Abstract
Context:
Glucocorticoids and inflammation inhibit bone formation; however, the impact on skeletal modeling is unknown.
Objectives:
The objectives of the study were to examine changes in bone mineral density (BMD) and cortical structure after Crohn disease (CD) diagnosis and identify associations with growth, glucocorticoids, and disease activity.
Design/Participants:
This was a prospective cohort study among 76 CD participants, aged 5–21 years. Tibia quantitative computed tomography trabecular BMD and cortical dimensions were obtained at diagnosis and 6 and 12 and a median of 42 months later; 51 completed the final visit.
Outcomes:
Sex, race, and age-specific Z-scores were generated for outcomes based on more than 650 reference participants, and cortical dimension Z-scores were further adjusted for tibia length. Generalized estimating equations were used to model changes in Z-scores.
Results:
Disease activity improved over the study interval (P < .001). Trabecular BMD Z-scores improved over the first 6 months; increases were associated with improved disease activity (P < .001), younger age (P = .005), and increases in vitamin D levels (P = .02). Greater increases in tibia length were associated with greater increases in cortical area Z-scores (P < .001). Greater glucocorticoid doses and disease activity were significantly associated with failure to accrue cortical area and were more pronounced with greater linear growth (interaction P < .05). Mean (±SD) trabecular BMD (−1.0 ± 1.21) and cortical area (−0.57 ± 1.10) Z-scores at the final visit were significantly reduced.
Conclusions:
CD was associated with persistent deficits in trabecular BMD, although younger participants demonstrated a greater potential for recovery. In addition, greater linear growth was associated with a greater recovery of cortical dimensions, especially among participants with less glucocorticoid exposure and inflammation. These data suggest that younger age and concurrent growth provide a window of opportunity for skeletal recovery.
During growth, skeletal modeling is characterized by sex-, race-, and maturation-specific increases in trabecular and cortical volumetric bone mineral density (BMD) and bone dimensions (1, 2). As long bones increase in length, bone formation by osteoblasts on the periosteum results in increases in bone width and strength. Simultaneously, bone resorption by endocortical osteoclasts expands the medullary cavity. Sex and race differences in bone modeling during development contribute to life-long differences in bone strength (3).
Glucocorticoids are highly effective and widely prescribed for the treatment of a myriad childhood diseases. It is well established that glucocorticoids result in sustained reductions in bone formation due to decreased osteoblast differentiation, function, and life span (4, 5) and increases in bone resorption by osteoclasts (4). Proinflammatory cytokines, such as TNF-α and IL-6 have similar adverse effects on bone formation (6, 7) and resorption (8, 9). The growing skeleton may be particularly vulnerable to the detrimental effects of chronic inflammation and glucocorticoids on bone metabolism, resulting in life-long insufficiency in bone structure.
Crohn disease (CD) is a chronic inflammatory condition of the gastrointestinal tract associated with defective innate immune regulation. We previously established an incident pediatric CD cohort to document the effects on peripheral quantitative computed tomography (pQCT) measures of volumetric BMD and cortical structure prior to initiation of glucocorticoid therapy and to examine changes in bone outcomes over the subsequent year (10). We reported substantial deficits in trabecular BMD and cortical dimensions at diagnosis. Although trabecular BMD improved over the first 6 months, no further improvements were observed over the subsequent 6 months, and cortical dimensions failed to improve commensurate with linear growth despite significant improvements in muscle area. We were unable to demonstrate associations of glucocorticoid exposure or disease activity with changes in these outcomes.
The relatively short duration of that study limited our ability to examine the independent effects of glucocorticoid exposure, disease activity, and linear growth on bone modeling. Therefore, we extended follow-up to include an additional visit a median of 42 months after diagnosis. The objectives of this extension study were to identify determinants of long-term changes in trabecular and cortical outcomes, including glucocorticoid exposure, linear growth, disease activity, muscle area, and vitamin D levels. We postulated that the glucocorticoid exposure and greater disease activity would impair skeletal modeling during growth.
Materials and Methods
Study participants
Incident CD patients, aged 5–21 years, were eligible for enrollment and were recruited from the Children's Hospital of Philadelphia and gastroenterologists in the community. CD patients with other chronic illnesses or medications potentially affecting growth and nutrition were excluded. Baseline visits were completed within 2 weeks of diagnosis and follow-up visits 6 and 12 months later. A long-term follow-up (LTFU) visit was completed a median of 42 months (range 23–54 months) after diagnosis. This study was limited to the 76 participants with at least 2 visits; 51 completed the LTFU visit. Changes in body composition (11) and vitamin D metabolism (12) through the LTFU visit have been reported. The CD participants were compared with a concurrent reference population of more than 650 healthy children and adolescents recruited from general pediatric clinics in the greater Philadelphia area (1, 10). Study approval was obtained from the institutional review board. Informed consent was obtained from participants 18 years of age or older and assent as well as parental consent in those younger than 18 years.
