Abstract
The interaction of DNA with Mn2+ was studied in absorbance and optical activity in the electronic and vibrational regions. Based on the data, several stages of the interaction were identified. Con formational transition towards the C-form of DNA was observed in solution at the molar ratio Mn2+/DNA-phosphates between 0.1 and 1.5. The exact ratio depended on the ionic strength and increased with increasing NaCl concentration. Although manganese interacted with the phosphates and bases of DNA at higher metal concentrations, it is unlikely that direct chelation occurred. A model for the interaction between manganese ions and DNA mediated by water is suggested destabilizing the double helix and partially breaking the hydrogen bonds between the base pairs. At high Mn2+ concentrations DNA aggregation was observed.
INTRODUCTION
Interaction of different metal ions with DNA has been studied for many years. Interest in these ions, which are known to be involved in DNA functioning in the living cell, has increased recently. Some of the metals interact directly with DNA, while others act as cofactors for a number of nuclear proteins. This study deals with the interaction of manganese(II) ions with DNA in aqueous solution and in the presence of non-histone chromatin protein HMGB1 (High Mobility Group protein 1). Mn2+ ions are able to change the enzymatic activity of some nuclear proteins. The well-known example is DNase I, which cuts both DNA strands in the presence of Mn2+. Manganese was found to be an essential element for a number of DNA-binding proteins, which possess structural rather than enzymatic activity (1,2). Several structural non-histone proteins significantly influence the activity of some of the regulatory factors that use manganese ions as a cofactor. One of these proteins is HMGB1, well known for its remarkable DNA-binding activity. To understand the molecular mechanisms it is necessary to have a picture of the metal ion interaction with DNA. It was demonstrated that manganese is involved in interactions with phosphate oxygens and with the bases of DNA (3,4,5). However, the particular mechanism is still uncertain and there is experimental evidence supporting different models. In our study we combined infrared (IR) and ultraviolet (UV) absorption and circular dichroism (CD) spectroscopy, which seems to be the first time that vibrational CD (VCD) and electronic CD (ECD) were used together to study this system. Based on the data a model of Mn–DNA interaction is proposed.
MATERIALS AND METHODS
Calf thymus DNA (Sigma) was used with further sonication as described below. All non-organic salts were of spectroscopic grade (Alfa Aesar), except sodium cacodylate (98% grade, Sigma Ultra). All aqueous solutions were prepared using double distilled deionized water. Heavy water (Sigma, 99.9% D2O) was used for the IR and VCD experiments.
DNA–Mn2+ complexes were obtained by direct mixing of equal volumes of the DNA and MnCl2/NaCl solutions of appropriate concentrations. The final solutions always had 500 µg/ml DNA and a [Mn]/[P] molar ratio (R) between 1/150 and 165/1 (0.01–250 mM MnCl2 absolute concentration, where [Mn] and [P] are the molar concentrations of manganese and DNA phosphates, respectively). The samples were incubated at room temperature for at least 20 min before the measurements.
All UV spectra of the complexes were recorded with a Jasco 715 spectropolarimeter (Jasco Corp., Japan) in 1 mm quartz cells. The spectra are the averages of three sequential accumulations for each sample between 200 and 320 nm using the standard Jasco software. The ECD spectra are presented in terms of mdeg rather than Δε. While this is contrary to common practice, the corresponding optical activities cannot be calculated since the spectra are superpositions taken simultaneously of DNA and protein having different concentrations.
In the DNA protonation experiment, the UV spectra were taken between 180 and 320 nm. The concentration of HCl was increased drop-wise up to 1 mM, and the spectra were taken immediately after addition of the acid.
For IR, initial stock solutions of DNA and metal ions were prepared in aqueous cacodylate buffer (10–2 M, pH 6.5 ± 0.5). Complete deuteration was achieved by lyophilizing and redissolving all solutions three times in D2O. The final concentration of the DNA stock solutions in D2O was determined by the absorption at 260 nm. DNA samples were contained in a demountable cell (International Crystal Laboratories, Inc.) composed of two BaF2 windows separated by a 50 µm Teflon spacer. DNA stock solution (30 µl) was deposited on one of the cell windows, and the metal ion solution (15 µl) of appropriate concentration added drop-wise, with continuous stirring using the tip of the micropipette, and covered with the second window. The final concentra tion of DNA in the sample was 40 mg/ml ([P] = 0.13 M). Mn2+ ion concentration was varied between 0 and 1.3 M (0–10 [Mn]/[P]). The temperature of the cell was maintained at 23 ± 0.5°C with a copper-constantan thermocouple (OMEGA Technology Co.).
The VCD and IR spectra were measured simultaneously in the range of 1800–750 cm–1 in D2O with the VCD instrument described elsewhere (6). A total of 4000 ac scans was accumulated (55 min scan time) ratioed against 500 dc scans for the VCD spectra, and 500 dc scans for the absorption spectra, all at 8 cm–1 resolution. The VCD spectra were corrected for polarization artefacts by subtracting the spectra of the solvent at the same conditions. The absorption and VCD spectra were interpolated with a factor 8 according to the 4-point cubic spline algorithm supplied with the Lab Calc package (Lab Calc, 1992). Several sets of IR and VCD spectra of DNA with Mn2+ ions were recorded independently at different times. The objective of one set was to record the changes in the spectra as they are affected by temperature (7,8), and for the other to observe the influence of Mn2+ ions on DNA structure in the presence of HMGB1 proteins (Polyanichko et al., in preparation).
High molecular weight DNA solutions were sonicated at 0°C (ice-chilled) for a total of 5 min. The molecular weight of the DNA samples was checked by agarose gel-electrophoresis from which the maximum length of the fragments was determined to be ∼550 bp by scanning the gel-electrophoretic page.
