Abstract
Diploid Aegilops umbellulata and Ae. comosa and their natural allotetraploid hybrids Ae. biuncialis and Ae. geniculata are important wild gene sources for wheat. With the aim of assisting in alien gene transfer, this study provides gene-based conserved orthologous set (COS) markers for the U and M genome chromosomes. Out of the 140 markers tested on a series of wheat-Aegilops chromosome introgression lines and flow-sorted subgenomic chromosome fractions, 100 were assigned to Aegilops chromosomes and six and seven duplications were identified in the U and M genomes, respectively. The marker-specific EST sequences were BLAST-ed to Brachypodium and rice genomic sequences to investigate macrosyntenic relationships between the U and M genomes of Aegilops, wheat and the model species. Five syntenic regions of Brachypodium identified genome rearrangements differentiating the U genome from the M genome and from the D genome of wheat. All of them seem to have evolved at the diploid level and to have been modified differentially in the polyploid species Ae. biuncialis and Ae. geniculata. A certain level of wheat–Aegilops homology was detected for group 1, 2, 3 and 5 chromosomes, while a clearly rearranged structure was showed for the group 4, 6 and 7 Aegilops chromosomes relative to wheat. The conserved orthologous set markers assigned to Aegilops chromosomes promise to accelerate gene introgression by facilitating the identification of alien chromatin. The syntenic relationships between the Aegilops species, wheat and model species will facilitate the targeted development of new markers specific for U and M genomic regions and will contribute to the understanding of molecular processes related to allopolyploidization.
Introduction
The genus Aegilops is closely related to Triticum and contains 23 species, 12 of them with U and/or M genomes [1]. The allotetraploid species Ae. biuncialis Vis. (2n = 4× = 28, UbUbMbMb) and Ae. geniculata Roth. (2n = 4× = 28, UgUgMgMg), which originated from the natural hybridization of the diploids Ae. comosa Sm. in Sibth. & Sm. (2n = 2× = 14, MM) and Ae. umbellulata Zhuk. (2n = 2× = 14, UU), have the greatest ecological adaptability [1]. These species represent an outstanding reservoir of useful genes and alleles responsible for tolerance to pests and diseases, including the stem rust strain UG99, and abiotic stresses such as salt, drought, frost and heat stress [2]–[9]. These species have also been reported to carry alleles affecting the nutritional and bread making quality of wheat [10], [11]. These traits are attractive candidates for transfer into bread wheat by interspecific hybridization. Over the past decades, extensive research efforts have been made to introgress Aegilops chromatin into wheat, resulting in a range of addition, substitution and translocation lines between bread wheat and Ae. comosa, Ae. umbellulata, Ae. geniculata and Ae. biuncialis [2], [3]. As a result of the introgression process, several genes for resistance to rusts and powdery mildew (Lr9, Lr57, Sr34, Yr8, Yr40, Pm 29) have been transferred into hexaploid wheat from the U and M genomes of Aegilops [2], [3].
The identification of alien chromatin in the wheat genome is an essential part of the pre-breeding process and determines the efficiency of gene transfer efforts. The cytogenetic methods used most commonly to detect Aegilops chromatin in the wheat genetic background [2], [12], [13] are powerful techniques, but they tend to be less efficient in identifying small introgressions when the goal is to screen a large population. At the same time, only a small number of cost-effective molecular markers specific for the U and M genomes are available [14]–[17], a fact that limits the high-throughput marker-assisted selection of T. aestivum - Aegilops introgression lines. The shortage of suitable DNA markers also slows the development of high density genetic and physical maps, the mapping of favourable agronomic traits and the map-based positional cloning of genes.
Until now, only a few wheat-Aegilops translocations have been used in breeding programmes and the introgression of favourable agronomic traits from wild relatives to cultivated wheat remains difficult due to undesirable linkage drag and yield reduction [18], [2]. The utilisation of interspecific translocations in the breeding process is only successful if the introgressed alien chromosome segment compensates for the loss of the wheat chromatin [2]. Compensating wheat-alien translocations are likely to be developed from wheat and alien chromosomes having a strong homoeologous relationship due to the similar gene order along the introgressed chromosome. The collinearity between the homoeologous wheat and Aegilops chromosomes could be interrupted by genome rearrangements occurring independently in wheat and Aegilops after their evolutionary divergence [19], [20]. For example, Zhang et al. [21] identified at least eleven rearrangements that differentiate the D genome of wheat from that of Ae. umbellulata. Therefore, it is extremely important to establish syntenic relationships between the wheat and Aegilops chromosomes and to map the breakpoints of genome rearrangements in the U and M genomes relative to wheat.
Rice has been considered as a model system for the Triticeae species because of its small genome size (1C = 389 Mb) and the availability of the genome sequence [22], [23]. The comparative mapping of cereal genomes has provided evidence of a high level of conservation of gene order across regions spanning many megabases (i.e. macrocolinearity) [22]. However, the colinearity between rice and Triticeae species frequently breaks down at micro level due to translocations, deletions and duplications [24], [25] leading to increased interest in the genome of the wild grass, Brachypodium distachyon. This was proposed as a better model organism for structural and functional genomics in cereals because of its biological features (such as self-fertility, inbreeding annual life cycle of less than 4 months, small size, undemanding growth requirements, high capacity for plant regeneration via somatic embryogenesis and resistance to several cereal-adapted pests and diseases), small genome size (1C = 272 Mb) and its closer phylogenetic position to the tribe Triticeae [26]–[29]. The genomic sequence of Brachypodium distachyon has recently become available [30], allowing a deeper comparison of syntenic regions between crop species and Brachypodium as a reference.
Comparative genomic and phylogenetic studies between the Triticeae/Aegilops taxa and the model systems rice and Brachypodium identified a set of genes conserved throughout evolution in both sequence and copy number. This set of >1000 conserved genes, referred to as conserved orthologous set (COS) markers, was identified by the in silico comparison of the rice, wheat and Brachypodium EST databases (http://www.wgin.org.uk/resources/Markers/TAmarkers.php; http://www.modelcrop.org/cos_markers) [31]. The COS markers were designed over the exon-intron boundaries of genes conserved between the model and target species. The markers are potentially highly polymorphic, as they span the introns, which have an increased frequency of polymorphisms relative to the exons (6.07 SNP/kb versus 3.00 SNP/kb in introns and exons, respectively, in rice) [32]. These markers define orthologous regions, thus enabling the comparison of regions on the chromosomes of related species. It was shown that COS markers are highly transferable between species such as rice, wheat, maize, sorghum and barley [33]. Wheat-specific COS markers are also transferable to Aegilops, as demonstrated by Howard et al. [34], who mapped a major QTL controlling the content of B-type starch granules on chromosome 4S in Ae. peregrina. Burt and Nicholson [35] used COS markers to map the eyespot resistance gene Pch1 originating from Ae. ventricosa in hexaploid wheat. Therefore, the COS markers have potential for the identification of alien chromatin introgressed from various species of Aegilops into hexaploid wheat, and also to identify the chromosomal locations of orthologous regions in the U and M genomes relative to wheat using rice and Brachypodium as references.
The aim of the present study was to assign COS markers to U and M genome chromosomes with the help of a series of wheat-Aegilops disomic addition, substitution and translocation lines and using subgenomic DNA samples obtained by flow cytometric sorting of well-defined groups of U and/or M genome chromosomes [17]. A further aim was to compare the Aegilops genomes with wheat by identifying orthologous chromosomal regions in the U and M genomes relative to wheat (D genome) using rice and Brachypodium as references.
Materials and Methods
Plant Materials
The assignment/identification of the COS markers on the U and M genome chromosomes of diploid and allotetraploid Aegilops species (Ae. umbellulata, Ae. comosa, Ae. biuncialis and Ae. geniculata) was carried out on wheat-Aegilops introgression lines and on flow-sorted subgenomic DNA fractions with well-defined chromosomal content.
The parental wheat (Triticum aestivum L.) genotypes (Chinese Spring, Mv9kr1) of the wheat-Aegilops introgression lines and the wheat genotype Mv25, which were used for the first backcross during the production of wheat-Ae. biuncialis additions, were used as control. The parental Aegilops genotypes of the introgression lines (Ae. umbellulata JIC2010001, Ae. comosa JIC2110001, Ae. biuncialis MvGB642 and Ae. geniculata TA2899) and the genoptypes used for the production of flow-sorted subgenomic DNA fractions in previous work [17] (Ae. umbellulata MvGB470, Ae. comosa MvGB1039, Ae. biuncialis MvGB382 and Ae. geniculata AE1311/00) were also included in the present study.
The wheat (Chinese Spring)/Ae. umbellulata (JIC2010001) addition lines 1U, 2U, 4U, 5U, 6U and 7U, the wheat (Chinese Spring)/Ae. comosa (JIC2110001) addition lines 2M, 3M, 4M, 5M, 6M and 7M, and the substitution 6M(6A) were supplied from the John Innes Centre germplasm collection, Norwich, UK by Dr. Steve Reader. The partial set of wheat (Mv9kr1)/Ae. biuncialis (MvGB642) addition lines 1Ub, 1Ub6Ub, 3Ub, 2Mb, 3Mb and 7Mb [12], and the substitution 3Mb(4B) and the centric fusion 3Mb.4BS, both obtained from a cross between Mv9kr1/Ae. biuncialis (MvGB642) 3Mb addition × Chinese Spring ph1b mutant [36], were produced in Martonvásár. The wheat (Chinese Spring)-Ae. geniculata (TA2899) addition lines 1Ug, 2Ug, 3Ug, 4Ug, 5Ug, 6Ug, 7Ug, 1Mg, 2Mg, 3Mg, 5Mg, 6Mg and 7Mg [37] were provided by Dr. Bernd Friebe (Kansas State University, Manhattan, Kansas).