Anthropometry, pubertal development, and race
Height was measured using a stadiometer (Holtain, Crymych, United Kingdom) and weight with a digital scale (Scaletronix, White Plains, New York). Tanner stage was assessed by self-assessment questionnaire (13, 14). Race was identified by the parent or participant according to National Institutes of Health categories.
CD characteristics
CD diagnosis was confirmed by endoscopic, histological, and clinical parameters. CD activity was assessed using the Pediatric CD Activity Index (PCDAI) based on symptoms (30%), physical examination (30%), laboratory parameters (20%), and growth (20%), with scores ranging from 0 to 100 (15). Disease activity was categorized as none (1–10), mild (11–30), and moderate to severe (>30). Disease characteristics, medications, and nutritional supplements were recorded. Participants were provided with glucocorticoid diaries that were reviewed at each visit. Intravenous methylprednisolone doses were converted to prednisone equivalents, and the mean glucocorticoid dose in each participant (milligrams per kilogram per day prednisone equivalents) was tabulated over each interval between visits.
Peripheral quantitative computed tomography
Scans were obtained in the left tibia using a Stratec XCT2000 device (Orthometrix, White Plains, New York) with a 12 detector unit, voxel size 0.4 mm, slice thickness 2.3 mm, and scan speed 25 mm/sec. Bone measurements were obtained 3% proximal to the distal physis for trabecular volumetric BMD (milligrams per cubic centimeter) and at the 38% diaphysis for cortical volumetric BMD, (milligrams per cubic centimeter), periosteal and endosteal circumference (millimeters), and cortical cross sectional area (square millimeters). In vivo studies demonstrated that the pQCT trabecular BMD and diaphyseal cortical area were highly correlated with fracture load (R = 0.75 and R = 0.93, respectively) (16). Muscle area (cubic millimeters) was assessed at the 66% site. The manufacturer's hydroxyapatite phantom was scanned daily. In our laboratory, the coefficient of variation ranged from 0.5% to 1.6% for pQCT outcomes.
Laboratory measurements
Measures at each visit included serum erythrocyte sedimentation rate (ESR) (millimeters per hour) and albumin (grams per deciliter) levels using standard methods in the clinical laboratory. Serum 25-hydroxy vitamin D [25(OH)D] levels were measured by I25RIA, with a coefficient of variation of 2.2% (17).
Statistical analysis
Analyses were performed using Stata 11.0 (Stata Corp, College Station, Texas). P < 0.05 was considered significant and 2-sided tests used throughout. Continuous variables were expressed as means ± SD or median (range). Group differences between CD and reference participants were assessed using a Student's t test or a Wilcoxon rank-sum test. Changes within CD patients were tested using the paired t test or the Wilcoxon signed-rank test. Differences in proportions were tested using the χ2 test. Correlations between continuous variables were assessed by Pearson or Spearman correlations, as appropriate.
Age- and sex-specific height and BMI Z-scores were generated using national data (18). pQCT outcomes were converted to race- and sex-specific Z-scores using the method of smooth L curve, mean, and coefficient of variation (Chartmaker Program, version 2.3, Tyne and Wear, United Kingdom) based on the reference participants (19). This method accounts for the nonlinearity, heteroscedasticity, and skew of bone data in growing children. The BMD outcomes were assessed relative to age. The cortical geometry outcomes were highly correlated with tibia length (all P < .0001); therefore, Z-scores for these parameters were generated relative to age and further adjusted for tibia length for age Z-score (20, 21). Changes in pQCT Z-scores were assessed with generalized estimating equations (GEE) (22). The GEE models examined changes in pQCT Z-scores between study visits. The following covariates were tested in all models: Z-score at the start of each interval, age, sex, race, and visit number. Models for changes in bone outcomes evaluated associations with glucocorticoids (milligrams per kilogram per day) and increases in tibia length (millimeters) within each interval, PCDAI score, and 25(OH)D level at the beginning of each interval and changes in PCDAI and 25(OH)D over each interval. Models for changes in cortical BMD Z-score included changes in cortical area Z-scores and interval tibia growth to examine the effects of new bone formation. Models for changes in cortical dimensions Z-scores tested the association with baseline and change in muscle area Z-score over each interval.
Increases in tibia length were highly correlated with increases in absolute cortical dimensions (eg, R = 0.82 for periosteal circumference, P < .0001). Therefore, the models for changes in cortical dimensions tested for an interaction between tibia growth and glucocorticoid exposure as well as tibia growth and PCDAI score. These models determined whether growth modified the associations of glucocorticoids or disease activity with changes in cortical dimension Z-scores.
Results
Participant characteristics
Seventy-eight CD participants enrolled at diagnosis and 76 completed at least 1 subsequent visit (Table 1). Fifty-one completed the LTFU visit. Baseline age, sex, maturation, height Z-score, and PCDAI scores did not differ between those who did vs did not complete the LTFU visit.
