Abstract
Context:
The exact mechanisms responsible for increased plasma triglyceride (TG) concentration in obese people are unclear, and it is not known whether excess energy intake per se is involved in the pathophysiology of this abnormality.
Objective:
The purpose of our study was to examine how excess energy intake from a balanced diet for 1 day affects very-low-density lipoprotein (VLDL)-TG kinetics and its putative regulators hepatic insulin sensitivity and plasma free fatty acid availability.
Subjects and Design:
We used stable isotope-labeled tracer methods to evaluate glucose and lipid kinetics in 8 overweight and obese men (age, 38 ± 3 years; body mass index, 33.7 ± 1.7 kg/m2; means ± SEM) on 2 occasions (randomized crossover design): once, the day after they consumed a balanced diet that provided an amount of energy that matched their energy expenditure, and another time, the day after they consumed a balanced diet that provided 30% excess calories. Eight healthy, lean men (34 ± 1 years; 22.5 ± 0.6 kg/m2) were studied under isocaloric conditions only to provide a reference for normal lipid kinetics.
Results:
VLDL-TG and VLDL-apolipoprotein B-100 (apoB-100) concentrations and secretion rates were significantly greater (P < .01) in overweight/obese compared with lean men. Hypercaloric, compared with isocaloric, feeding in overweight/obese men increased glucose rate of appearance in plasma (904 ± 21 vs 873 ± 26 μmol/min), the hepatic insulin resistance index (10.9 ± 2.2 vs 8.3 ± 1.8), and VLDL–apoB-100 concentration and secretion rate (1.91 ± 0.24 vs. 1.53 ± 0.13 nmol/min), whereas VLDL–apoB-100 plasma clearance rate, VLDL-TG secretion and plasma clearance rates, and free fatty acid rate of appearance in plasma were not affected by overfeeding.
Conclusion:
One day of moderate overfeeding (30% excess energy intake) stimulates hepatic glucose and VLDL–apo B-100 secretion rates but has no effect on hepatic and adipose tissue fatty acid metabolism in overweight/obese men.
It is well established that obesity, which develops in response to an imbalance in energy intake and energy expenditure, is associated with increased plasma triglyceride (TG) concentrations (both total and very-low-density lipoprotein [VLDL]-TG) (1–3). The exact mechanisms responsible for the development of hypertriglyceridemia in obese people are less clear, and it is not known whether excess energy intake per se is involved in the pathophysiology of this abnormality. Results from long-term overfeeding studies are difficult to interpret because of the changes in body fat mass and the accompanying changes in fatty acid release into plasma, ectopic fat accumulation, and insulin sensitivity as people become obese (4–9). Fatty acids (derived from both plasma and splanchnic tissues) and insulin are major regulators of hepatic VLDL-TG secretion rates (10–12). The results from short-term overfeeding studies are inconclusive, in large part because dietary macronutrient content was manipulated along with dietary energy intake. Several studies report an increase in VLDL-TG secretion rate and plasma concentration after 4 to 7 days of consuming a hypercaloric high-carbohydrate diet (13–16), whereas several days of overfeeding with a high-fat diet actually lowered VLDL-TG concentration (15, 17).
The purpose of our study was to examine how excess energy intake from a balanced diet for 1 day affects hepatic insulin sensitivity (evaluated by using the hepatic insulin resistance index (18)), plasma free fatty acid (FFA) availability and hepatic VLDL-TG and VLDL–apolipoprotein B-100 (apoB-100) secretion rates. To this end, we measured plasma substrate and insulin concentrations and used stable isotope-labeled tracer methods to evaluate glucose and lipid kinetics in overweight and obese men on 2 occasions: once, the day after they consumed a balanced diet that provided an amount of energy that matched their energy expenditure, and another time, the day after they consumed a balanced diet that provided 30% excess calories. To provide some insight into potential mediators of changes in insulin sensitivity and lipid kinetics, we also measured the plasma concentrations of adipokines (high–molecular-weight adiponectin, visfatin, and resistin) that are known regulators of glucose and lipid metabolism (19, 20) and β-hydroxybutyrate rate of appearance in plasma, an index of (mostly hepatic) fatty acid oxidation. A group of age-matched, healthy, lean men was studied under isocaloric conditions only to provide a reference for normal lipid kinetics.
Subjects and Methods
Subjects and prestudy testing
Eight lean and 8 overweight and obese, age-matched men (Table 1) who had been sedentary (<1.5 hours exercise/wk) and weight-stable (<2 kg change) for at least 6 months participated in the study. Written informed consent was obtained from all subjects before participation in the study, which was approved by the Institutional Review Board of Washington University School of Medicine in St Louis, Missouri.
Table 1.
Age, Body Composition, and Basic Metabolic Characteristics of Lean and Overweight/Obese Subjectsa
| Lean | Overweight/Obese | P Value | |
|---|---|---|---|
| Age, y | 34 ± 1 | 38 ± 3 | .25 |
| Body mass index, kg/m2 | 23 ± 1 | 34 ± 2 | <.01 |
| Body mass, kg | 76 ± 2 | 105 ± 7 | <.01 |
| Fat-free mass, kg | 64 ± 2 | 75 ± 4 | .02 |
| Body fat, % total body mass | 16 ± 2 | 28 ± 2 | <.01 |
| Resting energy expenditure, kJ/d | 6779 ± 208 | 8931 ± 759 | .02 |
| Resting energy expenditure, kJ/kg FFM/d | 106 ± 3 | 119 ± 8 | .14 |
| Plasma insulin and substrate concentrations | |||
| Insulin, μU/mL | 3.1 ± 0.2 | 9.4 ± 2.0 | <.01 |
| Glucose, mmol/L | 4.96 ± 0.12 | 5.05 ± 0.05 | .49 |
| Total cholesterol, mmol/L | 4.36 ± 0.29 | 4.73 ± 0.24 | .35 |
| HDL-cholesterol, mmol/L | 1.07 ± 0.04 | 1.01 ± 0.07 | .49 |
| LDL-cholesterol, mmol/L | 2.81 ± 0.27 | 2.92 ± 0.25 | .77 |
| TG, mmol/L | 1.06 ± 0.12 | 1.81 ± 0.26 | .02 |
| VLDL-TG, mmol/L | 0.50 ± 0.06 | 1.32 ± 0.25 | <.01 |
| VLDL-apoB-100, nmol/L | 65 ± 8 | 117 ± 13 | <.01 |
Abbreviation: HDL, high-density lipoprotein.