Chromosome Sorting and Amplification of Subgenomic DNA Samples
Flow cytometric chromosome sorting from individual peaks (I–IV) on flow karyotypes of Ae. umbellulata (MvGB470), Ae. comosa (MvGB1039), Ae. biuncialis (MvGB382) and Ae. geniculata (AE1311/00) and the determination of the chromosome content of flow-sorted fractions by FISH were carried out as described by Molnár et al. [17]. The assignment of chromosomes to peaks on flow karyotypes of individual Aegilops species is summarized in Table 1. In order to prepare template DNA for PCR with COS markers, chromosomes were sorted in batches of 25–50,000 (equivalent to 20–40 ng) into 40 µl of sterile deionized water in 1.5 ml tubes. The sorted chromosomes were treated with proteinase K and their DNA was amplified by multiple displacement amplification (MDA) using an Illustra GenomiPhi V2 DNA Amplification Kit (GE Healthcare, Chalfont St. Giles, United Kingdom) as described by Šimková et al. [38].
Table 1. Chromosome content of subgenomic DNA samples prepared from chromosomes flow-sorted from peaks on flow karyotypes of Ae. umbellulata (MvGB470), Ae. comosa (MvGB1039), Ae. biuncialis (MvGB382) and Ae. geniculata (AE1311/00).
| Subgenomic DNA samples | Chromosome content* | |
| Species | Flow karyotype peak | |
| Ae. umbellulata | I | 1U |
| II | 6U | |
| III | 3U | |
| IV | 2U, 4U, 5U, 7U | |
| Ae. comosa | I | 1M, 4M |
| II | 2M, 6M | |
| III | 2M, 5M | |
| IV | 3M, 7M | |
| Ae. biuncialis | I | 1Ub |
| II | 3Ub, 6Ub, 2Mb, 3Mb, 4Mb, 6Mb | |
| III | 2Ub, 5Ub,4Ub, 7Ub, 1Mb, 3Mb, 5Mb | |
| IV | 7Mb | |
| Ae. geniculata | I | 1Ug, 6Mg |
| II | 3Ug, 4Ug, 6Ug | |
| III | 2Ug, 5Ug, 7Ug, 2Mg, 4Mg, 5Mg | |
| IV | 1Mg, 3Mg, 7Mg | |
Chromosomes were assigned to peaks in which they occurred at the highest frequency (Molnár et al. 2011b).
COS Marker Analysis
DNA preparation and genotyping was carried out as described by Howard et al. [34] using the following templates; wheat-Aegilops genetic stocks, parental wheat (Chinese Spring, Mv9kr1, Mv25) and Aegilops (Ae. umbellulata JIC2010001, Ae. comosa JIC2110001; Ae. biuncialis MvGB642; Ae. geniculata TA2899) genotypes and the Aegilops genotypes used for the flow cytometric analysis (Ae. umbellulata MvGB470, Ae. comosa MvGB1039, Ae. biuncialis MvGB382 and Ae. geniculata AE1311/00).
A total of 140 markers (whose primer sequences and PCR conditions were summarised in Table S1) potentially covering wheat homoeologous groups I–VII were chosen from two publicly available COS marker collections, the Wheat Genetic Improvement Network (WGIN) (http://www.wgin.org.uk/resources/Markers/TAmarkers.php) and Tools and Resources (TR) collections (http://www.modelcrop.org/cos_markers). When the chromosomal locations of the markers were not available in the D genome of hexaploid wheat, the source EST sequences of the COS markers were searched from the GrainGenes database (http://wheat.pw.usda.gov/cgi-bin/westsql/map_locus.cgi?t=estacc&q=).
Reverse PCR primers were directly labelled with a fluorescent dye (6-FAM) and the following programmes performed on an MJ Research Tetrad PTC-225 Thermal Cycler (Waltham, Massachusetts) were used to amplify PCR products from 10 ng of genomic DNA in 10 µl reactions. WGIN: 95°C (15 min), 39 cycles of (95°C (0.5 min), 58°C (0.5 min), 72°C (0.5 min)), hold at 72°C (5 min) then at 10°C. TR: 94°C (10 min), 16 cycles of (95°C (0.5 min), 58°C (1 min), decreasing by 0.5°C per cycle to 50°C, 72°C (1 min)), 25 cycles of (94°C (0.5 min), 50°C (1 min), 72°C (1 min)), hold at 15°C. The fragment analysis of PCR products was carried out on a POP7 column attached to a 3730×l DNA Analyzer (Applied Biosystems, USA). The results were analysed using GeneMapper v4.0.
Sequence Analysis
To compare the orthologous regions defined by the COS markers between D genome of T. aestivum, U and M genomes of Aegilops species and rice and Brachypodium, a physical map was constructed showing the physical positions of the COS markers on the chromosomes of rice and Brachypodium as reference. To identify the physical positions of the markers, the EST source sequences of the COS markers (shown as Accession No. in Tables S2, S3 and S4) were downloaded from The Institute of Genomic Research (TIGR) database (http://plantta.jcvi.org/index.shtml) and used as queries in BLASTn searches to identify homologues in the assembled genomic sequences of Brachypodium distachyon and Oryza sativa (Brachypodium distachyon v1.0 [30]; Oryza sativa Japonica Group, The IRGSP pseudomolecules, Build 4.0, GenBank Assembly ID: CA_000005425.2) using the EnsemblPlants Database (http://plants.ensembl.org/). As the best hits were considered the hits with the highest score value and characterized by their BLAST parameters E-value, % of Identity and Alignment length (Tables S2, S3 and S4).Throughout the study, BLAST hits with E-values smaller than 2.8e−08, Identity % >58.44 and Alignment length >100 bp were considered as significant (Tables S2, 3 and 4).
The start genomic positions of the best hits in Brachypodium and rice were used to construct physical maps of the COS markers. The lengths (in bp) of Brachypodium and rice chromosomes as well as the start genomic positions of the best hits of the ESTs were converted to pixels and the physical maps of the COS markers were designed.
Results
Assignment of COS Markers to U and M Chromosomes
A set of COS markers specific to different ESTs was mapped to Aegilops chromosomes using wheat-Aegilops introgression lines carrying the U- and M-genome chromosomes of diploid Aegilops species (Ae. umbellulata and Ae. comosa) and their allotetraploid hybrids (Ae. biuncialis and Ae. geniculata). Subgenomic DNA samples amplified from each of the flow-karyotype peaks of the four goatgrass species and representing individual chromosomes or groups of chromosomes were also used (Table 1).
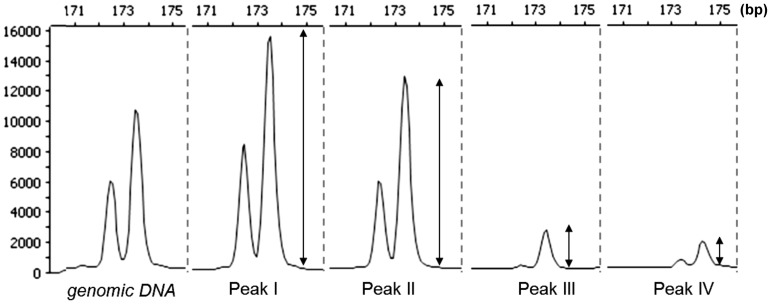
Of the 140 COS markers, 133 showed PCR products in the wheat genotypes (Chinese Spring, Mv9kr1 and Mv25) or in at least one of the eight Aegilops genotypes, while seven markers did not amplify any product. The 133 markers resulted in 822 PCR products (range: 1–5 PCR products/marker/genotype, mean: 2.04 PCR products; Table S5) with different sizes on the eight genotypes of the four Aegilops species, 492 (59.85%) of which showed size polymorphism relative to wheat, while 330 (40.15%) were non-polymorphic. Out of the 492 polymorphic PCR products, 295 (59.95%) products of 89 COS markers could not be unambiguously assigned to Aegilops chromosomes because a relevant wheat-Aegilops addition line bearing the polymorphic locus was not available and the locus was located in a subgenomic DNA sample representing more chromosomes. 197 (40.04%) polymorphic PCR products were assigned to Aegilops chromosomes. The majority of these (159 products) were assigned using wheat-Aegilops introgression lines, while the others could be assigned using flow-sorted chromosomes (Table S6). Because each of the Aegilops chromosomes has a major location in one of the peaks on a flow-karyotype (Table 1), the yield of PCR products was different on the peak-specific subgenomic DNA samples of the species. Therefore, the highest PCR yield was observed in the peak where the locus-carrying chromosome has its major location (Figure 1). For example, the marker X1N, specific for group 1 chromosomes of wheat, produced a 173 bp PCR amplicon with continuously decreasing yield in the Ae. umbellulata flow karyotype peaks I, II, III and IV (no amplicon in peak IV) (Figure 1), where the 1U chromosome content was 98.9%, 25.8%, 4.32% and 0%, respectively (Table 1). Based on the yield differences between the subgenomic samples, 25 polymorphic PCR products were also located on Aegilops chromosomes, while the genomic positions of 13 fragments were identified simultaneously by introgression lines and by subgenomic DNA samples. Of the 330 non-polymorphic PCR products, 35 could also be assigned using subgenomic DNA samples.
Figure 1. Differences in the yield of a 173 bp PCR product of the X1N marker amplified from the genomic DNA and subgenomic DNA samples from peaks I–IV on the flow karyotype of Aegilops umbellulata (MvGB470).
