Abstract
Individual differences in the sensitivity to fentanyl, a widely used opioid analgesic, lead to different proper doses of fentanyl, which can hamper effective pain treatment. Voltage-activated Ca2+ channels (VACCs) play a crucial role in the nervous system by controlling membrane excitability and calcium signaling. Cav2.3 (R-type) VACCs have been especially thought to play critical roles in pain pathways and the analgesic effects of opioids. However, unknown is whether single-nucleotide polymorphisms (SNPs) of the human CACNA1E (calcium channel, voltage-dependent, R type, alpha 1E subunit) gene that encodes Cav2.3 VACCs influence the analgesic effects of opioids. Thus, the present study examined associations between fentanyl sensitivity and SNPs in the human CACNA1E gene in 355 Japanese patients who underwent painful orofacial cosmetic surgery, including bone dissection. We first conducted linkage disequilibrium (LD) analyses of 223 SNPs in a region that contains the CACNA1E gene using genomic samples from 100 patients, and a total of 13 LD blocks with 42 Tag SNPs were observed within and around the CACNA1E gene region. In the preliminary study using the same 100 genomic samples, only the rs3845446 A/G SNP was significantly associated with perioperative fentanyl use among these 42 Tag SNPs. In a confirmatory study using the other 255 genomic samples, this SNP was also significantly associated with perioperative fentanyl use. Thus, we further analyzed associations between genotypes of this SNP and all of the clinical data using a total of 355 samples. The rs3845446 A/G SNP was associated with intraoperative fentanyl use, 24 h postoperative fentanyl requirements, and perioperative fentanyl use. Subjects who carried the minor G allele required significantly less fentanyl for pain control compared with subjects who did not carry this allele. Although further validation is needed, the present findings show the possibility of the involvement of CACNA1E gene polymorphisms in fentanyl sensitivity.
Introduction
Voltage-activated Ca2+ channels (VACCs) mediate Ca2+ entry into cells in response to membrane depolarization and play a crucial role in the nervous system by controlling membrane excitability and calcium signaling [1]. VACCs are composed of a major pore-forming subunit (α1A-I and α1S) and multiple auxiliary subunits (α2-δ, β, and γ). Molecular characterizations have determined that the α1E subunit encodes Cav2.3 (R-type) VACCs [2], [3]. Cav2.3 VACCs are reported to be distributed throughout the central and peripheral nervous systems, including pain pathways [4], [5]. Furthermore, Cav2.3 knockout mice have been reported to show functional deficits in pain perception [6]. Thus, Cav2.3 VACCs may be hypothesized to contribute to pain transmission.
Opioid analgesics, such as fentanyl and morphine, are widely used for the treatment of moderate to severe pain. However, the analgesic efficacy of opioids is well known to vary widely among individuals [7]. Individual differences may be related to various genetic and nongenetic factors, including gender, age, ethnic origin, hepatic or renal function, and mental status [8]. Several studies that used mice that lack the μ-opioid receptor (MOP) [9], [10], [11] have shown that analgesia produced by opioids crucially depends on the level of MOP expression. Furthermore, several single-nucleotide polymorphisms (SNPs) in the OPRM1 (opioid receptor, mu-1) gene, which encodes the human MOP protein, have been reported to lead to differences in the analgesic efficacy of opioids [12].
Voltage-activated Ca2+ channels have also been considered to play important roles in the analgesic effects of and tolerance to opioids. Ca2+ influx modulators have been shown to affect both the antinociceptive effects of and tolerance to morphine [13], [14], [15]. Moreover, Cav2.3 knockout mice show enhanced analgesic effects of morphine [16]. One of the supraspinal analgesic mechanisms by which opioids are known to disinhibit the endogenous descending antinociceptive pathway is via inhibition of γ-aminobutyric acid (GABA) neurons in the periaqueductal grey (PAG). The relatively high expression level of Cav2.3 in the PAG has also been reported [6]. Although the precise mechanism is still unknown, Cav2.3 in the PAG could affect the activity of the endogenous descending antinociceptive pathway by regulating the release of GABA or other endogenous neurotransmitters. Thus, the expression level of or functional changes in Cav2.3 may cause differences in the analgesic efficacy of opioids.