RESULTS
UV experiment
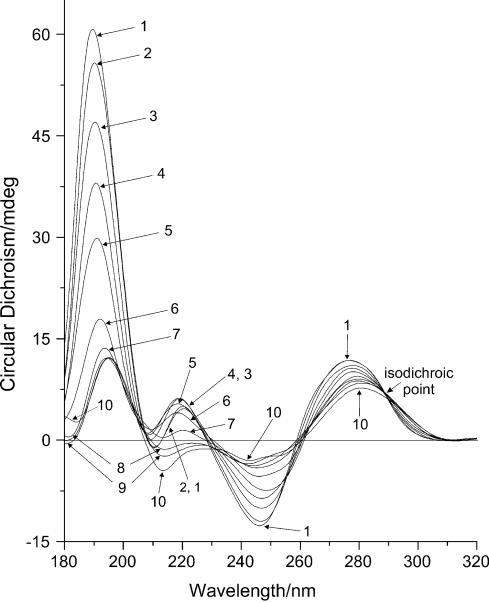
All series of ECD spectra show similar behaviour of the systems (Figs 1,2,3), although several differences can be observed. The most significant changes in optical activity occurred for the major positive band with maximum at 277 nm. The intensity of this band decreased gradually with increasing Mn2+ concentration and finally dropped to ∼0.4 times the initial value. In the process, the maximum shifted to longer wavelengths reaching 283 nm accompanied by changes of the band shape with a second maximum emerging at 273 nm. Both the change in shape and the maximum shift occurred at some intermediate [Mn]/[P] ratio depending on the NaCl concentration. The ECD spectra reflect the changes in intensity at 280 nm with increasing manganese ion concentration at particular NaCl concentrations (Fig. 4).
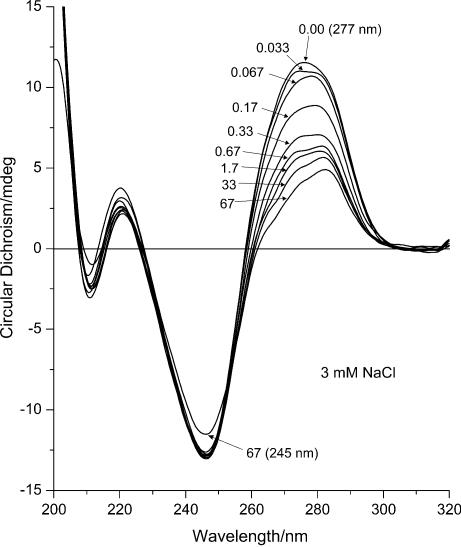
Figure 1.
ECD (mdeg) of DNA–Mn2+ complexes at 3 mM NaCl. The curves represent DNA spectra at different manganese concentrations in sequence: 1, 0 mM MnCl2; 2, 0.05 mM (R = 0.033); 3, 0.10 mM (R = 0.067); 4, 0.25 mM (R = 0.17); 5, 0.50 mM (R = 0.33); 6, 1.00 mM (R = 0.67); 7, 2.50 mM (R = 1.7); 8, 50.00 mM (R = 33); 9, 100 mM (R = 67) MnCl2.
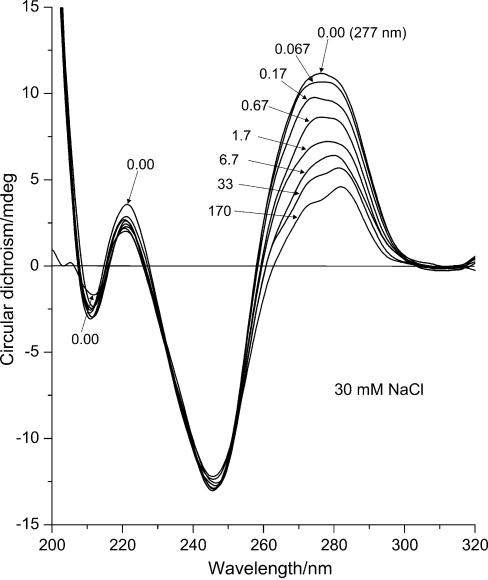
Figure 2.
ECD (mdeg) of DNA–Mn2+ complexes at 30 mM NaCl. The curves represent DNA spectra at different manganese concentrations in sequence: 1, 0 mM MnCl2; 2, 0.10 mM (R = 0.067); 3, 0.25 mM (R = 0.17); 4, 1.00 mM (R = 0.67); 5, 2.50 mM (R = 1.7); 6, 10.00 mM (R = 6.7); 7, 50.00 mM (R = 33); 8, 250 mM (R = 170) MnCl2.
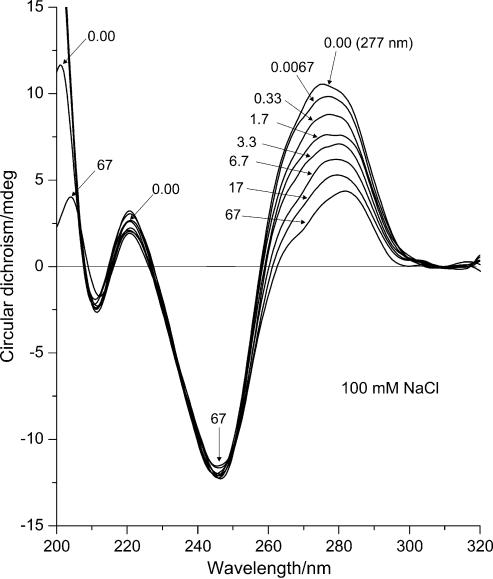
Figure 3.
ECD (mdeg) of DNA–Mn2+ complexes at 100 mM NaCl. The curves represent DNA spectra at different manganese concentrations in sequence: 1, 0 mM MnCl2; 2, 0.01 mM MnCl2 (R = 0.0067); 3, 0.5 mM (R = 0.33); 4, 2.50 mM (R = 1.7); 5, 5.00 mM (R = 3.3); 6, 10.00 mM (R = 6.7); 7, 25.00 mM (R = 17); 8, 100 mM (R = 67) MnCl2.
