Abstract
Context:
Thyroid cancer is the most common endocrine malignancy, but due to its rare occurrence in the pediatric population, the cancer risk of childhood thyroid nodules is incompletely defined, and optimal management of children with suspected nodules is debated.
Objective:
The aim was to study the presenting features and cancer risk of sporadic childhood thyroid nodules using a standardized clinical assessment and management plan.
Design and Setting:
Boston Children's Hospital and Brigham and Women's Hospital collaborated to create a multidisciplinary pediatric thyroid nodule clinic and implement a standardized assessment plan. Upon referral for a suspected nodule, serum TSH was measured and hypothyrotropinemic patients underwent 123I scintigraphy. All others underwent thyroid ultrasonography, and if this confirmed nodule(s) ≥ 1 cm, ultrasound-guided fine-needle aspiration was performed. Medical records were retrospectively reviewed and compared to a control population of 2582 adults evaluated by identical methods.
Patients and Results:
Of 300 consecutive children referred for the initial evaluation of suspected thyroid nodules from 1997 to 2011, 17 were diagnosed with autonomous nodules by scintigraphy. Neck ultrasonography performed in the remainder revealed that biopsy was unnecessary in over half, either by documenting only sub-centimeter nodules or showing that no nodule was present. A total of 125 children met criteria for thyroid biopsy, which was performed without complication. Their rate of cancer was 22%, significantly higher than the adult rate of 14% (P = .02).
Conclusions:
Neck ultrasonography and biopsy were key to the evaluation of children with suspected thyroid nodules. Although the relative cancer prevalence of sonographically confirmed nodules ≥ 1 cm is higher in pediatric patients than adults, most children referred for suspected nodules have benign conditions, and efforts to avoid unnecessary surgery in this majority are warranted.
Thyroid cancer is the most common endocrine malignancy and usually presents as a thyroid nodule. Early diagnosis and treatment improve outcome, and this concept may be especially important in children who, compared to adults, more commonly develop regional lymph node and pulmonary metastases (1, 2). Accurate identification of benign nodules is an equally important diagnostic goal so as to avoid unnecessary surgery and the risks of operative complications (1, 3–5).
Neck ultrasonography and fine-needle aspiration are diagnostic mainstays in the evaluation of adults with thyroid nodules, and the American Thyroid Association recommends the application of these tools in children (6). However, some experts dispute their appropriateness in this age group due to concerns about the trauma of biopsy and the risks of repeated sedation. This controversy is further fueled by wide variability in the reported cancer prevalence of childhood nodules because estimates as high as 70% have been used to justify referring children directly for surgery (7–12). Unfortunately, most pediatric series are small and retrospective, with widely variable diagnostic methods and inclusion criteria.
The goals of our study were to assess the value of diagnostic tests and to measure pediatric cancer prevalence in thyroid nodules ≥ 1 cm in size. To do this, we performed a retrospective analysis of 300 consecutive children referred to our multidisciplinary pediatric thyroid clinic from 1997 to 2011 for the initial evaluation of suspected thyroid nodules.
Patients and Methods
Study design
We reviewed the hospital records of all pediatric patients (defined as ≤ 18 y of age) referred for suspected nodules and compared their features to our recently published population of 2582 adults (13). All patients were evaluated by a standard diagnostic algorithm (Figure 1). Serum TSH was measured in all children referred for suspected thyroid nodules. Hypothyrotropinemic patients underwent 123I scintigraphy to assess for nodule autonomy. All others were seen in our multidisciplinary thyroid nodule clinic for combined evaluation by a radiologist and an endocrinologist. Sixty minutes before each evaluation, a nurse applied EMLA cream over the thyroid bed and completed a standardized history intake form (14).
Figure 1.
Standard diagnostic algorithm. UG-FNA, ultrasound-guided fine-needle aspiration.
Thyroid ultrasonography was performed with a 5 to 18 MHz transducer. The UltraSTAR structured data entry system was used to input nodule features, including size, cystic content, and calcifications (15). Lymph nodes were considered enlarged when width exceeded 7 mm and were categorized as sonographically abnormal when the fatty hilum was lost or intranodal calcifications or echogenic regions were seen.
Biopsies were collected via fine-needle aspiration performed by the endocrinologist, with ultrasound guidance by the radiologist. Biopsy sites were cleansed with alcohol and injected with sc 1% lidocaine before aspiration. For each nodule, 4 to 6 aspirates were collected with 1.5-in, 25-gauge needles attached to 10-ml syringes and pooled in CytoLyt solution for ThinPrep (13). Specimens with at least 6 groups of 10 or more follicular cells were considered adequate and were categorized according to the Bethesda Thyroid Cytopathology Reporting System (16). The cytology categorization system and interpreting cytopathology department was constant through the entirety of this study.