Human Cav2.3 is encoded by the CACNA1E (calcium channel, voltage-dependent, R type, alpha 1E subunit) gene, which is located on chromosome 1q25-31. Many SNPs have been identified in the CACNA1E gene, and some of these SNPs have been reported to be associated with type 2 diabetes [17], [18]. Unknown is whether genetic polymorphisms in the CACNA1E gene have any association with pain sensitivity or opioid analgesia. In contrast to animal studies that use standardized pain tests, the analgesic effects of opioids in humans are usually evaluated in patients with actual pain, particularly cancer pain or acute postoperative pain [12]. Patients with acute postoperative pain following standardized surgical procedures may be more optimal subjects for investigating gene-opioid effect relationships [7], [19]. Therefore, the present study examined whether SNPs in the CACNA1E gene affect pain sensitivity and the analgesic effects of fentanyl, one of the most commonly used opioid analgesics, evaluated by a standardized pain test and fentanyl requirements in healthy Japanese subjects who underwent uniform surgical procedures.
Materials and Methods
Ethics Statement
The study protocol was approved by the Institutional Review Board, Tokyo Dental College, Chiba, Japan, and the Institutional Review Board, Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan. Written informed consent was obtained from all of the patients and from parents if required.
Patients
Enrolled in the study were 355 healthy patients (American Society of Anesthesiologists Physical Status I, age 15–52 years, 125 males and 230 females) who were scheduled to undergo cosmetic orthognathic surgery (mandibular sagittal split ramus osteotomy) for mandibular prognathism at Tokyo Dental College Suidoubashi Hospital. Patients with chronic pain, those taking pain medication, and those who had experienced Raynaud’s phenomenon were excluded.
Preoperative Cold Pressor-induced Pain Test
Patients were premedicated with oral diazepam, 5 mg, and oral famotidine, 150 mg, 90 min before the induction of anesthesia. Patients had an intravenous (i.v.) line on the forearm on their nondominant side. The temperature in the operating room was maintained at 26°C. The cold pressor-induced pain test was then performed before and 3 min after an i.v. bolus injection of fentanyl, 2 µg/kg, as previously described [19], [20]. Briefly, crushed ice cubes and cold water were blended 15 min before the test in a 1 L isolated tank, and the mixture was stirred immediately before each test to ensure uniform temperature distribution (0°C) within the tank. The dominant hand was immersed up to the wrist. Patients were instructed to keep the hand calm in the ice-cold water and withdraw it as soon as they perceived any pain. All of the patients were administered the test by the same investigator. The baseline latency to pain perception, defined as the time of immersion of the hand in the ice water, before an i.v. injection of fentanyl (PPLpre) was recorded. A cut-off point of 150 s was set to avoid tissue damage. The hand was warmed with a hair dryer as soon as it was withdrawn from the ice water until the sensation of cold was completely abolished. Patients then received i.v. fentanyl, 2 µg/kg. Three minutes after the injection, the pain perception latency of the dominant hand (PPLpost) was measured again. The analgesic effect of fentanyl in the preoperative cold pressor-induced pain test was evaluated simply as the difference between PPLpost and PPLpre (PPLpost - PPLpre).
Anesthesia and Surgery
After the cold pressor-induced pain test ended, general anesthesia was induced with a target-controlled infusion (TCI) of propofol using a TCI pump (TE-371, Terumo, Tokyo, Japan). Vecuronium, 0.1 mg/kg, was administered to facilitate nasotracheal intubation. After the induction of anesthesia, 10 ml of venous blood was sampled for the preparation of DNA specimens. General anesthesia was maintained with propofol at a target blood concentration of 4–6 µg/ml. Vecuronium was administered at a rate of 0.08 mg/kg/h. The lungs were ventilated with oxygen-enriched air. Local anesthesia was performed on the right side of the surgical field with 8 ml of 2% lidocaine that contained epinephrine, 12.5 µg/ml, and right mandibular ramus osteotomy was performed. Local anesthesia was then performed on the left side, and left mandibular ramus osteotomy was performed. The bilateral mandibular bone segments were fixed in appropriate positions. Whenever systolic blood pressure or heart rate exceeded +20% of the preinduction value during surgery, i.v. fentanyl, 1 µg/kg, was administered.