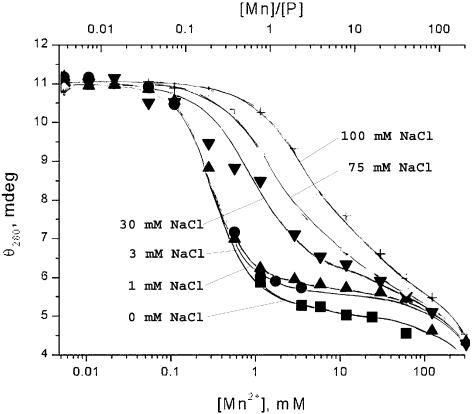
Figure 4.
Optical activity of DNA–Mn2+ complexes (ECD, elipticity θ at 280 nm in mdeg) versus MnCl2 concentration (mM, bottom scale), and molar ratio of Mn2+ to DNA phosphate (R, top scale) at different NaCl concentrations: square, 0 mM NaCl; round spot, 1 mM NaCl; triangle, 3 mM NaCl; inverted triangle, 30 mM NaCl; diamond, 75 mM NaCl; plus sign, 100 mM NaCl.
In contrast to the major positive ECD peak, the major negative band with minimum at 245 nm remained unchanged. This band at maximum NaCl concentration is ∼2.5 times more intense than the positive lobe and hence resembles the spectrum of C-DNA rather than B-DNA (9). Upon further increasing the MnCl2 concentration the optical activity of the samples decreased significantly (data not shown), which is due to DNA aggregation and precipitation rather than changes to its structure as was suggested previously (10).
There are no significant differences in the 205–230 nm region. The systems with low salt concentration, i.e. <3 mM NaCl (data not shown), demonstrate a similar behaviour as the 3 mM NaCl series (Fig. 1). For the systems with 3 mM NaCl concentration and greater, the minor positive and negative bands become symmetric, i.e. the intensity of the positive band decreases while that of the negative band increases until it reaches the same magnitude. This shift, which is similar to the behaviour of the bands upon increasing the ionic strength of the solutions, likely indicates the stabilization of the double helix.
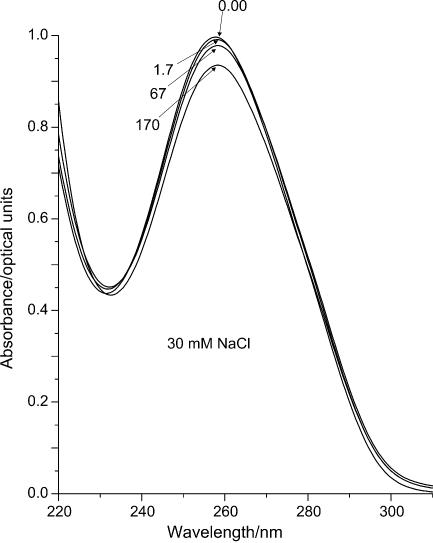
The UV absorption spectra of DNA show only minor intensity changes (Fig. 5 for 30 mM NaCl; for all other NaCl concentrations the spectra are similar and are not shown). A small decrease of the absorption upon increasing the Mn2+ concentration suggests stabilization of the double helical structure due to the higher concentration of counter ions in the solution. There is also a slight shift (within 2–3 nm) of the maximum towards longer wavelength. The drop of the intensity of the last spectrum in the series, viz., 250 mM (R = 170) MnCl2, is also quite typical and corresponds to the slight precipitation that started in the solution.
Figure 5.
Typical UV absorbance spectra of DNA–Mn2+ complexes in 30 mM NaCl at different Mn2+ concentrations: 1, 0 mM MnCl2; 2, 2.50 mM MnCl2 (R = 1.7); 3, 100 mM MnCl2 (R = 67); 4, 250 mM MnCl2 (R = 170).
IR experiment
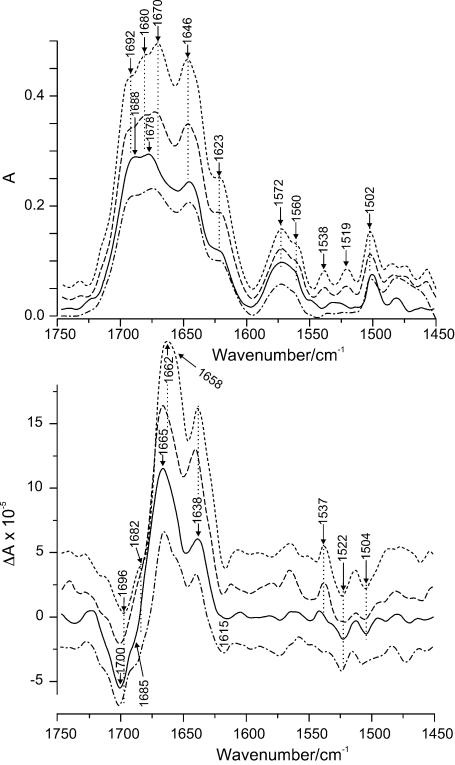
The absorption and VCD spectra of DNA without metal ions in the region of the carbonyl and in-plane ring modes of the bases (Fig. 6) correspond with those of the B-form (8,11–13). Interaction with Mn2+ ions leads to some informative changes in the spectra particularly for the VCD couplets. (A couplet is a pair of positive and negative peaks arising from through-space coupling of characteristic vibrations of molecular groups located in spatial proximity to one another.) As the Mn2+ concentration increases, the intensity of the spectra generally drops slightly at first and then increases significantly. This behaviour is likely due to the stabilization of the DNA double helix at the beginning, which is similar to the behaviour in the UV experiment (14,15). Further intensity increases thereafter are due to stronger interaction of the manganese ions with particular chemical groups of the DNA.
Figure 6.
IR absorbance (top) and VCD (bottom) spectra of DNA–Mn2+ complexes in carbonyl range at different molar ratios, R, of Mn2+ to DNA phosphates: solid, DNA, R = 0; dash-dot-dash, R = 0.9; long dashes, R = 3.8; short dashes, R = 10.
