Abstract
Anti-thyroglobulin antibodies are commonly identified in patients with differentiated follicular cell-derived thyroid cancer. When present, they interfere with the measurement of thyroglobulin (Tg), which is the primary biochemical marker used for disease surveillance, creating challenges in monitoring patients for residual or recurrent disease. Moreover, there is variability in measuring anti-Tg antibodies according to the different assays, such that not all patients with anti-Tg antibodies are identifiable on a single assay system. The persistence of anti-Tg antibodies, especially if levels are rising, may indicate persistent, recurrent, or progressive thyroid cancer. In contrast, declining anti-Tg antibody levels may indicate reduced tumor burden or the absence of disease. In this review, we will explore in a case-based manner the data supporting monitoring and treatment paradigms for patients with anti-Tg antibodies and will stress areas where more evidence is needed to better inform clinicians regarding the management of patients with this challenging situation.
Accreditation and Credit Designation Statements.
The Endocrine Society is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. The Endocrine Society has achieved Accreditation with Commendation.
The Endocrine Society designates this JCEM Journal-based CME activity for a maximum of 1 AMA PRA Category 1 CreditsTM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Learning Objectives
Upon completion of this educational activity, participants should be able to:
Compare the effectiveness of assays in measuring anti-Tg antibodies, including the use of single and multiple assays in detecting all interfering antibodies.
Appropriately monitor anti-Tg antibodies as a surrogate marker of disease course in patients with thyroid cancer.
Recommend radiographic imaging in thyroid cancer patients with positive anti-Tg antibodies.
Disclosure Policy
Authors, editors, and Endocrine Society staff involved in planning this JCEM Journal-based CME activity are required to disclose to The Endocrine Society and to learners any relevant financial relationship(s) of the individual or spouse/partner that have occurred within the last 12 months with any commercial interest(s) whose products or services are discussed in the CME content. The Endocrine Society has reviewed all disclosures and resolved all identified conflicts of interest.
The following author reported no relevant financial relationships:
Fadi Nabhan, M.D., has no relevant financial relationships.
The following author reported relevant financial relationships:
Matthew D. Ringel, M.D., has previously served on a medical advisory board for Veracyte and has been a member of the Board of the International Thyroid Oncology Group.
The following JCEM Editors reported relevant financial relationships:
The Editor-in-Chief, Leonard Wartofsky, M.D., is a Consultant for Asurogen, Genzyme, and IBSA, and is on the Speaker's Bureau for Genzyme. Kenneth Burman, M.D., is a Consultant for Medscape and UpToDate; a Reviewer for the Endocrine Fellows Foundation; and has received Institutional Grants for Research from Amgen, Eisei, and Pfizer. Samuel Dagogo-Jack, M.D., is a Consultant for Merck and Novo Nordisk; a Grantee for the American Diabetes Association, AstraZeneca, Boehringer Ingelheim, National Institutes of Health, and Novo Nordisk; and a Grant Reviewer for the American Diabetes Association and National Institutes of Health. Silvio Inzucchi, M.D., is a Consultant/Advisor for Boehringer Ingelheim, Genentech, Janssen, Merck, and Takeda; has DSMB Activity with Amgen, Esai, and Gilead; and receives CME support from Abbott, Amylin, Boeringher-Ingelheim, Merck, and Takeda. Kieren Mather, M.D., received an Investigator-initiated Grant from Novo Nordisk. Lynnette Nieman, M.D., is an Author/Editor for UpToDate, and receives Research Support from HRA-Pharmaceutical.
The following JCEM Editors reported no relevant financial relationships: Paolo Beck-Peccoz, M.D.; David Ehrmann, M.D.; David Handelsman, Ph.D.; Michael Kleerekoper, M.D.; Merrily Poth, M.D.; Constantine Stratakis, M.D.
Endocrine Society staff associated with the development of content for this activity reported no relevant financial relationships.
Acknowledgement of Commercial Support
JCEM Journal-based CME activities are not supported by grants, other funds, or in-kind contributions from commercial supporters.
Instructions
The estimated time to complete each JCEM Journal-based CME activity, including review of material, is 1 hour. Instructions for completing this activity can be found at https://www.endocrine.org/education-and-practice-management/continuing-medical-education/journal-cme.
If you have questions about this JCEM Journal-based CME activity, please direct them to education@endocrine.org.
