Abstract
Pyruvate dehydrogenase kinases (PDK1-4) play a critical role in the inhibition of the mitochondrial pyruvate dehydrogenase complex especially when blood glucose levels are low and pyruvate can be conserved for gluconeogenesis. Under diabetic conditions, the Pdk genes, particularly Pdk4, are often induced, and the elevation of the Pdk4 gene expression has been implicated in the increased gluconeogenesis in the liver and the decreased glucose utilization in the peripheral tissues. However, there is no direct evidence yet to show to what extent that the dysregulation of hepatic Pdk genes attributes to hyperglycemia and insulin resistance in vivo. To address this question, we crossed Pdk2 or Pdk4 null mice with a diabetic model that is deficient in hepatic insulin receptor substrates 1 and 2 (Irs1/2). Metabolic analyses reveal that deletion of the Pdk4 gene had better improvement in hyperglycemia and glucose tolerance than knockout of the Pdk2 gene whereas the Pdk2 gene deletion showed better insulin tolerance as compared to the Pdk4 gene inactivation on the Irs1/2 knockout genetic background. To examine the specific hepatic effects of Pdks on diabetes, we also knocked down the Pdk2 or Pdk4 gene using specific shRNAs. The data also indicate that the Pdk4 gene knockdown led to better glucose tolerance than the Pdk2 gene knockdown. In conclusion, our data suggest that hepatic Pdk4 may be critically involved in the pathogenesis of diabetes.
Introduction
Mitochondrial pyruvate dehydrogenase complex (PDC) plays an essential role in glucose metabolism by converting pyruvate to acetyl-CoA in glycolysis [1]. The activity of PDC is not only allosterically regulated by acetyl-CoA and NADH, but also by covalent modifications such as phosphorylation that is controlled by four pyruvate dehydrogenase kinases (Pdks) and two pyruvate dehydrogenase phosphatases (Pdps) [1]. Pdks have differential tissue distribution: Pdk1 is abundant in the heart and is expressed at a low level in other organs; Pdk2 is ubiquitously expressed in most tissues; Pdk3 is abundant in testis and is expressed at a low level in other organs; Pdk4 is highly expressed in the heart and skeletal muscle and is also expressed at an intermediate level in the liver, lung, and kidney [2]–[6]. Among these Pdks, Pdk4 is highly inducible by starvation and it is also elevated under insulin resistance [6]–[15]. Systemic Pdk4 knockout leads to hypoglycemia after the prolonged starvation [16]. In contrast, Pdk2 null mice only manifest a moderate reduction in blood glucose under non-fasted conditions [17]. When challenged by a high-fat diet, Pdk4 knockout mice exhibit lower blood glucose levels and better glucose tolerance relative to the control wild-type mice [18].
The Pdk4 gene expression can be suppressed by insulin under normal physiological conditions [10]. Insulin receptor substrates (Irs) play an essential role in the insulin signal transduction through a direct mediation of insulin receptor activities [19]. There are four Irs genes in mammals, and among them, Irs1 and Irs2 are ubiquitously expressed. Mouse genetic data have shown that deletion of Irs1 and Irs2 genes in the mouse liver (IrsLDKO) causes severe insulin resistance and early onset of diabetes [20], [21]. It is also noticed that Pdk4 gene expression is highly induced in the liver of the IrsLDKO mice due to the impairment of insulin signaling [20]; however, it is not clear that, to what extent, the elevated Pdk4 contributes to the diabetes in the IrsLDKO mice. To address this question, we genetically inactivated Pdk2 or Pdk4 in the IrsLDKO mice. Our results indicate that Pdk4 indeed plays a more significant role in the development of hyperglycemia and glucose intolerance in this hepatic insulin resistance model.
Materials and Methods
Animals
Irs1 and Irs2 floxed mice and Pdk2/4 null mice were generated as previously described [16], [17], [20]. Transgenic mice that carry a Cre coding sequence plus the Albumin gene promoter were purchased from the Jackson Laboratory. For insulin stimulation, animals were anesthetized before a bolus of 5 units of insulin (human regular insulin — humulin R, Eli Lilly) was injected via vena cava for 3 min.
Ethics statement
All procedures were performed in accordance with the Guide for Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Institutional Animal Use and Care Committee of Indiana University School of Medicine (study 10322).
Blood chemistry and metabolic analysis
Blood glucose levels were measured using a glucose meter under ad libitum (fed) or overnight 16-hour fasting. Serum insulin was measured using commercial assay kits (ALPCO). Glucose and insulin tolerance tests were performed as previously described [22], and 2 g glucose and 1 unit human regular insulin per kg body weight were used, respectively.
Immunoblot analysis
Liver tissue was homogenized in the lysis buffer (50 mM Hepes, pH 7.5, 150 mM NaCl, 10% Glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 10 mM Sodium Pyrophosphate, 100 mM Sodium Fluoride and freshly added 100 uM Sodium Vanadate, 1 mM PMSF, 10 ug/ml Aprotinin, and 10 ug/ml Leupeptin). Protein extracts were resolved on an SDS-PAGE gel and transferred to nitrocellulose membrane. Proteins were probed using the following antibodies: Irs1 and Irs2 (Millipore), Pdk2, Pdk4, β-actin and Actinin (Santa Cruz Biotechnology), total and phosphorylated Akt and Erk (Cell Signaling Technology). Protein signals were detected by incubation with HRP-conjugated secondary antibodies, followed by ECL detection reagent (Thermo Fisher Scientific Inc.).
