Abstract
Dedicated neuronal circuits enable animals to engage in specific behavioral responses to environmental stimuli. We demonstrate here that hypoxic stress enhances gustatory sensory perception via novel circuitry in the nematode Caenorhabditis elegans. The hypoxia-inducible transcription factor HIF-1 upregulates serotonin (5-HT) expression in specific sensory neurons not normally required for chemosensation. 5-HT subsequently signals to promote hypoxia-enhanced sensory perception using a metabotropic G-protein-coupled receptor, SER-7, in an unusual peripheral neuron, the M4 motor neuron. M4 relays this information back into the central nervous system via the FMRFamide-related neuropeptide FLP-21 and its cognate receptor NPR-1. Thus, physiological detection of hypoxia results in the activation of an additional, previously unrecognized circuit for processing sensory information that is not required for sensory processing under normoxic conditions.
INTRODUCTION
The ability of organisms to react and adapt to stress is essential for survival. In response to specific stressors, a battery of neurotransmitters, neuropeptides and steroid stress mediators may be deployed to enact the appropriate physiological and behavioral modifications1–3. One potent stress modulator in humans is serotonin (5-HT)4. In the nematode C. elegans 5-HT also modulates a variety of stress responses (heat, infection, starvation) in addition to cognitive behaviors (chemosensation, learning and memory)5–8. However, the neural circuits that underlie 5-HT-mediated stress responses are poorly characterized in any system to date and little molecular detail is known about how specific stressors couple to 5-HT-mediated signal transduction in the nervous system.
We describe here a novel circuit for the processing of sensory information that is activated upon stressful environmental conditions. In a previous study, we noted that hypoxia alters axon guidance and cell migration9. In the course of this neuroanatomical analysis, we observed, and describe here in detail, hypoxia-induced changes in the production of 5-HT. We show that hypoxia-induced alterations in 5-HT synthesis result in the activation of a novel pathway for processing gustatory information, thereby increasing the sensory acuity of the animal. Such behavioral plasticity may provide survival advantages under specific, stressful environmental conditions. Some lines of evidence indicate that the direct coupling of hypoxia to the production of 5-HT, and the use of alternate neuronal circuits may be phylogenetically conserved.
RESULTS
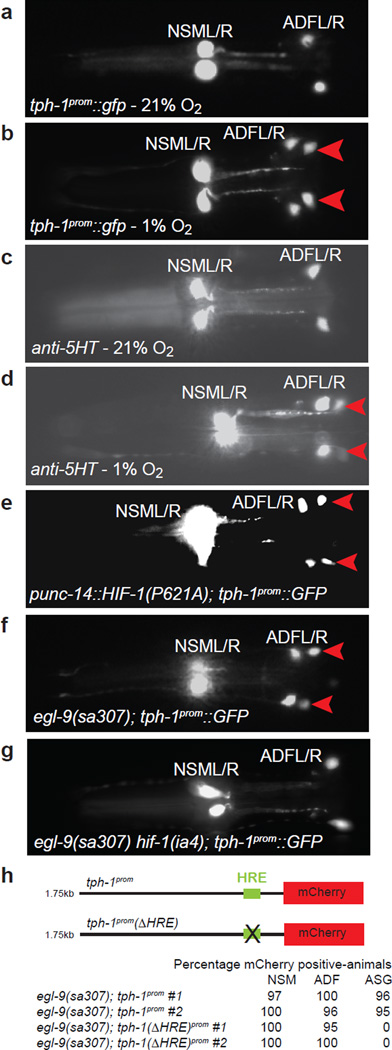
The rate-limiting enzyme of 5-HT biosynthesis is tryptophan hydroxylase, encoded by the tph-1 locus in C. elegans10. We found that under low oxygen conditions a gfp-based reporter which monitors tph-1 transcription (tph-1prom∷gfp) shows specific alterations in its expression pattern (Fig. 1). Under normoxic conditions (21% O2), tph-1prom∷gfp is robustly expressed in the head of the adult hermaphrodite in the NSM and ADF neurons (in 100% of animals; n=141), and weakly expressed in the ASG sensory neurons of a small percentage (5%; n=141) of animals (Fig. 1a and data not shown). We found that after exposure to 1% O2 for 12 hours, tph-1prom∷gfp expression is strongly upregulated in the ASG neurons (100% of animals; n= 78; Fig. 1b and data not shown). The already robust expression of tph-1prom∷gfp in NSM and ADF is also further augmented under hypoxic conditions (Suppl. Fig. 1). Anti-5-HT immunofluorescence staining of hypoxic animals also revealed induction of 5-HT expression compared to normoxic control animals (Fig. 1c, d).
