of the flow and correlate these observations with patient MV subsets. These analyses will additionally provide the ability to assess the effect of in vitro chamber geometry on observed gradients, which may contribute to the observed differences in in vitro- and in vivo-derived Doppler gradients.
There are certain limitations associated with the techniques used in this study. The challenges associated with fabricating flexible MV models resulted in modeling the MVs in a rigid middiastolic configuration and submitting these models to steady flow testing. This modeling therefore represents a snapshot in time, during which the peak transmitral velocity and pressure gradient are observed. If flexible models mimicking the increased leaflet stiffness due to MAC were utilized, we believe the pressure drop under steady flow would be similar to that achieved with the rigid models. Since continuous-wave Doppler measures the peak velocity along a chosen vector and the in vitro working fluid was titrated to match the speed of sound in blood, we believe no differences in performance exist between measuring the transmitral pressure gradient in the rigid chamber and selected subjects.
During segmentation, the user is required to select points that outline the valves, and this resulted in discrepancies between the subjects and prototyped valves. The resultant measurement errors were generally small, although relative measurement error was substantial for posterior leaflet height. Decreasing the distance between each segmented slice and improving interpolation at the leaflets' free edges could improve MV segmentation. Furthermore, the results are limited to only three valves. In future studies, the sample size will be extended to a larger MAC patient population to understand the extremes of these measurements and possible errors associated with them.
This study provides a novel experimental platform to rapid prototype the diastolic geometry of calcified mitral valves and to test the effect of valve geometry on Doppler-derived transmitral pressure gradients. Upon the testing of additional normal and calcific MVs, these data may contribute to an improved clinical understanding of MAC-related mitral stenosis. Moreover, they provide the ability to statistically evaluate between measurement locations, flow rates, and valve geometries for Doppler-derived pressure gradients. Determining these end points will contribute to greater clinical understanding for the diagnosis of MAC patients and understanding the use and application of Doppler echocardiography to estimate transmitral pressure gradients.
Acknowledgment
This study was partially supported by a research grant awarded from the National Institutes of Health (R01 HL090661-02) and a donation from Tom and Shirley Gurley. We would like to acknowledge the intellectual contributions of Christopher M. Haggerty.
Contributor Information
Andrew W. Siefert, The Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, GA 30332
Gregg S. Pressman, Einstein Medical Center, Department of Internal Medicine, Division of Cardiology, Philadelphia, PA 19141
Ajit P. Yoganathan, e-mail: ajit.yoganathan@bme.gatech.edu, The Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, GA 30332.
References
- [1]. Muddassir, S. M. , and Pressman, G. S. , 2007, “Mitral Annular Calcification as a Cause of Mitral Valve Gradients,” Int. J. Cardiol., 123, pp. 58–62. 10.1016/j.ijcard.2006.11.142 [DOI] [PubMed] [Google Scholar]
- [2]. Pressman, G. S. , Agarwal, A. , Braitman, L. E. , and Muddassir, S. M. , 2010, “Mitral Annular Calcium Causing Mitral Stenosis,” Am. J. Cardiol., 105, pp. 389–391. 10.1016/j.amjcard.2009.09.042 [DOI] [PubMed] [Google Scholar]
- [3]. Jesri, A. , Braitman, L. E. , and Pressman, G. S. , 2008, “Severe Mitral Annular Calcification Predicts Chronic Kidney Disease,” Int. J. Cardiol., 128, pp. 193–196. 10.1016/j.ijcard.2007.05.015 [DOI] [PubMed] [Google Scholar]