Adenovirus-mediated gene knockdown in vivo
Gene-specific shRNAs were designed using the BLOCK-iT RNAi Designer (Invitrogen) and cloned using a BLOCK-iT system (Invitrogen). The target template sequences are the followings: shGFP, 5′-GCATCAAGGTGAACTTCAAGA-3′; shPdk2, 5′-GGCTCTTCAGCTACATGTACT-3′; and shPdk4, 5′-GGAAGGAATCAAAGCACTTTA-3′. Adenoviruses were prepared following the standard procedure. Mice were injected with adenoviruses (1×109 pfu/animal) via tail vein as previously described [23]. Three days post-injection, glucose tolerance tests were performed. Five days post-injection, insulin tolerance tests were performed. On day 7 post-injection, animals were fasted overnight for 16 hours before tissues were collected for further analysis.
Real-time PCR
Liver RNA isolation was performed as previously described (6). Quantitative RT-PCR (RT-qPCR) was performed in two steps: first, cDNA was synthesized using a cDNA synthesis kit (Applied Biosystems Inc.); second, cDNA was analyzed by real-time PCR using SYBR Green Master Mix (Promega). Primer sequences for the specific genes are as follows: Ppia forward 5′-CACCGTGTTCTTCGACATCA-3′; Ppia reverse 5′-CAGTGCTCAGAGCTCGAAAGT-3′; Pdk2 forward 5′- TGGAAAGCTCCGAGTTCAGT; Pdk2 reverse 5′- GGAGACTGGCACTCACCACT-3′; Pdk4 forward 5′- GATTGACATCCTGCCTGACC-3′; Pdk4 reverse 5′- CATGGAACTCCACCAAATCC-3′; Pck1 forward ATCATCTTTGGTGGCCGTAG; Pck1 reverse TGATGATCTTGCCCTTGTGT; G6pc forward TCGGAGACTGGTTCAACCTC; G6pc reverse TCACAGGTGACAGGGAACTG.
Statistics
Data are presented as means ± SEM. Student's t-test (2-way) was performed to test significance between two groups. P<0.05 was considered as a statistical significance.
Results
Inactivation of Pdk2 or Pdk4 improves glucose homeostasis in IrsLDKO mice
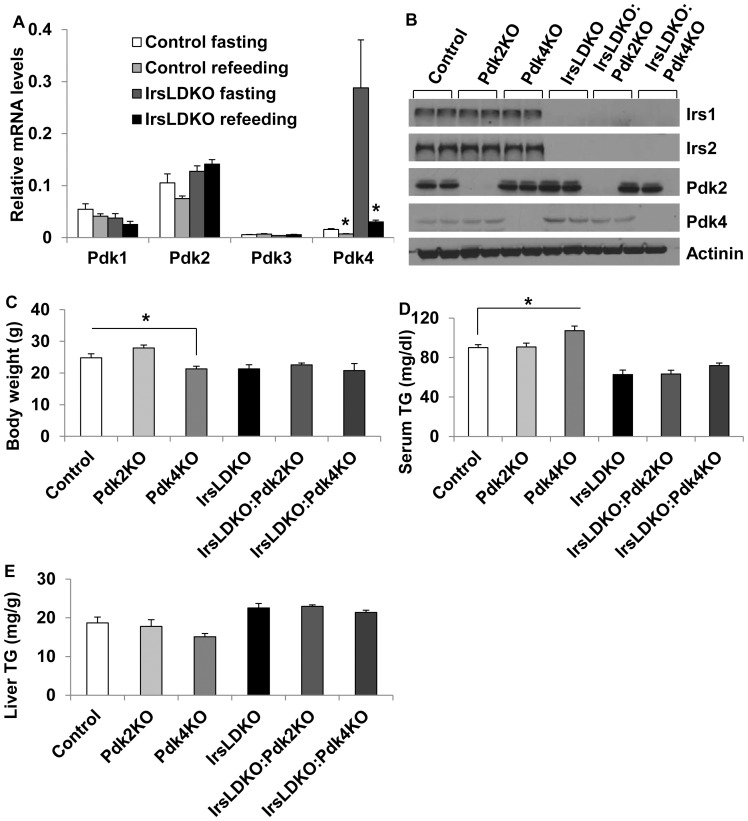
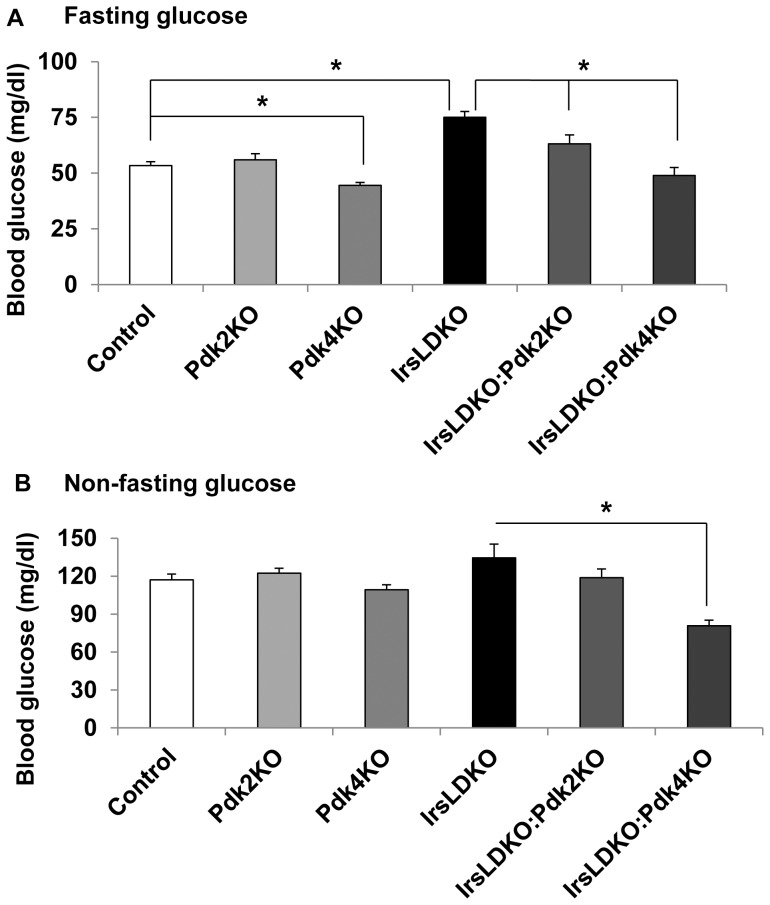
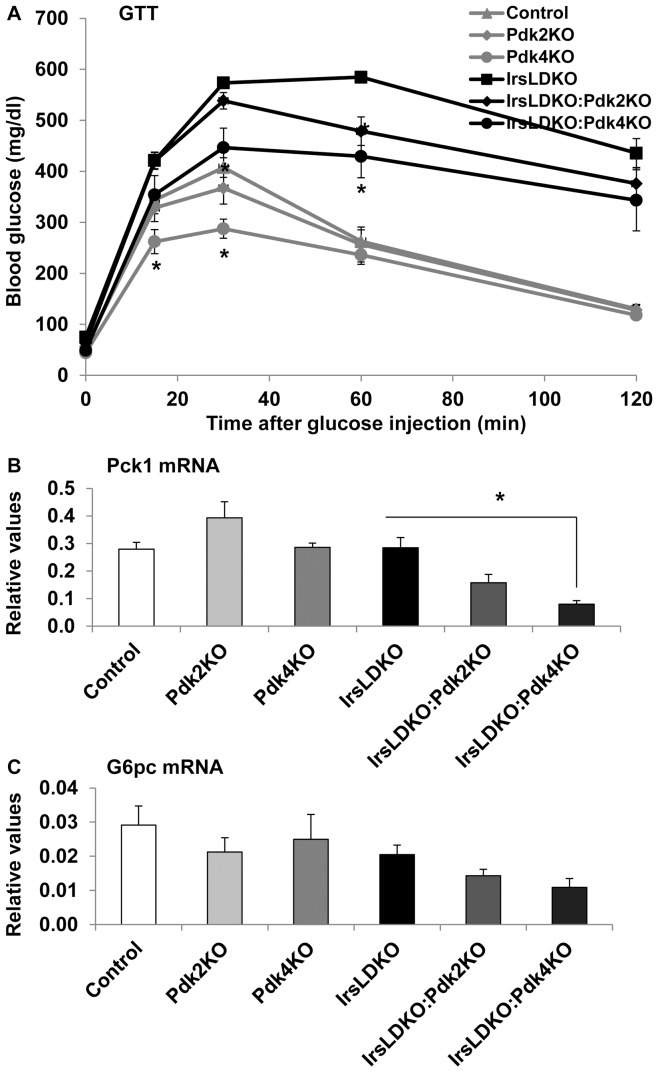
It has been previously reported that hepatic Irs1 and Irs2 play a crucial role in glucose homeostasis because simultaneous deletions of both genes in the liver (IrsLDKO) lead to diabetes in mice [20], [21]. Since Pdks can promote hepatic gluconeogenesis, it is possible that they contribute to the development of hyperglycemia in the IrsLDKO mice. To test this hypothesis, we first analyzed expression of all four Pdk genes in the liver of control wild-type and IrsLDKO mice after an overnight 16-hour fasting or immediately followed by 4-hour refeeding. According to mRNA analysis, Pdk2 