Activity release date: August 2013
Activity expiration date: August 2015
Case Presentation
A 36-year-old woman was seen in follow-up for further management of papillary thyroid cancer (PTC). She initially presented with a right lobe thyroid nodule in 2002. Fine-needle aspiration revealed a follicular neoplasm, and she underwent a right hemithyroidectomy that revealed a 4.6-cm follicular variant of PTC with perivascular lymphatic invasion and lymphocytic thyroiditis. She had completion thyroidectomy that revealed lymphocytic thyroiditis and was then treated with 157 mCi of I-131 therapy after levothyroxine (L-T4) withdrawal. Anti-Tg antibodies were elevated at the time of treatment, and Tg levels were undetectable. Pre- and post-therapy whole body radioiodine scans revealed uptake in the thyroid bed with no evidence of regional or distant metastases. The patient was placed on TSH-suppressive doses of L-T4 and was monitored thereafter with a combination of neck ultrasound, TSH, Tg, and anti-Tg antibody levels with persistently positive anti-Tg antibodies. She also had a 4-mCi I-131 whole body scan after L-T4 withdrawal in 2004 with no uptake. Since 2006, the Tg and anti-Tg antibody measurements have been performed in the same laboratory using a single assay system (Immulite 2000 and L2KTG, respectively; Siemens, Deerfield, Illinois). Tg levels have been measured yearly since 2006 and have been persistently undetectable. The anti-Tg antibody levels are as follows (IU/ml): February 2006, 209; October 2006, 159; September 2007, 162; January 2009, 152; July 2010, 74; August 2011, 53; and August 2012, 37.6 (reference range, <40; lower limit of detection, <20). Neck ultrasound was performed periodically without evidence of disease, most recently in 2012. Chest computed tomography (CT) scans without iv contrast in 2010 and 2012 revealed 2 stable tiny lung nodules that were not felt to be consistent with metastases. Thus, at this time the patient has dropping anti-Tg antibodies and no certain radiographic or functional evidence of residual thyroid cancer.
Frequency and Measurement of Anti-Tg Antibodies
Thyroglobulin (Tg) is a critical biochemical marker used to monitor patients with well-differentiated forms of follicular cell-derived thyroid cancer (differentiated thyroid cancer [DTC]). Tg measurement can be affected by the presence of anti-Tg antibodies causing inaccurate results, thereby limiting its usefulness in patients with circulating anti-Tg antibodies (1, 2). The frequency of these antibodies in patients with DTC varies, but it is approximately 20–25% depending on the assay used and the particular study population (3–5). The prevalence of anti-Tg antibodies in patients with DTC therefore appears to be higher than the approximately 10% frequency reported in the general population (6). The difference in antibody prevalence in DTC populations may in part be related to the use of different anti-Tg antibody assays or differences in the frequency of lymphocytic thyroiditis. Latrofa et al (7) reported that among the thyroid cancer patient samples, the prevalence of anti-Tg antibodies was higher in those with DTC and lymphocytic thyroiditis (29.2–50%) than in those with DTC alone (1.9–6.7%), depending on the assays used. In a second study by this same group of investigators (8), the discordant results between anti-Tg antibody assays were felt to be related to 1 or more of several factors, including the assay method (immunometric assay [IMA] vs RIA), when individuals had lower levels of antibody, and differences in Tg epitope heterogeneity. The effects of anti-Tg antibodies on measured Tg levels vary according to the type of Tg assay. Anti-Tg antibodies tend to cause an underestimation of Tg when IMA is used, whereas they can cause either an under- or overestimation of RIA measurements (5). Currently in the United States, most commercial laboratories use IMAs to measure Tg. Importantly, with this type of assay, undetectable Tg levels in the presence of anti-Tg antibodies may not correlate with the absence of disease.