The region between 1710 and 1620 cm–1 consists of strong bands arising from the carbonyl stretching modes of thymine, guanine and cytosine. Although the complex band is best described as a superposition of all these modes, some more specific assignments can be suggested (8,13,16). After the addition of MnCl2 the absorption peak of pure DNA at 1688 cm–1 due to C2=O2 stretching of thymine shifts to 1692 cm–1, while the corresponding negative VCD peak at 1700 cm–1 shifts to 1696 cm–1. If there is a positive lobe for this mode, its position has not yet been identified clearly. The absorption maximum at 1678 cm–1 of DNA alone, which likely is a superposition of the C=O stretch of guanine and cytosine, splits into two peaks with increasing Mn2+ concentration with maxima at 1680 and 1670 cm–1 accompanied by a significant intensity increase. The first of these two peaks can be attributed to C=O stretching of guanine. Its negative VCD component corresponds to the shoulder at 1685 cm–1, which shifts to 1682 cm–1 and becomes more pronounced. The positive VCD peak at 1665 cm–1 is broadened with maximum at 1662 cm–1 and with an apparent shoulder emerging at ∼1658 cm–1. These changes likely arise from a superposition of two couplets, one due to guanine corresponding to the absorption maximum at 1680 cm–1 and the other to the absorption 1670 cm–1.
An absorption at 1646 cm–1, which is due either to an in-plane ring vibration of cytosine or C4=O4 of thymine or both, and the band at 1623 cm–1 arising from a ring in-plane vibration of adenine, thymine and cytosine (8,13) show no significant wave number shifts but do increase in intensity. The corresponding VCD couplet due to adenine and thymine at 1638(+)/1619(–) cm–1 also shows only minor changes, namely a slight intensity increase and a shift of the negative peak to ∼1615 cm–1. No changes are observed ∼1590 cm–1, where the in-plane ring modes including the C=N stretch of the guanine occur, and the interaction of different ligands with N7 of guanine is usually expressed (8,17). The wide absorption band with maximum at 1572 cm–1, which actually is a superposition of three peaks attributed to vibrations of the purines, also shows a slight increase. It begins to split into two peaks at 1572 and 1560 cm–1, which may be ascribed to C–N(CD2) and C=N stretch of guanine, respectively (18). Two new peaks appear in absorption at 1538 and 1519 cm–1, which normally occur only weakly. They were assigned to ring in-plane vibrations of cytosine (8,18,19). Both become more pronounced in the presence of manganese ions. A corresponding VCD couplet for the former develops at 1537(+)/∼1522(–) cm–1 and becomes particularly prominent at the highest Mn concentration, whereas the position for the latter is not clearly distinguishable. Finally, the absorption at 1502 cm–1 due to C=N stretching of cytosine increases in intensity (8,17,18).
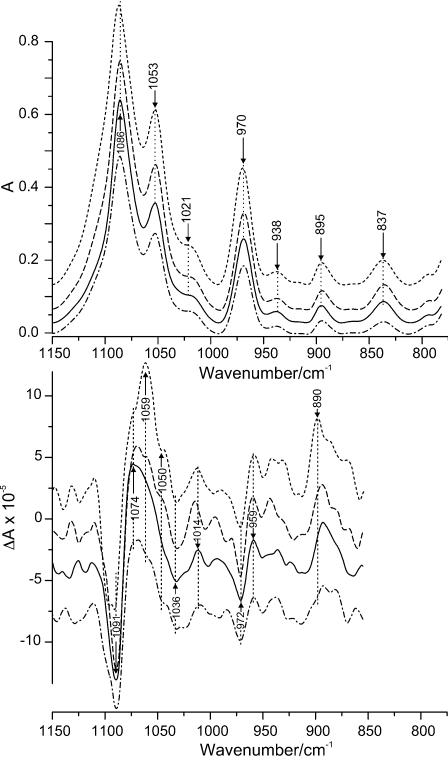
The strongest absorption in the phosphate backbone region arises from the symmetric vibration of the O=P=O group (Fig. 7). The maximum of this band is located at 1086 cm–1. It does not change position significantly upon interaction with Mn2+ ions, nor does the corresponding VCD couplet at 1091(–)/1074(+) cm–1. However, the intensities of both features are sensitive to metal ion binding as they grow gradually with Mn2+ concentration. The next peak at 1053 cm–1 arises from the C–O stretch of the sugar. This mode is also stable in wave number and becomes more intense with Mn2+ concentration. The corresponding VCD displays changes to a much greater extent. In the spectrum of alone DNA the positive lobe is very broad without distinct features. In the presence of Mn2+, this lobe develops into a distinct shoulder at 1074 cm–1 and further into a distinct and comparatively strong couplet at 1059(+)/1050(–) cm–1. No changes of note occur for the deoxyribose absorption at 1021 cm–1, whereas two couplets emerge at ∼1045(+)/1036(–) and ∼1025(–)/1014(+) cm–1, which increase slightly in intensity. Neither the sugar absorption at 970 cm–1 nor its corresponding couplet at 972(–)/959(+) cm–1 show noticeable changes as a result of the interaction with Mn2+. The peaks at 938, 895 and 837 cm–1, known as the B-DNA markers (19,20), are stable with only minor intensity changes confirming that the DNA structure remains within the B-family when interacting with the metal ions. Although the VCD spectrum shows several other features below ∼950 cm–1, they have to be viewed with caution due to decreasing light throughput of the photoelastic modulator below ∼900 cm–1.
Figure 7.
IR absorbance (top) and VCD (bottom) spectra of DNA–Mn2+ complexes in phosphate range at different molar ratio, R, of Mn2+ to DNA phosphates: solid, DNA, R = 0; dash-dot-dash, R = 0.9; long dashes, R = 3.8; short dashes, R = 10.