In total, 232 (197 polymorphic and 35 non-polymorphic) PCR products of 100 COS markers were assigned to Aegilops chromosomes (Table S6). A significant number of PCR products detected in the tetraploid species Ae. biuncialis and Ae. geniculata had similar size to the products observed with the corresponding markers in their diploid progenitors. Interestingly, the ratio of non-polymorphic to polymorphic products in the diploid progenitors and their allotetraploid hybrids was higher in the case of the U genome (of the U genome- specific PCR amplicons 80.5% and 77.7% were non-polymorphic in Ae. biuncialis and Ae. geniculata, respectively, relative to Ae. umbellulata) than in the M genome (55.5% and 65.7% in Ae. biuncialis and Ae. geniculata relative to Ae. comosa) (Table S6).
Some markers produced similar sized amplicons both in the diploid progenitors and in the tetraploids Ae. biuncialis and Ae. geniculata, but the chromosomal location of the locus could not be identified due to the fact that the sets of addition lines were incomplete. When the chromosomal location of a locus could be determined unambiguously in at least one species (in the diploid progenitor, or in Ae. biuncialis or Ae. geniculata) and the highest product yield in the other two species was detected in the subgenomic DNA sample containing the same chromosome, it was concluded that the locus was located on the same chromosome in all three Aegilops species. For example the X2N marker produced a 558 bp PCR fragment in Ae. umbellulata (AE740/03), Ae. biuncialis (MvGB382) and Ae. geniculata (TA2899 and AE1311/00), which was also found in the 2Ug wheat-Ae. geniculata disomic addition line and on the subgenomic samples specific for peaks IV and III of the flow-karyotype containing the 2U and 2Ub chromosomes of Ae. umbellulata and Ae. biuncialis, respectively. As a consequence, it was suggested that the X2N marker detects loci on chromosomes 2U and 2Ub of Ae. umbellulata and Ae. biuncialis as well as in Ae. geniculata.
Some PCR products could be detected on more than one Aegilops chromosome, so 156 loci were assigned unambiguously to the U genome chromosomes. 30 loci (19.23%) were located on group 1 chromosomes, 8 (5.12%) on group 2, 44 (28.20%) on group 3, 21 (13.46%) on group 4, 10 (6.41%) on group 5, 28 (17.94%) on group 6, and 15 (9.61%) on group 7. Out of the 132 loci assigned to the M genome chromosomes, 4 loci (3.03%) were located on group 1 chromosomes, 27 (20.45%) on group 2, 47 (35.60%) on group 3, 3 (2.27%) on group 4, 8 (6.06%) on group 5, 19 (14.39%) on group 6 and 24 loci (18.18%) on group 7 chromosomes. Some markers assigned to Aegilops chromosomes showed different chromosomal location in the allopolyploid species relative to the diploid ancestors. The proportion of these markers was significantly higher (27.9%) in the M genome (12 out of 43 markers assigned to chromosomes in Ae. comosa and one of the allopolyploid Aegilops sp.) than in the U genome (6 of the 74 assigned markers ― 8.1%).
Aegilops chromosome-specific markers with a significant level (≥2 bp) of length polymorphism between the parental wheat and Aegilops genotypes were considered to be suitable for the marker-assisted selection of new wheat-Aegilops introgression lines in prebreeding programmes (Table 2). In this study, 169 polymorphic loci of 51 markers covering all 7 homoeologous groups of the U and M genomes were found to be suitable for the high-throughput detection of diploid and allotetraploid Aegilops chromosomes.
Table 2. COS markers showing polymorphic (≥2 bp) PCR amplicons between wheat and Aegilops species are considered as suitable for the marker-assisted introgression into hexaploid wheat of the U and M genome chromosomes from Ae. umbellulata (UU) and Ae. comosa (MM) and from Ae. biuncialis and Ae. geniculata.
| diploid progenitors | Ae. Biuncialis | Ae. geniculata | |
| 1U | X1B(224), X2B(162) | X1B(226), X2B(162), X tr248(208) | X1B(226), X2B(162) |
| 2U | X2N * (558), X2P * (292), Xtr146(303), Xtr451(192) | X2N * (558), Xtr146 * (303), Xtr451 * (262) | X2N(558), X2P(292), Xtr146(303), Xtr451(262) |
| 3U | X3J(205), Xtr62(180), Xtr63(545), Xtr80(429), Xtr83(360), | X3J(205), Xtr62(180), Xtr63(545), Xtr77(364), Xtr80(429),Xtr83(360) | X3J(205), Xtr62(180), Xtr63(545), Xtr80(429), Xtr83(360) |
| 4U | X6J * (236), Xtr72(179), Xtr76(179), Xtr92(231), Xtr102(318), Xtr103(270) | X6J * (236), Xtr72 * (179), Xtr76 * (179), Xtr92 * (231), Xtr103 * (270), | X6J(236), Xtr72(179), Xtr76(179), Xtr92(231), Xtr102(318), Xtr103(270), Xtr129(300), |
| 5U | X5I * (270), X5Q * (311), Xtr128(214), Xtr131(470), Xtr248 * (208) | X5I * (270), X5Q * (311), Xtr128 * (214), Xtr131 * (470) | X5I(270), X5M(199), X5Q(311), X5S(443), Xtr128(214), Xtr131(470), Xtr248(208) |
| 6U | X2I(226), X4C(385), X4G(239), X6A(250), Xtr77(363), Xtr90(290), Xtr91(287), Xtr400(127) | X2U(351), X2I(230), X4C(385), X6A(250), Xtr91(287) | X4C * (385), Xtr90 * (290), Xtr91 * (287) |
| 7U | X3B * (234), X7C * (327), X7I(248), Xtr4(266) | X3B * (234), X7C * (327), X7I * (248), Xtr4 * (266) | X3B(234), X6A(277), X7C(327), Xtr4(271, 281) |
| 1M | X2B * (163) | X2B * (163) | X1J(207), X2B(163) |
| 2M | X1J * (228), Xtr146(381), | X1J * (228), X2R * (267), Xtr72(168), Xtr76(168), Xtr131(356), Xtr134(250), | X1J(228), X2I(230), X2R(267), |
| 3M | Xtr62(178), Xtr63(444), Xtr67(351), Xtr73(473), Xtr80(487), Xtr83(356), Xtr85 * (226) | Xtr62 * (178), Xtr63 * (444), Xtr76(168), Xtr72(168), Xtr80 * (487), Xtr83 * (356), Xtr85(226), Xtr131(356), Xtr134(250), Xtr471(263) | Xtr62 * (178), Xtr63 * (444), Xtr80 * (487), Xtr83 * (356), Xtr85 * (226), Xtr146(381), |
| 4M | Xtr88(407) | Xtr88 * (407), | Xtr88 * (407), |
| 5M | X5Q * (311), Xtr128(212), Xtr471(209), Xtr764(214) | X5Q * (311), Xtr471 * (209), Xtr764 * (214) | X5A(245), X5Q(311), Xtr128(210), Xtr471 * (209), Xtr764(214) |
| 6M | X6J * (236), Xtr93(477), Xtr103(261), Xtr104(406), Xtr112(390) | X6J * (236), Xtr103 * (261), | X6J(236), Xtr93(475), Xtr103(261), Xtr104(406) |
| 7M | X7C * (328), X7I(249, 312) | X6A(262), X7C(328), X7I(249, 312) | X6A(250), X7C * (328), X7I(249, 312) |
The size (in bp) of the chromosome-specific loci is shown in brackets. Asterisks indicate the loci with predicted chromosomal location when the PCR amplicon was specific for the U or M genomes and could be determined unambiguously in at least one Aegilops species (in the diploid progenitor, or in Ae. biuncialis or Ae. geniculata) and when the highest PCR product yield in the other two species was detected in the subgenomic DNA sample containing the same chromosome.
Loci with predicted chromosomal location.
Duplications in the U and M Genome of Diploid and Polyploid Aegilops
The chromosomal location of COS markers revealed several intragenomic duplications in the diploid and polyploid Aegilops species (Table 3). In the case of the U genome, six duplications were detected. Three (1U/3U, 4U/7U, 6U/7U) were found in the diploid progenitor Ae. umbellulata and in one tetraploid Aegilops. One duplication (3U/4U) was detected separately in Ae. umbellulata and Ae. geniculata by markers Xtr76 and X7T, respectively, while two species-specific duplications (1Ug/2Ug/7Ug, 4Ug/5Ug) were found in Ae. geniculata.
Table 3. Duplications in the U and M genomes identified in diploid progenitors (Ae. umbellulata and Ae. comosa) and in their tetraploid hybrids Ae. biuncialis and Ae. geniculata by COS markers assigned to Aegilops chromosomes.
| Marker | Diploid progenitor | Ae. biuncialis | Ae. geniculata | |
| U genome | X6N | 1U/3U | 1Ub/3Ub | 1Ug/2Ug/7Ug |
| Xtr76 | 3U/4U | – | 3Ug/4Ug | |
| X7T | – | – | 3Ug/4Ug | |
| Xtr61 | 4U/7U | – | 4Ug/7Ug | |
| X6A | 6U/7U | 6Ub/7Ub | – | |
| X5M | – | – | 4Ug/5Ug | |
| M genome | X1J | – | – | 1Mg/2Mg |
| X6N | – | 2Mb/3Mb/7Mb | 1Mg/2Mg/6Mg | |
| Xtr150 | – | 2Mb/7Mb | 2Mg/7Mg | |
| X7L | 4M/7M | – | – | |
| X7I | 7M/7M | 7Mb/7Mb | 7Mg/7Mg | |
| X4E | – | 2Mb/3Mb | – | |
| X4G | – | 2Mb/3Mb | – | |
| X4I | – | 2Mb/3Mb | – | |
| X4O | – | 2Mb/3Mb | – | |
| X4Q | – | 2Mb/3Mb | – | |
| X4S | – | 2Mb/3Mb | – | |
| Xtr72 | – | 2Mb/3Mb | – | |
| Xtr76 | – | 2Mb/3Mb | – | |
| Xtr29 | – | 2Mb/3Mb | – | |
| Xtr131 | – | 2Mb/3Mb | – | |
| Xtr134 | – | 2Mb/3Mb | – |
In the M genome, seven different duplications were detected in the diploid (Ae. comosa) and the allotetraploids Ae. biuncialis and Ae. geniculata (Table 3). Some were species- specific, like the 1Mg/2Mg duplication for Ae. geniculata, the 4M/7M duplication for Ae. comosa and the massive 2Mb/3Mb duplication detected by 11 COS markers for Ae. biuncialis. Two duplications were detected in more than one species, such as the 2M/7M duplication in Ae. biuncialis and Ae. geniculata and the 7M/7M duplication in all the three M genome species.