Concordance of Positive Anti-Tg Antibodies Among Different Assays
As noted above, Latrofa et al (8) reported a variable correlation for anti-Tg antibody levels when the same samples were measured using 6 different assays (3 IMAs and 3 RIAs). The correlation was higher between assays using the same methodology and also in patients with lymphocytic thyroiditis (8). This discordance also was reported by Spencer et al (9), who noted that among 143 patients in whom positive anti-Tg antibodies were detected using a semi-automated RIA, only 35–62.2% were detected on 1 or more of 3 IMAs. In clinical practice, this variability presents a challenge when determining whether an individual patient has an accurate Tg measurement and also whether anti-Tg antibody levels are used as a biochemical marker of tumor progression or persistence. It is important to recognize that the lower limit of detection of anti-Tg antibodies is a possible partial cause of the discordance. Indeed, when the “cutoff” was lowered to the lowest limit of accurate assay detection, rather than the reference range for an individual assay (often used to identify patients with chronic lymphocytic thyroiditis), greater concordance was observed (10). Thus, for patients with thyroid cancer, it is our approach to use the lower limit of detection, rather than the lower part of the normal range to define the presence of anti-Tg antibodies. This should also be determined by individual laboratories. As noted above, another potential cause of discordance is the heterogeneity of Tg epitopes to which the antibodies are directed and their recognition in the different anti-Tg antibody assays (8, 10). For these reasons, when managing patients with proven or suspected DTC who have undetectable Tg and anti-Tg antibody results, it is reasonable to repeat the measurements using a different method to enhance confidence that undetected anti-Tg antibodies are not the cause of the discordance.
Alternative Methods of Detecting Tg Antibody Interference
If there is concern regarding inaccuracy of Tg measurement, recovery assays to detect interference in Tg measurement have been advocated. Indeed, a reduced recovery result often correlates with the presence of interference (11). However, the routine use of recovery assays has been limited due to the relative insensitivity compared with measurement of anti-Tg antibodies (5). Nonetheless, recovery assays may have a role in selected situations. A second, less common type of antibody, heterophile antibodies against Tg, can also occur (12). These are nonhuman antibodies that cross-react with Tg. In comparison with human anti-Tg antibodies, heterophile antibodies tend to elevate IMA results for Tg and do not typically cause positive anti-Tg antibody measurements (13). Heterophile antibodies are suspected when results do not match clinical course or radiographic findings, such as when Tg levels are elevated in the absence of identifiable residual or recurrent thyroid tissue, when Tg levels are inexplicably variable despite stable TSH levels, and when there is no rise of Tg level with TSH stimulation. This type of interfering antibody can often be detected using proprietary antiheterophile antibody tubes, by performing recovery assays, or by performing serial dilutions of Tg samples if levels are high enough to be reliably measured.
Assays That Are Unaffected by Anti-Tg Antibody Interference
Peptide immunoaffinity enrichment in concert with liquid chromatography–tandem mass spectrometry has been used to measure Tg, and it appears not to be influenced by the presence of anti-Tg antibodies (14, 15). At this time, this method appears to lack adequate sensitivity to detect low Tg levels in the ranges afforded by conventional Tg immunoassays. Nonetheless, this approach holds great promise in the future as more sensitive assays are developed. A second approach evaluated by several groups has been to detect thyroid or thyroid cancer-specific mRNA or DNA transcripts from circulating blood. A number of technical limitations to RNA isolation, stability, and detection; difficulty separating thyroid cell-mediated transcription from “illegitimate” transcription of mRNAs in nonthyroid cells; and evidence that protected mRNA transcripts circulate in peripheral blood from many tissues make this approach challenging for patients with small volume disease in whom the assay is most likely to be useful (16). Other approaches, such as measuring circulating DNA methylation (17) and the detection of mutant BRAF in patients with BRAF mutation-positive PTC, have been reported (18). These methods, as well as those reported for other solid tumors (eg, circulating tumor cell detection and exosome-derived microRNA detection) are incompletely studied in DTC, and their use is limited to research protocols at this time.
Management of Patients with DTC Who Are Anti-Tg Antibody Positive
Patients with anti-Tg antibodies at the time of diagnosis
Whether the presence of anti-Tg antibodies should influence a decision on administering I-131 after thyroidectomy is not well-studied. A potential advantage of I-131 treatment in these patients is the theoretical possibility that this treatment will eliminate thyroid tissue, the antigen source for anti-Tg antibodies, thereby leading to antibody disappearance. This has not been prospectively evaluated, and it is also not known whether it will improve the clinical outcomes. Overall, we believe that the current data do not support the hypothesis that the presence of anti-Tg antibodies alone should primarily drive the indication or approach to treatment with I-131, but rather that this should be considered along with other clinical and pathological data in the decision-making process. The presence of the antibodies leads to greater reliance on the pathology, postsurgical ultrasound, and diagnostic scan results (if performed) to inform therapeutic decisions.