is the most abundant among four Pdks in the liver of wild-type mice whereas Pdk4 was induced most in the fasted IrsLDKO livers (Figure 1A). Interestingly, refeeding could still suppress the hepatic Pdk4 gene expression in the IrsLDKO mice (Figure 1A). To further investigate the role of Pdk2 and Pdk4 in the pathogenesis of diabetes in the IrsLDKO mice, we deleted either the Pdk2 or Pdk4 gene on the IrsLDKO genetic background (Figure 1B). While Pdk4 knockout mice were significantly smaller than control wild-type mice, Pdk4 deletion had no effect on the body weight of growth-retarded IrsLDKO mice (Figure 1C). Neither Pdk2 nor Pdk4 deletion had any significant effect on serum or hepatic triglycerides in the IrsLDKO mice (Figure 1, D and E). We then monitored blood glucose levels in control wild-type, single, double, and triple knockout mice. Deletion of Pdk2 or Pdk4 on the IrsLDKO genetic background (IrsLDKO:Pdk2KO and IrsLDKO:Pdk4KO, respectively) significantly lowered blood glucose levels under the fasting conditions (Figure 2A). In contrast, only IrsLDKO:Pdk4KO mice showed a significant decrease in blood glucose levels under the non-fasting conditions (Figure 2B). Additionally, deletion of either Pdk2 or Pdk4 also improved glucose metabolism during glucose tolerance tests, but ablation of the Pdk4 gene had a more significant effect (Figure 3A). Gene expression analysis also revealed a significant decrease in Pck1 (phosphoenoylpyruvate carboxykinase 1) but not G6pc (glucose-6-phosphatase, catalytic) mRNAs, suggesting that hepatic gluconeogenesis might be reduced in the IrsLDKO:Pdk4KO mice (Figure 3, B and C).
Figure 1. Knockout of the Pdk genes in wild-type and IrsLDKO mice.
A, Control wild-type and IrsLDKO mice (n = 3) were fasted overnight for 16 hours and half of them were fed for 4 hours immediately after the fasting. Pdks gene expression in the liver was analyzed by real-time PCR and data were normalized to an internal control gene — Ppia. B, Western blot analysis of liver lysates from control and knockout mice. C, Body weight measurements in control and knockout mice (n = 6–20). D, Serum triglycerides (TG) were measured in overnight fasted control and knockout mice (n = 6–8). E, Liver TG analysis in control and knockout mice (n = 6–8). Pdk2KO, Pdk2 knockout; Pdk4KO, Pdk4 knockout; IrsLDKO, Irs1/2 liver-specific double knockout. Data are presented as means ± SEM. *, P<0.05 relative to corresponding controls.