Relationship of U and M Genomes Relative to Rice, Brachypodium and Wheat
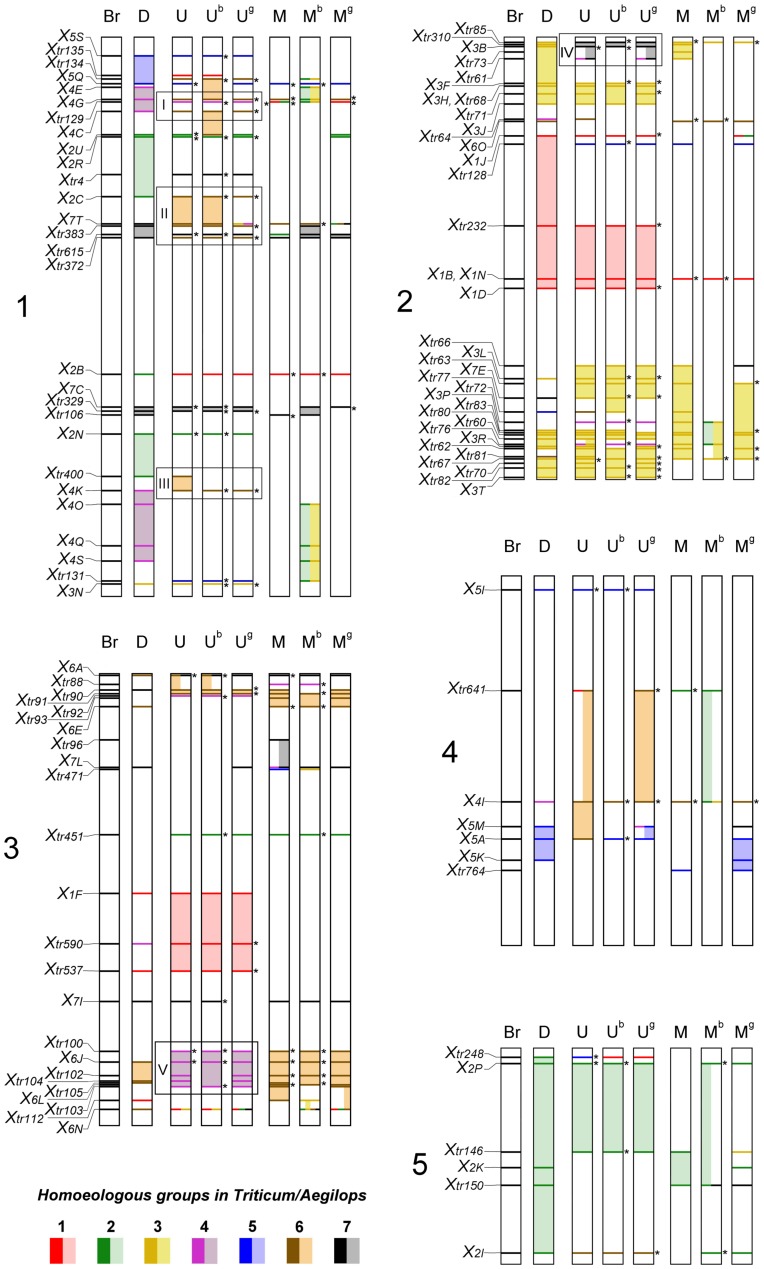
The source EST sequences of the 100 COS markers identified on the Aegilops chromosomes were aligned to the rice and Brachypodium sequence databases using BLASTn to identify the genomic positions of the markers in the model species. The genomic distribution of the marker-specific EST sequences in Brachypodium and rice and the parameters of the BLAST hits are detailed in Tables S3 and S4. Using the chromosomal length data and the start positions of the best hits, a physical map locating the markers for Brachypodium and rice was constructed (Figure 2, Figure S1). Figure 2 provides an overview, from the Brachypodium genome perspective, of the genome relationships between the model species and the wheat and Aegilops species at the resolution level of the Trtiticeae/Aegilops chromosomes.
Figure 2. Brachypodium–wheat–Aegilops orthologous relationships from the genomic perspective of Brachypodium distachyon.
The physical positions of the source ESTs of the COS markers are indicated on the Brachypodium chromosomes (Left). Each marker assigned to chromosomes of the wheat D genome or to the chromosomes of Ae. umbellulata (U), Ae. comosa (M), Ae. biuncialis (Ub, Mb) and Ae. geniculata (Ug, Mg) is colour-coded according to the homoeologous groups of Triticum/Aegilops chromosomes. Gaps between two markers assigned to the same Triticum/Aegilops chromosomes are filled in to show synteny (lighter colours). Blocks (designated I–V) indicate Brachypodium genomic regions related to the regions in the U genomes involved in evolutionary genome rearrangements relative to the wheat D genome or to M genomes. When a marker mapped to more than one wheat or Aegilops chromosome, other colour-coded locations are positioned adjacent to the first one. Asterisks indicate the predicted chromosomal location of a locus when the PCR amplicon was specific for the U or M genomes and could be determined unambiguously in at least one Aegilops species (in the diploid progenitor, or in Ae. biuncialis or Ae. geniculata) and when the highest PCR product yield in the other two species was detected in the subgenomic DNA sample containing the same chromosome.
The marker coverage of rice (R) chromosomes R1, R2 and R3 (with 30, 18 and 14 markers per chromosome, respectively) was better than that of the remaining chromosomes (R4–R12). Similar results were obtained for the Brachypodium (Br) chromosomes, where 28, 36 and 24 markers were mapped on chromosomes Br1, Br2 and Br3, respectively, while Br4 and Br5 were represented by 7 and 6 markers (Figure 2). The chromosomal locations of the orthologous genes indicated similar structural relationships between the model genomes (Brachypodium or rice) and wheat (D genome), as described previously [39], [30]. For example, COS markers specific for wheat (W) chromosome group W3 were located largely on rice chromosome R1, whereas R2 and R3 were generally related to W6 and W4. Moreover, some wheat chromosomes showed homology to two rice chromosomes; for instance, W2 was related to R4 and R7, W1 to R5 and R10 and W7 to R6 and R8. Wheat chromosome 1 was also related to Brachypodium chromosomes Br2 and Br3, W2 to Br1 and Br5, W3 to Br2, W4 and W5 to Br1 and Br4, W6 to Br3 and W7 to Br1 and Br3.
In general the homology of the U and M chromosomes of diploid and tetraploid Aegilops species to rice and Brachypodium was similar to that of wheat (Figure 2, Table 4). Thus, group 3 Aegilops chromosomes (Ae) were related mainly to R1 and Br2, whereas the Ae1 chromosomes (1U) showed homology to R5 and R10 and to Br2 and Br3.
Table 4. Syntenic genome relationships between the chromosomes of U and M genomes in the diploid progenitors Ae. umbellulata and Ae. comosa and their allotetraploid hybrids Ae. biuncialis and Ae. geniculata, and the chromosomes of rice (R) and Brachypodium (Br).
| diploid progenitors | Ae. biuncialis | Ae. geniculata | ||||
| Rice | Brachy | Rice | Brachy | Rice | Brachy | |
| 1U | R2*, R3*, R5, R7*, R9*, R10, R11* | Br1, Br2, Br3, Br4* | R2*, R3*, R4*, R5, R7*,R9*,R10 | Br1, Br2, Br3, Br5* | R2*, R4*, R5, R7*, R9*,R10 | Br1*, Br2, Br3, Br5* |
| 2U | R4, R7, R8* | Br1, Br3*, Br5 | R4, R7, R8* | Br1, Br3*, Br5 | R2*, R4, R7, R8* | Br1, Br3, Br5 |
| 3U | R1, R2*, R6* | Br1*, Br2, Br3* | R1, R2*, R6* | Br1*, Br2, Br3* | R1, R6 | Br1*, Br2 |
| 4U | R1, R2, R3* | Br1*, Br2, Br3 | R1, R2, R3* | Br1*, Br2, Br3 | R1, R2, R3*, R6*, R9* | Br1, Br2, Br3, Br4* |
| 5U | R3, R4*, R12* | Br1, Br2*, Br4*, Br5* | R3, R9*, R12* | Br1, Br2*, Br4 | R3, R9, R12* | Br1, Br2*, Br4 |
| 6U | R1, R2, R3, R4*, R6, R7, R9*, R11 | Br1, Br2, Br3, Br4, Br5* | R2, R3, R4*, R6, R7, R11* | Br1, Br3, Br4*, Br5* | R2, R3, R4*, R6, R7*, R11 | Br1, Br3, Br4, Br5* |
| 7U | R1, R2*, R6, R7*, R8*, R10* | Br1, Br2, Br3 | R1, R2*, R6, R7*, R8*, R10* | Br1, Br2, Br3 | R1, R2*, R6, R7*, R8*, R10 | Br1, Br2, Br3 |
| 1M | R3*, R5*, R7* | Br1, Br2* | R5*, R7* | Br1*, Br2* | R2*, R3*, R5, R7* | Br1, Br2, Br3* |
| 2M | R3*, R4, R8*, R10*, R11* | Br1, Br3*, Br4*, Br5 | R1, R2*, R3, R4, R8*, R11 | Br1, Br2, Br3, Br4, Br5 | R2*, R4, R5*, R6*, R7*, R8* | Br1, Br2*, Br3, Br5 |
| 3M | R1 | Br2 | R1, R2, R3, R8*, R11* | Br1, Br2, Br3, Br4* | R1, R4* | Br2, Br4*, Br5* |
| 4M | R2*, R8* | Br3 | R2* | Br3* | ||
| 5M | R3, R8*, R9* | Br1*, Br2*, Br3*, Br4* | R3* | Br1* | R3, R9 | Br1*, Br2*, Br4 |
| 6M | R1*, R2, R3*, R6*, R11* | Br1, Br2*, Br3, Br4* | R1*, R2, R6* | Br1*, Br2*, Br3 | R1*, R2, R3*, R6*, R11* | Br1?, Br2*, Br3, Br4* |
| 7M | R2, R6*, R8 | Br1, Br3 | R2, R3*, R4*, R6, R8, R10* | Br1, Br3, Br5* | R1*, R4*, R6, R8, R9*, R10* | Br1, Br2*, Br3, Br4*, Br5* |
Bold letters indicate rice or Brachypodium genomic regions represented by at least three markers.