Following patients with anti-Tg antibodies
In the setting of anti-Tg antibodies, there has been growing evidence that the antibody levels themselves may serve as a surrogate biochemical marker of disease persistence and response to therapy. However, the timing of testing and the duration to see a maximal response appear to differ from Tg levels in patients without anti-Tg antibodies. For example, there may be an initial transient rise in anti-Tg antibodies after radioactive iodine treatment (19). Also, it has been shown that the eventual disappearance of Tg antibodies takes approximately 2–3 years on average (19–21). It is important to recognize that due to the variability between assay systems, monitoring levels requires the use of a consistent assay and preferably consistent laboratories performing the assays. In the published studies as well as in our clinical experience, some patients have very slowly dropping anti-Tg antibody levels that can take much longer than 3 years to resolve. The variability in the rate of disappearance of antibodies may reflect the heterogeneity in the population studied and may be influenced by factors such as the duration and height of anti-Tg antibody levels and potential differences in patients with underlying chronic lymphocytic thyroiditis vs those with tumor-associated lymphocytic infiltrates. Nonetheless, there is evidence that decrement in anti-Tg antibodies after I-131 therapy may predict rates of residual thyroid cancer. For example, Kim et al (22) reported that patients who were positive for anti-Tg antibodies that either became negative or had > 50% decline vs the pretreatment value over 6–12 months after I-131 therapy had a lower recurrence rate than patients with lesser reductions or increased anti-Tg antibodies (22). The expected change in anti-Tg antibodies after total thyroidectomy if radioactive iodine is not administered is less clear, particularly if residual normal thyroid tissue is present. However, in these patients, if anti-Tg antibodies remain positive, following the trend also may be helpful. It may also be important to identify the presence of residual nonmalignant thyroid tissue in patients with persistent stable anti-Tg antibodies by ultrasound to allow for follow-up.
There is no consensus on the extent of the workup that should be performed for patients with persistently elevated anti-Tg antibodies. One approach could be that if anti-Tg antibodies remain detectable without dropping or if levels are rising, this should prompt an evaluation similar to a patient with persistent or rising Tg in the absence of antibodies. However, the likelihood of finding thyroid cancer in this setting and the best methods for disease localization are not firmly established. It is our approach to modify the strategy applied to individual patients based on their overall risk of recurrent or persistent disease.
Several studies have addressed this question in relatively small groups of patients. Pacini et al (23) included 25 patients with undetectable serum Tg levels and positive anti-Tg antibodies in a study of the most sensitive methods to monitor patients with early-stage thyroid cancer. TSH-stimulated Tg was performed along with diagnostic whole body scan (DxWBS) and neck ultrasound in this group of patients. Some also received I-131 treatment and had reported post-therapy whole body scan. In response to TSH stimulation, serum Tg changed from undetectable to detectable in 4 of 25 (16.0%) patients, whereas it remained undetectable in 21 of 25 patients. In the 4 patients whose Tg levels rose with stimulation, DxWBS was negative in all; however, in 1 of the 4, uptake was identified by post-therapy whole body scan, and in another patient disease was identified by neck ultrasound. In the 21 patients whose Tg did not change with stimulation, DxWBS identified disease in 3 patients, and neck ultrasound identified neck metastases in 2 patients (23). Thus, in this group of thyroid cancer patients with positive anti-Tg antibodies, TSH stimulation of Tg did not predict the presence or absence of residual or recurrent thyroid cancer.
Rosario et al (24) also used stimulated Tg, ultrasound of the neck, or DxWBS in 136 patients with positive anti-Tg antibodies with undetectable Tg without stimulation to investigate for the presence of disease. All these patients underwent total thyroidectomy and received radioiodine. The presence of disease was defined by elevated Tg with stimulation, positive DxWBS, or positive disease on neck ultrasound. Using these criteria, disease was present in 17 of 136 patients (12.5%). Seven of these 17 patients who were proven to have disease by either ultrasound or DxWBS had undetectable Tg with TSH stimulation. However, neck ultrasound and DxWBS were negative in 7 patients in whom Tg increased with stimulation, and 2 of these were later found to have disease when chest CT and fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT were done (24). This may indicate that the frequency of disease could have been underestimated because those other imaging tests such as CT scan or PET scan were not universally performed if stimulated Tg was undetectable and neck ultrasound and DxWBS were negative for disease (24). It also underscores the lack of a role for TSH stimulation of Tg in the setting of positive anti-Tg antibodies. Finally, Ozkan et al (25) recently evaluated FDG PET/CT in detecting recurrent thyroid cancer in patients with isolated detectable anti-Tg antibody level with negative serum Tg and whole body I-131 scan. Forty-eight patients were identified, and 17 with concomitant lymphocytic thyroiditis were excluded. Of the remaining 31 patients, FDG PET/CT was positive in 16, including 15 in neck lymph nodes and 1 on bone. The overall sensitivity and specificity for detecting recurrent disease were 75 and 76%, respectively. However, only a subset of patients also had other imaging, and those with lymphocytic thyroiditis were excluded; therefore, it is difficult to draw a firm conclusion on the role of PET/CT in this population compared with routine imaging at this time (25).