Figure 2. Deletion of the Pdk4 gene improves hyperglycemia in IrsLDKO mice.
A, Blood glucose was measured in overnight fasted control and knockout mice. B, Blood glucose was measured in ad libitum fed control and knockout mice. Data are presented as means ± SEM, n = 8–23. *, P<0.05 relative to corresponding controls.
Figure 3. Ablation of Pdks improves glucose tolerance in IrsLDKO mice.
A, Glucose tolerance tests (GTT) were performed in age-matched control and knockout mice (n = 8–12). B and C, Expression of gluconeogenic genes Pck1 and G6pc was analyzed in the liver of overnight fasted control and knockout mice (n = 3). Data are presented as means ± SEM. *, P<0.05 relative to corresponding controls.
Deletion of Pdk2 or Pdk4 improves insulin resistance in IrsLDKO mice
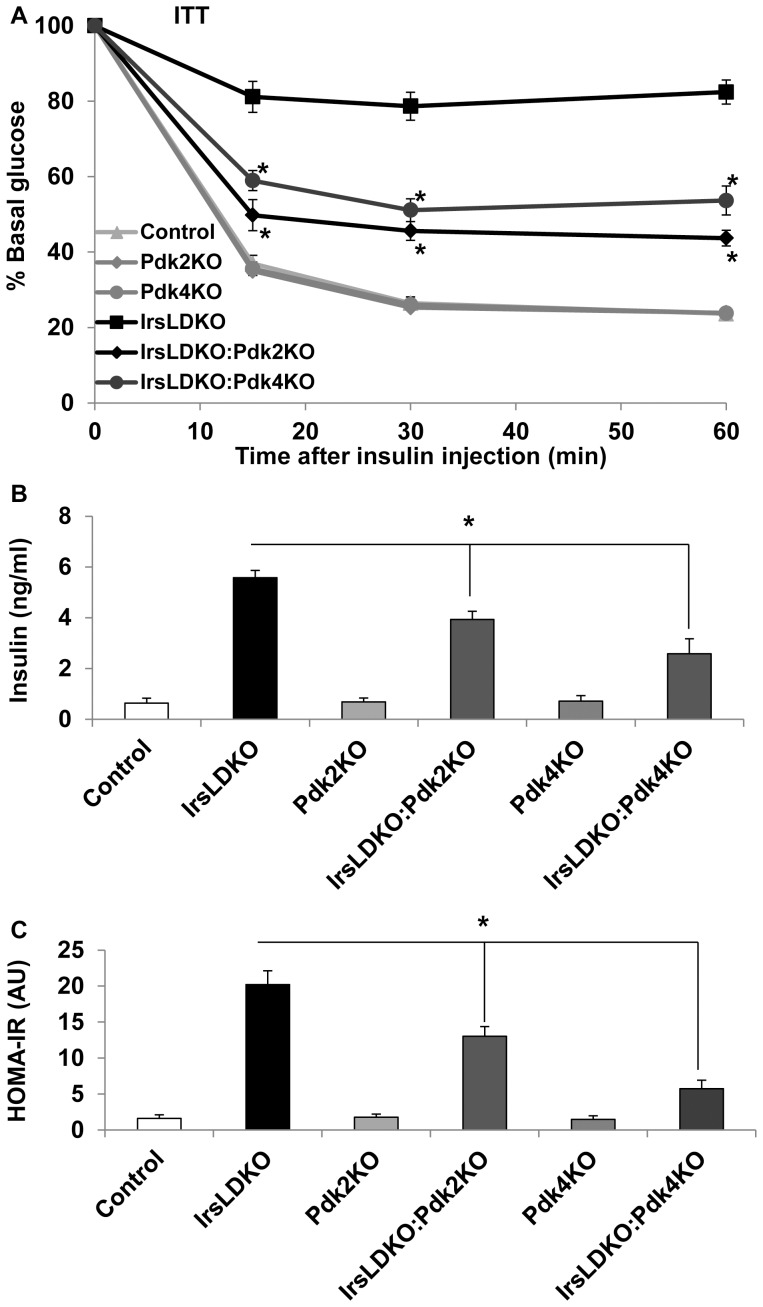
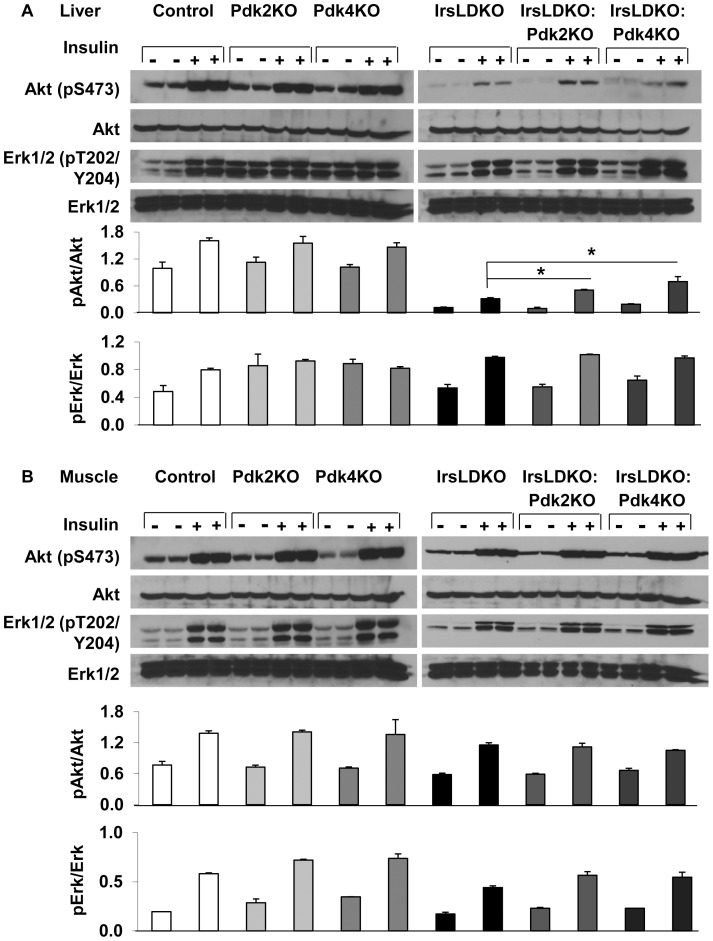
To examine whether Pdk2 or Pdk4 knockout might affect insulin resistance in IrsLDKO mice, we performed insulin tolerance tests. The data showed that either Pdk2 or Pdk4 gene deletion remarkably improved insulin tolerance in the IrsLDKO mice (Figure 4A). In addition, fasting plasma insulin levels were also significantly reduced in IrsLDKO:Pdk2KO and IrsLDKO:Pdk4KO mice (Figure 4B). The HOMA-IR (homeostatic model analysis-insulin resistance) analysis indicated improved insulin resistance in those triple knockout mice (Figure 4C). In order to understand molecular changes during insulin action, we also analyzed insulin signaling in the liver and skeletal muscle. Akt phosphorylation (Ser473) was moderately elevated in the liver of IrsLDKO:Pdk2KO and IrsLDKO:Pdk4KO mice, and Erk1/2 phosphorylation (Thr202/Tyr204) was not significantly changed in the IrsLDKO:Pdk4KO or IrsLDKO:Pdk2 livers as compared to IrsLDKO livers (Figure 5A). No significant changes in insulin signaling were observed in the skeletal muscle of IrsLDKO:Pdk2KO or IrsLDKO:Pdk4KO mice in comparison to IrsLDKO mice (Figure 5B).