Table 1.
Participant and Disease Characteristics
| Visit |
||||
|---|---|---|---|---|
| Baseline | 6 Months | 12 Months | LTFU | |
| n | 76 | 71 | 66 | 51 |
| Age, y, mean ± SD (range) | 12.6 ± 2.8 (5.5–18.0) | 13.1 ± 2.7 (6.0–18.6) | 13.6 ± 2.6 (6.6–19.0) | 15.9 ± 2.9 (9.4–21.2) |
| Male, n, % | 42 (55) | 39 (55) | 36 (55) | 29 (57) |
| Black race, n, % | 7 (9) | 5 (7) | 4 (6) | 4 (8) |
| Tanner stage 1 or 2, n, % | 42 (55) | 32 (45) | 20 (30) | 8 (16) |
| PCDAI, n, % | ||||
| No active disease (<10) | 3 (4) | 30 (48) | 38 (68) | 41 (85) |
| Mild (11–30) | 26 (36) | 28 (44) | 17 (30) | 7 (15) |
| Moderate to severe (>30) | 43 (60) | 5 (8) | 1 (2) | 0 (0) |
| Albumin, g/dL, median (range) | 3.4 (1.8–4.7) | 3.8 (2.4–4.5) | 4.0 (3.2–4.9) | 4.2 (3.0–5.3) |
| ESR, mm/h, median (range) | 25 (1–128) | 15 (1–46) | 16 (0–60) | 8 (0–51) |
| 25(OH)D, ng/mL, median (range) | ||||
| Nonblack race | 22.7 (7.6–45.9) | 32.7 (9.3–74.9) | 28.3 (7.1–47.0) | 38.1 (9.9–72.2) |
| Black race | 13.0 (4.5–19.1) | 31.2 (20.0–43.5) | 31.2 (20.0–43.5) | 31.2 (20.0–43.5) |
| Supplements and medications, n (%) | ||||
| Vitamin D ≥ 200 IU/d | 38 (51) | 46 (68) | 38 (58) | 24 (48) |
| Interval glucocorticoidtherapy | 44 (61) | 21 (31) | 16 (31) | |
| Glucocorticoid (mg/kg·d) amongthose treated during the interval | 0.19 (0.02–0.86) | 0.15 (0.05–0.36) | 0.04 (0.003–0.19) | |
| Methotrexate | 4 (6) | 6 (9) | 11 (21) | |
| 6-Mercaptopurine | 28 (39) | 33 (49) | 31 (60) | |
| Azathioprine | 4 (6) | 5 (7) | 3 (6) | |
| Infliximab | 5 (7) | 9 (13) | 13 (25) | |
| Enteral feeds | 4 (6) | 3 (4) | 8 (15) | |
Results are presented as means ± SD and range.
Clinical course
Disease activity, medications, and laboratory results are summarized in Table 1. At enrollment, 60% of participants had moderate to severe disease activity. PCDAI, ESR, and serum albumin levels improved (P < .001) and glucocorticoid doses declined (P < 001). At the final visit, 85% and 15% had no active disease or mild disease, respectively. Vitamin D levels improved, as described (12).
pQCT outcomes
Table 2 summarizes pQCT and anthropometry Z-scores in CD participants at each visit, limited to those with a LTFU visit to facilitate comparisons across visits. The asterisk indicates differences (P < .05) compared with reference participants.
Table 2.
pQCT and Anthropometry Z-Scores
| Visit |
12 Months to LTFU P Value | Baseline to LTFU P Value | ||||
|---|---|---|---|---|---|---|
| Baseline | 6 Months | 12 Months | LTFU | |||
| pQCT | ||||||
| Trabecular BMD | −1.47 ± 1.19a | −1.18 ± 1.16a | −1.04 ± 1.30a | −1.01 ± 1.21a | .88 | .0001 |
| (−4.62 to 1.79) | (−4.13 to 1.31) | (−3.85 to 2.25) | (−3.28 to 2.40) | |||
| Cortical BMD | −0.25 ± 1.06 | 0.04 ± 1.16 | −0.21 ± 1.12 | −1.06 ± 1.06a | .0001 | <.0001 |
| (−3.40 to 2.45) | (−2.45 to 2.28) | (−2.76 to 1.71) | (−3.35 to 1.95) | |||
| Cortical area | −0.83 ± 1.18a | −1.06 ± 1.17a | −0.93 ± 1.07a | −0.57 ± 1.10a | .002 | .03 |
| (−5.17 to 1.43) | (−5.17 to 0.82) | (−4.02 to 0.76) | (−3.01 to 1.56) | |||
| Periosteal circumference | −0.22 ± 0.92 | −0.37 ± 0.90a | −0.34 ± 0.85a | −0.22 ± 0.83 | .06 | .98 |
| (−2.82 to 1.51) | (−2.89 to 1.41) | (−2.41 to 1.36)a | (−1.74 to 1.60) | |||
| Endosteal circumference | 0.60 ± 0.91a | 0.59 ± 0.88a | 0.52 ± 0.91a | 0.38 ± 0.92a | .03 | .004 |
| (−0.87 to 3.36) | (−0.74 to 3.31) | (−1.33 to 3.18) | (−1.50 to 2.74) | |||
| Muscle area | −1.04 ± 1.12a | −0.76 ± 1.03a | −0.75 ± 0.99a | −0.47 ± 0.97a | .005 | <.0001 |
| (−3.68 to 0.86) | (−2.98 to 2.14) | (−2.92 to 1.05) | (−3.50 to 1.33) | |||
| Anthropometry | ||||||
| Height | −0.22 ± 1.06a | −0.36 ± 1.00a | −0.28 ± 0.95a | −0.07 ± 1.00a | .04 | .0995 |
| (−3.17 to 1.81) | (−3.01 to 1.39) | (−2.65 to 1.38) | (−2.11 to 2.21) | |||
| BMI | −0.54 ± 1.25a | −0.13 ± 1.00a | −0.01 ± 0.91a | 0.14 ± 1.04 | .07 | <.0001 |
| (−2.75 to 2.18) | (−2.26 to 2.22) | (−2.31 to 1.95) | (−2.95 to 2.15) | |||
Results are presented as means ± SD and range.