In general, these studies suggest that neck ultrasound, perhaps in combination with other imaging based on risk of metastatic disease determined by risk features of individual patients, should be performed in an effort to identify disease in patients with persistent or rising anti-Tg antibodies. However; it remains uncertain whether the degree of elevation of anti-Tg antibodies or the degree of change in levels correlates with radiographic disease, and further studies to better inform appropriate diagnostic testing in this area are needed. If suspicious lesions are detected on imaging, fine-needle aspiration for cytology can be performed if indicated, and Tg can be measured on fine-needle aspiration samples. Although it is not certain whether anti-Tg antibodies can alter Tg levels in nodes, the data support its usefulness as a diagnostic test in this population (26–28).
Clinical Approach to DTC Patients With Anti-Tg Antibodies
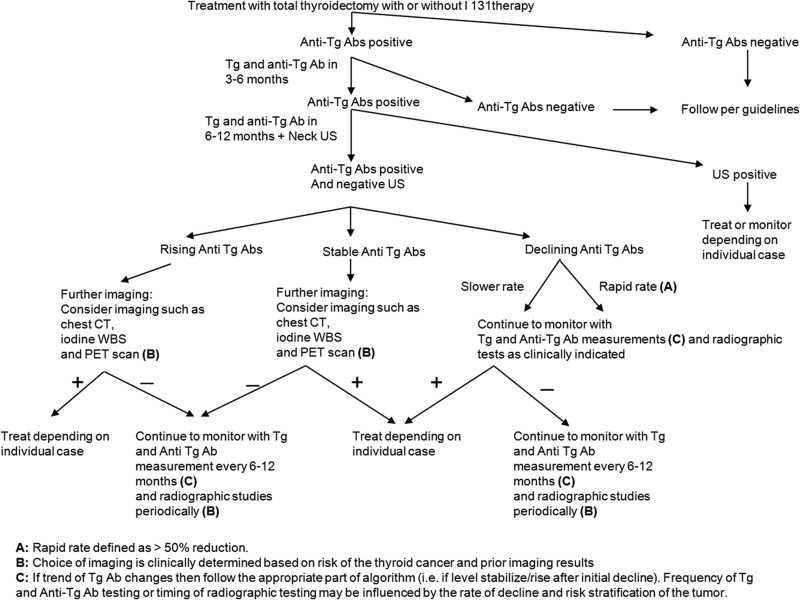
The example case in this manuscript represents only 1 of several patterns of Tg antibodies that are seen in clinical practice. Our approach is based on the pattern of change in anti-Tg antibody levels or correlation with clinical or radiographic findings tailored to the clinical-pathological risk of an individual patient. It is important to recognize that anti-Tg antibody levels can fluctuate; thus, clear identification of a trend requires repeat lab tests over time. The common situations we encounter and the approach to imaging are described below and in Figure 1.
Figure 1.
Suggested algorithm for patients with circulating anti-Tg antibodies. Ab, antibody; US, ultrasound.
1. Patients with declining anti-Tg antibodies
As exemplified in the case report, we typically perform active surveillance (ie, TSH suppression based on the initial tumor stage and periodic imaging) when anti-Tg antibodies are declining. For patients with low-risk PTC, periodic neck ultrasound seems most appropriate for imaging. In patients with low-risk FTC without metastatic disease on post-therapy whole body scan if treated with I-131, neck ultrasound still may be useful to monitor presumed residual normal thyroid tissue depending on the comfort level and experience of the ultrasound operator. We typically limit the use of DxWBS and/or chest CT to patients with declining antibodies for targeted imaging of known metastases on prior imaging.