:Genomic regions represented by one marker.
Five large-scale chromosomal rearrangements (I–V) identified by more than one marker were detected on the Aegilops genomes relative to the wheat D genome and also on the U genome relative to the M genome (Figure 2). The first of these (Rearrangement I), the region spanning from X4G to X4C on Br1 (or R3) and related to W4, was located on 6U and on various chromosomes of the M genome (6M, 2Mb–3Mb, 1Mg in Ae. comosa, Ae. biuncialis and Ae. geniculata, respectively). Another Br1 (or R6 and R7) region from X2C to Xtr372 (Rearrangement II), related to W2 (X2C) and W7 (X7T, Xtr383, Xtr372), was identified on the group 6 chromosomes of the U genome and also on group 6 (X7T) and 7 (Xtr383-Xtr372) of the M genome. A further region on Br1 (or R3 and R7), defined by the markers Xtr400 and X4K (Rearrangement III) which are related to W2 and W4, respectively, was also found on the 6U chromosomes of Ae. umbellulata and in some cases on the polyploid Aegilops species.
The Xtr85-X3B region on the distal part of the short arm of Br2 (or R1) (Rearrangement IV), which is related to the group 3 chromosomes of wheat and to the M genomes, was homoeologous with the 7U chromosome in the diploid and polyploid Aegilops. The massive region spanning from Xtr100 to Xtr103 on the long arm of Br3 (or R2) (Rearrangement V) was homologous with the group 6 chromosomes of wheat and the M genome (in diploid and polyploid Aegilops species), whereas it was related to the 4U chromosomes of the three Aegilops species. Additional genome rearrangements detected by single markers were also found in the U and M genomes relative to each other and to wheat.
Syntenic Relationship of U and M Genomes Relative to Wheat
The markers whose EST sequences could be located on wheat allowed the direct investigation of syntenic relationships between wheat and the U and M genomes of Aegilops. Table 5 summarises the conserved genomic regions while Table S7 shows the syntenic relationship established based on COS marker positions (Figure 2) between the U and M genomes of diploid and tetraploid Aegilops species relative to wheat.
Table 5. Conserved genomic regions between the D genome of hexaploid wheat and the chromosomes of the U and M genomes of the diploid species Ae. umbellulata and Ae. comosa and their allotetraploid hybrids, Ae. biuncialis and Ae. geniculata.
| Group of Aegilops chr. | U genome | M genome | ||||
| Ae. umbellulata | Ae. biuncialis | Ae. geniculata | Ae.Comosa | Ae. biuncialis | Ae. geniculata | |
| 1 | W1 (6) | W1 (6) | W1 (6) | W1 (1) | W1 (1) | W1 (1) |
| W2 (1) | W2 (2) | W2 (2) | W2 (1) | W2 (1) | W2 (1) | |
| W4 (1) | W4 (1) | W4 (1) | ||||
| 2 | W2 (4) | W2 (3) | W2 (4) | W2 (1) | W2 (2) | W2 (5) |
| 3 | W3 (15) | W3 (13) | W3 (14) | W3 (6) | W1 (1) | W3 (5) |
| W6 (1) | W5 (1) | W7 (1) | W5 (1) | W3 (2) | ||
| W7 (1) | W7 (1) | |||||
| 4 | W6 (2) | W6 (1) | W6 (2) | – | – | – |
| 5 | W2 (1) | W5 (3) | W5 (4) | W5 (1) | W5 (1) | W5 (3) |
| W5 (3) | ||||||
| 6 | W2 (3) | W2 (3) | W2 (2) | W1 (1) | W6 (4) | W6 (4) |
| W4 (5) | W4 (4) | W4 (4) | W4 (2) | W7 (1) | W7 (1) | |
| W5 (2) | W7 (4) | W7 (3) | W6 (4) | |||
| W7 (4) | W7 (2) | |||||
| 7 | W3 (2) | W3 (2) | W3 (2) | W6 (1) | W6 (1) | W2 (1) |
| W7 (3) | W7 (3) | W6 (1) | W7 (3) | W7 (6) | W5 (1) | |
| W7 (3) | W6 (1) | |||||
| W7 (4) | ||||||
The number of COS markers representing the wheat orthologous regions is shown in brackets.
Discussion
Assignment of COS Markers to U and M Chromosomes
In the present study 94.3% of the COS markers produced amplicons in at least one Aegilops species, indicating the high transferability of the conserved orthologous set markers between the related species. The good transferability of COS markers was also reported for Ae. peregrina and Ae. ventricosa by Howard et al. [34] and Burt and Nicholson [35], respectively. The present results also indicate that the transferability of COS markers is better than other types of molecular markers such as SSRs, where transferability of wheat-specific markers was 80.3% (for Ae. geniculata), 79.62% (for Ae. biuncialis) and 54.1% for one of the species, Ae. umbellulata, Ae. comosa, Ae. biuncialis and Ae. geniculata [40], [16], [17]. High transferability between the species could be explained by the sequence conservation of the primer target sites of COS markers, which could be less variable than those of genomic simple sequence repeat markers (SSR).
Wheat SSR markers have been used widely for the molecular characterization of various Aegilops species, including Ae. biuncialis and Ae. geniculata [14], [16], [40]–[42]. Previous studies assigned 33 SSR and 37 sequence-specific amplified polymorphism (S-SAP) markers to U and M chromosomes [15]–[17], [40]. This work significantly increased the number of U and M genome-specific markers by identifying the Aegilops-specific chromosomal location for 100 COS markers. One hundred and sixty nine loci of 51 markers covering all 7 chromosomes of the U and M genomes resulted in polymorphic amplicons relative to wheat, so they are potentially useful markers for detecting Aegilops chromosomes in bread wheat.
The results also confirmed previous observations on the suitability of MDA-amplified chromosomal DNA for molecular marker analysis [38], [17] and indicate that flow-sorted chromosomes can be used for the physical mapping of molecular markers, especially when a complete set of cytogenetic stocks representing the whole chromosome complements is not available. Furthermore, the possibility of purifying chromosomes in Aegilops species [17] opens a way for next-generation survey sequencing to identify low-copy and genic sequences for the development of new markers, including SSR, ISBP, COS and SNP, for genotyping by sequencing of different accessions and for the high-resolution analysis of synteny and the characterization of structural chromosome differences between wheat and its progenitors and relatives [43], [44].
Relationships between the Genomes of Diploid and Tetraploid Aegilops Species
The theory of pivotal–differential evolutionary patterns in Aegilops species suggested by Zohary and Feldman [45] states that the pivotal U genomes remain essentially unchanged during allopolyploid speciation, while the differential M genomes have accumulated substantial modifications as compared with the parental genome [45]–[47]. Consistently with this theory, the inactivation of major NORs on the 1M and 6M chromosomes, the redistribution of 5S rDNA sites, and the loss of minor 18S–26S rDNA loci were observed in Ae. geniculata and Ae. biuncialis relative to Ae. comosa [48], [49], [12], [20]. The amplification, elimination and redistribution of highly repetitive DNA sequences after allopolyploidisation were also more pronounced in the M genome than in the U genome [49], [20]. In the present study, 77.7% and 80.5% of the amplicons assigned to the U genome were non-polymorphic in the allopolyploid species Ae. geniculata and Ae. biuncialis relative to the diploid progenitor Ae. umbellulata, a ratio higher than that observed for M genome loci, which remained unchanged in the allopolyploid species (55.5% in Ae. biuncialis and 65.7% in Ae. geniculata relative to Ae. comosa). These results suggest that, besides the structural chromosomal modifications observed at the macro level, micro level changes may also have happened more frequently in the M genome during or after allopolyploid speciation. The intron regions of the genes, which show a higher frequency of polymorphism in the M genome relative to the U genome, might also be involved in genomic changes related to allopolyploidization.