P < .05 compared with reference participants. Data in the table are limited to those CD participants with results at baseline and LTFU, including 48 for trabecular BMD, 51 for cortical BMD, and 50 for cortical dimension.
Trabecular BMD
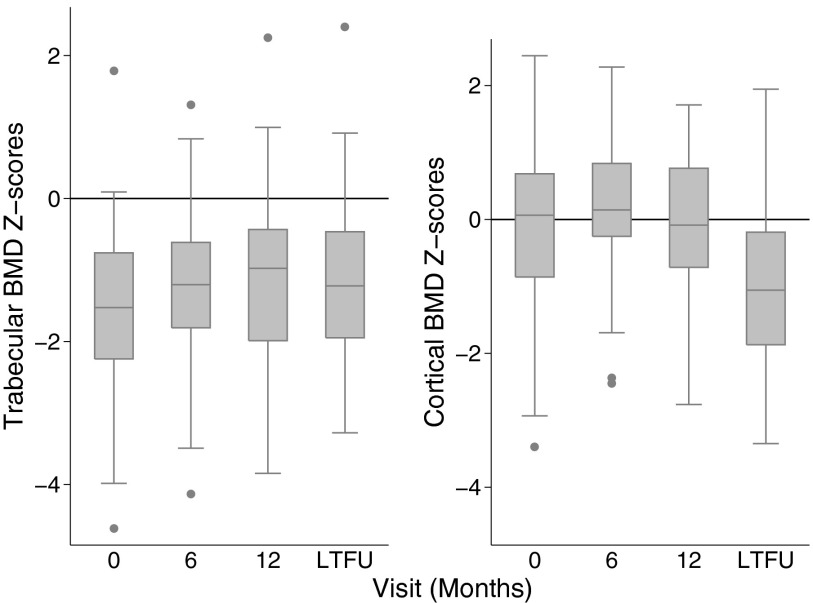
As previously described, trabecular BMD Z-scores were significantly lower in CD participants at diagnosis compared with the reference participants and increased significantly over the first 6 months (P = .001) (10). There was no evidence that age at diagnosis was associated with subsequent glucocorticoid exposure, (r = −0.07, P = .53). Z-scores did not improve further through LTFU (Figure 1) and remained on average, significantly lower compared with reference participants.
Figure 1.
Trabecular and cortical volumetric BMD Z-scores among CD participants at each visit, limited to the participants with Z-scores at all 4 study visits.