Fig. 1.
5-HT expression is altered in hypoxia through direct HIF-1 regulation of tph-1
(A–B) tph-1prom∷gfp expression in normoxia (A) and after exposure to 1% O2 for 12 hours (B). Using a reporter strain that expresses tph-1prom∷gfp at low-levels (injected at 2ng/µl) we found that tph-1prom∷gfp is also upregulated in the ADF and NSM neurons after hypoxic exposure (see Suppl. Fig. 1). (C–D) Anti-5-HT immunofluorescence of a normoxic animal (C) and an animal after exposure to 1% O2 for 12 hours (D). (E–G) tph-1prom∷gfp is ectopically expressed in ASGL/R under conditions that stabilize hif-1, achieved through either mutating the residue in HIF-1 required for its degradation (E) or in egl-9 mutants (F); the latter effect genetically depends on hif-1 (G). Red arrowheads point to the ASG chemosensory neurons. Ventral views, anterior to the left. (H) HIF-1-induced tph-1 reporter gene expression (induction achieved through preventing the degradation of HIF-1 in egl-9 mutants) is abolished upon deletion the canonical HIF-1 binding site (“HRE”'; 6 bp deletion). # = independent transgenic lines (n = 87–122).
To investigate how the cell-type specific alteration in tph-1 expression is brought about, we turned to the phylogenetically conserved HIF-1 protein, a bHLH-PAS domain-containing transcription factor which mediates many transcriptional responses to hypoxic stress11. In normoxic conditions, HIF-1 protein is degraded12. Degradation is initiated through hydroxylation of a specific proline residue in HIF-1 by the conserved oxygen-dependent prolyl 4-hydroxylase EGL-912. The E3 ubiquitin ligase VHL-1 (von Hippel-Lindau tumor suppressor protein) recognizes the hydroxylated proline and targets HIF-1 for ubiquitin-mediated proteasomal degradation13. Hypoxic conditions inhibit the hydroxylation of HIF-1, thereby stabilizing the protein and allowing it to transcriptionally regulate the expression of a host of target genes involved in promoting survival and adaptation to hypoxic stress13,14. To test whether hypoxic induction of tph-1prom∷gfp is regulated in a HIF-1-dependent manner, we analyzed tph-1prom∷gfp expression under conditions that disrupt HIF-1 protein degradation in normoxic conditions. First, we used transgenic animals in which we express, under control of a neuronal promoter, a mutated form of HIF-1, in which the proline in the conserved LXXLAP motif required for degradation of HIF-1 by the egl-9/vhl-1-dependent degradation pathway is altered to an alanine 9,12. These transgenic animals display the same upregulation of tph-1prom∷gfp as observed under hypoxic conditions (Fig. 1e). Second, the induction of tph-1prom∷gfp under hypoxic conditions is phenocopied under normoxic conditions in egl-9 and vhl-1 mutant backgrounds, in which HIF-1 fails to be degraded (Fig. 1F and data not shown). Furthermore, reduction of hif-1 function in these mutant backgrounds abolishes tph-1prom∷gfp induction demonstrating that induction is dependent on HIF-1 function (Fig. 1g and data not shown).