Outcomes and statistical analysis
Patient features, including age, gender, and laboratory results, were obtained from hospital records and entered into the REDCap data capture tool (17). Potential associations between subject features and thyroid cancer risk were examined by Fisher's exact test or χ2 analysis for categorical variables and by Wilcoxon test for continuous variables and multiple logistic regression for joint assessment of 2 or more variables. SAS software (SAS Institute Inc, Cary, North Carolina) was used, and P values < .05 were considered significant. Research was approved by our institutional review boards.
Results
Stratification of 334 children referred for suspected new nodules
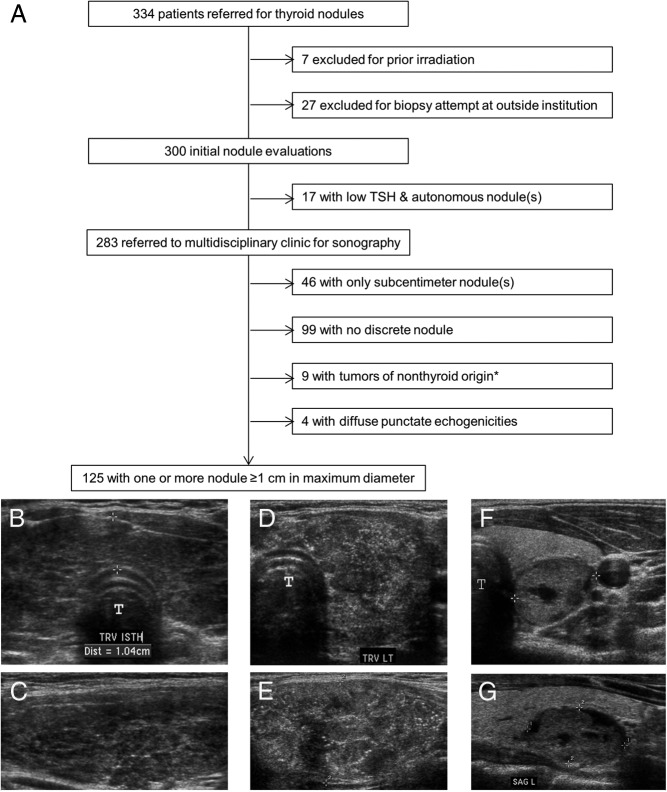
A total of 433 patients no more than 18 years of age were seen in our thyroid nodule program from July 1997 to March 2011. Of these, we excluded 68 who were referred for familial cancer syndromes and 31 others referred after previous thyroid surgery. The remaining 334 children were referred for suspected new nodules, detected by palpation or as incidental radiographic findings (Figure 2). We excluded 7 children with prior neck irradiation and, to avoid referral bias, also excluded 27 others referred after biopsy (either completed or attempted) at outside institutions.
Figure 2.
Study population (A) and representative ultrasonography (B–G). A, Patients were stratified by serum TSH and imaging. *, Tumors of nonthyroid origin included 2 thyroglossal duct cysts, 1 Burkitt lymphoma, 1 high-grade peripheral nerve sheath tumor, 1 undifferentiated sarcoma, 1 benign parathyroid cyst, 1 renal clear cell sarcoma metastasis, 1 ganglioneuroma, and 1 ectopic thymic rest. Three patient ultrasound studies are shown. B and C, Transverse (B) and sagittal (C) images of Hashimoto's thyroiditis, characterized by diffuse heterogeneity of the entire gland and a thickened isthmus (calipers; 1.04 cm) without a discrete nodule. D and E, Transverse (D) and sagittal (E) images of the diffuse sclerosing variant of papillary thyroid cancer, characterized by innumerable punctate echogenicities without a focal lesion. F and G, Transverse (F) and sagittal (G) images of a well-defined, complex (<25% cystic) nodule (calipers) with surrounding normal thyroid. T, trachea.
The remaining 300 patients were considered initial nodule evaluations. Nineteen children were directed to 123I scintigraphy for hypothyrotropinemia, and this diagnosed autonomous nodules in 17. Four patients elected to undergo thyroidectomy with benign operative pathology. The remainder elected medical treatment, and 3 received 131I therapy after reaching adulthood as definitive therapy for hyperthyroidism.
The residual 283 children were evaluated by ultrasonography in our multidisciplinary nodule clinic. Although all were referred for biopsy of clinically suspected nodules, imaging revealed that biopsy was unnecessary in half, either because their nodules were < 1 cm in maximum diameter (n = 46) or because there was no discrete nodule (n = 99). Within the latter group, 62% (61 of 99) had diffusely heterogeneous echotexture (Figure 2, B and C), and Hashimoto's thyroiditis was diagnosed in 74% (45 of 61) of these patients by documenting elevated serum thyroid autoantibody titers.