Data are mean ± SEM.
All subjects completed a comprehensive medical examination, including a detailed history and physical examination, a resting electrocardiogram, standard blood tests, and an oral glucose tolerance test. None of the subjects had evidence of chronic illness or significant organ dysfunction (eg, diabetes mellitus or cirrhosis) or were taking medications known to affect metabolism.
Total body fat mass and fat-free mass were determined by using dual-energy x-ray absorptiometry (QDR 1000/w; Hologic, Waltham, Massachusetts). Resting metabolic rate was determined after 30 minutes of bed rest by using online expiratory gas exchange analysis (TrueOne 2400; ParvoMedics, Sandy, Utah); the total daily energy requirement for weight maintenance in ambulatory but otherwise sedentary subjects was assumed to be 1.25 × resting metabolic rate (21).
Experimental design
Overweight and obese men completed 2 identical stable isotope-labeled tracer infusion studies each: one after an isocaloric day (ie, energy intake matched total daily energy requirement for weight maintenance) and another after a hypercaloric day (ie, energy intake exceeded total daily energy requirement for weight maintenance by 30%) in the Clinical Research Unit. Lean men completed only 1 (isocaloric) study. The 2 studies for overweight/obese subjects were carried out in randomized order, approximately 4 weeks apart. All subjects were instructed to consume their regular diet and to refrain from alcohol and exercise for at least 3 days before each study. They were admitted to the Clinical Research Unit in the late afternoon, the day before the iso- or hypercaloric study day, consumed a standard dinner between 6:00 and 7:00 pm, and then fasted, except for water, until the next morning.
Tracer infusion study
At 6:00 am on the morning, a catheter was inserted into an arm vein for the infusion of stable isotope-labeled tracers, and a second catheter was inserted into a vein of the contralateral hand, which was warmed to 55°C by using a thermostatically controlled box to obtain arterialized blood samples. The sampling catheter was kept open with a slow, controlled infusion of 0.9% NaCl solution (30 mL/h). At 7:00 am (time 0), a bolus of [1,1,2,3,3-2H5]glycerol (75 μmol/kg body weight), dissolved in 0.9% NaCl solution, was administered, and continuous infusions of [6,6-2H2]glucose (0.22 μmol/kg body weight/min; priming dose, 18 μmol/kg body weight) dissolved in 0.9% NaCl solution, [2,4-13C2]β-hydroxybutyrate (0.03 μmol/kg body weight/min) dissolved in 0.9% NaCl solution, [2,2-2H2]palmitate (0.03 μmol/kg body weight/min) dissolved in 25% albumin solution, and [5,5,5-2H3]leucine (0.12 μmol/kg body weight/min; priming dose, 7.2 μmol/kg body weight) dissolved in 0.9% NaCl solution were started. The bolus amounts, infusion rates, and duration of infusions for glycerol, leucine, glucose, and palmitate have previously been validated and are standard in our laboratory (22–24); the β-hydroxybutyrate infusion rate was based on the results from pilot studies (unpublished data). The glucose and β-hydroxybutyrate tracer infusions were maintained for 4 hours, and palmitate and leucine tracers were infused for 12 hours.
Blood samples were obtained immediately before administering the tracers at 5, 15, 30, 60, 90, 120, 150, 180, 210, and 240 minutes after starting the tracer infusions and then hourly for another 8 hours to determine 1) plasma insulin, adipokine, and substrate concentrations; 2) VLDL-TG and VLDL–apoB-100 concentrations; 3) glycerol, leucine, β-hydroxybutyrate, palmitate, and glucose tracer-to-tracee ratios (TTRs) in plasma, and 4) glycerol and palmitate TTRs in VLDL-TG and leucine TTR in VLDL–apoB-100.
Isocaloric and hypercaloric study day diets
For each feeding day (isocaloric and hypercaloric), subjects consumed 3 meals (breakfast, lunch, and dinner served at 8:30 am, 1:30 pm, and 6:30 pm, respectively) that contained equal amounts of energy and together provided 70% of total daily energy requirement for weight maintenance. During the isocaloric study, subjects consumed 2 snacks (one at 11:00 am and another at 4 pm) that provided 15% of their total daily energy requirement each and provided the additional energy required for weight maintenance; during the hypercaloric trial, subjects consumed 2 snacks that provided 30% of total daily energy requirement each so that total energy intake exceeded total daily energy requirement by 30% (ie, 11 094 ± 952 vs. 14 625 ± 1088 kJ were consumed in the isocaloric and hypercaloric trials, respectively). Each meal and snack contained ∼55% of total energy as carbohydrate, 30% as fat, and 15% as protein. Subjects were required to consume all of the food provided to them and were allowed to drink only water or noncaloric, caffeine-free beverages. An example of one subject's food and beverage intake is presented in Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Physical activity during the isocaloric and hypercaloric study days
During the day, subjects sat in a chair or rested in bed and were asked to walk around the Clinical Research Unit for 5 min every hour between 7 am and 7 pm. After dinner (7 pm), subjects rested in bed and fasted until the completion of the tracer infusion study the next day.
Sample collection, processing, and analyses
Blood samples were collected in chilled tubes containing either heparin (to determine glucose and insulin concentrations) or EDTA (all other analyses). Samples were placed in ice, and plasma was separated by centrifugation within 30 minutes of collection. Aliquots of fresh plasma were kept at 4°C for isolation of the VLDL fraction and measurement of total plasma apoB-100 concentration. The remaining plasma samples were stored at −80°C until final analyses were performed. The VLDL fraction was isolated from plasma by ultracentrifugation in a 50.4 Ti rotor (Beckman Instruments Inc, Palo Alto, California) at 100 000g for 16 hours at 10°C (2). The top layer, containing VLDL, was removed by tube slicing (Beckman Instruments). Aliquots of recovered VLDL fraction were kept in the refrigerator for immediate measurement of VLDL–apoB-100 concentration. The remaining samples were stored at −80°C until final analyses were performed.