2. Patients with rising anti-Tg antibodies or those who become positive for anti-Tg antibodies after being negative
These patients are more concerning for progressive or recurrent thyroid cancer, and a more aggressive approach to imaging is generally performed. In our practice, the approach is similar to that of patients with elevated Tg levels, which usually include neck ultrasound, CT scans, DxWBS, or PET/CT scans depending on the stage of the tumor, prior imaging, the presence of poorly differentiated features, or other features. In general, we do not perform empiric I-131 therapy if imaging tests are unrevealing, other than in highly selected high-risk situations.
3. Patients whose anti-Tg antibody levels have reached a plateau after declining
When anti-Tg antibodies stop declining, it is unclear whether this correlates with a residual benign thyroid tissue or thyroid cancer or whether it is related to the immune response. Depending on the clinical scenario, these patients should be evaluated as for patient group 1 or 2 above. It is important also to monitor stable anti-Tg antibodies over an extended period of time to be confident the level is not changing.
4. Patients who are considered biochemically free of disease because of undetectable Tg levels in the absence of anti-Tg antibodies but who have suspicious or proven disease on imaging
In this situation, there are several possibilities: inaccurate Tg because of failure to detect anti-Tg antibodies that are interfering; a tumor secreting Tg that is not measured by the assay; or a tumor that does not express or secrete Tg. In such cases, it may be helpful to immunostain the tumor for Tg. In cases of DTC or a confirmed Tg-expressing tumor, the primary option is to run the same sample on different assays, optimally including 1 that uses a different method. If thyroid cancer is confirmed and the Tg is undetectable with both a low and high TSH, Tg levels will be insensitive for monitoring that particular patient.
5. Patients who have no evidence of disease clinically or radiographically but have erratic Tg levels that do not rise with TSH stimulation
This situation raises suspicion for the presence of heterophile antibodies against Tg. These can be directly measured or assessed using serial dilutions of the Tg samples.
6. Patients who have negative anti-Tg antibodies on 1 assay but then are positive by a different assay
In this scenario, it is important to confirm the results of both assays by repeating the test. Then one can consider a recovery study to prove whether anti-Tg antibodies are truly present and whether they are interfering. However, when it is not clear whether anti-Tg antibodies are truly positive, the general approach would be similar to other patients with positive anti-Tg antibodies in whom there is greater reliance on imaging testing.
Areas Where Further Information Is Needed to Optimize Clinical Management
Improving the accuracy of antibody assays and developing more standardized systems to allow for comparison of results.
Determining a reference range for anti-Tg antibodies that would be accurately measurable and would correlate clinically with the presence or absence of disease in patients with thyroid cancer.
Clarifying whether the presence of anti-Tg antibodies at diagnosis should influence decisions regarding treating patients with I-131 after surgery.
Identifying appropriate cutoff levels or cutoff times of antibody disappearance that would correlate with the likelihood of detecting disease so imaging intensity can be individualized.
Determining whether antibody persistence or levels convey different risks of cancer persistence or progression in patients with chronic lymphocytic thyroiditis vs those with tumor-associated lymphocytic infiltrates.
Clarifying the sensitivity and specificity of imaging paradigms for patients with persistent or rising anti-Tg antibody levels.
Developing sensitive biomarkers of thyroid cancer that are not altered by circulating anti-Tg antibody levels.
Summary
Patients with elevated anti-Tg antibodies should initially be treated in a way similar to other thyroid cancer patients based on clinical and pathological characteristics. If radioactive iodine therapy is indicated based on pathology staging and other characteristics, it may take up to a few years before the anti-Tg antibodies become undetectable. For patients with low-risk DTC who are not treated with I-131, the levels should be monitored, and imaging can be performed based on the pattern of antibody level change. It is important whenever possible to employ a consistent anti-Tg antibody assay over time to enable comparison of results. It is also important for clinicians to consider that anti-Tg antibodies may not be detected by 1 assay in situations where there is a mismatch between clinical findings of disease and undetectable Tg and anti-Tg antibodies. Rising levels of anti-Tg antibodies may be a harbinger of cancer progression and warrant further assessment. Dropping levels may be associated with reduced tumor burden, and persistent stable levels may warrant imaging, depending on the clinical situation. The best imaging approach is uncertain, and more data are needed in this area. If recurrent or residual disease is localized, then treatment or active surveillance should be employed as for other patients with thyroid cancer.
Back to the Patient
Although it is not possible to definitively state that our patient is in a complete biochemical remission, it is reassuring that the anti-Tg antibody level is declining and there is no obvious residual thyroid cancer on imaging 10 years from her diagnosis. We will continue to perform periodic neck ultrasound and, if her anti-Tg antibodies become undetectable, will consider performing a TSH-stimulated Tg level.