Nine patients were diagnosed with tumors of nonthyroid origin that were typically adjacent to rather than within the thyroid gland. Four other children had no discrete mass but displayed a dramatically abnormal and distinctive sonographic pattern of diffusely scattered, punctate echogenicities without acoustic shadowing, involving a large area of the gland (Figure 2, D and E). In all 4 of these patients, aspirates were positive for papillary carcinoma, and operative pathology showed the diffuse sclerosing variant of papillary thyroid cancer.
Features of 125 patients with 1 or more nodules ≥ 1 cm in maximum diameter
The final group of 125 patients all had 1 or more nodules ≥ 1 cm in size (Figure 2, F and G). Children evaluated for a suspected thyroid nodule were more commonly adolescents, and the female:male ratio was 5.2 (Figure 3). With the exception of purely cystic lesions, biopsy was offered for all nodules ≥ 1 cm (even when multiple nodules were present in the same thyroid gland). Biopsies were well tolerated and, with the exception of 2 children under 6 years of age, were performed without sedation. There were no biopsy complications requiring emergency department evaluation or admission.
Figure 3.
Age and gender distribution of benign and cancerous nodules.
Children with cytological abnormalities concerning for cancer and those with nodules ≥ 4 cm in maximum diameter (regardless of cytology) were referred for surgery. The latter recommendation recognizes the potentially decreased sensitivity of even optimal biopsy in very large nodules (18, 19). Overall, 28 children were diagnosed with thyroid cancer, corresponding to a 22% cancer rate in pediatric patients with discrete nodules ≥ 1 cm. The variables of age, gender, lobe involved, Hashimoto's thyroiditis, and nodule number per patient are shown in Table 1. None of these variables were significantly associated with cancer risk.
Table 1.
Features of 125 Children Confirmed to Have at Least One Nodule ≥1 Centimeter and Their Association With Cancer Risk
| Patient Characteristics | Patients, n (% of Total) | n (% of Category) |
Pa | |
|---|---|---|---|---|
| Benign | Cancer | |||
| All | 125 | 97 (78) | 28 (22) | |
| Gender | .39 | |||
| Female | 105 (84) | 83 (79) | 22 (21) | |
| Male | 20 (16) | 14 (70) | 6 (30) | |
| No. of nodules ≥ 1 cm per patient | .10b | |||
| 1 | 101 (81) | 75 (74) | 26 (26) | |
| 2 | 16 (13) | 16 (100) | 0 (0) | |
| 3 | 4 (3) | 2 (50) | 2 (50) | |
| 5 | 2 (2) | 2 (100) | 0 (0) | |
| 8 | 1 (1) | 1 (100) | 0 (0) | |
| 10 | 1 (1) | 1 (100) | 0 (0) | |
| Hashimoto's thyroiditisc | .13 | |||
| Present | 19 (15) | 12 (63) | 7 (37) | |
| Absent | 106 (85) | 85 (80) | 21 (20) | |
| Lobe involved | .44 | |||
| Bilateral | 17 (14) | 15 (88) | 2 (12) | |
| Isthmus | 1 (1) | 1 (100) | 0 (0) | |
| Left lobe | 48 (38) | 39 (81) | 9 (19) | |
| Right lobe | 59 (47) | 42 (71) | 17 (29) | |
Testing hypothesis of equal proportions in the 2 samples by Fisher's exact test.
Comparing patients with a single nodule to those with 2 or more nodules by Fisher's exact test.
Hashimoto's thyroiditis was defined by documentation of elevated serum autoantibodies against either thyroperoxidase or thyroglobulin.
Characteristics of 136 nodules biopsied
In the above group of 125 patients, a total of 136 nodules were biopsied. Mean nodule size was 2.5 cm (median, 2.3 cm), and 37% (50 of 136) were more than 50% cystic. Based upon standards established in adult patients, repeat biopsy was offered whenever initial cytology was nondiagnostic or atypical, and these data were used to derive the final cytology results in Table 2 (13, 16). Cancer diagnosis (defined by operative pathology) showed a direct relationship to nodule size and an inverse relationship to cystic content (Table 2). The sonographic abnormalities of calcifications and abnormal lymph nodes associated strongly with cancer but had low sensitivity (0.07–0.36) due to their rare occurrence. Of note, whereas the presence of nonspecific lymph node enlargement (without abnormal lymph node appearance) was associated with significantly higher cancer risk (34 vs 17%), most of these nodules were benign. In multivariable logistic regression analysis of all sonographic features, the independent associations of nodule size and cystic content with cancer both remained significant (P = .0007 or < .0001, respectively).