Basal (0 and 2 hours averaged) plasma glucose concentration was determined on an automated glucose analyzer (Yellow Spring Instruments Co, Yellow Springs, Ohio). Basal (0 and 2 hours averaged) plasma insulin concentration was measured by using a chemiluminescent immunometric assay (Immulite 1000; Diagnostic Products Corp, Los Angeles, California). Basal (0 and 2 hours averaged) plasma β-hydroxybutyrate concentration was determined by using a colorimetric enzymatic kit (Cayman Chemical, Ann Arbor, Michigan).
Commercially available ELISAs were used to measure basal (average value of 2 samples during the first 30 minutes of the study) high–molecular-weight adiponectin (ALPCO Diagnostics, Salem, New Hampshire), visfatin (Phoenix Pharmaceuticals, Inc, Burlingame, California), and resistin (R&D Systems, Minneapolis, Minnesota) concentrations in plasma. Basal (0 and 2 hours averaged) plasma FFA concentrations were quantified by gas chromatography (HP 5890 series II GC; Hewlett-Packard, Palo Alto, California) (25). Total plasma and VLDL-TG concentrations (0, 2, 4, 6, 8, 10, and 12 hours, data averaged) were determined by using a colorimetric enzymatic kit (Sigma Chemicals, St. Louis, Missouri). Total plasma and VLDL–apoB-100 concentrations (0, 2, 4, 6, 8, 10, and 12 hours, data averaged) were measured by using an immunoturbidity assay kit (Kamiya Biomedical, Seattle, Washington).
The TTRs of plasma free glycerol (all samples), glucose (0–4 hours), palmitate (all samples), and leucine (all samples), and the TTRs of glycerol and palmitate in VLDL-TG (all samples) and leucine in VLDL–apoB-100 (all samples) were determined by using electron-impact gas-chromatography mass-spectrometry (MSD 5973 system; Agilent, Palo Alto, California) (2, 25). Heptafluorobutyric (HFB) anhydride was used to form heptafluorobutyric derivatives of glycerol and glucose in plasma and glycerol in VLDL-TG. The t-butyldimethylsilyl derivative was prepared for the analysis of plasma leucine, and the N-heptafluorobutyryl n-propyl ester derivative was prepared for leucine in VLDL–apoB-100 (2, 25). β-Hydroxybutyrate TTR (0–4 hours) was measured by using positive chemical ionization and ions of mass to charge ratio of 333 and 335 for the unlabeled and labeled t-butyldimethylsilyl β-hydroxybutyrate, respectively. Plasma free palmitate and palmitate in VLDL-TG (all samples) were analyzed as methyl esters.
Calculations
Palmitate, glucose, and β-hydroxybutyrate rates of appearance (Ra) in plasma were calculated by dividing the tracer infusion rate by the respective average plasma TTR between 120 and 240 minutes (steady state); total FFA Ra was calculated based on the proportional contribution of palmitate to total plasma FFA concentration (26).
The hepatic insulin resistance index (HIRI) was calculated by dividing the product of glucose Ra (in micromoles per minute) and plasma insulin concentration (in microunits per milliliter) by 1000 (18).
The fractional turnover rate (FTR) of VLDL-TG was determined by fitting the TTR time courses of glycerol in plasma and glycerol in VLDL-TG to a compartmental model (22). The absolute rate of VLDL-TG secretion (in micromoles per minute) was calculated as FTR × C × PV, where C is the concentration of VLDL-TG in plasma and PV is the plasma volume, which was estimated to be 0.055 L/kg fat-free mass (27), because VLDL is restricted to plasma (28). The VLDL-TG plasma clearance rate (milliliters per minute) was calculated by dividing the VLDL-TG secretion rate (micromoles per minute) by the VLDL-TG concentration (in micromoles per minute) in plasma.
The relative contribution of systemic plasma FFA to the VLDL-TG fatty acid pool was calculated by fitting the palmitate TTR in plasma and VLDL-TG to a compartmental model (2). The systemic plasma fatty acid pool includes fatty acids from the systemic circulation that are taken up by the liver and directly incorporated into VLDL-TG or temporarily incorporated into rapidly turning over intrahepatic and ip TG stores before incorporation into VLDL-TG. The remaining fatty acids in VLDL-TG (nonsystemic fatty acids) are derived from pools of fatty acids that are not labeled with tracer during the infusion period, including 1) preexisting lipid stores in the liver and retroperitoneal fat depots, 2) lipolysis of plasma lipoproteins taken up by the liver, and 3) hepatic de novo lipogenesis.
The FTR of VLDL–apoB-100 was calculated by fitting the TTR time courses of leucine in plasma and leucine in VLDL–apoB-100 to a compartmental model (2). The total rate of VLDL–apoB-100 secretion by the liver (indicative of the secretion rate of VLDL particles because each VLDL particle contains a single molecule of apoB-100) (29) and VLDL–apoB-100 plasma clearance rate were calculated based on the FTR and plasma concentration of VLDL–apoB-100, as described for VLDL-TG.
Statistical analysis
Differences in lipid kinetics between lean and overweight/obese men (isocaloric conditions) were evaluated by using Student's t test for independent samples. Substrate kinetics from the isocaloric and hypercaloric trials in overweight/obese men were compared by using Student's paired t test (for normally distributed data) or Wilcoxon's signed-rank test (for skewed data); normality of the distributions was assessed by using the Kolmogorov-Smirnov test. A P value ≤ .05 was considered statistically significant. Results are presented as means ± SEM or median (quartiles). All analyses were carried out with SPSS version 20 for Windows (IBM, Armonk, New York).