DISCUSSION
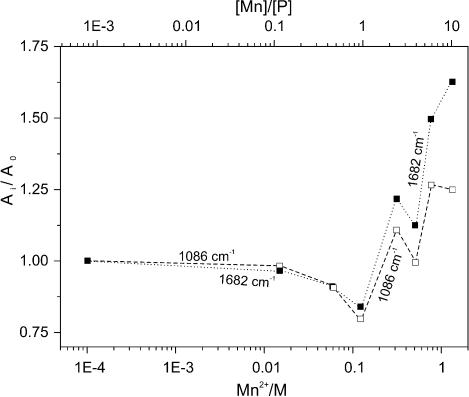
Both the IR (Fig. 8) and UV (Fig. 5) data show that DNA absorbance slightly decreases at low [Mn]/[P] ratio (R). At the same time, both minor bands ∼220 nm in the ECD spectrum become more negative on the CD scale while their shapes remain unchanged (Fig. 1). This is especially pronounced at low NaCl concentrations, which suggests that these changes reflect a slight stabilization of the DNA structure with increasing ionic strength. At this stage no shift in the position of the band maxima occurred either in UV or in the carbonyl region of the IR spectra, indicating that there is no spectral evidence of manganese interaction with the DNA bases. Based on these two observations, we conclude that the observed effects are due only to the interaction between manganese cations and the phosphate oxygens (8). There are no other changes in the spectra up to R ≈ 0.1.
Figure 8.
Relative intensity of IR absorptions in the presence of Mn2+ concentration compared with the intensities without MnCl2 (Ai/Ao): solid squares, carbonyl band at 1682 cm–1; open squares, symmetric phosphate (PO2–) band at 1086 cm–1.
When R reaches the level of 0.1, the optical activity in UV at 277 nm and the IR absorbance of DNA begin to change simultaneously (Figs 1 and 8), whereas the UV absorbance and CD at 245 nm stay unchanged (Figs 1 and 5). This signals the beginning of conformational rearrangements in the DNA structure. According to the CD data, this rearrangement demonstrates the behaviour of a phase transition (Fig. 4). The shape of the CD spectrum of the final state resembles the CD spectrum of C-form of DNA (9). The exact R values at which the transition starts and ends depend on the ionic strength of the solution. In the presence of NaCl, the mid-point of the transition curve shifts towards higher R values (Fig. 4). This shift demonstrates almost linear behaviour with increasing ionic strength up to ∼90 mM NaCl, after which the behaviour changes significantly. At low NaCl concentrations, the transition takes place between R = 0.1 and 1.0. This matches exactly the range in which a significant decrease of intensity in the IR spectra occurs (Fig. 8).
Further increasing the manganese concentration (1 < R < 20) at low NaCl concentrations produces virtually no changes in ECD intensity (Fig. 4) indicating the end of the conformational transition. However, the shape and intensity of the positive ECD band changes significantly. The single maximum at 277 nm splits into two peaks at 273 and 283 nm (Fig. 1). The maximum of the DNA absorbance band shifts 1–2 nm to longer wavelengths (Fig. 5). These changes indicate that the electronic structure of the guanine ring is affected as a result of the interaction with the N7 atom. However, it is not the result of a direct interaction of the metal ions with the guanine bases. According to the protonation measurements with DNA (Fig. 9), direct protonation on N7(G) leads to a significant shift of the absorption and ECD bands towards longer wavelengths. This shift is clearly marked by the presence of the isodichroic point in the spectra of the titration series (289 nm in this set of measurements). The process is accompanied by a strong decrease of the negative ECD intensity at 245 nm. None of these spectral features could be observed upon the interaction with manganese ions. Although there are slight changes in electronic structure of the bases as well as in the vibrational modes as shown below, there still are no significant conformational changes in DNA. This is confirmed by the previous studies of DNA viscosity and rigidity in solutions in the presence of Mn2+ ions (21,22). In these experiments it was shown that in this range of manganese concentration, neither intrinsic viscosity nor persistence length of DNA depend on metal ion concentration.
Figure 9.
ECD (mdeg) of DNA upon the gradual increase of HCl concentration from 0 mM (curve 1) to 1 mM (curve 10). The numbers of the curves reflect the order of the spectra corresponding to the gradual increase of HCl concentration.
The shapes of the VCD features in the base and phosphate regions are modified appreciably upon the further increase of Mn2+ concentration indicating a significant extent of metal ion interaction with both the bases and phosphate groups. The most significant changes in the carbonyl region can be attributed to the vibrational modes of guanine and cytosine. The maximum at 1678 cm–1 shifts to 1670 cm–1 and splits into two peaks at 1680 and 1670 cm–1 making it possible to distinguish between the vibrations of C=O of guanine and of cytosine upon interaction with Mn2+ ions. There are two reasons for this to occur: (i) an interaction of some ligand with the guanine ring at N7 when cytosine is not involved in the complexation, and/or (ii) an interaction of the metal ions with the C=O group of cytosine. Both processes can take place at this Mn2+ concentration. The first can arise because N7 of guanine has a high affinity for metal ions and is easily accessible in the major groove. The second would be due to fluctuant opening of the GC base pair exposing C=O of cytosine normally involved in base pair hydrogen bonding (23). Moreover, as Mn2+ ion concentration increases, chelation between phosphate oxygens and the bases can occur. Such a binding mode is unfavourable for the stability of the base pairs thereby inducing more GC openings and opportunities for Mn2+ binding to C=O of cytosine. This idea is supported by the increase of the first absorption peak of guanine followed by the relative intensity increase of the C=O peak of cytosine. The increase of the latter is accompanied by a shift of the positive VCD peak at 1665–1662 cm–1, which is likely due to an increase in optical activity of the C=O mode of cytosine and changes the intensity of the overlapping VCD bands. The change of the vibrations of the guanine ring is also confirmed by the better definition of the peak at 1560 cm–1. However, there are no changes in the vicinity of 1590 cm–1 essential for concluding that a direct interaction of the metal ions with N7(G) occurs (17). A further increase of the intensity of the bands at 1086, 1053 and 970 cm–1 reflects a significant influence on the vibrations in the phosphate backbone.