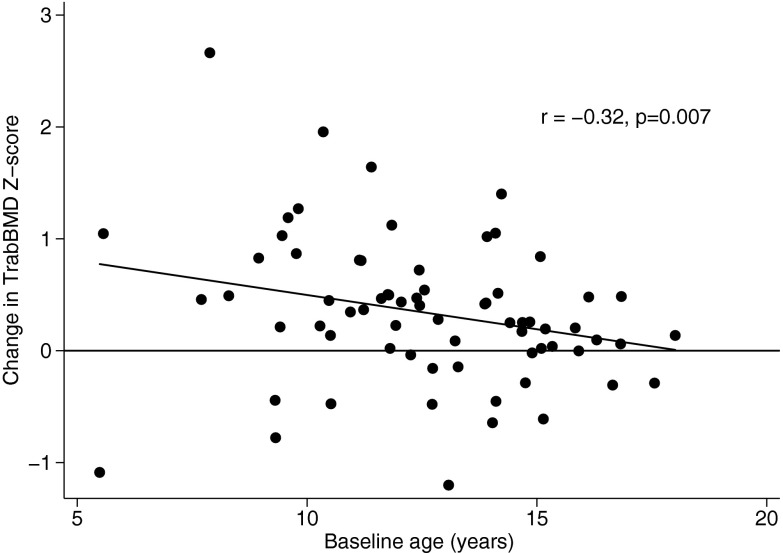
The multivariable GEE model over the entire study demonstrated that increases in trabecular BMD Z-scores were significantly greater during the first 6 months, compared with subsequent intervals (P = .03). Younger age was associated with greater increases in BMD Z-score (P = .005), and this was most pronounced during the first 6 months (test for interaction, P = .036) (Figure 2). Lower PCDAI at the start of each interval [β = −.01, 95% confidence interval (CI) −0.02, −0.002; P = .02] and greater declines in PCDAI over each interval (β = −.02, 95% CI −0.03, −0.01; P < .001) were independently associated with greater improvements in trabecular BMD Z-score. Increases in 25(OH)D levels were independently associated with increases in trabecular BMD Z-scores (β = .10 per 10 ng/mL greater 25(OH)D level, 95% CI 0.02, 0.20; P = .02). Glucocorticoid dose, sex, and tibia growth were not related to changes in trabecular BMD Z-scores.
Figure 2.
Association between change in trabecular BMD Z-score over the first 6-month interval and baseline age in CD participants.
Cortical BMD
At diagnosis, cortical BMD Z-scores were not significantly different compared with reference participants (10). Cortical BMD Z-scores increased significantly over the first 6 months (P = .003) and subsequently declined. Cortical BMD Z-scores were markedly lower compared with reference participants at the final visit (Table 2 and Figure 1).
In the multivariable GEE model, greater increases in tibia length [β = −.20/cm (95% CI −0.21, −0.14); P < .001] and greater increases in cortical area Z-scores [β = −.48 (95% CI −0.65, −0.31); P < .001] were independently associated with greater declines in cortical BMD Z-scores. Greater glucocorticoid exposure over the study interval was independently associated with greater increases in cortical BMD Z-scores [β = .72 per mg/kg·d (95% CI 0.03, 1.41); P = .04]. PCDAI scores, age, sex, and 25(OH)D levels were not associated with changes in cortical BMD Z-score.
Cortical dimensions
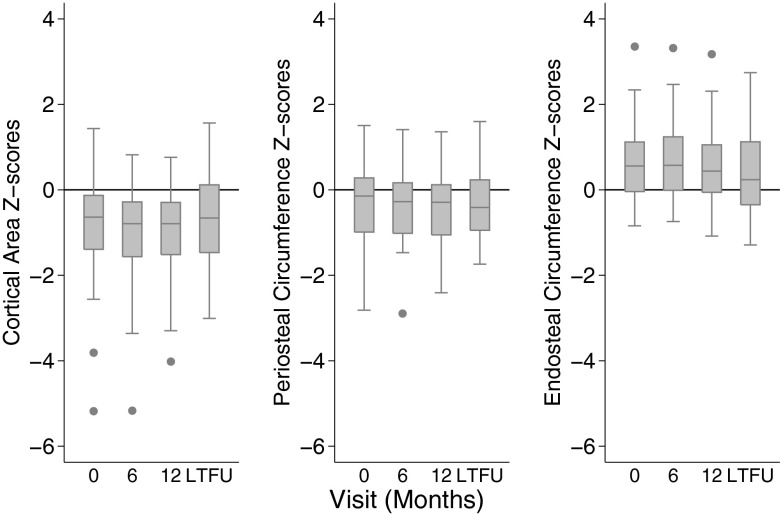
At diagnosis, cortical area Z-scores were significantly lower and endosteal circumference Z-scores significantly greater in CD participants compared with reference participants. Over the first 6 months, both cortical area and periosteal circumference Z-scores decreased significantly (P = .001 and P = .0001, respectively), whereas endosteal circumference Z-scores remained unchanged. However, from 12 months to the LTFU, cortical area Z-scores improved, predominantly due to slowed endosteal expansion in addition to marginal increases in periosteal circumference Z-scores (Figure 3). At the LTFU visit, cortical area and endosteal deficits persisted.
Figure 3.
Cortical dimension Z-scores including; cortical area, periosteal circumference, and endosteal circumference Z-scores among CD participants at each visit, limited to the participants with Z-scores at all 4 study visits.
In the multivariable GEE model of changes in cortical dimension Z-scores, greater increases in tibia length were associated with greater improvements in cortical area (increase) and endosteal circumference (decrease) Z-scores (both, P < .001). Greater glucocorticoid exposure (P = .008) and disease activity (P = .001) were associated with declines in cortical area Z-score. Furthermore, there was a negative interaction between growth and glucocorticoid exposure (P = .047) and between growth and PCDAI (P = .035). That is, greater glucocorticoid exposure and greater disease activity were associated with lesser improvements in cortical area Z-scores, and these associations were significantly more pronounced with greater increases in tibia length. Similar patterns were observed for periosteal circumference.