Table 2.
Characteristics of 136 Nodules Biopsied and Their Association With Cancer Risk
| Nodule Characteristics | Nodules, n (% of Total) | n (% of Category) |
Pa | |
|---|---|---|---|---|
| Benign | Cancer | |||
| All | 136 | 108 (79) | 28 (21) | |
| Size in mm, median (range)b | 23.0 (10–64) | 22.5 (10–61) | 29.5 (10–64) | .004 |
| Cystic content | <.0001 | |||
| 0% (solid) | 37 (27) | 16 (43) | 21 (57) | |
| <25% | 19 (14) | 15 (79) | 4 (21) | |
| 25–50% | 30 (22) | 28 (93) | 2 (7) | |
| 50–75% | 18 (13) | 18 (100) | 0 (0) | |
| >75% | 32 (24) | 31 (97) | 1 (3) | |
| Calcifications | <.0001 | |||
| Present | 9 (7) | 0 (0) | 9 (100) | |
| Absent | 127 (93) | 108 (85) | 19 (15) | |
| Nonspecific lymph node enlargement | .07 | |||
| Present | 29 (21) | 19 (66) | 10 (34) | |
| Absent | 107 (79) | 89 (83) | 18 (17) | |
| Abnormal lymph node appearance | .03 | |||
| Present | 4 (3) | 1 (25) | 3 (75) | |
| Absent | 132 (97) | 107 (81) | 25 (19) | |
| Final cytology | <.0001 | |||
| Benign | 86 (63) | 84 (98) | 2 (2)c | |
| Atypical | 10 (7) | 6 (60) | 4 (40) | |
| Suspicious for follicular neoplasm | 6 (4) | 0 (0) | 6 (100) | |
| Suspicious for PTC | 10 (7) | 6 (60) | 4 (40) | |
| Positive for PTC | 11 (8) | 0 (0) | 11 (100) | |
| Nondiagnostic | 13 (10) | 12 (92) | 1 (8) | |
Abbreviation: PTC, papillary thyroid cancer.
Comparing malignant to benign by Fisher exact test.
By continuous measure, malignant nodules were significantly larger than benign by Wilcoxon test. Mean ± SD values were 24.8 ± 12.2 mm for all nodules; 23.0 ± 10.9 mm for benign nodules; and 31.7 ± 14.7 mm for malignant nodules.
The 2 cytologically benign nodules diagnosed as cancers were encapsulated follicular variants of papillary carcinoma, one 5.4-cm nodule that was empirically resected for large size and one 2.3-cm nodule that was rebiopsied at a surveillance visit 2 years after initial presentation and then referred to surgery for new cytological abnormalities on the repeat biopsy. With the exception of 3 follicular carcinomas (2 with preoperative cytology that was “suspicious for follicular neoplasm” and one with cytology that was “suspicious for PTC”), all thyroid cancers were papillary carcinomas on operative pathology.
Final cytology and patient outcome
The Bethesda System for Reporting Thyroid Cytopathology was used to categorize biopsies as nondiagnostic, benign, malignant, atypia of undetermined significance, suspicious for follicular neoplasm, or suspicious for malignancy. The last 3 categories are considered indeterminate abnormalities in adult series (16).
The most common final cytology result was “benign” (63%; Table 2). Fourteen of these nodules were surgically resected for large size ≥ 4 cm (n = 5); large nodule number within a multinodular goiter rendering long-term surveillance impractical (n = 4); or parental desire to obtain definitive pathology (n = 5). With the exception of one 5.4-cm nodule found to be an encapsulated follicular variant of papillary carcinoma, all others had benign histology. Two of these patients with benign nodules were noted to have incidental, solitary papillary microcarcinomas (0.1 and 0.3 cm) that were remote from the biopsy sites. In 1 adolescent who presented with both a cytologically benign left-sided nodule and a cytologically abnormal right-sided nodule, thyroidectomy showed a benign left-sided nodule and a right-sided papillary carcinoma, indicating that both biopsy results were predictive.
The remaining patients with cytologically benign nodules deferred surgery and agreed to long-term surveillance with annual neck ultrasonography. Forty patients elected to have this surveillance performed at our center, and following the standard of repeating biopsy for nodule growth or other significant interval change, 9 nodules were rebiopsied over a median follow-up of 3.2 years (range, 1.0 to 9.8 y). Only one 2.3-cm nodule was abnormal on repeat biopsy (suspicious for follicular neoplasm), and its operative pathology showed an encapsulated follicular variant of papillary thyroid cancer. All other diagnostic repeat aspirates performed for interval growth were benign.