Figure 4. Inactivation of Pdks improves insulin sensitivity in IrsLDKO mice.
A, Insulin tolerance tests (ITT) were performed in age-matched control and knockout mice (n = 8–20). B, Fasting plasma insulin was analyzed in age-matched control and knockout mice (n = 5–9). C, HOMA-IR (homeostatic model assessment-insulin resistance) was analyzed using fasting glucose and insulin data. Data are presented as means ± SEM. *, P<0.05 relative to corresponding controls.
Figure 5. Insulin signaling analysis in the control and knockout mice.
A and B, Animals were stimulated with 5 units of human insulin (saline as a vehicle control) for 3 min before liver and skeletal muscle samples were collected for Akt and Erk phosphorylation analyses. Western blot signals were quantified using the Quantity One software (Bio-Rad). Data are presented as means ± SEM. *, P<0.05 relative to corresponding controls.
Liver-specific knockdown of Pdk2 or Pdk4 in IrsLDKO mice
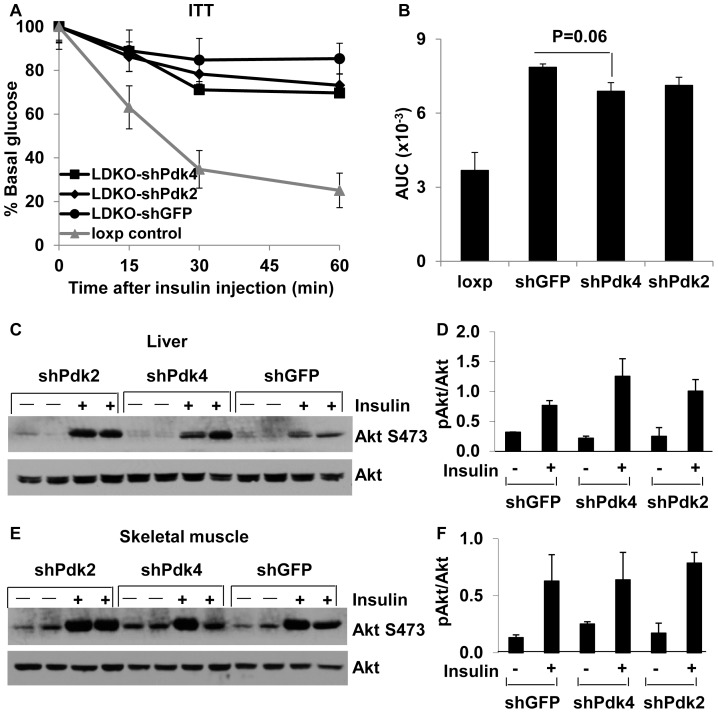
In order to directly assess the role of hepatic Pdks in glucose homeostasis, we used adenovirus-mediated shRNAs to knock down Pdk2 or Pdk4 specifically in the liver. Pdk2 and Pdk4 mRNA levels were reduced 85% and 60%, respectively (Figure 6A). Interestingly, although the Pdk4 knockdown efficiency was less than that of Pdk2, glucose tolerance was improved only in the Pdk4 knockdown mice in the last two time points during the glucose tolerance tests and the area under curve was significantly decreased in the Pdk4 knockdown group (Figure 6, B and C). Insulin tolerance tests did not reveal a significant improvement in either Pdk2 or Pdk4 knockdown mice although Pdk4 knockdown had a trend of improvement of insulin resistance (Figure 7, A and B). Insulin signaling analysis did not reveal any significant improvement in Akt phosphorylation in the liver or skeletal muscle of shPdk2 or shPdk4 mice as compared to shGFP control mice (Figure 7, C–F).
Figure 6. Hepatic Pdk4 knockdown moderately improves glucose tolerance in IrsLDKO mice.