Acknowledgments
M.D.R. is supported by National Institutes of Health Grants PO1 CA124570-01 and R01 CA152066.
Disclosure Summary: M.D.R. has previously served on a medical advisory board for Veracyte and has been a member of the Board of the International Thyroid Oncology Group. F.N. has nothing to disclosure.
Footnotes
- CT
- computed tomography
- DTC
- differentiated thyroid cancer
- DxWBS
- diagnostic whole body scan
- FDG
- fluorodeoxyglucose
- IMA
- immunometric assay
- L-T4
- levothyroxine
- PET
- positron emission tomography
- PTC
- papillary thyroid cancer
- Tg
- thyroglobulin.
References
- 1. American Thyroid Association Surgery Working Group, American Association of Endocrine Surgeons, American Academy of Otolaryngology-Head and Neck Surgery, American Head and Neck Society; Carty SE, Cooper DS, Doherty GM, et al. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid. 2009;19:1153–1158 [DOI] [PubMed] [Google Scholar]
- 2. Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W; European Thyroid Cancer Taskforce European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787–803 [DOI] [PubMed] [Google Scholar]
- 3. Pacini F, Mariotti S, Formica N, et al. Thyroid autoantibodies in thyroid cancer: incidence and relationship with tumour outcome. Acta Endocrinol (Copenh). 1988;119:373–380 [DOI] [PubMed] [Google Scholar]
- 4. Kumar A, Shah DH, Shrihari U, Dandekar SR, Vijayan U, Sharma SM. Significance of antithyroglobulin autoantibodies in differentiated thyroid carcinoma. Thyroid. 1994;4:199–202 [DOI] [PubMed] [Google Scholar]
- 5. Spencer CA, Takeuchi M, Kazarosyan M, et al. Serum thyroglobulin autoantibodies: prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1998;83:1121–1127 [DOI] [PubMed] [Google Scholar]
- 6. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489–499 [DOI] [PubMed] [Google Scholar]
- 7. Latrofa F, Ricci D, Montanelli L, et al. Lymphocytic thyroiditis on histology correlates with serum thyroglobulin autoantibodies in patients with papillary thyroid carcinoma: impact on detection of serum thyroglobulin. J Clin Endocrinol Metab. 2012;97:2380–2387 [DOI] [PubMed] [Google Scholar]
- 8. Latrofa F, Ricci D, Montanelli L, et al. Thyroglobulin autoantibodies in patients with papillary thyroid carcinoma: comparison of different assays and evaluation of causes of discrepancies. J Clin Endocrinol Metab. 2012;97:3974–3982 [DOI] [PubMed] [Google Scholar]
- 9. Spencer C, Petrovic I, Fatemi S. Current thyroglobulin autoantibody (TgAb) assays often fail to detect interfering TgAb that can result in the reporting of falsely low/undetectable serum Tg IMA values for patients with differentiated thyroid cancer. J Clin Endocrinol Metab. 2011;96:1283–1291 [DOI] [PubMed] [Google Scholar]
- 10. Pickett AJ, Jones M, Evans C. Causes of discordance between thyroglobulin antibody assays. Ann Clin Biochem. 2012;49:463–467 [DOI] [PubMed] [Google Scholar]
- 11. Persoon AC, Links TP, Wilde J, Sluiter WJ, Wolffenbuttel BH, van den Ouweland JM. Thyroglobulin (Tg) recovery testing with quantitative Tg antibody measurement for determining interference in serum Tg assays in differentiated thyroid carcinoma. Clin Chem. 2006;52:1196–1199 [DOI] [PubMed] [Google Scholar]
- 12. Preissner CM, O'Kane DJ, Singh RJ, Morris JC, Grebe SK. Phantoms in the assay tube: heterophile antibody interferences in serum thyroglobulin assays. J Clin Endocrinol Metab. 2003;88:3069–3074 [DOI] [PubMed] [Google Scholar]
- 13. Giovanella L, Keller F, Ceriani L, Tozzoli R. Heterophile antibodies may falsely increase or decrease thyroglobulin measurement in patients with differentiated thyroid carcinoma. Clin Chem Lab Med. 2009;47:952–954 [DOI] [PubMed] [Google Scholar]