Lower PCDAI (P = .03) at the start of each interval and less glucocorticoid exposure over the study interval (P = .04) were associated with improvement (decreases) in endosteal circumference Z-scores. These effects were not significant when both covariates were included in the model. There was no evidence of an interaction with growth.
Female sex was associated with smaller increases in periosteal (P = .01) and cortical area Z-scores (P < .01). Participant age and 25(OH)D levels were not associated with changes in cortical dimension Z-scores.
Assessment of the functional muscle bone unit
CD participants had significant muscle deficits at diagnosis with modest improvement over the study interval and persistent deficits at LTFU, consistent with our prior report using whole-body dual-energy x-ray absorptiometry (DXA) lean body mass (11). The GEE models demonstrated that higher muscle area Z-scores at the start of each interval were positively and significantly associated with greater increases in cortical area [β = .12 (95% CI 0.05,0.20) P = .001] and periosteal circumference [β = .06 (95% CI 0.01, 0.10) P = .014] Z-scores and greater decreases in endosteal circumference [β = −.04 (95% CI −0.07, −0.01) P = .009] Z-scores. The associations between muscle and the changes in cortical dimensions did not vary with growth (nonsignificant interaction).
Discussion
This study demonstrates that CD was associated with significant and persistent deficits in trabecular volumetric BMD and with impaired modeling on the periosteal and endosteal surface of cortical bone (with consequent deficits in cortical area) despite marked improvements in disease activity. The observation that younger children had greater increases in trabecular BMD in the first 6 months after diagnosis suggests a greater potential for recovery in younger children. Age at diagnosis was not associated with subsequent glucocorticoid exposure; therefore, greater improvements in the younger participants were not explained by differences in glucocorticoid dose. The lack of an association between glucocorticoid dose and changes in trabecular BMD Z-scores may have been due to the effects of glucocorticoids to improve disease activity, which was associated with improvements in trabecular BMD Z-score. With regard to cortical area Z-scores, the positive association between changes in tibia length and cortical area Z-scores was attenuated by greater concurrent glucocorticoid exposure. That is, less glucocorticoid exposure was associated with greater recovery of the cortical area, especially in the setting of linear growth. This is consistent with a study demonstrating catch-up of cortical bone area after the cessation of dexamethasone in growing rabbits (23).
This study advances previous studies in multiple ways. First, pQCT provides 3-dimensional measures of trabecular and cortical volumetric BMD as well as measures of cortical dimensions that are highly correlated with failure load (16). In contrast, DXA is a 2-dimensional technique that cannot distinguish between superimposed cortical and trabecular bone or evaluate cortical structure (24). Second, to our knowledge, this is the first quantitative computed tomography study to assess long-term (>1 year) changes in BMD and cortical structure in any childhood chronic disease, with one exception. Werkstetter et al (25) examined a mixed incident/prevalent cohort of children with inflammatory bowel disease (CD and ulcerative colitis) over a median of 2.6 years. Analyses were limited to the first and last visit, and Z-scores for cortical geometry were calculated relative to height without adjustment for age. The study did not demonstrate glucocorticoid effects or examine the impact of growth. The authors reported low trabecular BMD and preserved cortical area. As noted by the authors, the lack of cortical deficits may have been due to correction for height alone and the potential comparison of pubertal inflammatory bowel disease participants with healthy prepubertal controls of the same height. We previously demonstrated the bias introduced by this approach in DXA studies and advocated for adjustment of DXA Z-scores for height Z-scores (20). Our robust reference population in this pQCT study facilitated an adjustment for age, sex, and race along with an adjustment for tibia length for age Z-score, analogous to our DXA approach.
The baseline trabecular BMD deficits in our incident CD cohort illustrate the impact of the underlying disease (10). The subsequent changes after the initiation of treatment suggest that inflammation and malnutrition are primary determinants of trabecular deficits; trabecular BMD Z-scores improved significantly over the first 6 months despite glucocorticoid therapy in the majority, improvements in PCDAI scores and vitamin D levels were associated with improvement in trabecular BMD Z-scores, and greater glucocorticoid exposure was not associated with changes in trabecular BMD Z-scores independent of disease severity.
This is the third chronic pediatric disease in which we observed that glucocorticoid therapy was associated with greater cortical BMD Z-scores (26, 27). We hypothesize that this is due to glucocorticoid actions to suppress bone formation with less deposition of new matrix and a resultant accumulation of older cortical bone with greater secondary mineralization. In this study, we observed increases in cortical BMD Z-scores over the first 6-month interval when glucocorticoid doses were greatest. In the subsequent intervals, cortical BMD Z-scores decreased significantly. We attributed this to accrual of new, less well-mineralized bone as cortical area expanded. This is consistent with our prior observations during recovery from chemotherapy in childhood leukemia in which catch-up in cortical area was associated with marked reductions in cortical BMD (21). A similar pattern was reported in the setting of GH therapy (28).