- [4]. Baumgartner, H. , Hung, J. , Bermejo, J. , Chambers, J. B. , Evangelista, A. , Griffin, B. P. , Lung, B. , Otto, C. M. , Pellikka, P. A. , and Quiñones, M. , 2009, “Echocardiographic Assessment of Valve Stenosis: EAE/ASE Recommendations for Clinical Practice,” Eur. J. Echocardiogr., 10, pp. 1–25. 10.1093/ejechocard/jen303 [DOI] [PubMed] [Google Scholar]
- [5]. Iung, B. , Cormier, B. , Ducimetiere, P. , Porte, J. M. , Nallet, O. , Michel, P. L. , Acar, J. , and Vahanian, A. , 1996, “Immediate Results of Percutaneous Mitral Commissurotomy: A Predictive Model on a Series of 1514 Patients,” Circulation, 94, pp. 2124–2130. 10.1161/01.CIR.94.9.2124 [DOI] [PubMed] [Google Scholar]
- [6]. Wilkins, G. T. , Weyman, A. E. , Abascal, V . M. , Block, P. C. , and Palacios, I . F. , 1988, “Percutaneous Balloon Dilatation of the Mitral Valve: An Analysis of Echocardiographic Variables Related to Outcome and the Mechanism of Dilatation,” Br. Heart J., 60, pp. 299–308. 10.1136/hrt.60.4.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Nishimura, R. A. , Rihal, C. S. , Tajik, A. J. , and Holmes, Jr., D. R. , 1994, “Accurate Measurement of the Transmitral Gradient in Patients With Mitral Stenosis: A Simultaneous Catheterization and Doppler Echocardiographic Study,” J. Am. Coll. Cardiol., 24, pp. 152–158. 10.1016/0735-1097(94)90556-8 [DOI] [PubMed] [Google Scholar]
- [8]. Nielsen, S. L. , Nygaard, H. , Fontaine, A. A. , Hasenkam, J. M. , He, S. , Anderson, N. T. , and Yoganathan, A. P. , 1999, “Chordal Force Distribution Determines Systolic Mitral Leaflet Configuration and Severity of Functional Mitral Regurgitation,” J. Am. Coll. Cardiol., 33, pp. 843–853. 10.1016/S0735-1097(98)00627-5 [DOI] [PubMed] [Google Scholar]
- [9]. Llanera, M. R. , Nance, M. L. , Streicher, J. T. , Lima, J. A. C. , Savino, J. S. , Bogen, D. K. , Deac, R. F. P. , Ratcliffe, M. B. , and Edmunds, Jr., L. H. , 1994, “Large Animal Model of Ischemic Mitral Regurgitation,” Ann. Thorac. Surg., 57, pp. 432–439. 10.1016/0003-4975(94)91012-X [DOI] [PubMed] [Google Scholar]
- [10]. Dixon, J. A. , and Spinale, F. G. , 2009, “Large Animal Models of Heart Failure: A Critical Link in the Translation of Basic Science to Clinical Practice,” Circ. Heart Fail., 2, pp. 262–271. 10.1161/CIRCHEARTFAILURE.108.814459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Carpentier, A. , Adams, D. H. , and Filsoufi, F. , 2010, “Pathophysiology, Preoperative Valve Analysis, and Surgical Indications,” Carpentier's Reconstructive Valve Surgery: From Valve Analysis to Valve Reconstruction, 1st ed., Carpentier A., Adams D. H., and Filsoufi F., eds., Saunders-Elsevier, Maryland Heights, MO, pp. 43–53. [Google Scholar]
- [12]. Rausch, M. K. , Bothe, W. , Kvitting, J. P. E. , Swanson, J. C. , Ingels, Jr., N. B. , Miller, D. C. , and Kuhl, E. , 2011, “Characterization of Mitral Valve Annular Dynamics in the Beating Heart,” Ann. Biomed. Eng., 39, pp. 1690–1702. 10.1007/s10439-011-0272-y [DOI] [PubMed] [Google Scholar]
- [13]. Liddicoat, J. R. , Mac Neill, B. D. , Gillinov, A. M. , Cohn, W. E. , Chin, C. , Prado, A. D. , Pandian, N. G. , and Oesterle, S. N. , 2003, “Percutaneous Mitral Valve Repair: A Feasibility Study in an Ovine Model of Acute Ischemic Mitral Regurgitation,” Cathet. Cardiovasc. Interv., 60, pp. 410–416. 10.1002/ccd.10662 [DOI] [PubMed] [Google Scholar]
- [14].National Research Council of the National Academies, “Guide for the Care and Use of Laboratory Animals,” National Institutes of Health Publication 85–23, Rev. 1996, Bethesda, Md: National Institutes of Health.
- [15]. Allison, M. A. , Cheung, P. , Criqui, M. H. , Langer, R. D. , and Wright, C. M. , 2006, “Mitral and Aortic Annular Calcification Are Highly Associated With Systemic Calcified Atherosclerosis,” Circulation, 113, pp. 861–866. 10.1161/CIRCULATIONAHA.105.552844 [DOI] [PubMed] [Google Scholar]
- [16]. Barasch, E. , Gottdiener, J. S. , Larson, E. K. M. , Chaves, P. H. M. , Newman, A. B. , and Manolio, T. A. , 2006, “Clinical Significance of Calcification of the Fibrous Skeleton of the Heart and Aortosclerosis in Community Dwelling Elderly. The Cardiovascular Health Study (CHS),” Am. Heart J., 51, pp. 39–47. 10.1016/j.ahj.2005.03.052 [DOI] [PubMed] [Google Scholar]