Little is known about the underlying mechanisms of selective alterations in M-genome chromosomes in (allo)polyploid species. Segmental and single gene duplications were reported to play an important role in the evolution of polyploid species, permitting the functional diversification of paralogues leading to plant adaptation and speciation [50]. In the present study, the number of duplications which were absent in diploid ancestors was higher in the M genomes of Aegilops allotetraploids (five duplications detected by fifteen markers) than in the U genomes (three duplications detected by three COS markers) (Table 3). These results suggest that the molecular process of gene duplication is more frequent in the M genome than in the U genome in allotetraploid Aegilops species. Two mechanisms were proposed for gene duplication which are linked to the activity of transposable elements (TE) [51], [52]. According to Wicker et al. [52] genes can be captured and copied together with a transposable element to a new location, which has also been documented in allopolyploid plant species [53]. The other mechanism is double-strand break repair involving synthesis-dependent strand annealing, which accompanies the TE insertion [51]. However, earlier studies on the presence of SINE elements in diploid and tetraploid Aegilops species with U and M genomes did not support the role of transposable elements in the selective alteration of M genomes during allopolyploidization [15]. The possibilities that allopolyploidization triggered the loss of duplicated genes more frequently in the U genomes, leading to the observation of an apparently lower number of duplicated loci, cannot be excluded. Clearly, a more detailed investigation of allopolyploidization-related changes in the U and M genome chromosomes is needed to explain the distinct difference in the number of duplicated loci on the U and M genomes and to obtain information on the molecular mechanism of their selective alteration.
Relationships between Aegilops, Model Species and Wheat
Previously, wheat-Ae. umbellulata macrosynteny was investigated by mapping wheat RFLP markers on the U genome chromosomes of Ae. umbellulata [21], [54], [55]. The present work extended the comparative analysis of wheat and Aegilops genomes to the M genome of diploid Ae. comosa, allowing the U and M genomes in polyploid Ae. biuncialis and Ae. geniculata to be investigated in relation to wheat, Brachypodium and rice. The wheat-Brachypodium and wheat-rice genome relationships obtained by the physical location of marker-represented orthologue genes on Brachypodium and rice were consistent with previous data reported on syntenic relationships after the sequencing and assembly of the genomes of Brachypodium and rice [30], [23]. The physical maps allowed the detection of macrosyntenic relationships between Aegilops and the model species. In this respect, previous results indicated that rice chromosome 10 (R10) was inserted into R5 to form Triticeae chromosome 1, R7 was inserted into R4 to form Triticeae chromosome 2, and R8 was inserted into R6 to form Triticeae chromosome 7 [56], [50]. These chromosomal rearrangements were also detected in wheat and in ryegrass chromosomes 1 and 7 [50], [57]. In the present study, the relationship between the R5-R10-R5 insertion and chromosome 1U was also detected, but it was not confirmed for chromosome 1M, where mainly R5 and R7 regions were present (Table 4). In the case of group 2 Aegilops chromosomes, the R4-R7-R4 insertion was indicated on 2U chromosomes, but the 2M chromosomes were related mainly to R4 and R8. Finally, the R6-R8-R6 insertion was detected for both the 7U and 7M chromosomes. These results suggest that genome rearrangements derived from common ancestors appear to characterize the U genome of the Aegilops species, but were only partly valid for the M genomes.
From an agronomic point of view, the macrosyntenic relationships between Aegilops and the model species provide useful background information for the targeted development of markers specific for the Aegilops chromosome regions responsible for important agronomic traits [35].
Physical maps of COS markers also allowed the investigation of relationships between the U and M genomes and between wheat and Aegilops species in the genomic perspective of Brachypodium and rice. Besides the relatively close relationship between the U and M genomes, the chromosomal location of Brachypodium and rice syntenic regions in wheat and Aegilops genomes detected five genome rearrangements differentiating the U and M genomes. All of them seem to have evolved at the diploid level and to have been modified differentially in the polyploid species Ae. biuncialis and Ae. geniculata. Three rearrangements (I, II and III) were connected to chromosome 6U, one (IV) to 7U and one (V) to 4U. Interestingly, in three of the five rearrangements (II, IV and V), the genomic regions involved were located on chromosomes in the same homoeologous group in wheat and in the M genomes of Ae. comosa, Ae. biuncialis and Ae. geniculata, while they were located on different homoeologous group chromosomes in the U genome of diploid and polyploid Aegilops species. These results suggest that the M genome is more closely related to the wheat D genome than the U genome.
The relationship between the U genome of Ae. umbellulata and the D genome of wheat was investigated by Zhang et al. [21], who mapped 79 wheat RFLP markers on wheat cv. Chinese Spring–Ae. umbellulata addition lines and 68 on an Ae. umbellulata segregating mapping population. This work revealed at least eleven rearrangements that differentiate the D genome of wheat from that of Ae. umbellulata [21]. Using a comparable level of marker resolution, the present study generally showed similar U-D homologous relationships to those reported in the previous work. It was found that 1U was related mainly to W1, though the presence of small fragments related to W2 and W4 was also indicated. The relationship of chromosome 1M to W1 and W2 was also detected, and chromosomes 2U and 2M were again found to be related to W2. Zhang et al. [21] showed that 3U was closely related to W3 and suggested the presence of a fragment related to W7 but no experimental evidence was presented. The close relationship of W3 to 3U, and also to 3M, was supported by the present work. Moreover, one marker indicated the presence of a fragment related to W7, which is consistent with the results of Yang et al. [58], who suggested that a translocation may exist between Ae. umbellulata chromosomes 3U and 7U. Due to the low number of markers, relationships could only be detected between 4U and W6 and between 5U/5M and W5. The present results confirmed the highly rearranged structure of chromosome 6U and indicated a rearranged structure for the 6M chromosomes for the first time. At the diploid and tetraploid levels, fragments related to W2, W4 and W7 were detected on 6U, as also reported by Zhang et al. [21]. Two markers also indicated the homology of Ae. umbellulata 6U with W5, but due to the low number of markers, no experimental evidence could be found for the presence of a fragment related to W6. The present study indicated homology between chromosome 6M and W6 and W7 in diploid and tetraploid Aegilops species and a relationship with W1 and W4 in Ae. comosa. Chromosome 7U contains regions syntenic with W3 and W7, consistently with the previous results [21], while the 7M chromosomes were related mainly to W6 and W7 in diploid and tetraploid Aegilops.
Evolutionary genome rearrangements are considered to be a common phenomenon in most plant taxa, including the Triticeae, and to be one of the most important evolutionary driving forces for the formation of new species. Genome shuffling can be triggered by polyploidization events and is the main reason for synteny breakage in grasses since their divergence from a common ancestor. Such mosaic synteny blocks were found in rye and ryegrass when their genomes were compared with wheat [59], [57] and they were also formed during the evolution of barley and hexaploid wheat [60], [61]. Based on the comparison of orthologous regions from rice, maize, sorghum and Brachypodium, Murat et al. [62] proposed that chromosome shuffling events were driven by non-random centric double-strand break repair processes. The centromeric/telomeric illegitimate recombination between non-homologous chromosomes results in nested chromosome fusions, followed by additional structural changes (inversions and repeat invasions) and the formation of synteny break points [62], [63]. By investigating the hardness locus in diploid and polyploid wheat species, Chantret et al. [64] detected various genome rearrangements and suggested that illegitimate DNA recombination is one of the major evolutionary mechanisms leading to various genomic rearrangements. Recently, it became clear that retrotransposons have a definitive role in these processes [65], [66]. Genome rearrangements are also thought to induce gene duplications which lead to the pseudogenization (functionless paralogues), concerted evolution (conservation of function for paralogues), subfunctionalization (complementary function of paralogues) and neofunctionalization (novel function of paralogues) of new alleles [62]. As the investigated Aegilops species are closely related to Triticum, it can be concluded that similar mechanisms took part in the evolution of the U and M genomes. Genome rearrangements in Aegilops, which were also formed frequently after allopolyploid speciation [20], could have been triggered by gene duplication events, as detected in this study (Table 3). After functional divergence, these duplicated loci may serve as raw material for evolution and represent potentially useful alleles for increasing the genetic diversity of bread wheat. It should be noted that the present comparisons of Aegilops genomes with wheat and model species were based on 100 orthologous genes. Ae. tauschii, whose D genome is of similar size (5.1 pg DNA/1C) to Ae. umbellulata (5.05 pg DNA/1C) and Ae. comosa (6.18 pg DNA/1C), has approximately 36,000 genes [67], [68], so the coverage in the present work cannot be more than 0.0027×, allowing only macro level comparison. In the near future, the shotgun sequencing of individual U and M genome chromosomes isolated by flow sorting [17] will result in a much deeper comparative genome analysis of Aegilops (http://www.wheatgenome.org/Projects/Complimentary-Projects/Wild-Relatives) and will provide more detailed information about evolutionary rearrangements and polyploidization-related processes in Aegilops U and M genomes.
Conclusions
Major efforts are underway to improve wheat yield and quality under stress conditions by increasing genetic diversity in breeding materials. Various Aegilops species have already been used as sources of new alleles for wheat breeding through interspecific hybridization. The conserved orthologous set markers assigned here to Aegilops chromosomes promise to accelerate gene introgression by facilitating the identification of alien chromatin. The analysis of complex polygenic traits such as earliness, abiotic stress tolerance and nutritional quality will also be accelerated, contributing to sustainable increases in wheat yields. Finally, the syntenic relationships between the Aegilops species, wheat and model species established in this work will facilitate the targeted development of new markers specific for U and M genomic regions and will contribute to the understanding of allopolyploidization-related molecular processes.
Supporting Information
Rice–wheat– Aegilops orthologous relationships from the genomic perspective of Oryza sativa . The physical positions of the source ESTs of the COS markers are indicated on the rice chromosomes (Left). Each marker assigned to chromosomes of the wheat D genome or to chromosomes of Ae. umbellulata (U), Ae. comosa (M), Ae. biuncialis (Ub, Mb) and Ae. geniculata (Ug, Mg) is colour-coded according to the homoeologous groups of Triticum/Aegilops chromosomes. When a marker mapped to more than one wheat or Aegilops chromosome, other colour-coded locations are positioned adjacent to the first one. Asterisks indicate the predicted chromosomal location of a locus when the PCR amplicon was specific for the U or M genomes and could be determined unambiguously in at least one Aegilops species (in the diploid progenitor, or in Ae. biuncialis or Ae. geniculata) and when the highest PCR product yield in the other two species was detected in the subgenomic DNA sample containing the same chromosome.