Results
Substrate and hormone concentrations and VLDL-TG and VLDL–apoB-100 secretion rates in lean and overweigh t/obese men
Plasma insulin, total TG, VLDL-TG, and VLDL–apoB-100 concentrations were more than 70% greater (all P ≤ .02) in overweight/obese compared with lean men (Table 1). Plasma glucose and cholesterol concentrations were not significantly different in lean and overweight/obese men (Table 1).
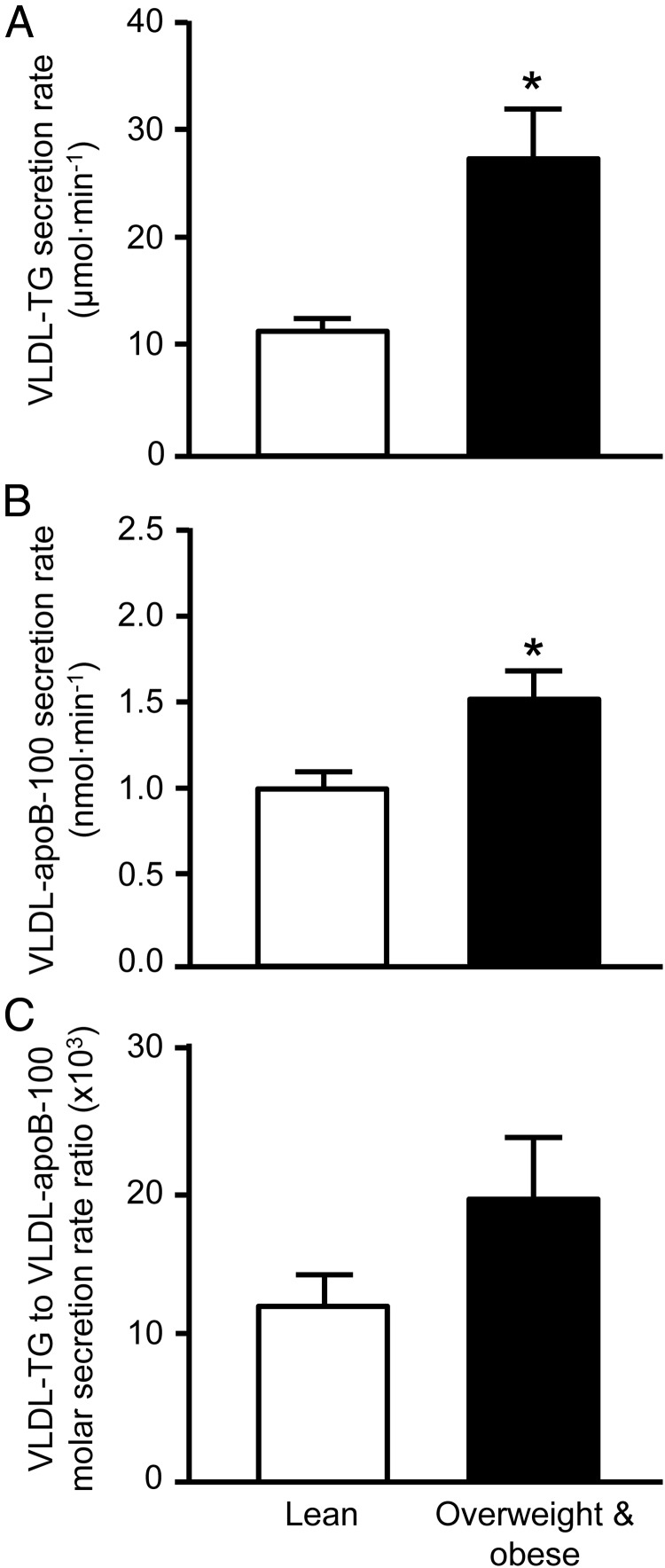
VLDL-TG and VLDL–apoB-100 secretion rates were ∼130% and 50% greater in overweight/obese compared with lean men (P < .01; Figure 1), respectively; the molar ratio of VLDL-TG to VLDL–apoB-100 secretion rates, an index of the average TG content of newly secreted VLDL particles, was ∼60% greater in overweight/obese men, but the difference did not reach significance (P = .13; Figure 1). The contribution of fatty acids from systemic to total VLDL-TG production was greater in lean than overweight/obese men (80% ± 5% and 62% ± 7%, respectively; P = .04), whereas, accordingly, the contribution of nonsystemic fatty acid sources was less. The plasma clearance rates of VLDL-TG (25.4 ± 2.4 vs. 22.4 ± 1.7 mL/min) and VLDL–apoB-100 (18.2 ± 2.6 vs. 14.4 ± 2.0 mL/min) were not significantly different between lean and overweight/obese men.
Figure 1.
A–C, VLDL-TG (A) and VLDL–apoB-100 (B) secretion rates into plasma and the molar ratio of VLDL-TG to VLDL–apoB-100 secretion rates (C), an index of the average TG content of newly secreted VLDL particles, in lean and overweight/obese men. Data are means ± SEM. *, Value in the overweight/obese men is significantly different from the corresponding value in lean men, P < .01.
Effect of overfeeding in overweight/obese men
Substrate and hormone concentrations
Plasma insulin concentration was ∼30% greater after overfeeding compared with isocaloric feeding (Table 2). Plasma glucose, FFA, β-hydroxybutyrate, total plasma TG, and VLDL-TG concentrations were not different in the hypercaloric and isocaloric conditions, but total plasma apoB-100 concentration tended to be greater (P = .09) and VLDL–apoB-100 concentration was significantly greater (P < .001) after hypercaloric than isocaloric feeding (Table 2). The plasma concentrations of high–molecular-weight adiponectin, visfatin, and resistin were not affected by overfeeding (Table 2).
Table 2.