To assess whether the HIF-1-dependent regulation of tph-1 expression is direct, we examined the tph-1 promoter region for hypoxia response elements (HRE) - biochemically defined binding sites of HIF-115. The tph-1 promoter indeed contains a HRE (TACGTG) 180bp upstream from its translational start site. This site is conserved in the tph-1 genes of other Caenorhabditis species and functional HREs are also present in vertebrate TPH loci 16. To ask whether HIF-1 directly activates tph-1 we deleted the HRE in the context of the tph-1 upstream regulatory sequence fused to a red fluorescent protein, mCherry (Fig. 1h). When expressed in wild-type animals both tph-1prom∷mCherry and tph-1prom(ΔHRE)∷mCherry transgenes are expressed in a wild-type manner i.e. in the NSM and ADF neurons in the head of the worm (data not shown). However, in an egl-9 mutant background in which HIF-1 is stabilized, only the tph-1prom∷mCherry transgenic lines but not the tph-1prom(ΔHRE)∷mCherry lines showed induced expression in the ASG neurons (Fig. 1h). These data suggest that in C. elegans HIF-1 binds directly to the tph-1 promoter via the HRE to drive 5-HT production in hypoxic conditions. In vertebrates, hypoxia-induced upregulation of 5-HT also occurs in specific regions of the brain required for processing information on oxygen status, pointing to possible conserved mechanisms, yet the functional significance of 5-HT upregulation in these specific brain regions is not known17.
Laser ablation studies have shown that an attractive response to gustatory cues is primarily mediated through the ASE gustatory neurons18. The ADF, ASG and ASI sensory neurons are not required for gustatory behavior in the presence of ASE, but the minor and residual response to gustatory cues observed in ASE-ablated animals is eliminated upon co-ablation of ADF, ASG and ASI18. The potential of ASG and ADF to be involved in gustatory behavior under specific circumstances prompted us to hypothesize that hypoxia-induced 5-HT upregulation in ASG and ADF may enhance the ability of animals to engage in a gustatory response. To test this hypothesis, we first assayed whether hypoxia can modulate gustatory behavior. Using conventional gradient chemotaxis assays18, we indeed found that exposure of wild-type adult hermaphrodites to 1% O2 for 24 hours enhanced their ability to move up a NaCl gradient (Fig. 2a). This suggests that hypoxic exposure enhances the ability of animals to sense their environment, perhaps to enable them to escape from stressful conditions. We term this adaptive stress response "hypoxia-enhanced sensory perception" (HESP).
Fig. 2.
C. elegans enhances sensory perception after hypoxic stress via HIF-1 and 5-HT in a neuron-specific manner. (A) Hypoxic exposure of C. elegans wild-type adult hermaphrodites enhances their ability to respond to 500mM and 250mM NaCl. (B) Hypoxic exposure of che-1(ot66) mutant adult animals (which lack functional ASE neurons and therefore do not chemotax well under normoxic conditions) significantly enhances their ability to chemotax towards 2.5M NaCl. This behavior is dependent on 5-HT and HIF-1 as it is abolished in che-1(ot66); tph-1(mg280) and che-1(ot66); hif-1(ia4) double mutant animals. Transgenic expression of hif-1 in ADF and ASG, but not the NSM neurons, rescues the loss of HESP in the che-1(ot66); hif-1(ia04) double mutant (# = independent transgenic lines). Based on previous ablation data18, we therefore hypothesize that the ADF and ASG neurons do have a latent ability to sense chemosensory cues, and that hypoxia-induced 5-HT production allows the sensory information to be relayed to downstream neurons (M4 etc.). Promoters used for cell specific rescue were ops-1 (ASG)37, srh-142 (ADF)38 and ceh-2 (NSM)39. Statistical significance was assessed using the t-test (**P<0.05, ***P<0.005, n.s. = not statistically significant from control). Black bars, normoxia; gray bars 24 hour incubation at 1% O2 as adults.