Thus, our data demonstrate good agreement with previous results (21,22,24) that indicate possible chelation between phosphate oxygens and the bases in DNA. However, they favour indirect water-mediated Mn2+ interaction with DNA rather than direct coordination of the ions to DNA. According to the previous studies of proton relaxation enhancement (5), not >20% of the manganese ions are involved in direct interactions with DNA phosphates. The other 80% are placed in the second and further hydration layers, but their influence on the DNA structure is still appreciable. This indirect influence is possible because of the strong dependence of the DNA conformation on the arrangement of the surrounding water molecules. Substitution of the water molecules by Mn2+ ions leads to the remarkable changes in the structure of the hydration layers and hence the DNA conformation. Therefore, the spectral changes in the phosphate region described above are caused by indirect interaction with Mn2+ via water molecules. Qualitatively, similar changes in the carbonyl region can then be explained in the same way. Interactions mediated by water molecules will cause only slight changes in the electronic structure of the bases, which will result neither in significant shifts of absorption maxima nor in the appearance of new bands. The presence of the polar molecules associated with the heavy and charged manganese ions, is able to change the vibrational modes of the bases, but not enough for the weak band at 1590 cm–1 to arise in the IR absorbance spectrum. The possibility of this approach is supported by several previous experiments (25–28), where distortions of DNA hydration layer upon the interaction with Mn2+ ions were reported.
Upon further increasing the manganese ion concentration the behaviour of the system changes as is evident from the IR spectra. Whereas the curve for the symmetric phosphate absorption reaches a plateau at R > 6, no such a plateau can be noted for the carbonyl curve (Fig. 8). This may indicate saturation of the phosphate-binding sites at R > 6, while no saturation of the sites on the bases may occur due to the involvement of new binding sites on the bases at high metal ion concentration. The latter effect may possibly be due to partial destabilization of the double helix. The intensity increase of the absorption bands is accompanied by an increasing amplitude of the corresponding VCD couplets as the concentration of Mn2+ increases (Fig. 6). Moreover, the appearance and further gradual increase of the absorbance bands at 1538 and 1519 cm–1 as well as the corresponding couplet at 1537(+)/1522(–) cm–1 in the VCD spectrum indicate that there are some N3(C) atoms involved in the interaction with the metal ions (17,29). Since this atom is involved in base pairing in the double helix, these peaks reflect the formation of single-stranded regions in DNA. The interaction with N3(C) affects the vibrational modes of the cytosine ring and results in a significant increase of both the absorbance at 1500 cm–1 and the VCD couplet at 1504(–)/1483(+) cm–1. The further separation of the guanine/cytosine peaks in absorption and the increase of their relative intensity might occur for the same reason, namely, a destabilization of the double helical structure could lead to the interaction of the Mn2+ ions with the carbonyl groups, which were involved before in hydrogen bonding. The enhancement of the purine peaks at 1623 and 1572 cm–1 might indicate possible involvement of this base in this kind of interaction at high manganese concentrations. This confirms the assumption about an increasing possibility for metal coordination to a base and a phosphate at the same time, which is unfavourable for double helix stability. However, no noticeable decrease of the VCD signal in the spectrum of DNA at R = 10 as compared with that without metal ions can be seen. DNA denaturation in the whole range of metal ion concentrations studied here can therefore be ruled out. This is confirmed by the shape of the absorption spectrum of DNA at R = 10 and by absence of a shift of the sugar absorption at 1053 cm–1 (11,14). It is known that Mn2+ ions at high concentrations significantly decrease the DNA melting temperature due to the destabilization of the double helix (30–32). Therefore, some extent of opening of base pairs cannot be ruled out completely; however significant DNA denaturation can definitely be excluded.
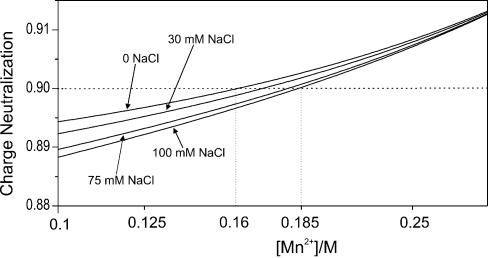
At the values of R > 20, the spectral intensity as detected by UV measurements begins to change again (Fig. 4). Since both absorbance and optical activity of the system drop in the whole range, this is likely the beginning of DNA aggregation and further precipitation at R > 100, but not a new DNA conformation with low optical activity, as was suggested previously (10). According to the approach suggested by Wilson and Bloomfield (33), DNA condensation can occur when at least 90% of negative phosphate charges are neutralized by counterions. These authors suggested a procedure for calculating numerically the charge neutralization based on the polyelectrolyte theory (34). Application of the approach to our system is illustrated in Figure 10, where the phosphate charge neutralization as it varies with [Mn2+] is calculated for different NaCl concentrations. Accordingly, the 90% neutralization level is achieved between 160 and 185 mM MnCl2 depending on sodium chloride concentration, which is in good agreement with our data.
Figure 10.
Degree of charge neutralization of DNA phosphates upon the increase of Mn2+ concentration in solution at different NaCl concentrations: 1, no NaCl added; 2, 30 mM NaCl; 3, 75 mM NaCl; 4, 100 mM NaCl.