Postoperative Pain Management
At the end of the surgery, rectal diclofenac sodium, 50 mg, and i.v. dexamethasone, 8 mg, were administered at the request of surgeons to prevent postoperative orofacial edema/swelling. After emergence from anesthesia and tracheal extubation, droperidol, 1.25 mg, was administered i.v. to prevent nausea/vomiting, and i.v. patient-controlled analgesia (PCA) with a fentanyl-droperidol combination (2 mg fentanyl and 5 mg droperidol diluted in normal saline in a total volume of 50 ml) commenced using a CADD-Legacy PCA pump (Smiths Medical Japan, Tokyo, Japan). A bolus dose of fentanyl, 20 µg, on demand and a lockout time of 10 min were set. Continuous background infusion was not employed. Droperidol was coadministered with fentanyl to prevent nausea/vomiting because our preliminary study showed a high incidence (up to 30%) of nausea/vomiting with PCA fentanyl in young females. Patient-controlled analgesia continued for 24 h postoperatively. In the case of treatment-refractory adverse effects or inadequate analgesia, PCA was discontinued, and rectal diclofenac sodium, 50 mg, was prescribed as a rescue analgesic as required (two patients required a rescue analgesic only once). The intensity of spontaneous pain was assessed 3 and 24 h postoperatively using a 100-mm visual analog scale (VAS), with 0 mm indicating no pain and 100 mm indicating the worst pain imaginable. Intraoperative fentanyl use and postoperative PCA fentanyl use during the first 24 h postoperative period were recorded. Doses of fentanyl administered intraoperatively and postoperatively were normalized to body weight. Additionally, perioperative fentanyl use was calculated as the sum of intraoperative fentanyl use and postoperative fentanyl use because the analgesic effect of the intermediate-acting opioid fentanyl, administered pre- and intraoperatively, could outlast the duration of surgery and thus affect postoperative fentanyl use, especially in patients who received large doses of fentanyl intraoperatively. Therefore, in the present study, we considered perioperative fentanyl use an appropriate indicator of fentanyl analgesia in addition to postoperative fentanyl use.
Genotyping Procedures and Linkage Disequilibrium Analysis
Genomic DNA was extracted from whole-blood samples using standard procedures. The extracted DNA was dissolved in TE buffer (10 mM tris-HCl, 1 mM ethylenediaminetetraacetic acid, pH 8.0). The DNA concentration was adjusted to 5–50 ng/µl for genotyping individual SNPs or 100 ng/µl for whole-genome genotyping using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE).
For the analysis of SNPs within and around the CACNA1E gene region, genotype data from whole-genome genotyping were used. Briefly, whole-genome genotyping was performed using Infinium assay II and an iScan system (Illumina, San Diego, CA) according to the manufacturer’s instructions. Five kinds of BeadChips were used to genotype 40, 67, 6, 119, and 123 samples, respectively: HumanHap300 (total markers: 317,503), HumanHap300-Duo (total markers: 318,237), Human610-Quad v1 (total markers: 620,901), Human1M v1.0 (total markers: 1,072,820), and Human 1M-Duo v3 (total markers: 1,199,187). Some BeadChips included a number of probes specific to copy number variation markers, but most were for SNP markers on the human autosome or sex chromosome. Approximately 300,000 SNP markers were commonly included in all of the BeadChips. After the whole-genome genotyping, the data for genotyped samples were analyzed using BeadStudio or GenomeStudio with the Genotyping module v3.3.7 (Illumina) to evaluate the quality of the results, and the genotype data for all of the SNPs with CACNA1E gene annotation were extracted. In the data-cleaning process, markers that had “Cluster sep” values (i.e., an index of genotype cluster separation) less than 0.4 and three genotype clusters that were not separate from one another were excluded from the subsequent association study.
Single-nucleotide polymorphisms for the association studies were selected based on recently advanced tagging strategies [21], [22], [23]. To identify relationships between the SNPs used in the study, linkage disequilibrium (LD) analysis was performed in 223 SNPs that were in the approximately 640 kbp region that contained the CACNA1E gene, among 1,199,187 markers of the Human 1M-Duo v3 Bead Chip for 100 samples using Haploview v.4.2 [24]. For the estimation of LD strength between the SNPs, the commonly used D′ and r2 values were pairwise calculated using the genotype dataset of each SNP. Linkage disequilibrium blocks were defined among the SNPs that showed “strong LD,” based on the default algorithm of Gabriel et al. [25], in which the upper and lower 95% confidence limits on D′ for strong LD were set at 0.98 and 0.7, respectively. Tag SNPs in the LD block were consequently determined using Tagger software with default settings, which is incorporated in Haploview and has been detailed in a previous report [23].