The lower cortical bone area was a result of significant expansion of endosteal dimensions coupled with variable reductions in periosteal expansion. In the rabbit study of dexamethasone effects on cortical accrual during growth, the marrow diameter was unaffected (23). The authors concluded that the major effect of glucocorticoid excess was not to stimulate cortical resorption but to inhibit periosteal bone formation. This is consistent with our results because significant periosteal deficits were not observed at diagnosis but increased over the first year in association with glucocorticoids. Of note, the decline in periosteal Z-scores did not represent a reduction in absolute circumference; rather, there was a failure to expand the periosteum commensurate with increases in tibia length. The endocortical deficits were pronounced at diagnosis and likely reflected cytokine effects to increase bone resorption. Muscle deficits may have also contributed to periosteal and endosteal deficits.
Numerous studies have demonstrated that cortical expansion in response to biomechanical loading (eg, playing vs nonplaying arm in tennis players) is maturity and growth dependent (29, 30). Ducher et al (29) reported that height velocity was a significant determinant of exercise-induced benefits in periosteal dimensions (P < .01), concluding that Tanner stages I-III represent the optimal time to enhance bone mass. We hypothesize that the potential for recovery of periosteal dimensions in childhood disease is greatest during growth, with the corollary that the risk for progressive deficits is also greatest during growth. This is consistent with our report that changes in periosteal circumference Z-scores in children with nephrotic syndrome were inversely associated with growth velocity (27).
This study is subject to multiple limitations. First, the lack of bone biopsy data precluded measures of bone turnover and mineralization, trabecular microarchitecture, and cortical porosity. Bone biomarkers have been proposed as surrogates of bone turnover; however, poor growth results in marked reductions in bone biomarkers, confounding their interpretation in childhood disease (31). Second, the study may have been subject to bias as a result of loss to follow-up; however, participant characteristics did not differ between those who did vs did not complete the LTFU visit. Third, this long-term study was initiated in an era when biological therapies were not yet in widespread use; however, the results still speak more broadly to the impact of glucocorticoids and inflammation on the growing skeleton. Fourth, we did not include measures of dietary intake or physical activity; therefore, we cannot assess the contributions of these disease-related risk factors.
In summary, this study suggests that childhood chronic diseases are characterized by a window of vulnerability during growth and development as glucocorticoids and inflammation impair trabecular and cortical modeling. Future studies are needed to leverage the window of opportunity for interventions to promote bone accrual during growth and maturation.
Acknowledgments
We greatly appreciate the dedication and enthusiasm of the children and their families who participated in this study.
This work was supported by National Institutes of Health Grants R01-DK060030, R01-HD040714, K24-DK076808, K23-DK082012, UL1RR024134, and UL1TR000003; University of Ottawa, Faculty of Medicine; North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition; and the Society for Pediatric Research/American Pediatric Society Student Research Program.
Current address for M.T.: Merck, Sharp, & Dohme Corp, North Wales, Pennsylvania. Her research contribution was completed while she was affiliated with the Children's Hospital of Philadelphia.
Disclosure Summary: The authors disclose no conflicts.
Footnotes
- BMD
- bone mineral density
- CD
- Crohn disease
- CI
- confidence interval
- DXA
- dual-energy x-ray absorptiometry
- ESR
- erythrocyte sedimentation rate
- GEE
- generalized estimating equation
- LTFU
- long-term follow-up
- 25(OH)D
- 25-hydroxy vitamin D
- PCDAI
- Pediatric CD Activity Index
- pQCT
- peripheral quantitative computed tomography.