We next sought to establish the molecular and cellular basis of this behavior. We found that the ASE primary gustatory neurons are not required for HESP. In che-1 mutant animals, the ASE neurons are present but not functional19 and, consequently, che-1 mutants show barely any response in a NaCl gradient (Fig. 2b). However, when che-1 mutants are exposed to 1% O2 for 24 hours their ability to respond to NaCl is remarkably enhanced (Fig. 2b). ASE-independent HESP is dependent on 5-HT and HIF-1 because che-1; tph-1 and che-1; hif-1 double mutant animals do not display the hypoxia-induced sensory response. To test whether the neurons that upregulate 5-HT expression under hypoxic conditions are responsible for HESP we performed neuron-specific rescue experiments. We re-expressed hif-1 cDNA in the ASG, ADF or NSM neurons in a che-1; hif-1 double mutant background, using the ops-1, srh-142 and ceh-2 promoters, respectively (Fig. 2b). We found that expression of HIF-1 in the ASG or ADF, but not NSM neurons rescued HESP in the che-1; hif-1 double mutant. These data suggest that a hypoxia-induced increase in 5-HT levels in the ADF and ASG sensory neurons enhances gustatory perception. To test whether an increase in 5-HT must be provided by a specific cellular source to generate an enhanced gustatory response, we exogenously added 5-HT to the culture medium. Adult worms exposed to exogenous 5-HT indeed display an enhanced gustatory response (Suppl. Fig. 2). Taken together, our results indicate that an overall increase in 5-HT levels - induced by hypoxic conditions - that improve gustatory performance.
To further map the circuit of 5-HT action during HESP, we sought to identify the 5-HT receptor(s) that mediate this stress response. A survey of mutant strains of all previously identified 5-HT receptors revealed that only SER-7, a 5-HT7-like metabotropic receptor, is required for HESP (Fig. 3a). SER-7 was previously shown to be required for peristaltic movements of the pharyngeal isthmus and 5-HT-stimulated egg-laying20,21, but has not previously been implicated in controlling sensory responses. SER-7 is expressed in a very small number of peripheral neurons in the pharynx, one being the M4 pharyngeal motorneuron20. Expression of ser-7 under control of an M4-specific regulatory element from the ser-7 promoter22 or under control of the M4-specific ceh-28 promoter23 rescues the HESP defect of ser-7 mutant animals (Fig. 3b). The role of M4 and ser-7 in promoting hif-1-mediated HESP is independent of the motor function of M4 and ser-7. First, the hif-1 system, which is responsible for the HESP response, has no impact on M4-mediated motor control of the pharynx24. Second, decreases in food intake alone, observed upon disruption of M4 motor neuron function or deletion of ser-7, does not induce the HESP response (data not shown).
Fig. 3.
SER-7, a 5-HT7-like metabotropic receptor, acts in the M4 pharyngeal motorneuron to induce hypoxia-enhanced sensory perception. (A) Loss of ser-7 but not ser-1, ser-2, ser-3, ser-4 causes defects in HESP to a 250mM NaCl gradient. (B) Expression of the ser-7 cDNA exclusively in the M4 neuron with the M4-specific ser-7 and ceh-28 promoter fragments restores HESP in the ser-7(tm1325) mutant. Statistical significance was assessed using the t-test (**P<0.05, ***P<0.005). Black bars, normoxia; gray bars 24 hour incubation at 1% O2 as adults.
The only previously reported role for the M4 motor neuron is the control of pharyngeal muscle contraction, a function essential for survival of the animal 25. An additional role for this motor neuron in sensory plasticity is unexpected and suggests the existence of long-range signals not just from the sensory apparatus into the pharynx (via 5-HT), but also from the pharynx back into the circuit that eventually controls sensory cue-elicited locomotory behavior. Neuropeptides are attractive candidates for such long-range signaling molecules and, indeed, the M4 motor neuron contains, unlike other pharyngeal motor neurons, darkly-staining vesicles which may release such neuropeptides 26. Surveying the reported gene expression profile of the M4 neuron, we noted the expression of the FMRFamide flp-21 27. To test whether flp-21 may be involved in M4-mediated HESP, we analyzed flp-21 knockout animals. These animals display wild-type gustatory behavior under normoxic conditions, but fail to display an HESP response (Fig. 4). The HESP defect in the flp-21(ok889) mutant is rescued when flp-21 cDNA is re-expressed specifically in the M4 neuron (Suppl. Fig. 3).