Among patients with abnormal cytology (defined as malignant, atypia of undetermined significance, suspicious for follicular neoplasm, or suspicious for malignancy), one 17-year-old female with a 2.6-cm cystic nodule and mild cytological atypia suggestive of benign cyst-lining cells declined surgery, and she remains well after 9 years of surveillance imaging (20). All other patients with abnormal cytology underwent surgery, and their histological diagnoses are shown in Table 2. Forty-two percent had initial near-total thyroidectomy, and the remainder had lobectomy as their initial procedure. In the latter group, 41% (12 of 29) subsequently underwent completion thyroidectomy for the diagnosis of cancer on lobectomy pathology. No permanent hypoparathyroidism or vocal cord paralysis was observed. Altogether, 28 thyroid cancers were diagnosed by pathology, with cervical lymph node metastases in 46% (13 of 28), including all 11 patients with malignant nodule cytology. Distant (pulmonary) metastases were present upon diagnosis in 1 adolescent.
Comparison of children and adults with thyroid nodules
Our diagnostic algorithm and technical methods were constant over the 14-year study interval and identical to those used to evaluate adults at the Brigham and Women's Hospital. Of note, since 1995, all primary care physicians in the Brigham and Women's Hospital system have been asked to refer adult patients to the thyroid clinic upon either the clinical suspicion or the incidental imaging detection of a thyroid nodule, so even the referral criteria and system were identical to our pediatric cohort (13, 21). This permitted a controlled comparison of pediatric data to our recently reported adult statistics (Table 3), which revealed a higher cancer prevalence in children (22%) compared to adults (14%; P = .02) with discrete nodules ≥ 1 cm (13). Pediatric nodules were also larger at presentation and more often solitary.
Table 3.
Comparison of 125 Children and 2582 Adults With Nodules ≥1 Centimeter
| Patient Characteristics | Children | Adults | Pa |
|---|---|---|---|
| Gender | .21 | ||
| Female | 105 (84) | 2266 (88) | |
| Male | 20 (16) | 316 (12) | |
| Thyroid cancer | .02 | ||
| Patients with thyroid cancer | 28 (22) | 368 (14) | |
| Patients without thyroid cancer | 97 (78) | 2214 (86) | |
| No. of nodule(s) per patient | <.0001 | ||
| 1 | 101 (81) | 1473 (57) | |
| 2 | 16 (13) | 592 (23) | |
| 3 | 4 (3) | 276 (11) | |
| ≥4 | 4 (3) | 241 (9) | |
| Size of nodules, mm | .02 | ||
| 10–14 | 61 (35) | 1438 (31) | |
| 15–19 | 20 (12) | 996 (22) | |
| 20–24 | 25 (14) | 667 (15) | |
| 25–29 | 26 (15) | 469 (10) | |
| 30–39 | 26 (15) | 593 (13) | |
| ≥40 | 15 (9) | 421 (9) |
Data are expressed as number (percent).
Testing hypothesis of equal proportions in the 2 samples by Fisher's exact test or χ-squared test. Adult patient data were adapted from Yassa et al (13), modified to exclude tumors of nonthyroid origin.
Discussion
The primary goal of this paper is to report our experience in the diagnostic approach to childhood thyroid nodules and their subsequent management. The results of our study indicate that the cancer risk of sporadic thyroid nodules is approximately 1.6-fold higher in children (22%) compared to adults (14%). In this pediatric population, application of a focused assessment plan based upon adult consensus guidelines (6) was well tolerated and avoided unnecessary procedures in most patients evaluated.
In contrast to the 5 to 15% cancer rate reported for adults with thyroid nodules, reports of cancer prevalence in children with thyroid nodules vary widely, ranging from 3 to 70% even among the most comprehensive series (7, 11, 12, 22–29). As mentioned, this wide range of cancer prevalence has fueled debate regarding initial management and led many experts to recommend that children with nodules proceed directly to thyroidectomy without biopsy or other preoperative testing. Efforts to resolve this controversy may be aided by understanding the factors responsible for this variability in the pediatric literature. Although discordance between pediatric reports is generally attributed to their small size, variability in inclusion criteria is another important contributor. Our study has shown that, whereas cancer risk is indeed higher in children than in adults, over one-third of patients referred for clinically suspected nodules have no discrete mass by ultrasonography. Therefore, pediatric series that include young adults up to 21 years of age or palpable nodules that are not radiographically confirmed underestimate cancer risk (23–27). Conversely, our study has also demonstrated that over 75% of childhood nodules are benign. Thus, series that limit inclusion to operated nodules may overestimate cancer risk by excluding individuals who deferred surgery in the context of reassuring biopsies (7, 11). Tertiary pediatric centers are further prone to overestimation of cancer prevalence if they fail to control for their population's medical complexity (enrichment for individuals with genetic or radiation-associated cancer risk) or for referral bias (11, 22, 30). The critical impact of the latter is illustrated by the seminal paper of Hayles et al (10), which reported a 70% cancer rate for pediatric nodules on the basis of 21 children with cancer but noted that 20 of these patients were referred to their center only after the diagnosis of carcinoma had already been established by biopsy. To minimize these biases and focus on sporadic childhood nodules, we restricted our cohort to patients no more than 18 years of age and excluded all individuals with familial thyroid cancer syndromes, previous neck irradiation, or prior biopsy attempts at outside institutions.