Statistical Analysis
Parametric and nonparametric data are expressed as mean ± SD and median [interquartile range], respectively. The statistical analysis was performed using IBM SPSS v.20.0.0 software (IBM, Tokyo, Japan). In the present study, none of the clinically measured endpoints that were related to pain sensitivity (i.e., PPLpre) or fentanyl analgesia (i.e., analgesia measured with the preoperative cold pressor test, perioperative fentanyl use, and VAS scores at 3 and 24 h postoperatively) were normally distributed. Therefore, nonparametric analyses, including the Mann-Whitney U-test, Kruskal-Wallis test (with Steel-Dwass multiple comparison tests), or Spearman’s rank correlation test, were used to detect possible associations between any of the clinical or genomic parameters (e.g., sex, age, and genotypes of the Tag SNP) and clinical endpoints related to pain sensitivity or the analgesic effects of fentanyl. Many factors other than the genotypes of the screened SNPs (e.g., age and sex) may also influence the analgesic effects of fentanyl. Therefore, when a significant association between a genotype and clinical endpoint was found, factors other than genotype were compared between genotypes using nonparametric analyses according to the types of data to evaluate whether the genotype groups were controlled by other factors that might affect pain sensitivity, the analgesic effects of fentanyl, or fentanyl requirements, including age and sex. Furthermore, we conducted additional multivariate covariate analyses using the Stepwise method (independent variables: SNP genotypes, sex, and age), although the nonparametric distributions of most of our data were not suitable for the application of such parametric techniques. Values of p<0.05 were considered statistically significant. The sample size of the present nonparametric data was higher than the estimated size that possesses statistical power (1 minus type II error probability) of 99% for the Cohen’s conventional “medium” effect size of 0.25, when power analysis was performed for analysis of variance with three genotype groups using G*Power v.3.1.3 [26].
Results
First, to identify the LD blocks in the approximately 640 kbp region that contains the CACNA1E gene, 223 SNPs among 1,199,187 markers included in the whole-genome genotyping (Human 1M-Duo v3 Bead Chip) were tested using genomic samples from 100 Japanese patients (Fig. S1). A total of 13 LD blocks, LD1-13, were observed within and around the CACNA1E gene region (the exon, intron, and approximately 3 kbp 5′-flanking region and 50 kbp 3′-flanking region of the CACNA1E gene [approximately 380 kbp]; Fig. 1), and 42 Tag SNPs were selected in this region (Table 1). In the preliminary study, we analyzed associations between the genotypes of 42 Tag SNPs and perioperative fentanyl use using the same 100 samples. Among these SNPs, only one SNP (rs3845446) in LD11 had a significant association with perioperative fentanyl use (p = 0.032, Kruskal-Wallis test; Table 1 and 2). Furthermore, the rs3845446 SNP also had a significant association with perioperative fentanyl use (p = 0.033, Kruskal-Wallis test; Table 2) in the confirmatory study using the other 255 samples. Therefore, we further analyzed the associations between the genotypes of the rs3845446 SNP and all of the clinical data using a total of 355 samples. The genotype distributions of the rs3845446 SNP in the patients were AA (168 [47.3%]), AG (148 [41.7%]), and GG (39 [11.0%]). This genotype frequency was in Hardy-Weinberg equilibrium.
Figure 1. Genomic scheme of the CACNA1E gene.
Boxes represent exons (black boxes indicate coding regions of major expression type of Cav2.3 [Cav2.3c (α1E-3), NM_000721.3]; gray box indicates exon in the reported major splicing variant isoform [Cav2.3e (α1Ee), NM_001205293.1]; white boxes indicate untranslated regions) [33].
Table 1. Allelic frequencies and associations with perioperative fentanyl use of SNPs in 13 LD blocks in the CACNA1E gene.