Fig. 4.
FLP-21 acts with NPR-1 to control hypoxia-enhanced sensory perception. Mutations in the neuropeptide, FLP-21, and its receptor NPR-1 cause defects in the HESP response. Expression of npr-1 cDNA under the gcy-32 promoter (AQR, PQR and URX expression)40 and not under the ncs-1 promoter (other npr-1-expressing neurons)40 is sufficient to restore the HESP response in npr-1(ad609) animals. Ablation of the oxygen-sensing neurons AQR/PQR/URX (strain qaIs2241)7 also causes defects in HESP, providing further evidence for a role of these neurons in this behavior. Note that non-wild type genetic backgrounds shown here not only fail to display HESP, but that chemotaxis in normoxia is enhanced relative to wild type, while under hypoxic conditions, chemotaxis drops to the level of wild-type animals under normoxic condition. This observation is consistent with the previously made notion that npr-1 and the AQR/PQR/URX neurons promote exploratory behavior under normoxic conditions; this exploratory drive is abolished upon lowering oxygen33. Statistical significance was assessed using the t-test (**P<0.05, ***P<0.005). Black bars, normoxia; gray bars 24 hour incubation at 1% O2 as adults.
FLP-21 is a ligand for the neuropeptide Y receptor-related protein NPR-1 28. npr-1 has been implicated in a number of different behavioral paradigms, including foraging, aggregation and aerotaxis behavior7,29,30. Consistent with FLP-21 signaling through NPR-1 in the context of hypoxia enhanced gustation, we found that npr-1 mutant animals fail to display a HESP response (Fig. 4). npr-1 is expressed in a number of head neurons, including the AQR, PQR and URX neurons, which have been previously implicated in aerotaxis behaviors7,31. We found that expression of npr-1 under the control of an AQR/PQR/URX-specific promoter rescues the HESP defects of npr-1, while npr-1 expression with a driver for other npr-1 positive neurons fails to rescue the mutant phenotype (Fig. 4). Confirming the importance of the npr-1-expressing AQR/PQR/URX neurons, we furthermore found that transgenic animals in which the AQR, PQR and URX neurons are ablated by ectopic expression of the cell-death activator gene egl-1 7 also fail to display a HESP response (Fig. 4). Another gustatory plasticity paradigm, in which extended pre-exposure to NaCl modulates responses to NaCl also involves the AQR, PQR and URX neurons 32, suggesting that these neurons are common integrators of upstream sensory information. Even though it is not clear how npr-1 and the neurons expressing npr-1 may signal to downstream neurons to control chemosensory behavior, npr-1 activity can couple to locomotory outputs, as alterations in npr-1 activity affect locomotory speed 33, which in turn may affect the ability of an animal to migrate toward a chemosensory cue. This strategy would be an effective supplement to the strategy employed by the normal, normoxic chemosensory circuit which controls relative turning probability 34.
DISCUSSION
Our data, schematically summarized in Suppl. Fig. 4, indicate that hypoxic stress in C. elegans results in the deployment of an additional previously unrecognized circuit for mediating gustatory responses, which appears to have no detectable role under normoxic conditions. This response differs from the aerotaxis neuronal circuit which becomes simplified and less flexible after a hypoxic insult 35. Rather than altering intrinsic features of the circuit normally involved in gustation, animals engage, for the processing of chemosensory information, a novel set of neurons that is not required for chemotaxis under normoxic conditions. The deployment of a pharyngeal motor neuron in this 5-HT mediated circuitry is particularly unexpected and a testament to the multi-functionality of individual neuron types. As a result, through the utilization of 5-HT and neuropeptide signaling in response to hypoxia, animals become hypersensitive to environmental cues. We propose that hypoxia-induced enhanced gustatory perception forms part of a more widespread escape response that enables an animal to be more responsive to other cues in its environment under unfavorable conditions. Even though the hypoxia-mediated upregulation of 5-HT expression appears to be mostly restricted to the chemosensory system, it is conceivable that hypoxia may also lead to the upregulation of other neuromodulatory systems that may impinge on additional aspects of C. elegans behavior.