Thus, we have observed two possible effects of manganese ions on DNA structure: (i) the neutralization of the negative charge of the phosphate backbone and stabilization of the double helical structure and (ii) the prevention of DNA renaturation by interaction with sites of the bases not involved in base pairing. Destabilization of the double helix and ‘freezing’ those parts that are already distorted may be of great biological importance. It may be a general mechanism for Mn2+ interaction as a cofactor for a number of regulatory and architectural factors, which need Mn2+ ions as a cofactor. For example, it was recently reported that the effect of manganese ions on the dynamics of translesion DNA synthesis could be explained assuming the ability of Mn2+ to both stabilize and destabilize DNA structure (35). However, there were no data confirming this possibility. The ability of the manganese ions to disturb the structure of the DNA hydration layer and to interact via water molecules should also be taken into account as a possible mechanism for hydrogen bonding influence. According to the recent structural studies in some cases when enzymatic activity is facilitated by Mn2+, the ions establish interaction not only with amino acids of the enzyme’s active centre but also with water molecules (36). The importance of this mechanism arises from the fact that the coordination number of Mn2+ is six, but very often the ions coordinate with only four amino acids and/or phosphate groups (36–38). The other two possibilities for coordination occur usually via water molecules. In cases of some particular nuclease reactions (37), the ions either interact with phosphates or are exposed to the active centre of the enzyme, which in the case of the nucleases is in the proximity of the DNA. Another example of the functioning of manganese deals with the well known architectural protein HMGB1. In the presence of Mn2+ this protein can increase the activity of the RAG1/2 complex (3). The HMGB-Box proteins are well known for their ability to recognize different kinds of distortions of DNA including hairpins, which are critical for the functioning of the RAG1/2 protein, for whose formation Mn2+ can be involved. However, our further studies of the interaction between HMGB1 and DNA in the presence of manganese ions suggest that the functioning of the HMGB protein at some stages of recombination events differ from its usual properties. Based on the results obtained in the present study, the influence of manganese on HMGB1/DNA interaction and biological function is discussed elsewhere.
Acknowledgments
ACKNOWLEDGEMENTS
The authors wish to acknowledge financial support from the Alberta Heritage Foundation for Medical Research (AHFMR) (Postdoctoral Fellowship to V.V.A.), the Russian Foundation for Basic Research (A.M.P., E.V.C. and V.I.V., grants 01-04-49196, 02-04-81012 Bel, 00-15-97824 and MAC 00-15-97824), and the Natural Sciences and Engineering Research Council of Canada (H.W.).
REFERENCES
- 1.Santagata S., Aidinis,V. and Spanopoulou,E. (1998) The effect of Me2+ cofactors at the initial stages of V(D)J recombination. J. Biol. Chem., 273, 16325–16331. [DOI] [PubMed] [Google Scholar]
- 2.Shockett P.E. and Schatz,D.G. (1999) DNA hairpin opening mediated by the RAG1 and RAG2 proteins. Mol. Cell. Biol., 19, 4159–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sissoeff I., Grisvard,J. and Guille,E. (1976) Studies of metal ions—DNA interactions: specific behaviour of reiterative DNA sequences. Prog. Biophys. Mol. Biol., 31, 165–199. [DOI] [PubMed] [Google Scholar]
- 4.Granot J., Feigon,J. and Kearns,D.R. (1982) Interaction of DNA with divalent metal ions. I. 31P-NMR studies. Biopolymers, 21, 181–201. [DOI] [PubMed] [Google Scholar]
- 5.Granot J., Feigon,J. and Kearns,D.R. (1982) Interaction of DNA with divalent metal ions. II. Proton relaxation enhancement studies. Biopolymers, 21, 203–218. [DOI] [PubMed] [Google Scholar]
- 6.Tsankov D., Eggimann,T. and Wieser,H. (1995) Alternative design for improved FT-IR/VCD capabilities. Appl. Spectrosc. Rev., 49, 132–138. [Google Scholar]
- 7.Andrushchenko V.V. (2000) VCD and IR spectroscopic study of metal ion induced structural changes of synthetic and natural nucleic acids at different temperatures. Ph.D. Thesis. The University of Calgary, Canada.
- 8.Andrushchenko V., van de Sande,H. and Wieser,H. (2003) DNA interaction with Mn2+ ions at elevated temperatures: VCD evidence of DNA aggregation. Biopolymers, 69, 529–545. [DOI] [PubMed] [Google Scholar]
- 9.Ivanov V.I., Minchenkova,L.E., Schyolkina,A.K. and Poletayev,A.I. (1973) Different conformations of double-stranded nucleic acid in solution as revealed by circular dichroism. Biopolymers, 12, 89–110. [DOI] [PubMed] [Google Scholar]
- 10.Patil S.D. and Rhodes,D.G. (2000) Influence of divalent cations on the conformation of phosphorothioate oligodeoxynucleotides: a circular dichroism study. Nucleic Acids Res., 28, 2439–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuboi M. (1974) Infrared and Raman spectroscopy. In Ts’o,P.O.P. (ed.), Basic Principles in Nucleic Acid Chemistry. Academic Press, New York, Vol. I, pp. 400–451. [Google Scholar]
- 12.Taillandier E., Liquier,J. and Taboury,J.A. (1985) Infrared spectral studies of DNA conformations. In Clark,R.J.H. and Hester,R.E. (eds), Advances in Infrared and Raman Spectroscopy. Wiley Heyden, New York, Vol. 12, pp. 65–114. [Google Scholar]