Although our analysis is retrospective, it is worth noting that certain features of our clinical practice resulted in unusually uniform data collection. We instituted a standardized assessment and management plan to guide serum testing and imaging decisions (31). We also used standardized data forms to obtain medical histories and the UltraSTAR structured data entry system to record sonographic features (15). Variability was further reduced by limiting pediatric cases to a small core of identically trained providers, with 95% of the ultrasounds in this study performed by 4 radiologists and 94% of the biopsies performed by 3 endocrinologists. All biopsies were interpreted within a single cytopathology department. Collectively, these practices ensured the consistent collection of targeted data in every subject, and because our methods matched those used to evaluate adults at the Brigham and Women's Hospital, a controlled comparison was possible. To our knowledge, this report is the first to accomplish this goal.
In the analysis of presenting features, no single patient feature was significantly associated with increased cancer risk. Larger multicenter studies, as are currently being organized by Pediatric Task Forces of the American Thyroid Association, may better address features such as the number of nodules per gland and the presence of coexisting Hashimoto's thyroiditis that approached statistical significance in this cohort. When comparing sonographic characteristics of benign and malignant nodules in our series, cancers were more likely to be solid in nature and larger in size. The sonographic abnormalities of calcifications and abnormal lymph nodes were also strongly associated with cancer, but their low incidence limits their sensitivity. Ultimately, no presenting sonographic feature offered sufficient negative predictive value to forego biopsy, so we recommend aspiration of all nodules ≥ 1 cm, unless the lesion is completely cystic or documented to be autonomous by scintigraphy.
All children in this series with malignant nodule cytology were confirmed at pathology to have papillary thyroid cancer that was metastatic to neck lymph nodes. Based upon this experience, we recommend near-total thyroidectomy for children with nodules that are ≥ 1 cm and cytologically positive for malignancy, consistent with current consensus recommendations for adult patients (6). Focusing on the opposite extreme, benign cytology was similarly predictive (98%) with only 2 known false-negatives, a 13-year-old with a 5.4-cm nodule and an 18-year-old male who was rebiopsied with indeterminate cytology at a surveillance visit 2 years after initial presentation. Both had encapsulated follicular variants of papillary thyroid cancer, a major cause of false-negative cytology in reported adult series (32). They were without metastases and were successfully treated. As in much larger adult series, the fact that the vast majority of patients with benign cytology do not have surgery prevents an absolute assessment of the accuracy of biopsy (6, 13). Based upon our experience, we continue to recommend deferral of surgery for children with benign cytology, as long as their nodules are < 4 cm in size and their families commit to long-term surveillance. Further cytology research in larger populations is needed to determine whether the cancer risk of specific indeterminate cytological categories differs between children and adults. In the interim, we recommend lobectomy for children with indeterminate cytological abnormalities and unilateral nodules.
We found our diagnostic assessment plan to be both useful and appropriate. An initial serum TSH was a helpful screen that avoided the expense and radiation exposure of radionuclide imaging in most children referred for suspected nodules and also predicted nodule autonomy in most hypothyrotropinemic patients directed to 123I scintigraphy. Ultrasonography was extremely useful; it determined that biopsy was unnecessary in half the children referred for suspected nodules, either by documenting only sub-centimeter nodules or showing that there was no nodule. The latter finding has been previously described in adults (21), and it illustrates the limited specificity of thyroid palpation for the diagnosis of nodules. The nearly 50% incidence of autoimmune thyroiditis we observed in such patients indicates that Hashimoto's thyroiditis can produce palpable changes that mimic nodules in children as well as adults. This is also supported by our recent report of pediatric thyroidectomies performed before preoperative imaging was standard, which revealed 6 children who underwent thyroidectomy for palpable nodules but were found to have autoimmune thyroiditis, without nodules, on operative pathology (33). Thus, neck ultrasonography should always be performed in children with clinically suspected nodules before contemplating surgery, to confirm the nodule's presence and to assess the less common possibilities of tumors of nonthyroid origin and the diffuse sclerosing variant of papillary thyroid cancer.