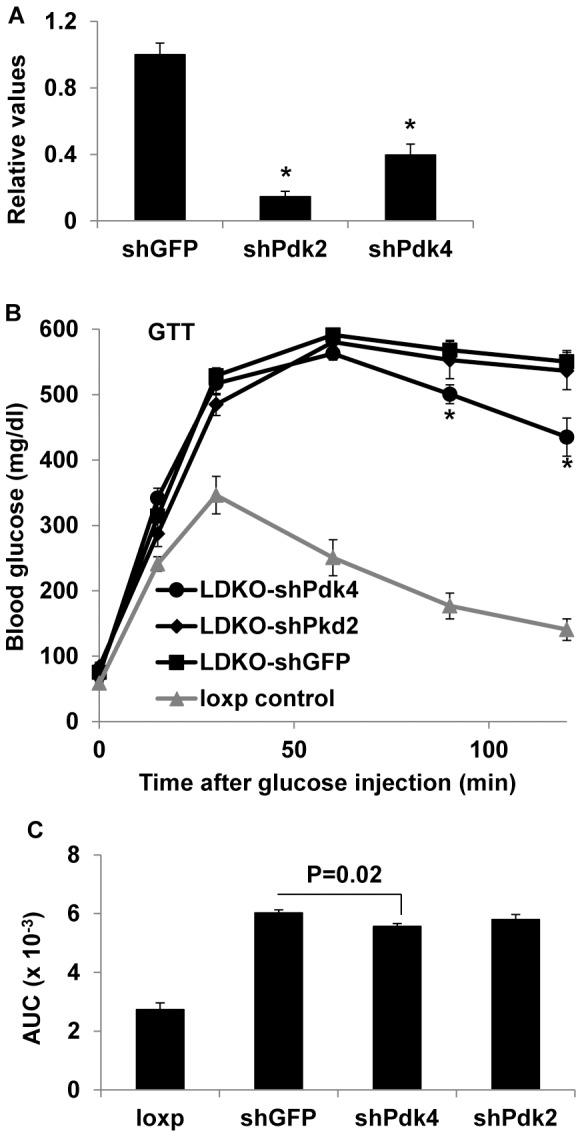
A, Gene knockdown efficiency was analyzed by real-time PCR in IrsLDKO livers transduced with shRNA adenoviruses against GFP (shGFP), Pdk2 (shPdk2), or Pdk4 (shPdk4). B, Glucose tolerance tests were performed in shRNA adenoviruses infected IrsLDKO mice. C, Area under curve analysis (AUC) was performed for the above glucose tolerance test data. Data are presented as means ± SEM, n = 4–5. *, P<0.05 relative to corresponding controls.
Figure 7. Pdk2 or Pdk4 knockdown has no significant effect on insulin tolerance in IrsLDKO mice.
A, Insulin tolerance tests were performed on IrsLDKO mice (n = 4–5) that were injected with shGFP, shPdk2, and shPdk4 adenoviruses. B, Area under curve was analyzed for the above ITT data. C–F, Akt phosphorylation was analyzed in the liver and skeletal muscle of mice injected with shGFP, shPdk2, and shPdk4 adenoviruses. Western blot signals were also quantified using the Quantity One software. Data are presented as means ± SEM. *, P<0.05 relative to corresponding controls.
Discussion
In this study, we directly assessed the involvement of Pdk2 and Pdk4 in the pathogenesis of diabetes. As Pdks regulate glucose homeostasis at least in two ways — inhibiting glycolysis and promoting gluconeogenesis, deletion of Pdk genes is expected to improve hyperglycemia and glucose tolerance. Indeed, both Pdk2 and Pdk4 ablations can improve glucose tolerance in the IrsLDKO mice, but only the Pdk4 gene inactivation lowers both fasting and non-fasting glucose levels whereas the Pdk2 gene deletion only decreases fasting blood glucose. This is quite intriguing because both Pdk2 and Pdk4 genes are ubiquitously expressed in most tissues and Pdk2 has been shown to be more potent for the inhibition of the PDC activity [3], [4], [17]. However, Pdk2 has also been shown to be more sensitive than other Pdks in response to allosteric regulators like pyruvate, NADH, and acetyl-CoA [24], [25]. Under non-diabetic conditions, Pdk2 deficiency causes a modest decrease in fed glucose levels whereas Pdk4 deficiency results in lower fasting glucose levels in mice as compared to the wild-type controls [17]. The differential effects can also be attributable to their respective modulation of the PDC activity since Pdk2 deficiency leads to increased PDC activity only in a fed state and Pdk4 deficiency affects the PDC activity in both fed and fasting states [17]. It seems that Pdk2 mainly regulates glucose utilization whereas Pdk4 may be involved in both hepatic gluconeogenesis and systemic glucose metabolism. In diabetic IrsLDKO mice that have severe hepatic insulin resistance, although glucose disposal may be decreased, the unsuppressed hepatic glucose production may be the major cause of hyperglycemia [26]. Under this condition, inactivation of the Pdk4 gene produces a better effect than that by the Pdk2 gene deletion largely because of a stronger role of Pdk4 in hepatic gluconeogenesis. This interpretation is supported by our data of the better glucose tolerance in the IrsLDKO:Pdk4 mice and the better insulin-stimulated glucose metabolism in the IrsLDKO:Pdk2 mice.
Although IrsLDKO mice are only deficient in hepatic Irs1 and Irs2, they manifest systemic insulin resistance as well [20], [21], which is indicated by decreased phosphorylation of Akt and Erk in the skeletal muscle (Figure 5B). In addition to the liver, the role of Pdks in other tissues including skeletal muscle and fat may be also critical for glucose homeostasis. This interpretation is consistent with our liver-specific knockdown of the Pdk4 gene since the Pdk4 knockdown only results in moderate changes in glucose tolerance in the IrsLDKO mice. But we should caution not to over-interpret the data due to the less ideal knockdown efficiency for the Pdk4 gene.