(TIF)
Primer sequences and anealing temperatures of the COS markers used in the present study.
(DOC)
Genomic positions of the non-polymorphic COS markers in rice and Brachypodium which were not assigned to Aegilops chromosomes.
(DOC)
Results of BLASTn search of source ESTs of COS markers assigned to Aegilops chromosomes in the rice genomic database.
(DOC)
Results of BLASTn search of source ESTs of COS markers assigned to Aegilops chromosomes in the Brachypodium genomic database.
(DOC)
PCR products of the COS markers in the genotypes of wheat and Aegilops species.
(DOC)
Assignment of COS markers to the chromosomes or to the peaks on flow karyotypes in Aegilops umbellulata, Ae. comosa, Ae. biuncialis and Ae. geniculata .
(DOC)
Syntenic relationship of U and M genomes relative to wheat.
(DOC)
Acknowledgments
The authors are grateful to Dr. Jarmila Číhalíková, Bc. Romana Šperková, and Zdenka Dubská for their assistance with chromosome sorting and DNA amplification.
Funding Statement
This work was funded by the Hungarian National Research Fund (PD83444), by TÁMOP-4.2.2.A-11-1-KONV-2012-0008, by a János Bolyai Research Scholarship from the Hungarian Academy of Sciences (for MI and CSA), by the Agrisafe Programme (EU-FP7-REGPOT-2007-1, No. 203288), by the Czech Science Foundation (grant award P501/12/G090), by the Ministry of Education, Youth and Sports of the Czech Republic and the European Regional Development Fund (Operational Programme Research and Development for Innovations No. ED0007/01/01) and by the United Kingdom Biotechnology and Biological Sciences Research Council. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Van Slageren MW (1994) Wild wheats: a monograph of Aegilops L. and Amblyopyrum (Jaub. & Spach) Eig (Poaceae). Wageningen, the Netherlands: Agricultural University, Aleppo, Syria: International Center for Agricultural Research in Dry Areas.
- 2. Friebe B, Jiang J, Raupp WJ, McIntosh RA, Gill BS (1996) Characterization of wheat alien translocations conferring resistance to diseases and pests: current status. Euphytica 71: 59–87. [Google Scholar]
- 3. Schneider A, Molnár I, Molnár-Láng M (2008) Utilisation of Aegilops (goatgrass) species to widen the genetic diversity of cultivated wheat. Euphytica 163: 1–19. [Google Scholar]
- 4. Shiferaw GA, Hoffmann B (2012) Recent advances and future perspectives in resistance breeding against Puccinia graminis f. sp. tritici, strain UG99. Acta Agron Hung 60: 71–86. [Google Scholar]
- 5.Waines JG, Rafi MM, Ehdaie B (1993) Yield components and transpiration efficiency in wild wheats. In: Damania AB editor. Biodiversity and Wheat Improvement. Chichester, UK: John Wiley and Sons. 173–186.
- 6. Rekika D, Monneveux P, Havaux M (1997) The in vivo tolerance of photosynthetic membranes to high and low temperatures in cultivated and wild wheats of the Triticum and Aegilops genera. J Plant Physiol 150: 734–738. [Google Scholar]
- 7. Colmer TD, Flowers TJ, Munns R (2006) Use of wild relatives to improve salt tolerance in wheat. J Exp Bot 57: 1059–1078. [DOI] [PubMed] [Google Scholar]
- 8. Molnár I, Gáspár L, Sárvári É, Dulai S, Hoffmann B, et al. (2004) Physiological and morphological responses to water stress in Aegilops biuncialis and Triticum aestivum genotypes with differing tolerance to drought. Funct Plant Biol 31: 1149–1159. [DOI] [PubMed] [Google Scholar]
- 9. Dulai S, Molnár I, Prónay J, Marschall M, Csernák Á, et al. (2005) Effects of drought on thermal stability of photosynthetic apparatus in bread wheat and in Aegilops species originating from various habitats. Acta Biol Szeged 49: 215–217. [Google Scholar]
- 10. Rawat N, Tiwari VK, Singh N, Randhawa GS, Singh K, et al. (2009) Evaluation and utilization of Aegilops and wild Triticum species for enhancing iron and zinc content in wheat. Genet Res Crop Evol 56: 53–64. [Google Scholar]
- 11. Kozub NA, Sozinov IA, Ksinias IN, Sozinov AA (2011) Allelic variation at high-molecular-weight glutenin subunit loci in Aegilops biuncialis Vis. Genetika 47: 1216–1222. [PubMed] [Google Scholar]
- 12. Schneider A, Linc G, Molnár I, Molnár-Láng M (2005) Molecular cytogenetic characterization of Aegilops biuncialis and its use for the identification of 5 derived wheat – Aegilops biuncialis addition lines. Genome 48: 1070–1082. [DOI] [PubMed] [Google Scholar]
- 13. Molnár I, Benavente E, Molnár-Láng M (2009) Detection of intergenomic chromosome rearrangements in irradiated Triticum aestivum – Aegilops biuncialis amphiploids by multicolour genomic in situ hybridization. Genome 52: 156–165. [DOI] [PubMed] [Google Scholar]
- 14. Peil A, Korzun V, Schubert V, Schumann E, Weber WE, et al. (1998) The application of wheat microsatellites to identify disomic Triticum aestivum–Aegilops markgrafii addition lines. Theor Appl Genet 96: 138–146. [Google Scholar]
- 15. Nagy ED, Molnár I, Schneider A, Kovács G, Molnár-Láng M (2006) Characterisation of chromosome-specific S-SAP markers and their use to study genetic diversity in Aegilops species. Genome 49: 289–296. [DOI] [PubMed] [Google Scholar]
- 16. Schneider A, Molnár I, Molnár-Láng M (2010) Selection of U and M genome-specific wheat SSR markers using wheat–Aegilops biuncialis and wheat–Ae. geniculata addition lines. Euphytica 175: 357–364. [Google Scholar]
- 17. Molnár I, Kubaláková M, Šimková H, Cseh A, Molnár-Láng M, et al. (2011b) Chromosome isolation by flow sorting in Aegilops umbellulata and Ae. comosa and their allotetraploid hybrids Ae. biuncialis and Ae. geniculata . PLoS ONE 6: e27708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang J, Friebe B, Gill BS (1994) Recent advances in alien gene transfer in wheat. Euphytica 73: 199–212. [Google Scholar]
- 19. Badaeva ED, Dedkova OS, Gay G, Pukhalskyi VA, Zelenin AV, et al. (2007) Chromosomal rearrangements in wheat: their types and distribution. Genome 50: 907–926. [DOI] [PubMed] [Google Scholar]
- 20. Molnár I, Cifuentes M, Schneider A, Benavente E, Molnár-Láng M (2011a) Association between simple sequence repeat-rich chromosome regions and intergenomic translocation breakpoints in natural populations of allopolyploid wild wheats. Ann Bot 107: 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang H, Jia J, Gale MD, Devos KM (1998) Relationships between the chromosomes of Aegilops umbellulata and wheat. Theor Appl Genet 96: 69–75. [Google Scholar]
- 22. Xu Y, McCouch SR, Zhang Q (2005) How can we use genomics to improve cereals with rice as a reference genome? Plant Mol Biol 59: 7–26. [DOI] [PubMed] [Google Scholar]
- 23. The International Rice Genome Sequencing Project (2005) The mapbased sequence of the rice genome. Nature 436: 793–800. [DOI] [PubMed] [Google Scholar]
- 24. Bennetzen JL, Ma J (2003) The genetic colinearity of rice and other cereals on the basis of genomic sequence analysis. Curr Opin Plant Biol 6: 128–133. [DOI] [PubMed] [Google Scholar]
- 25. Feuillet C, Keller B (2002) Comparative genomics in the grass family: molecular characterization of grass genome structure and evolution. Ann Bot 89: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Draper J, Mur LAJ, Jenkins G, Ghosh-Biswas GC, Bablak P, et al. (2001) Brachypodium distachyon. A new model system for functional genomics in grasses. Plant Physiol 127: 1539–1555. [PMC free article] [PubMed] [Google Scholar]
- 27. Foote T, Griffiths S, Allouis S, Moore G (2004) Construction and analysis of a BAC library in the grass Brachypodium sylvaticum and its use as a tool to bridge the gap between rice and wheat in elucidating gene content. Funct Integr Genomic 4: 26–33. [DOI] [PubMed] [Google Scholar]
- 28. Hasterok R, Marasek A, Donnison IS, Armstead I, Thomas A, et al. (2006) Alignment of the genomes of Brachypodium distachyon and temperate cereals and grasses using bacterial artificial chromosome landing with fluorescence in situ hybridization. Genetics 173: 349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luo MC, Deal KR, Akhunov ED, Akhunova AR, Anderson OD, et al. (2009) Genome comparisons reveal a dominant mechanism of chromosome number reduction in grasses and accelerated genome evolution in Triticeae . Proc Natl Acad Sci USA 106: 15780–15785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. The International Brachypodium Initiative (2010) Genome sequencing and analysis of the model grass Brachypodium distachyon . Nature 463: 763–768. [DOI] [PubMed] [Google Scholar]
- 31. Quraishi UM, Abrouk M, Bolot S, Pont C, Throude M, et al. (2009) Genomics in cereals: from genome-wide conserved orthologous set (COS) sequences to candidate genes for trait dissection. Funct Integr Genomics 9: 473–484. [DOI] [PubMed] [Google Scholar]
- 32. Yu J, Wang J, Lin W, Li S, Li H, et al. (2005) The genomes of Oryza sativa: A history of duplications. PLoS Biol 3: e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parida SK, Kumar ARK, Dalal V, Singh NK, Mohapatra T (2006) Unigene derived microsatellite markers for the cereal genomes. Theor Appl Genet. 112: 808–817. [DOI] [PubMed] [Google Scholar]