In our experience, thyroid biopsies were well tolerated and, with the exception of 2 children under 6 years of age, all patients in this report were biopsied without sedation by using local anesthetics and standard pediatric distraction techniques (34). Similar to other reports describing the safety of ultrasound-guided fine-needle aspiration, we observed no complications (23, 28, 35). However, we recommend ultrasound guidance for all pediatric biopsies to optimize accuracy and safety because the smaller size of pediatric patients increases the proximity of the thyroid gland to the esophagus, trachea, and great vessels.
In summary, pediatric patients with discrete thyroid nodules ≥ 1 cm in maximum diameter have greater relative cancer risk than adults. However, like adults, the vast majority of children referred for suspected nodules have either no nodule or benign conditions. In experienced hands, neck ultrasonography and ultrasound-guided fine-needle aspiration are safe and effective tools to rapidly diagnose pediatric thyroid cancer and to avoid the unnecessary risk and expense of surgery in children with benign entities.
Acknowledgments
We are indebted to the patients and families evaluated in this series and to Dr Christian Botte for his expertise and assistance in REDCap database design.
This work was supported by a donation from the Murray Family and by grants DK076099, DK007699 and DK007529 from the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
References
- 1. Waguespack SG, Francis G. Initial management and follow-up of differentiated thyroid cancer in children. J Natl Compr Canc Netw. 2010;8:1289–1300 [DOI] [PubMed] [Google Scholar]
- 2. Hung W, Sarlis NJ. Current controversies in the management of pediatric patients with well-differentiated nonmedullary thyroid cancer: a review. Thyroid. 2002;12:683–702 [DOI] [PubMed] [Google Scholar]
- 3. Rivkees SA, Mazzaferri EL, Verburg FA, et al. The treatment of differentiated thyroid cancer in children: emphasis on surgical approach and radioactive iodine therapy. Endocr Rev. 2011;32:798–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sosa JA, Tuggle CT, Wang TS, et al. Clinical and economic outcomes of thyroid and parathyroid surgery in children. J Clin Endocrinol Metab. 2008;93:3058–3065 [DOI] [PubMed] [Google Scholar]
- 5. Tuggle CT, Roman SA, Wang TS, et al. Pediatric endocrine surgery: who is operating on our children? Surgery. 2008;144:869–877; discussion 877 [DOI] [PubMed] [Google Scholar]
- 6. Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 7. Roy R, Kouniavsky G, Schneider E, et al. Predictive factors of malignancy in pediatric thyroid nodules. Surgery. 2011;150:1228–1233 [DOI] [PubMed] [Google Scholar]
- 8. Van Vliet G, Glinoer D, Verelst J, Spehl M, Gompel C, Delange F. Cold thyroid nodules in childhood: is surgery always necessary? Eur J Pediatr. 1987;146:378–382 [DOI] [PubMed] [Google Scholar]
- 9. Koch CA, Sarlis NJ. The spectrum of thyroid diseases in childhood and its evolution during transition to adulthood: natural history, diagnosis, differential diagnosis and management. J Endocrinol Invest. 2001;24:659–675 [DOI] [PubMed] [Google Scholar]
- 10. Hayles AB, Kennedy RL, Woolner LB, Black BM. Nodular lesions of the thyroid gland in children. J Clin Endocrinol Metab. 1956;16:1580–1594 [DOI] [PubMed] [Google Scholar]
- 11. Canadian Pediatric Thyroid Nodule (CaPTN) Study Group The Canadian Pediatric Thyroid Nodule Study: an evaluation of current management practices. J Pediatr Surg 2008;43:826–830 [DOI] [PubMed] [Google Scholar]
- 12. Saavedra J, Deladoey J, Saint-Vil D, et al. Is ultrasonography useful in predicting thyroid cancer in children with thyroid nodules and apparently benign cytopathologic features? Horm Res Paediatr. 2011;75:269–275 [DOI] [PubMed] [Google Scholar]
- 13. Yassa L, Cibas ES, Benson CB, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer. 2007;111:508–516 [DOI] [PubMed] [Google Scholar]
- 14. Ehrenstrom-Reiz G, Reiz S, Stockman O. Topical anaesthesia with EMLA, a new lidocaine-prilocaine cream and the Cusum technique for detection of minimal application time. Acta Anaesthesiol Scand. 1983;27:510–512 [DOI] [PubMed] [Google Scholar]