Taken together, our results provide novel insights into the molecular and cellular basis of 5-HT-mediated stress responses. The conservation of hypoxia-response elements in the tph genes in humans, the hypoxia-induced upregulation of 5-HT in specific regions of the vertebrate brain17 and alterations in the activity of the neuropeptide Y signaling system under hypoxic conditions36, suggest phylogenetically conserved mechanisms of coupling hypoxia, serotonin and neuropeptide signaling to produce alteration in behavioral responses.
Supplementary Material
ACKNOWLEDGMENTS
We thank A. Boyanov and G. Baison for technical assistance; Q. Chen for expert microinjection help, Iva Greenwald, Mario De Bono and members of the Hobert Laboratory for comments on the manuscript and the Caenorhabditis Genetics Center and C. Bargmann for providing strains. This work was supported by the Howard Hughes Medical Institute.
Footnotes
AUTHOR CONTRIBUTION. R.P. initiated this study and conducted all the experiments. R.P. and O.H. designed and discussed the experiments and wrote the paper.
REFERENCES
- 1.Joels M, Baram TZ. Nat Rev Neurosci. 2009;10(6):459. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Kloet ER, Joels M, Holsboer F. Nat Rev Neurosci. 2005;6(6):463. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS. Physiol Rev. 2007;87(3):873. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 4.Chaouloff F. J Psychopharmacol. 2000;14(2):139. doi: 10.1177/026988110001400203. [DOI] [PubMed] [Google Scholar]
- 5.Liang B, Moussaif M, Kuan CJ, et al. Cell Metab. 2006;4(6):429. doi: 10.1016/j.cmet.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Lu H, Bargmann CI. Nature. 2005;438(7065):179. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]
- 7.Chang AJ, Chronis N, Karow DS, et al. PLoS Biol. 2006;4(9):e274. doi: 10.1371/journal.pbio.0040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hukema RK, Rademakers S, Jansen G. Learn Mem. 2008;15(11):829. doi: 10.1101/lm.994408. [DOI] [PubMed] [Google Scholar]
- 9.Pocock R, Hobert O. Nat Neurosci. 2008;11(8):894. doi: 10.1038/nn.2152. [DOI] [PubMed] [Google Scholar]
- 10.Sze JY, Victor M, Loer C, et al. Nature. 2000;403(6769):560. doi: 10.1038/35000609. [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL. Trends Mol Med. 2001;7(8):345. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- 12.Epstein AC, Gleadle JM, McNeill LA, et al. Cell. 2001;107(1):43. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 13.Bishop T, Lau KW, Epstein AC, et al. PLoS Biol. 2004;2(10):e289. doi: 10.1371/journal.pbio.0020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen C, Nettleton D, Jiang M, et al. J Biol Chem. 2005;280(21):20580. doi: 10.1074/jbc.M501894200. [DOI] [PubMed] [Google Scholar]
- 15.Semenza GL, Jiang BH, Leung SW, et al. J Biol Chem. 1996;271(51):32529. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 16.Rahman MS, Thomas P. Neuroscience. 2009;158(2):751. doi: 10.1016/j.neuroscience.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Poncet L, Denoroy L, Dalmaz Y, et al. Brain Res. 1997;765(1):122. doi: 10.1016/s0006-8993(97)00520-9. [DOI] [PubMed] [Google Scholar]