References
- 1. Leonard MB, Elmi A, Mostoufi-Moab S, et al. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95:1681–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261 [DOI] [PubMed] [Google Scholar]
- 3. Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002;359:1841–1850 [DOI] [PubMed] [Google Scholar]
- 4. Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319–1328 [DOI] [PubMed] [Google Scholar]
- 5. Rauch A, Seitz S, Baschant U, et al. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell metabolism. 2010;11:517–531 [DOI] [PubMed] [Google Scholar]
- 6. Gilbert L, He X, Farmer P, et al. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2α A) is inhibited by tumor necrosis factor-α. J Biol Chem. 2002;277:2695–2701 [DOI] [PubMed] [Google Scholar]
- 7. Ahuja SS, Zhao S, Bellido T, Plotkin LI, Jimenez F, Bonewald LF. CD40 ligand blocks apoptosis induced by tumor necrosis factor α, glucocorticoids, and etoposide in osteoblasts and the osteocyte-like cell line murine long bone osteocyte-Y4. Endocrinology. 2003;144:1761–1769 [DOI] [PubMed] [Google Scholar]
- 8. Kudo O, Fujikawa Y, Itonaga I, Sabokbar A, Torisu T, Athanasou NA. Proinflammatory cytokine (TNFα/IL-1α) induction of human osteoclast formation. J Pathol. 2002;198:220–227 [DOI] [PubMed] [Google Scholar]
- 9. Kwan Tat S, Padrines M, Theoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-α/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15:49–60 [DOI] [PubMed] [Google Scholar]
- 10. Dubner SE, Shults J, Baldassano RN, et al. Longitudinal assessment of bone density and structure in an incident cohort of children with Crohn's disease. Gastroenterology. 2009;136:123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thayu M, Denson LA, Shults J, et al. Determinants of changes in linear growth and body composition in incident pediatric Crohn's disease. Gastroenterology. 2010;139:430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prosnitz AR, Leonard MB, Shults J, et al. Changes in vitamin D and parathyroid hormone metabolism in incident pediatric Crohn's disease. Inflamm Bowel Dis. 2013;19:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tanner J, Whitehouse R, Marshall W, Healy M, Goldstein H. Assessment of Skeletal Maturity and Prediction of Adult Height (TW2 Method). London, United Kingdom: Academic Press; 1975 [Google Scholar]
- 14. Morris NM, Udry J. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–280 [DOI] [PubMed] [Google Scholar]
- 15. Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn's disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439–447 [PubMed] [Google Scholar]
- 16. Kontulainen SA, Johnston JD, Liu D, Leung C, Oxland TR, McKay HA. Strength indices from pQCT imaging predict up to 85% of variance in bone failure properties at tibial epiphysis and diaphysis. J Musculoskelet Neuronal Interact. 2008;8:401–409 [PubMed] [Google Scholar]
- 17. Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol. 1997;282:174–186 [DOI] [PubMed] [Google Scholar]
- 18. Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60 [DOI] [PubMed] [Google Scholar]
- 19. Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44:45–60 [PubMed] [Google Scholar]
- 20. Zemel BS, Leonard MB, Kelly A, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95:1265–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mostoufi-Moab S, Brodsky J, Isaacoff EJ, et al. Longitudinal assessment of bone density and structure in childhood survivors of acute lymphoblastic leukemia without cranial radiation. J Clin Endocrinol Metab. 2012;97:3584–3592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fitzmaurice GM, Laird NM. Applied Longitudinal Analysis. 2nd ed Hoboken, NJ: John Wiley, Sons, Inc; 2011 [Google Scholar]
- 23. Gafni RI, McCarthy EF, Hatcher T, et al. Recovery from osteoporosis through skeletal growth: early bone mass acquisition has little effect on adult bone density. FASEB J. 2002;16:736–738 [DOI] [PubMed] [Google Scholar]
- 24. Prentice A, Parsons TJ, Cole TJ. Uncritical use of bone mineral density in absorptiometry may lead to size-related artifacts in the identification of bone mineral determinants. Am J Clin Nutr. 1994;60:837–842 [DOI] [PubMed] [Google Scholar]
- 25. Werkstetter KJ, Pozza SB, Filipiak-Pittroff B, et al. Long-term development of bone geometry and muscle in pediatric inflammatory bowel disease. Am J Gastroenterol. 2011;106:988–998 [DOI] [PubMed] [Google Scholar]
- 26. Terpstra AM, Kalkwarf HJ, Shults J, et al. Bone density and cortical structure after pediatric renal transplantation. J Am Soc Nephrol. 2012;23:715–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsampalieros A, Gupta P, Denburg MR, et al. Glucocorticoid effects on changes in bone mineral density and cortical structure in childhood nephrotic syndrome. J Bone Miner Res. 2013;28(3):480–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schweizer R, Martin DD, Schwarze CP, et al. Cortical bone density is normal in prepubertal children with growth hormone (GH) deficiency, but initially decreases during GH replacement due to early bone remodeling. J Clin Endocrinol Metab. 2003;88:5266–5272 [DOI] [PubMed] [Google Scholar]
- 29. Ducher G, Bass SL, Saxon L, Daly RM. Effects of repetitive loading on the growth-induced changes in bone mass and cortical bone geometry: a 12-month study in pre/peri- and postmenarcheal tennis players. J Bone Miner Res. 2011;26:1321–1329 [DOI] [PubMed] [Google Scholar]
- 30. Kontulainen S, Sievanen H, Kannus P, Pasanen M, Vuori I. Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: a peripheral quantitative computed tomography study between young and old starters and controls. J Bone Miner Res. 2002;17:2281–2289 [DOI] [PubMed] [Google Scholar]
- 31. Tuchman S, Thayu M, Shults J, Zemel BS, Burnham JM, Leonard MB. Interpretation of biomarkers of bone metabolism in children: impact of growth velocity and body size in healthy children and chronic disease. J Pediatr. 2008;153:484–490 [DOI] [PMC free article] [PubMed] [Google Scholar]