- 15. Bell DS, Greenes RA, Doubilet P. Form-based clinical input from a structured vocabulary: initial application in ultrasound reporting. Proc Annu Symp Comput Appl Med Care. 1992:789–790 [PMC free article] [PubMed] [Google Scholar]
- 16. Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132:658–665 [DOI] [PubMed] [Google Scholar]
- 17. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pinchot SN, Al-Wagih H, Schaefer S, Sippel R, Chen H. Accuracy of fine-needle aspiration biopsy for predicting neoplasm or carcinoma in thyroid nodules 4 cm or larger. Arch Surg. 2009;144:649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCoy KL, Jabbour N, Ogilvie JB, Ohori NP, Carty SE, Yim JH. The incidence of cancer and rate of false-negative cytology in thyroid nodules greater than or equal to 4 cm in size. Surgery. 2007;142:837–844; discussion 844.e1–e3 [DOI] [PubMed] [Google Scholar]
- 20. Faquin WC, Cibas ES, Renshaw AA. “Atypical” cells in fine-needle aspiration biopsy specimens of benign thyroid cysts. Cancer. 2005;105:71–79 [DOI] [PubMed] [Google Scholar]
- 21. Marqusee E, Benson CB, Frates MC, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Intern Med. 2000;133:696–700 [DOI] [PubMed] [Google Scholar]
- 22. Hoperia V, Larin A, Jensen K, Bauer A, Vasko V. Thyroid fine needle aspiration biopsies in children: study of cytological-histological correlation and immunostaining with thyroid peroxidase monoclonal antibodies. Int J Pediatr Endocrinol. 2010;2010:690108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Izquierdo R, Shankar R, Kort K, Khurana K. Ultrasound-guided fine-needle aspiration in the management of thyroid nodules in children and adolescents. Thyroid. 2009;19:703–705 [DOI] [PubMed] [Google Scholar]
- 24. Khozeimeh N, Gingalewski C. Thyroid nodules in children: a single institution's experience. J Oncol. 2011;2011:974125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amrikachi M, Ponder TB, Wheeler TM, Smith D, Ramzy I. Thyroid fine-needle aspiration biopsy in children and adolescents: experience with 218 aspirates. Diagn Cytopathol. 2005;32:189–192 [DOI] [PubMed] [Google Scholar]
- 26. Corrias A, Mussa A, Baronio F, et al. Diagnostic features of thyroid nodules in pediatrics. Arch Pediatr Adolesc Med. 2010;164:714–719 [DOI] [PubMed] [Google Scholar]
- 27. Kapila K, Pathan SK, George SS, Haji BE, Das DK, Qadan LR. Fine needle aspiration cytology of the thyroid in children and adolescents: experience with 792 aspirates. Acta Cytol. 2010;54:569–574 [DOI] [PubMed] [Google Scholar]
- 28. Moslavac S, Matesa N, Kusic Z. Thyroid fine needle aspiration cytology in children and adolescents. Coll Antropol. 2010;34:197–200 [PubMed] [Google Scholar]
- 29. Jamil F, McNally RJ, Richardson D, Ball S, Cheetham T. High likelihood of malignancy in young patients presenting with a thyroid nodule in Northern England [published online ahead of print April 13, 2013]. Clin Endocrinol (Oxf). doi:10.1111/cen.12082 [DOI] [PubMed] [Google Scholar]
- 30. Smith JR, Marqusee E, Webb S, et al. Thyroid nodules and cancer in children with PTEN hamartoma tumor syndrome. J Clin Endocrinol Metab. 2011;96:34–37 [DOI] [PubMed] [Google Scholar]
- 31. Rathod RH, Farias M, Friedman KG, et al. A novel approach to gathering and acting on relevant clinical information: SCAMPs. Congenit Heart Dis. 2010;5:343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sangalli G, Serio G, Zampatti C, Bellotti M, Lomuscio G. Fine needle aspiration cytology of the thyroid: a comparison of 5469 cytological and final histological diagnoses. Cytopathology. 2006;17:245–250 [DOI] [PubMed] [Google Scholar]
- 33. Scholz S, Smith JR, Chaignaud B, Shamberger RC, Huang SA. Thyroid surgery at Children's Hospital Boston: a 35-year single-institution experience. J Pediatr Surg. 2011;46:437–442 [DOI] [PubMed] [Google Scholar]
- 34. Koller D, Goldman RD. Distraction techniques for children undergoing procedures: a critical review of pediatric research. J Pediatr Nurs. 2012;27:652–681 [DOI] [PubMed] [Google Scholar]
- 35. Anne S, Teot LA, Mandell DL. Fine needle aspiration biopsy: role in diagnosis of pediatric head and neck masses. Int J Pediatr Otorhinolaryngol. 2008;72:1547–1553 [DOI] [PubMed] [Google Scholar]