Plasma Insulin, Adipokine, and Substrate Concentrations in Overweight/Obese Subjects on the Morning After the Isocaloric and Hypercaloric Study Daysa
| Isocaloric | Hypercaloric | P Value | |
|---|---|---|---|
| Insulin, μU/mL | 9.4 ± 2.0 | 12.1 ± 2.3 | .04 |
| Glucose, mmol/L | 5.05 ± 0.05 | 5.09 ± 0.10 | .69 |
| FFA, mmol/L | 0.35 ± 0.03 | 0.33 ± 0.04 | .35 |
| β-Hydroxybutyrate, mmol/L | 0.18 ± 0.02 | 0.20 ± 0.03 | .71 |
| High-molecular-weight adiponectin, μg/mL | 1.04 ± 0.20 | 1.11 ± 0.19 | .44 |
| Visfatin, ng/mL | 7.7 (5.4, 18.6) | 6.3 (4.7, 17.4) | .58 |
| Resistin, ng/mL | 7.1 (6.5, 7.8) | 7.1 (6.1, 7.7) | .89 |
| Total TG, mmol/L | 1.81 ± 0.26 | 1.92 ± 0.39 | .59 |
| VLDL-TG, mmol/L | 1.32 ± 0.25 | 1.42 ± 0.36 | .60 |
| Total apoB-100, nmol/L | 1480 ± 210 | 1741 ± 181 | .09 |
| VLDL-apoB-100, nmol/L | 117 ± 13 | 135 ± 13 | <.01 |
Data are mean ± SEM or median (quartiles).
Glucose, FFA, and β-hydroxybutyrate kinetics
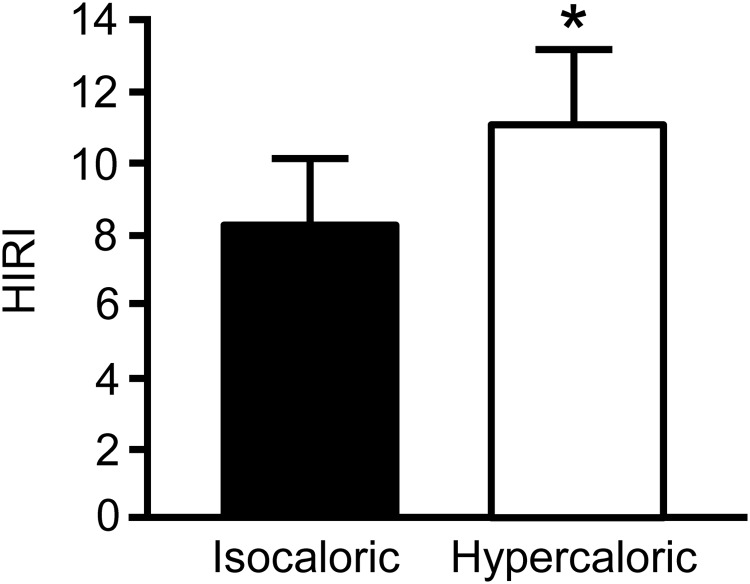
Glucose rate of appearance (Ra) (Table 3) and the HIRI (Figure 2), which assesses the rate of glucose production in relation to the prevailing insulin concentration, were both greater (P < .05) after hypercaloric than isocaloric feeding. There were no differences between trials in FFA or β-hydroxybutyrate Ra in plasma (Table 3).
Table 3.
Glucose, FFA, and β-Hydroxybutyrate Ra in Plasmaa
| Isocaloric | Hypercaloric | P Value | |
|---|---|---|---|
| Glucose, μmol/min | 873 ± 26 | 904 ± 21 | .01 |
| FFA, μmol/min | 383 ± 35 | 354 ± 37 | .37 |
| β-Hydroxybutyrate, μmol/min | 244 ± 52 | 222 ± 64 | .47 |
Data are mean ± SEM.
Figure 2.
HIRI on the morning after the isocaloric and hypercaloric study days in overweight/obese men. Data are means ± SEM. *, Value in the hypercaloric trial is significantly different from the corresponding value in the isocaloric trial, P < .05.
VLDL-TG and apoB-100 kinetics
Hypercaloric feeding had no effect on the secretion rate of VLDL-TG (Figure 3) but significantly increased VLDL–apoB-100 secretion rate (P < .05; Figure 3). Therefore, the molar ratio of VLDL-TG and VLDL–apoB-100 secretion rates, an index of the average TG content of newly secreted VLDL particles, was ∼25% smaller in the hypercaloric than the isocaloric trial (P < .05; Figure 3). The contribution of fatty acids from systemic and nonsystemic sources to total VLDL-TG production was not different between trials; systemic fatty acids accounted for 62% ± 7% and 66% ± 5% of all fatty acids in VLDL-TG in the isocaloric and hypercaloric trials, respectively (P = .59). The plasma clearance rates of VLDL-TG and VLDL–apoB-100 were not affected by hypercaloric feeding (VLDL-TG, 22.4 ± 1.7 and 20.8 ± 2.9 mL/min; VLDL–apoB-100, 14.4 ± 2.0 and 15.2 ± 2.3 mL/min in the isocaloric and hypercaloric trials, respectively).
Figure 3.
A–C, VLDL-TG (A) and VLDL–apoB-100 (B) secretion rates into plasma and the molar ratio of VLDL-TG to VLDL–apoB-100 secretion rates (C), an index of the average TG content of newly secreted VLDL particles, on the morning after the isocaloric and hypercaloric study days in overweight/obese men. Data are means ± SEM. *, Value in the hypercaloric trial is significantly different from the corresponding value in the isocaloric trial, P < .05.
Discussion
Obesity develops in response to excess food intake in relation to energy expenditure and is often associated with hypertriglyceridemia (1, 30), which results from an imbalance between hepatic VLDL-TG secretion and plasma clearance rates (2, 3, 31). In obese men, increased plasma VLDL-TG concentration is predominately due to increased VLDL-TG secretion (2, 3, 31), and our results confirm this finding. The goal of the present study was to find out whether excess energy intake (and the resulting positive energy balance) per se contributes to these abnormalities in VLDL-TG metabolism or whether they develop secondary to increased body fat stores and the accompanying increase in FFA release from adipose tissue and insulin resistance in obese persons (2, 3, 8, 9). To this end, we evaluated the acute (1 day) overfeeding-associated changes in hepatic insulin sensitivity, FFA appearance in plasma, and VLDL-TG and VLDL-apolipoprotein B-100 (apoB-100) kinetics in overweight and obese men and found that excess energy intake induced hepatic insulin resistance and stimulated hepatic VLDL–apoB-100 secretion but had no effect on plasma FFA availability, β-hydroxybutyrate production, hepatic VLDL-TG secretion, or VLDL–apoB-100 and VLDL-TG plasma clearance rates. These results indicate that hepatic glucose and apoB-100 metabolism are sensitive to acute (1 day) moderate increases in net energy balance in overweight/obese men, whereas hepatic and adipose tissue fatty acid/TG metabolism are not. Positive energy balance due to moderate excess energy intake for a single day therefore does not acutely exacerbate hypertriglyceridemia in obese men.