- 14. Hoofnagle AN, Becker JO, Wener MH, Heinecke JW. Quantification of thyroglobulin, a low-abundance serum protein, by immunoaffinity peptide enrichment and tandem mass spectrometry. Clin Chem. 2008;54:1796–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clarke NJ, Zhang Y, Reitz RE. A novel mass spectrometry-based assay for the accurate measurement of thyroglobulin from patient samples containing antithyroglobulin autoantibodies. J Investig Med. 2012;60:1157–1163 [DOI] [PubMed] [Google Scholar]
- 16. Ringel MD. Molecular detection of thyroid cancer: differentiating “signal” and “noise” in clinical assays. J Clin Endocrinol Metab. 2004;89:29–32 [DOI] [PubMed] [Google Scholar]
- 17. Hu S, Ewertz M, Tufano RP, et al. Detection of serum deoxyribonucleic acid methylation markers: a novel diagnostic tool for thyroid cancer. J Clin Endocrinol Metab. 2006;91:98–104 [DOI] [PubMed] [Google Scholar]
- 18. Chuang TC, Chuang AY, Poeta L, Koch WM, Califano JA, Tufano RP. Detectable BRAF mutation in serum DNA samples from patients with papillary thyroid carcinomas. Head Neck. 2010;32:229–234 [DOI] [PubMed] [Google Scholar]
- 19. Gorges R, Maniecki M, Jentzen W, et al. Development and clinical impact of thyroglobulin antibodies in patients with differentiated thyroid carcinoma during the first 3 years after thyroidectomy. Eur J Endocrinol. 2005;153:49–55 [DOI] [PubMed] [Google Scholar]
- 20. Chiovato L, Latrofa F, Braverman LE, et al. Disappearance of humoral thyroid autoimmunity after complete removal of thyroid antigens. Ann Intern Med. 2003;139:346–351 [DOI] [PubMed] [Google Scholar]
- 21. Thomas D, Liakos V, Vassiliou E, Hatzimarkou F, Tsatsoulis A, Kaldrimides P. Possible reasons for different pattern disappearance of thyroglobulin and thyroid peroxidase autoantibodies in patients with differentiated thyroid carcinoma following total thyroidectomy and iodine-131 ablation. J Endocrinol Invest. 2007;30:173–180 [DOI] [PubMed] [Google Scholar]
- 22. Kim WG, Yoon JH, Kim WB, et al. Change of serum antithyroglobulin antibody levels is useful for prediction of clinical recurrence in thyroglobulin-negative patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2008;93:4683–4689 [DOI] [PubMed] [Google Scholar]
- 23. Pacini F, Molinaro E, Castagna MG, et al. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:3668–3673 [DOI] [PubMed] [Google Scholar]
- 24. Rosario PW, Mineiro Filho AF, Lacerda RX, dos Santos DA, Calsolari MR. The value of diagnostic whole-body scanning and serum thyroglobulin in the presence of elevated serum thyrotropin during follow-up of anti-thyroglobulin antibody-positive patients with differentiated thyroid carcinoma who appeared to be free of disease after total thyroidectomy and radioactive iodine ablation. Thyroid. 2012;22:113–116 [DOI] [PubMed] [Google Scholar]
- 25. Ozkan E, Soydal C, Araz M, Aras G, Ibis E. The additive clinical value of 18F-FDG PET/CT in defining the recurrence of disease in patients with differentiated thyroid cancer who have isolated increased antithyroglobulin antibody levels. Clin Nucl Med. 2012;37:755–758 [DOI] [PubMed] [Google Scholar]
- 26. Snozek CL, Chambers EP, Reading CC, et al. Serum thyroglobulin, high-resolution ultrasound, and lymph node thyroglobulin in diagnosis of differentiated thyroid carcinoma nodal metastases. J Clin Endocrinol Metab. 2007;92:4278–4281 [DOI] [PubMed] [Google Scholar]
- 27. Baskin HJ. Detection of recurrent papillary thyroid carcinoma by thyroglobulin assessment in the needle washout after fine-needle aspiration of suspicious lymph nodes. Thyroid. 2004;14:959–963 [DOI] [PubMed] [Google Scholar]
- 28. Jeon MJ, Park JW, Han JM, et al. Serum antithyroglobulin antibodies interfere with thyroglobulin detection in fine-needle aspirates of metastatic neck nodes in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2013;98:153–160 [DOI] [PubMed] [Google Scholar]