- 13.Andrushchenko V., Leonenko,Z., Cramb,D., van de Sande,H. and Wieser,H. (2002) VCD and AFM study of DNA Interaction with Cr3+ ions. VCD and AFM evidence of DNA condensation. Biopolymers, 61, 243–260. [DOI] [PubMed] [Google Scholar]
- 14.Tajmir-Riahi H.A., Ahmad,R. and Naoui,M. (1993) Interaction of calf thymus DNA with trivalent La, Eu and Tb ions. Metal ion binding, DNA condensation and structural features. J. Biomol. Struct. Dyn., 10, 865–877. [DOI] [PubMed] [Google Scholar]
- 15.Tajmir-Riahi H.A., Naoui,M. and Ahmad,R. (1993) The effects of Cu2+ and Pb2+ on the solution structure of calf thymus DNA: DNA condensation and denaturation studied by Fourier transform IR difference spectroscopy. Biopolymers, 33, 1819–1827. [DOI] [PubMed] [Google Scholar]
- 16.Tsuboi M. (1969) Application of infrared spectroscopy to structure studies of nucleic acids. Appl. Spectrosc. Rev., 3, 45–90. [Google Scholar]
- 17.Fritzsche H. and Zimmer,C. (1968) Infrared studies of deoxyribonucleic acids, their constituents and analogues. 4. The binding sites of copper (II) in DNA. Eur. J. Biochem., 5, 42–44. [DOI] [PubMed] [Google Scholar]
- 18.Andrushchenko V.V., Wieser,H. and Bour,P. (2002) B-Z conformational transitions of nucleic acids monitored by vibrational circular dichroism. Ab initio interpretation of the experiment. J. Phys. Chem. B, 106, 12623–12634. [Google Scholar]
- 19.Taillandier E., Liquier,J. and Taboury,J.A. (1985) Infrared spectral studies of DNA conformations. In Clark,R.J.H. and Hester,R.E. (eds), Advances in Infrared and Raman Spectroscopy. Wiley Heyden, New York, Vol. 12, pp. 65–114. [Google Scholar]
- 20.Taillandier E. (1990) Nucleic acid conformations studied by vibrational spectroscopy. In Sarma,R.H. and Sarma,M.H. (eds), Structure and Methods, Adenine Press, New York, Vol. 3, pp. 73–78. [Google Scholar]
- 21.Kas'ianenko N.A., D’iakonova,N.E. and Frisman,E.V. (1989) A study of the molecular mechanism of DNA interaction with divalent metal ions. Mol. Biol. (Mosk.), 23, 975–982. [PubMed] [Google Scholar]
- 22.Kos'ianenko N.A., Selman-Housein Sosa,G., Uverskii,V.N. and Frisman,E.V. (1987) Effect of Mn2+ and Mg2+ ions on DNA conformation. Mol. Biol. (Mosk.), 21, 140–146. [PubMed] [Google Scholar]
- 23.Blagoi Yu.P., Galkin,V.L., Gladchenko,G.O., Kornilova,S.V., Sorokin,V.A. and Shkorbatov,A.G. (1991) Metal Complexes of Nucleic Acids in Solutions. Naukova Dumka, Kiev (in Russian), p. 272. [Google Scholar]
- 24.Eichhorn G.L. and Shin,Y.A. (1968) Interaction of metal ions with polynucleotides and related compounds. XII. The relative effect of various metal ions on DNA helicity. J. Am. Chem. Soc., 90, 7323–7328. [DOI] [PubMed] [Google Scholar]
- 25.Yamada A., Akasaka,K. and Hatano,H. (1976) Proton and phosphorus-31 magnetic relaxation studies on the interaction of polyriboadenylic acid with Mn2+. Biopolymers, 15, 1315–1331. [DOI] [PubMed] [Google Scholar]
- 26.VanSteenwinkel R., Campagnari,F. and Merlini,M. (1981) Interaction of Mn2+ with DNA as studied by proton-relaxation enhancement of solvent water. Biopolymers, 20, 915–923. [DOI] [PubMed] [Google Scholar]
- 27.Fujimoto B.S., Miller,J.M., Ribeiro,N.S. and Schurr,J.M. (1994) Effect of different cations on the hydrodynamic radius of DNA. Biophys. J., 67, 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanlon S., Chan,A. and Berman,S. (1978) Specific cation effects on conformational transitions of DNA in aqueous solutions. Biochim. Biophys. Acta, 519, 526–536. [DOI] [PubMed] [Google Scholar]
- 29.Zimmer C., Luck,G., Fritzsche,H. and Triebel,H. (1971) DNA-Copper(II) complex and the DNA conformation. Biopolymers, 10, 441–463. [DOI] [PubMed] [Google Scholar]
- 30.Yurgaitis A.P. and Lazurkin,Y.S. (1981) Mechanism of DNA denaturation in the presence of manganese ions. Biopolymers, 20, 967–975. [Google Scholar]
- 31.Knoll D.A., Fried,M.G. and Bloomfield,V.A. (1988) Heat-induced DNA aggregation in the presence of divalent metal salts. In Sarma,R.H. and Sarma,M.H. (eds), Structure and Expression. Adenine Press, New York, Vol. 2, pp. 123–145. [Google Scholar]
- 32.Duguid J., Bloomfield,V.A., Benevides,J. and Thomas,G.J.,Jr (1995) Raman spectroscopy of DNA-metal complexes. II. The thermal denaturation of DNA in the presence of Sr2+, Ba2+, Mg2+, Mn2+, Co2+, Ni2+ and Cd2+. Biophys. J., 69, 2623–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson R.W. and Bloomfield,V.A. (1979) Counterion-induced condensation of deoxyribonucleic acid. A light-scattering study. Biochemistry, 18, 2192–2196. [DOI] [PubMed] [Google Scholar]
- 34.Manning G.S. (1978) The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q. Rev. Biophys., 11, 179–246. [DOI] [PubMed] [Google Scholar]
- 35.Hays H. and Berdis,A.J. (2002) Manganese substantially alters the dynamics of translesion DNA synthesis. Biochemistry, 41, 4771–4778. [DOI] [PubMed] [Google Scholar]
- 36.Hadden J.M., Declais,A.-C., Phillips,S.E.V. and Lilley,M.J. (2002) Metal ions bound at the active site of the junction-resolving enzyme T7 endonuclease I. EMBO J., 21, 3505–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamagata A., Kakuta,Y., Masui,R. and Fukuyama,K. (2002) The crystal structure of exonuclease RecJ bound to Mn2+ ion suggests how its characteristic motifs are involved in exonuclease activity. Proc. Natl Acad. Sci. USA, 99, 5908–5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noble C.G. and Maxwell,A. (2002) The role of GyrB in DNA cleavage-religation reaction of DNA gyrase: a proposed two metal-ion mechanism. J. Mol. Biol., 318, 361–371. [DOI] [PubMed] [Google Scholar]