The effect of overfeeding on hepatic insulin sensitivity is well known. Significant increases in both plasma insulin concentration and endogenous glucose Ra have been reported after short-term (4–5 days) overfeeding of carbohydrates (32) as well as fat (17). Our results, which demonstrate a significant increase in hepatic insulin resistance after only 1 day of overfeeding on a mixed diet, confirm these findings and suggest that hepatic insulin resistance develops rapidly during periods of positive energy balance irrespective of dietary macronutrient composition. However, our results with regard to VLDL-TG kinetics differ from those obtained in previous short-term overfeeding studies. Several investigators demonstrated that short-term (4–7 days) overfeeding induced an increase in the rate of VLDL-TG secretion and plasma VLDL-TG concentration (13–16), whereas we found no effect of overfeeding on VLDL-TG kinetics and plasma concentration. The discrepancy in results could be due to the longer duration of overfeeding or the amount of excess energy provided in these (4–7 days and 35%–250% above requirements for weight maintenance) compared with our study (1 day and 30% above requirements for weight maintenance). However, we consider it to be more likely due to the fact that all of the extra energy in these previously published studies was provided as carbohydrates, whereas we provided the extra energy balanced between carbohydrates, protein, and fat. Dietary carbohydrate content is a major regulator of VLDL-TG secretion. We (33) and others (34, 35) have previously reported an increase in hepatic VLDL-TG secretion and plasma concentration even in response to an isocaloric high-carbohydrate vs high-fat diet. In fact, several investigators (15, 17) have reported a decrease in plasma VLDL-TG concentration after 5 to 7 days of overfeeding a high-fat diet. Together, these results suggest that VLDL-TG metabolism, at least in the short-term, appears to be more sensitive to dietary macronutrient composition than energy content and the alterations in VLDL-TG kinetics that are associated with obesity are not due to overfeeding per se. This concept is further supported by the findings from a short-term calorie restriction study, in which 1 day of consuming a 30% calorie-reduced, mixed diet had no effect on VLDL-TG kinetics and plasma concentrations (36).
The acute/short-term effect of excess caloric intake on VLDL–apoB-100 kinetics has, to our knowledge, never been evaluated. Furthermore, only 1 study has examined the effect of chronic (100 days) overfeeding on VLDL–apoB-100 concentration and consistent with the results from our study, found that it increased (4). A dissociation in the response of VLDL-TG and VLDL–apoB-100 kinetics to dietary interventions was first noted by Melish et al (34) who found that changing dietary macronutrient composition affected the VLDL-TG but not VLDL–apoB-100 secretion rate in men. In our study, overfeeding increased the VLDL–apoB-100 (ie, VLDL particle) but not VLDL-TG secretion rate. Such uncoordinated changes in hepatic TG and apoB-100 secretion affect the density (ie, TG content) of newly secreted VLDL particles, which can have significant effects on intravascular VLDL metabolism and contribute to atherosclerotic plaque formation. Our results indicate that overfeeding, acutely at least, leads to the secretion of (on average) smaller (and denser) VLDL particles. Gaw et al (37) and Packard et al (38) demonstrated that hepatic secretion of small rather than large VLDL particles is associated with increased plasma concentrations of total low-density lipoprotein (LDL) and small, dense LDL, which are thought to be particularly atherogenic (39). On the other hand, an increased plasma concentration of large, more TG-rich VLDL (rather than small, dense VLDL particles) is also associated with coronary artery disease (40). These results are not necessarily inconsistent with each other. The concentration and size of circulating VLDL is not only determined by the secretion rate and size of the newly secreted particle but reflects the balance between the number and TG content of VLDL secreted into the circulation and the rate of TG removal from circulating VLDL via lipoprotein lipase-mediated lipolysis, neutral lipid exchange with other lipoproteins (eg, high-density lipoprotein), conversion of VLDL to smaller, denser lipoprotein particles (eg, intermediate-density lipoprotein and LDL), and direct uptake of VLDL by tissues. Hence, increased production of (on average) smaller, denser VLDL particles and increased plasma concentration of large, TG-rich VLDL are not mutually exclusive. Optimizing VLDL kinetics to achieve cardiovascular health through diet appears to require attention to both dietary energy and macronutrient content.
The mechanisms responsible for the increase in VLDL–apoB-100 secretion after a day of overfeeding remain uncertain but are likely related to the overfeeding-induced insulin resistance in the liver. Insulin is an important regulator of hepatic apoB-100 turnover and VLDL particle assembly and secretion and limits VLDL particle secretion; impaired insulin signaling due to insulin resistance limits the inhibitory effect of insulin on apoB-100 degradation and promotes VLDL particle assembly and secretion of VLDL particles (41, 42). Hepatic VLDL-TG secretion, however, was not affected by the overfeeding-induced insulin resistance, although insulin is also involved in regulating hepatic VLDL-TG secretion. Insulin 1) suppresses hepatic VLDL-TG secretion via forkhead box O1-mediated reduction in microsomal transfer protein activity (43) as well as the insulin-mediated suppression of adipose tissue lipolysis and thus plasma fatty acid availability for VLDL-TG synthesis (12) and 2) stimulates de novo lipogenesis and VLDL-TG secretion (44–46). However, alterations in insulin sensitivity (evaluated by probing glucose metabolism) are not always associated with changes in hepatic VLDL-TG secretion (3, 47), most likely because hepatic VLDL-TG secretion is less sensitive to insulin than hepatic glucose production (48). The changes in plasma insulin concentration and hepatic insulin sensitivity in our study were small, and plasma fatty acid availability was not affected by overfeeding in our study; furthermore, any overfeeding-induced increase in de novo lipogenesis probably only minimally affected the total hepatic VLDL-TG secretion rate because de novo lipogenesis contributes little to total hepatic VLDL-TG secretion, and it has been demonstrated that 1 day of carbohydrate overfeeding (providing 150% energy in excess of requirements) increased the rate of de novo lipogenesis severalfold but did not alter total VLDL-TG secretion (49).