- 18.Bargmann CI, Horvitz HR. Neuron. 1991;7(5):729. doi: 10.1016/0896-6273(91)90276-6. [DOI] [PubMed] [Google Scholar]
- 19.Uchida O, Nakano H, Koga M, et al. Development. 2003;130(7):1215. doi: 10.1242/dev.00341. [DOI] [PubMed] [Google Scholar]
- 20.Hobson RJ, Hapiak VM, Xiao H, et al. Genetics. 2006;172(1):159. doi: 10.1534/genetics.105.044495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hapiak VM, Hobson RJ, Hughes L, et al. Genetics. 2009;181(1):153. doi: 10.1534/genetics.108.096891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hobson RJ, Geng J, Gray AD, et al. J Neurochem. 2003;87(1):22. doi: 10.1046/j.1471-4159.2003.01967.x. [DOI] [PubMed] [Google Scholar]
- 23.Ray P, Schnabel R, Okkema PG. Dev Neurobiol. 2008;68(4):421. doi: 10.1002/dneu.20599. [DOI] [PubMed] [Google Scholar]
- 24.Mehta R, Steinkraus KA, Sutphin GL, et al. Science. 2009;324(5931):1196. doi: 10.1126/science.1173507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avery L, Thomas JH. In: C.elegans II. Riddle DL, Blumenthal T, Meyer BJ, et al., editors. Cold Spring Harbor Laboratory Press; 1997. p. 679. [PubMed] [Google Scholar]
- 26.Albertson DG, Thomson JN. Philos Trans R Soc Lond B Biol Sci. 1976;275(938):299. doi: 10.1098/rstb.1976.0085. [DOI] [PubMed] [Google Scholar]
- 27.Kim K, Li C. The Journal of comparative neurology. 2004;475(4):540. doi: 10.1002/cne.20189. [DOI] [PubMed] [Google Scholar]
- 28.Rogers C, Reale V, Kim K, et al. Nat Neurosci. 2003;6(11):1178. doi: 10.1038/nn1140. [DOI] [PubMed] [Google Scholar]
- 29.Gloria-Soria A, Azevedo RB. Curr Biol. 2008;18(21):1694. doi: 10.1016/j.cub.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 30.de Bono M, Bargmann CI. Cell. 1998;94(5):679. doi: 10.1016/s0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- 31.Coates JC, de Bono M. Nature. 2002;419(6910):925. doi: 10.1038/nature01170. [DOI] [PubMed] [Google Scholar]
- 32.Hukema RK, Rademakers S, Dekkers MP, et al. EMBO J. 2006;25(2):312. doi: 10.1038/sj.emboj.7600940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung BH, Cohen M, Rogers C, et al. Curr Biol. 2005;15(10):905. doi: 10.1016/j.cub.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Pierce-Shimomura JT, Morse TM, Lockery SR. J Neurosci. 1999;19(21):9557. doi: 10.1523/JNEUROSCI.19-21-09557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang AJ, Bargmann CI. Proc Natl Acad Sci U S A. 2008;105(20):7321. doi: 10.1073/pnas.0802164105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poncet L, Denoroy L, Dalmaz Y, et al. Brain Res. 1996;733(1):64. doi: 10.1016/0006-8993(96)00539-2. [DOI] [PubMed] [Google Scholar]
- 37.Sarafi-Reinach TR, Melkman T, Hobert O, et al. Development. 2001;128(17):3269. doi: 10.1242/dev.128.17.3269. [DOI] [PubMed] [Google Scholar]
- 38.Sagasti A, Hobert O, Troemel ER, et al. Genes Dev. 1999;13(14):1794. doi: 10.1101/gad.13.14.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aspock G, Ruvkun G, Burglin TR. Development. 2003;130(15):3369. doi: 10.1242/dev.00551. [DOI] [PubMed] [Google Scholar]
- 40.Macosko EZ, Pokala N, Feinberg EH, et al. Nature. 2009;458(7242):1171. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.