The importance of Pdk4 in metabolism is evidenced by its dynamic gene expression in response to numerous factors, including insulin, glucocorticoid, fatty acids, bile acids, thyroid hormone, angiotensin II, retinoic acids, prolactin, growth hormone, adiponectin, epinephrine, thiazolidinediones, fibrates, statins, metformin, and others [7], [8], [13], [14], [22], [27]–[40]. Moreover, Pdk4 gene expression is often induced in the liver and skeletal muscle under insulin resistance and diabetes conditions [6]–[15]. From this and other gene knockout studies [17], [18], [41], [42], it seems likely that a selective inhibition of the Pdk4 activity may be useful to normalize glucose metabolism and improve insulin resistance.
In summary, hepatic Pdk4 gene expression is highly induced in diabetes. Inactivation of the Pdk4 gene can improve hyperglycemia, glucose tolerance, and insulin resistance in diabetic mice. Overall, our data suggest that Pdk4 may be a useful therapeutic target for type 2 diabetes.
Acknowledgments
We want to thank Dr. Dan Wei for his technical assistance in this work.
Funding Statement
This work was supported by the grants R00DK077505 (to X. C. Dong) from the National Institute of Diabetes And Digestive And Kidney Diseases. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Harris RA, Bowker-Kinley MM, Huang B, Wu P (2002) Regulation of the activity of the pyruvate dehydrogenase complex. Adv Enzyme Regul 42: 249–259. [DOI] [PubMed] [Google Scholar]
- 2. Sugden MC, Holness MJ (2003) Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab 284: E855–862. [DOI] [PubMed] [Google Scholar]
- 3. Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM (1998) Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J 329 (Pt 1) 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gudi R, Bowker-Kinley MM, Kedishvili NY, Zhao Y, Popov KM (1995) Diversity of the pyruvate dehydrogenase kinase gene family in humans. J Biol Chem 270: 28989–28994. [DOI] [PubMed] [Google Scholar]
- 5. Rowles J, Scherer SW, Xi T, Majer M, Nickle DC, et al. (1996) Cloning and characterization of PDK4 on 7q21.3 encoding a fourth pyruvate dehydrogenase kinase isoenzyme in human. J Biol Chem 271: 22376–22382. [DOI] [PubMed] [Google Scholar]
- 6. Wu P, Blair PV, Sato J, Jaskiewicz J, Popov KM, et al. (2000) Starvation increases the amount of pyruvate dehydrogenase kinase in several mammalian tissues. Arch Biochem Biophys 381: 1–7. [DOI] [PubMed] [Google Scholar]
- 7. McAinch AJ, Cameron-Smith D (2009) Adiponectin decreases pyruvate dehydrogenase kinase 4 gene expression in obese- and diabetic-derived myotubes. Diabetes Obes Metab 11: 721–728. [DOI] [PubMed] [Google Scholar]
- 8. Cadoudal T, Distel E, Durant S, Fouque F, Blouin JM, et al. (2008) Pyruvate dehydrogenase kinase 4: regulation by thiazolidinediones and implication in glyceroneogenesis in adipose tissue. Diabetes 57: 2272–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roche TE, Hiromasa Y (2007) Pyruvate dehydrogenase kinase regulatory mechanisms and inhibition in treating diabetes, heart ischemia, and cancer. Cell Mol Life Sci 64: 830–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kwon HS, Huang B, Unterman TG, Harris RA (2004) Protein kinase B-alpha inhibits human pyruvate dehydrogenase kinase-4 gene induction by dexamethasone through inactivation of FOXO transcription factors. Diabetes 53: 899–910. [DOI] [PubMed] [Google Scholar]
- 11. Kwon HS, Harris RA (2004) Mechanisms responsible for regulation of pyruvate dehydrogenase kinase 4 gene expression. Adv Enzyme Regul 44: 109–121. [DOI] [PubMed] [Google Scholar]
- 12. Sugden MC, Holness MJ (2002) Therapeutic potential of the mammalian pyruvate dehydrogenase kinases in the prevention of hyperglycaemia. Curr Drug Targets Immune Endocr Metabol Disord 2: 151–165. [PubMed] [Google Scholar]
- 13. Huang B, Wu P, Bowker-Kinley MM, Harris RA (2002) Regulation of pyruvate dehydrogenase kinase expression by peroxisome proliferator-activated receptor-alpha ligands, glucocorticoids, and insulin. Diabetes 51: 276–283. [DOI] [PubMed] [Google Scholar]
- 14. Wu P, Peters JM, Harris RA (2001) Adaptive increase in pyruvate dehydrogenase kinase 4 during starvation is mediated by peroxisome proliferator-activated receptor alpha. Biochem Biophys Res Commun 287: 391–396. [DOI] [PubMed] [Google Scholar]
- 15. Wu P, Sato J, Zhao Y, Jaskiewicz J, Popov KM, et al. (1998) Starvation and diabetes increase the amount of pyruvate dehydrogenase kinase isoenzyme 4 in rat heart. Biochem J 329 (Pt 1) 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeoung NH, Wu P, Joshi MA, Jaskiewicz J, Bock CB, et al. (2006) Role of pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) in glucose homoeostasis during starvation. Biochem J 397: 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jeoung NH, Rahimi Y, Wu P, Lee WN, Harris RA (2012) Fasting induces ketoacidosis and hypothermia in PDHK2/PDHK4-double-knockout mice. Biochem J 443: 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jeoung NH, Harris RA (2008) Pyruvate dehydrogenase kinase-4 deficiency lowers blood glucose and improves glucose tolerance in diet-induced obese mice. Am J Physiol Endocrinol Metab 295: E46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. White MF (2003) Insulin signaling in health and disease. Science 302: 1710–1711. [DOI] [PubMed] [Google Scholar]