Our study has several limitations: 1) we studied only overweight and obese men; 2) we evaluated the metabolic response to only a single day of overfeeding; 3) we evaluated only a single dose of excess energy intake; and 4) the amount of overfeeding in our study was relatively moderate. Therefore, it remains unclear whether longer duration or different degrees of mixed meal overfeeding would result in similar responses and/or whether similar responses to overfeeding would occur in other study populations (ie, lean subjects, women, etc,). The results from our study should therefore not be generalized.
In summary, the results from our study demonstrate that moderate excess energy intake rapidly (within a day) induces hepatic insulin resistance and stimulates VLDL particle secretion, whereas hepatic VLDL-TG secretion and FFA and β-hydroxybutyrate release into plasma are not affected by a single day of excess energy intake. A relatively modest (30%) positive energy balance due to excess energy intake per se therefore does not exacerbate hypertriglyceridemia in overweight/obese persons.
Acknowledgments
We thank Megan Steward and Rachel Burrows for help in subject recruitment, Freida Custodio and Jennifer Shew for their technical assistance, the staff of the Clinical Research Unit for their help in performing the studies, and the study subjects for their participation.
This publication was made possible by National Institutes of Health Grants HD 57796, DK 94483, DK 56341 (Nutrition and Obesity Research Center), RR024992 (Washington University School of Medicine Clinical Translational Science Award), and RR-00954 (Biomedical Mass Spectrometry Resource), and an American Heart Association fellowship award (0510015Z) to F.M.
This study was registered in ClinicalTrials.gov as trial number NCT00830999.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- apoB-100
- apolipoprotein B-100
- FFA
- free fatty acid
- FTR
- fractional turnover rate
- HIRI
- hepatic insulin resistance index
- LDL
- low-density lipoprotein
- Ra
- rate of appearance
- TG
- triglyceride
- TTR
- tracer-to-tracee ratio
- VLDL
- very-low-density lipoprotein.
References
- 1. Magkos F, Mohammed BS, Mittendorfer B. Effect of obesity on the plasma lipoprotein subclass profile in normoglycemic and normolipidemic men and women. Int J Obes (Lond). 2008;32:1655–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mittendorfer B, Patterson BW, Klein S. Effect of sex and obesity on basal VLDL-triacylglycerol kinetics. Am J Clin Nutr. 2003;77:573–579 [DOI] [PubMed] [Google Scholar]
- 3. Sørensen LP, Søndergaard E, Nellemann B, Christiansen JS, Gormsen LC, Nielsen S. Increased VLDL-triglyceride secretion precedes impaired control of endogenous glucose production in obese, normoglycemic men. Diabetes. 2011;60:2257–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Terán-García M, Després JP, Couillard C, Tremblay A, Bouchard C. Effects of long-term overfeeding on plasma lipoprotein levels in identical twins. Atherosclerosis. 2004;173:277–283 [DOI] [PubMed] [Google Scholar]
- 5. Samocha-Bonet D, Campbell LV, Mori TA, et al. Overfeeding reduces insulin sensitivity and increases oxidative stress, without altering markers of mitochondrial content and function in humans. PLoS ONE. 2012;7:e36320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh P, Somers VK, Romero-Corral A, et al. Effects of weight gain and weight loss on regional fat distribution. Am J Clin Nutr. 2012;96:229–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tam CS, Viardot A, Clément K, et al. Short-term overfeeding may induce peripheral insulin resistance without altering subcutaneous adipose tissue macrophages in humans. Diabetes. 2010;59:2164–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Conte C, Fabbrini E, Kars M, Mittendorfer B, Patterson BW, Klein S. Multiorgan insulin sensitivity in lean and obese subjects. Diabetes Care. 2012;35:1316–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prager R, Wallace P, Olefsky JM. In vivo kinetics of insulin action on peripheral glucose disposal and hepatic glucose output in normal and obese subjects. J Clin Invest. 1986;78:472–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106:15430–15435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lewis GF, Uffelman KD, Szeto LW, Steiner G. Effects of acute hyperinsulinemia on VLDL triglyceride and VLDL apoB production in normal weight and obese individuals. Diabetes. 1993;42:833–842 [DOI] [PubMed] [Google Scholar]
- 12. Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest. 1995;95:158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aarsland A, Chinkes D, Wolfe RR. Contributions of de novo synthesis of fatty acids to total VLDL-triglyceride secretion during prolonged hyperglycemia/hyperinsulinemia in normal man. J Clin Invest. 1996;98:2008–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ngo Sock ET, Lê KA, Ith M, Kreis R, Boesch C, Tappy L. Effects of a short-term overfeeding with fructose or glucose in healthy young males. Br J Nutr. 2010;103:939–943 [DOI] [PubMed] [Google Scholar]
- 15. Sobrecases H, Lê KA, Bortolotti M, et al. Effects of short-term overfeeding with fructose, fat and fructose plus fat on plasma and hepatic lipids in healthy men. Diabetes Metab. 2010;36:244–246 [DOI] [PubMed] [Google Scholar]
- 16. Nestel PJ, Carroll KF, Havenstein N. Plasma triglyceride response to carbohydrates, fats and caloric intake. Metabolism. 1970;19:1–18 [DOI] [PubMed] [Google Scholar]