- 34. Howard T, Rejab NA, Griffiths S, Leigh F, Leverington-Waite M, et al. (2011) Identification of a major QTL controlling the content of B-type starch granules in Aegilops . J Exp Bot 62: 2217–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burt C, Nicholson P (2011) Exploiting co-linearity among grass species to map the Aegilops ventricosa-derived Pch1 eyespot resistance in wheat and establish its relationship to Pch2 . Theor Appl Genet 123: 1387–1400. [DOI] [PubMed] [Google Scholar]
- 36. Molnár I, Molnár-Láng M (2010) GISH reveals different levels of meiotic pairing with wheat for individual Aegilops biuncialis chromosomes. Biologia Plant 54: 259–264. [Google Scholar]
- 37. Friebe B, Tuleen NA, Gill BS (1999) Development and identification of a complete set of Triticum aestivum –Ae. geniculata chromosome addition lines. Genome 42: 374–380. [Google Scholar]
- 38. Šimková H, Svensson JT, Condamine P, Hřibová E, Suchánková P, et al. (2008) Coupling amplified DNA from flow-sorted chromosomes to high-density SNP mapping in barley. BMC Genomics 9: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sorrells ME, La Rota M, Bermudez-Kandianis CE, Greene RA, Kantety R, et al. (2003) Comparative DNA sequence analysis of wheat and rice genomes. Genome Res 13: 1818–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zaharieva M, Suenaga K, William HM, Mujeeb-Kazi A (2003) Microsatellite markers for identification of Aegilops geniculata Roth. M- and U-genome chromosomes in wheat background. Annual Wheat Newsletter 49: 75–78. [Google Scholar]
- 41. Pestsova E, Korzun V, Goncharov NP, Hammer K, Ganal MW, et al. (2000) Microsatellite analysis of Aegilops tauschii germplasm. Theor Appl Genet 101: 100–106. [Google Scholar]
- 42. Lelley T, Stachel M, Grausguber H, Vollmann J (2000) Analysis of relationships between Aegilops tauschii and the D genome of wheat utilizing microsatellites. Genome 43: 661–668. [PubMed] [Google Scholar]
- 43. Berkman PJ, Lai K, Lorenc MT, Edwards D (2012) Next-generation sequencing applications for wheat crop improvement. Am J Bot 99: 365–371. [DOI] [PubMed] [Google Scholar]
- 44. Hernandez P, Martis M, Dorado G, Pfeifer M, Gálvez S, et al. (2012) Next-generation sequencing and syntenic integration of flow-sorted arms of wheat chromosome 4A exposes the chromosome structure and gene content. Plant J 69: 377–386. [DOI] [PubMed] [Google Scholar]
- 45. Zohary D, Feldman M (1962) Hybridization between amphiploids and the evolution of polyploids in the wheat Aegilops–Triticum group. Evolution 16: 44–61. [Google Scholar]
- 46. Feldman M (1965a) Further evidence for natural hybridization between tetraploid species of Aegilops section Pleionathera . Evolution 19: 162–174. [Google Scholar]
- 47. Feldman M (1965b) Chromosome pairing between differential genomes in hybrids of tetraploid Aegilops species. Evolution 19: 563–568. [Google Scholar]
- 48. Kimber G, Sallee PJ, Feiner MM (1988) The interspecific and evolutionary relationships of Triticum ovatum . Genome 30: 218–221. [Google Scholar]
- 49. Badaeva ED, Amosova AV, Samatadze TE, Zoshchuk SA, Shostak NG, et al. (2004) Genome differentiation in Aegilops. 4. Evolution of the U-genome cluster. Plant Syst Evol 246: 45–76. [Google Scholar]
- 50. Salse J, Bolot S, Throude M, Jouffe V, Piegu B, et al. (2008) Identification and characterization of shared duplications between rice and wheat provide new insight into grass genome evolution. Plant Cell 20: 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wicker T, Buchmann JP, Keller B (2010) Patching gaps in plant genomes results in gene movement and erosion of colinearity. Genome Res. 20: 1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wicker T, Mayer KFX, Gundlach H, Martis M, Steuernagel B, et al. (2011) Frequent gene movement and pseudogene evolution is common to the large and complex genomes of wheat, barley, and their relatives. Plant Cell 23: 1706–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bartoš J, Vlček Č, Choulet F, Džunková M, Cviková K, et al. (2012) Intraspecific sequence comparisons reveal similar rates of non-collinear gene insertion in the B and D genomes of bread wheat. BMC Plant Biol. 12: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gale MD, Devos KM (1998) Comparative genetics in the grasses. Proc Natl Acad Sci USA 95: 1971–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Devos KM, Gale MD (2000) Genome relationships: The grass model in current research. Plant Cell 12: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gale MD, Devos KM (1997) Comparative genetics in the grasses. Plant Mol Biol 35: 3–15. [PubMed] [Google Scholar]
- 57. Sim S, Chang T, Curley J, Warnke SE, Barker RE, et al. (2005) Chromosomal rearrangements differentiating the ryegrass genome from the Triticeae, oat, and rice genomes using common heterologous RFLP probes. Theor Appl Genet 110: 1011–1019. [DOI] [PubMed] [Google Scholar]
- 58. Yang Y-C, Tuleen NA, Hart GE (1996) Isolation and identification of Triticum aestivum L. em. Thell. cv Chinese Spring - T. peregrinum Hackel disomic chromosome-addition lines. Theor Appl Genet 92: 591–598. [DOI] [PubMed] [Google Scholar]
- 59. Devos KM, Atkinson MD, Chinoy CN, Francis HA, Harcourt RL, et al. (1993) Chromosomal rearrangements in the rye genome relative to that of wheat. Theor Appl Genet 85: 673–680. [DOI] [PubMed] [Google Scholar]
- 60. Mayer KFX, Martis M, Hedley PE, Šimková H, Liu H, et al. (2011) Unlocking the barley genome by chromosomal and comparative genomics. Plant Cell 23: 1249–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feuillet C, Salse J (2009) Comparative genomics in the Triticeae In: Feuillet C, Muehlbauer GJ, editors. Genetics and Genomics of the Triticeae, Plant Genetics and Genomics: Crops and Models 7. Springer Science+Business Media, LLC. Pp. 451–477.
- 62. Murat F, Xu JH, Tannier E, Abrouk M, Guilhot N, et al. (2010) Ancestral grass karyotype reconstruction unravels new mechanisms of genome shuffling as a source of plant evolution. Genome Res 20: 1545–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Salse J (2012) In silico archeogenomics unveils modern plant genome organisation, regulation and evolution. Curr Opin Plant Biol 15: 1–9. [DOI] [PubMed] [Google Scholar]
- 64. Chantret N, Salse J, Sabot F, Rahmand S, Bellec A, et al. (2005) Molecular basis of evolutionary events that shaped the hardness locus in diploid and polyploid wheat species (Triticum and Aegilops). Plant Cell 17: 1033–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bennetzen JL, Ma J, Devos KM (2005) Mechanisms of recent genome size variation in flowering plants. Ann Bot 95: 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wicker T, Yahiaoui N, Keller B (2007) Contrasting rates of evolution in Pm3 loci from three wheat species and rice. Genetics 177: 1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bennett MD, Leitch IJ (2012) Angiosperm DNA C-values database (release 8.0, Dec. 2012) http://www.kew.org/cvalues/.
- 68. Brenchley R, Spannag M, Pfeifer M, Barker GLA, D’Amore R, et al. (2012) Analysis of the bread wheat genome using whole-genome shotgun sequencing. Nature 491: 705–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rice–wheat– Aegilops orthologous relationships from the genomic perspective of Oryza sativa . The physical positions of the source ESTs of the COS markers are indicated on the rice chromosomes (Left). Each marker assigned to chromosomes of the wheat D genome or to chromosomes of Ae. umbellulata (U), Ae. comosa (M), Ae. biuncialis (Ub, Mb) and Ae. geniculata (Ug, Mg) is colour-coded according to the homoeologous groups of Triticum/Aegilops chromosomes. When a marker mapped to more than one wheat or Aegilops chromosome, other colour-coded locations are positioned adjacent to the first one. Asterisks indicate the predicted chromosomal location of a locus when the PCR amplicon was specific for the U or M genomes and could be determined unambiguously in at least one Aegilops species (in the diploid progenitor, or in Ae. biuncialis or Ae. geniculata) and when the highest PCR product yield in the other two species was detected in the subgenomic DNA sample containing the same chromosome.
(TIF)
Primer sequences and anealing temperatures of the COS markers used in the present study.
(DOC)
Genomic positions of the non-polymorphic COS markers in rice and Brachypodium which were not assigned to Aegilops chromosomes.
(DOC)
Results of BLASTn search of source ESTs of COS markers assigned to Aegilops chromosomes in the rice genomic database.
(DOC)
Results of BLASTn search of source ESTs of COS markers assigned to Aegilops chromosomes in the Brachypodium genomic database.
(DOC)
PCR products of the COS markers in the genotypes of wheat and Aegilops species.
(DOC)
Assignment of COS markers to the chromosomes or to the peaks on flow karyotypes in Aegilops umbellulata, Ae. comosa, Ae. biuncialis and Ae. geniculata .
(DOC)
Syntenic relationship of U and M genomes relative to wheat.
(DOC)