- 20. Dong XC, Copps KD, Guo S, Li Y, Kollipara R, et al. (2008) Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab 8: 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kubota N, Kubota T, Itoh S, Kumagai H, Kozono H, et al. (2008) Dynamic functional relay between insulin receptor substrate 1 and 2 in hepatic insulin signaling during fasting and feeding. Cell Metab 8: 49–64. [DOI] [PubMed] [Google Scholar]
- 22. Wei D, Tao R, Zhang Y, White MF, Dong XC (2011) Feedback regulation of hepatic gluconeogenesis through modulation of SHP/Nr0b2 gene expression by Sirt1 and FoxO1. Am J Physiol Endocrinol Metab 300: E312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xiong X, Tao R, DePinho RA, Dong XC (2012) The autophagy-related gene 14 (Atg14) is regulated by forkhead box O transcription factors and circadian rhythms and plays a critical role in hepatic autophagy and lipid metabolism. J Biol Chem 287: 39107–39114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bao H, Kasten SA, Yan X, Roche TE (2004) Pyruvate dehydrogenase kinase isoform 2 activity limited and further inhibited by slowing down the rate of dissociation of ADP. Biochemistry 43: 13432–13441. [DOI] [PubMed] [Google Scholar]
- 25. Klyuyeva A, Tuganova A, Popov KM (2008) Allosteric coupling in pyruvate dehydrogenase kinase 2. Biochemistry 47: 8358–8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo S, Copps KD, Dong X, Park S, Cheng Z, et al. (2009) The Irs1 branch of the insulin signaling cascade plays a dominant role in hepatic nutrient homeostasis. Mol Cell Biol 29: 5070–5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Attia RR, Connnaughton S, Boone LR, Wang F, Elam MB, et al. (2010) Regulation of pyruvate dehydrogenase kinase 4 (PDK4) by thyroid hormone: role of the peroxisome proliferator-activated receptor gamma coactivator (PGC-1 alpha). J Biol Chem 285: 2375–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Attia RR, Sharma P, Janssen RC, Friedman JE, Deng X, et al. (2011) Regulation of pyruvate dehydrogenase kinase 4 (PDK4) by CCAAT/enhancer-binding protein beta (C/EBPbeta). J Biol Chem 286: 23799–23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Connaughton S, Chowdhury F, Attia RR, Song S, Zhang Y, et al. (2010) Regulation of pyruvate dehydrogenase kinase isoform 4 (PDK4) gene expression by glucocorticoids and insulin. Mol Cell Endocrinol 315: 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Houten SM, Chegary M, Te Brinke H, Wijnen WJ, Glatz JF, et al. (2009) Pyruvate dehydrogenase kinase 4 expression is synergistically induced by AMP-activated protein kinase and fatty acids. Cell Mol Life Sci 66: 1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim YD, Kim YH, Tadi S, Yu JH, Yim YH, et al. (2012) Metformin inhibits growth hormone-mediated hepatic PDK4 gene expression through induction of orphan nuclear receptor small heterodimer partner. Diabetes 61: 2484–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kwon HS, Huang B, Ho Jeoung N, Wu P, Steussy CN, et al. (2006) Retinoic acids and trichostatin A (TSA), a histone deacetylase inhibitor, induce human pyruvate dehydrogenase kinase 4 (PDK4) gene expression. Biochim Biophys Acta 1759: 141–151. [DOI] [PubMed] [Google Scholar]
- 33. Lee JH, Kim EJ, Kim DK, Lee JM, Park SB, et al. (2012) Hypoxia induces PDK4 gene expression through induction of the orphan nuclear receptor ERRgamma. PLoS One 7: e46324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mori J, Alrob OA, Wagg CS, Harris RA, Lopaschuk GD, et al. (2013) ANG II causes insulin resistance and induces cardiac metabolic switch and inefficiency: a critical role of PDK4. Am J Physiol Heart Circ Physiol 304: H1103–1113. [DOI] [PubMed] [Google Scholar]
- 35. Motojima K, Seto K (2003) Fibrates and statins rapidly and synergistically induce pyruvate dehydrogenase kinase 4 mRNA in the liver and muscles of mice. Biol Pharm Bull 26: 954–958. [DOI] [PubMed] [Google Scholar]
- 36. Shin DJ, Joshi P, Hong SH, Mosure K, Shin DG, et al. (2012) Genome-wide analysis of FoxO1 binding in hepatic chromatin: potential involvement of FoxO1 in linking retinoid signaling to hepatic gluconeogenesis. Nucleic Acids Res 40: 11499–11509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stace PB, Fatania HR, Jackson A, Kerbey AL, Randle PJ (1992) Cyclic AMP and free fatty acids in the longer-term regulation of pyruvate dehydrogenase kinase in rat soleus muscle. Biochim Biophys Acta 1135: 201–206. [DOI] [PubMed] [Google Scholar]
- 38. Sugden MC, Bulmer K, Gibbons GF, Holness MJ (2001) Role of peroxisome proliferator-activated receptor-alpha in the mechanism underlying changes in renal pyruvate dehydrogenase kinase isoform 4 protein expression in starvation and after refeeding. Arch Biochem Biophys 395: 246–252. [DOI] [PubMed] [Google Scholar]
- 39. Thakran S, Sharma P, Attia RR, Hori RT, Deng X, et al. (2013) Role of sirtuin 1 in the regulation of hepatic gene expression by thyroid hormone. J Biol Chem 288: 807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. White UA, Coulter AA, Miles TK, Stephens JM (2007) The STAT5A-mediated induction of pyruvate dehydrogenase kinase 4 expression by prolactin or growth hormone in adipocytes. Diabetes 56: 1623–1629. [DOI] [PubMed] [Google Scholar]
- 41. Hwang B, Jeoung NH, Harris RA (2009) Pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) deficiency attenuates the long-term negative effects of a high-saturated fat diet. Biochem J 423: 243–252. [DOI] [PubMed] [Google Scholar]
- 42. Hwang B, Wu P, Harris RA (2012) Additive effects of clofibric acid and pyruvate dehydrogenase kinase isoenzyme 4 (PDK4) deficiency on hepatic steatosis in mice fed a high saturated fat diet. FEBS J 279: 1883–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]