- 17. Brøns C, Jensen CB, Storgaard H, et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J Physiol. 2009;587:2387–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 19. Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008;14:741–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;53(Suppl 1):S143–S151 [DOI] [PubMed] [Google Scholar]
- 21. James WP, McNeill G, Ralph A. Metabolism and nutritional adaptation to altered intakes of energy substrates. Am J Clin Nutr. 1990;51:264–269 [DOI] [PubMed] [Google Scholar]
- 22. Patterson BW, Mittendorfer B, Elias N, Satyanarayana R, Klein S. Use of stable isotopically labeled tracers to measure very low density lipoprotein-triglyceride turnover. J Lipid Res. 2002;43:223–233 [PubMed] [Google Scholar]
- 23. Smith GI, Atherton P, Reeds DN, et al. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin Sci (Lond). 2011;121:267–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Magkos F, Patterson BW, Mittendorfer B. Reproducibility of stable isotope-labeled tracer measures of VLDL-triglyceride and VLDL-apolipoprotein B-100 kinetics. J Lipid Res. 2007;48:1204–1211 [DOI] [PubMed] [Google Scholar]
- 25. Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res. 1999;40:2118–2124 [PubMed] [Google Scholar]
- 26. Mittendorfer B, Liem O, Patterson BW, Miles JM, Klein S. What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes. 2003;52:1641–1648 [DOI] [PubMed] [Google Scholar]
- 27. Boer P. Estimated lean body mass as an index for normalization of body fluid volumes in humans. Am J Physiol. 1984;247:F632-F-636 [DOI] [PubMed] [Google Scholar]
- 28. Reichl D. Lipoproteins of human peripheral lymph. Eur Heart J. 1990;11(Suppl E):230–236 [DOI] [PubMed] [Google Scholar]
- 29. Elovson J, Chatterton JE, Bell GT, et al. Plasma very low density lipoproteins contain a single molecule of apolipoprotein B. J Lipid Res. 1988;29:1461–1473 [PubMed] [Google Scholar]
- 30. McAdams MA, Van Dam RM, Hu FB. Comparison of self-reported and measured BMI as correlates of disease markers in US adults. Obesity. 2007;15:188–196 [DOI] [PubMed] [Google Scholar]
- 31. Mittendorfer B, Patterson BW, Klein S, Sidossis LS. VLDL-triglyceride kinetics during hyperglycemia-hyperinsulinemia: effects of sex and obesity. Am J Physiol Endocrinol Metab. 2003;284:E708–E715 [DOI] [PubMed] [Google Scholar]
- 32. Clore JN, Helm ST, Blackard WG. Loss of hepatic autoregulation after carbohydrate overfeeding in normal man. J Clin Invest. 1995;96:1967–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mittendorfer B, Sidossis LS. Mechanism for the increase in plasma triacylglycerol concentrations after consumption of short-term, high-carbohydrate diets. Am J Clin Nutr. 2001;73:892–899 [DOI] [PubMed] [Google Scholar]
- 34. Melish J, Le NA, Ginsberg H, Steinberg D, Brown WV. Dissociation of apoprotein B and triglyceride production in very-low-density lipoproteins. Am J Physiol. 1980;239:E354–E362 [DOI] [PubMed] [Google Scholar]
- 35. Blades B, Garg A. Mechanisms of increase in plasma triacylglycerol concentrations as a result of high carbohydrate intakes in patients with non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1995;62:996–1002 [DOI] [PubMed] [Google Scholar]
- 36. Bellou E, Siopi A, Galani M, et al. Acute effects of exercise and calorie restriction on triglyceride metabolism in women. Med Sci Sports Exerc. 2013;45:455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gaw A, Packard CJ, Lindsay GM, et al. Overproduction of small very low density lipoproteins (Sf 20–60) in moderate hypercholesterolemia: relationships between apolipoprotein B kinetics and plasma lipoproteins. J Lipid Res. 1995;36:158–171 [PubMed] [Google Scholar]
- 38. Packard CJ, Demant T, Stewart JP, et al. Apolipoprotein B metabolism and the distribution of VLDL and LDL subfractions. J Lipid Res. 2000;41:305–318 [PubMed] [Google Scholar]
- 39. Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43:1363–1379 [DOI] [PubMed] [Google Scholar]
- 40. Freedman DS, Otvos JD, Jeyarajah EJ, Barboriak JJ, Anderson AJ, Walker JA. Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease. Arterioscler Thromb Vasc Biol. 1998;18:1046–1053 [DOI] [PubMed] [Google Scholar]
- 41. Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2012;32:2104–2112 [DOI] [PubMed] [Google Scholar]
- 42. Vergès B. Abnormal hepatic apolipoprotein B metabolism in type 2 diabetes. Atherosclerosis. 2010;211:353–360 [DOI] [PubMed] [Google Scholar]
- 43. Kamagate A, Qu S, Perdomo G, et al. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest. 2008;118:2347–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Elam MB, Wilcox HG, Cagen LM, et al. Increased hepatic VLDL secretion, lipogenesis, and SREBP-1 expression in the corpulent JCR:LA-cp rat. J Lipid Res. 2001;42:2039–2048 [PubMed] [Google Scholar]
- 45. Park J, Lemieux S, Lewis GF, Kuksis A, Steiner G. Chronic exogenous insulin and chronic carbohydrate supplementation increase de novo VLDL triglyceride fatty acid production in rats. J Lipid Res. 1997;38:2529–2536 [PubMed] [Google Scholar]
- 46. Han S, Liang CP, Westerterp M, et al. Hepatic insulin signaling regulates VLDL secretion and atherogenesis in mice. J Clin Invest. 2009;119:1029–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nielsen S, Karpe F. Determinants of VLDL-triglycerides production. Curr Opin Lipidol. 2012;23:321–326 [DOI] [PubMed] [Google Scholar]
- 48. den Boer MA, Voshol PJ, Kuipers F, Romijn JA, Havekes LM. Hepatic glucose production is more sensitive to insulin-mediated inhibition than hepatic VLDL-triglyceride production. Am J Physiol Endocrinol Metab. 2006;291:E1360–E1364 [DOI] [PubMed] [Google Scholar]
- 49. Aarsland A, Wolfe RR. Hepatic secretion of VLDL fatty acids during stimulated lipogenesis in men. J Lipid Res. 1998;39:1280–1286 [PubMed] [Google Scholar]