| LDnumber | SNP name | Major:Minorallele | Allelicfrequency | p- value | LDnumber | SNP name | Major:Minorallele | Allelicfrequency | p- value |
| 1 | rs633143* | G:A | 0.100 | 0.433 | 6 | rs3766988 | A:G | 0.015 | – |
| rs2877651* | G:A | 0.090 | 0.491 | rs7513540 | G:T | 0.205 | – | ||
| rs556321 | A:G | 0.187 | – | rs3845444 | T:G | 0.205 | – | ||
| rs553042* | T:C | 0.365 | 0.646 | rs4306091 | A:G | 0.015 | – | ||
| rs12060765 | G:T | 0.090 | – | rs7540850 | C:T | 0.235 | – | ||
| rs943795 | T:C | 0.365 | – | rs2253388 | C:T | 0.235 | – | ||
| 2 | rs576006 | A:G | 0.309 | – | rs4651112 | G:A | 0.195 | – | |
| rs12405860* | A:G | 0.190 | 0.662 | 7 | rs12139677* | T:G | 0.370 | 0.402 | |
| rs558994* | G:A | 0.295 | 0.882 | rs3845445 | T:C | 0.045 | – | ||
| rs17494681* | C:T | 0.110 | 0.087 | rs2280866* | G:A | 0.305 | 0.522 | ||
| rs681271* | C:T | 0.485 | 0.927 | 8 | rs3767002* | C:T | 0.180 | 0.399 | |
| rs4126690* | C:A | 0.165 | 0.435 | rs16858051 | T:C | 0.015 | – | ||
| rs517209* | C:A | 0.460 | 0.468 | rs3753748* | G:A | 0.195 | 0.609 | ||
| 3 | rs589082 | C:A | 0.485 | – | rs3767003* | A:G | 0.240 | 0.957 | |
| rs10910948* | T:C | 0.120 | 0.218 | 9 | rs3767004* | G:A | 0.275 | 0.867 | |
| rs16857509 | A:G | 0.010 | – | rs704332* | T:C | 0.240 | 0.957 | ||
| rs625226* | A:G | 0.125 | 0.440 | rs704331* | A:G | 0.390 | 0.626 | ||
| rs4146634* | A:G | 0.485 | 0.783 | rs4652678* | T:C | 0.060 | 0.112 | ||
| rs2225875* | C:T | 0.205 | 0.103 | rs704329* | G:A | 0.455 | 0.990 | ||
| rs6681017 | C:T | 0.120 | – | rs4652679 | G:A | 0.060 | – | ||
| rs1933049 | G:A | 0.480 | – | rs697260 | G:A | 0.175 | – | ||
| rs12024842 | A:G | 0.270 | – | rs199922 | A:G | 0.045 | – | ||
| rs4652663 | T:C | 0.120 | – | rs199923 | G:A | 0.060 | – | ||
| rs11580052 | G:A | 0.485 | – | rs199930 | C:T | 0.060 | – | ||
| rs7524309 | A:G | 0.215 | – | rs2280868* | C:A | 0.185 | 0.796 | ||
| rs10797724 | G:A | 0.270 | – | 10 | rs473200* | C:T | 0.265 | 0.638 | |
| 4 | rs10797729* | G:A | 0.265 | 0.287 | rs601059 | C:T | 0.270 | – | |
| rs10797730* | C:T | 0.295 | 0.077 | rs704326* | C:T | 0.450 | 0.296 | ||
| rs1999838 | A:G | 0.295 | – | 11 | rs3845446* | A:G | 0.335 | 0.032 | |
| rs7511748 | T:G | 0.270 | – | rs3753752 | C:T | 0.335 | – | ||
| rs12135959 | G:A | 0.300 | – | rs2280869 | T:C | 0.335 | – | ||
| rs3856090 | C:T | 0.295 | – | 12 | rs12045458* | A:G | 0.105 | 0.727 | |
| rs10494540 | G:T | 0.300 | – | rs7513685* | G:A | 0.095 | 0.177 | ||
| rs10494541 | G:A | 0.005 | – | rs610100* | C:A | 0.490 | 0.341 | ||
| rs12138634 | G:T | 0.300 | – | rs480752* | C:A | 0.055 | 0.192 | ||
| rs12239392 | G:A | 0.030 | – | rs12130868* | C:T | 0.400 | 0.422 | ||
| rs2877652 | T:C | 0.030 | – | rs1281194 | A:G | 0.480 | – | ||
| rs6684423 | T:G | 0.031 | – | rs12136390 | C:T | 0.395 | – | ||
| rs3856094* | G:A | 0.250 | 0.246 | rs585315* | C:T | 0.395 | 0.601 | ||
| 5 | rs199943* | A:C | 0.050 | 0.244 | rs12071191 | G:A | 0.285 | – | |
| rs12071300 | T:G | 0.050 | – | rs486003 | G:A | 0.395 | – | ||
| 6 | rs199916* | G:A | 0.190 | 0.794 | rs695072 | T:C | 0.085 | – | |
| rs3820260 | C:T | 0.040 | – | rs13375273 | G:A | 0.490 | – | ||
| rs3766980* | C:T | 0.245 | 0.886 | rs4465155 | C:T | 0.425 | – | ||
| rs3753737* | A:G | 0.205 | 0.969 | rs7533297 | T:C | 0.405 | – | ||
| rs10910979 | C:T | 0.245 | – | rs7535666 | T:G | 0.110 | – | ||
| rs3845441 | T:C | 0.190 | – | 13 | rs598714* | A:C | 0.480 | 0.983 | |
| rs4652673 | A:G | 0.205 | – | rs677618* | T:C | 0.375 | 0.581 |
Tag SNPs in the LD blocks (selected using Tagger software with default settings; r2>0.8). p-value, association with perioperative fentanyl use (Kruskal-Wallis test).
Table 2. Association between the rs3845446 A/G SNP and perioperative fentanyl use in the preliminary and confirmatory study.
| Genotypes | Numbers | Perioperative fentanyl use (Median) | p-value | |
| Preliminary study | AA | 46 | 6.80 | 0.032 |
| AG | 41 | 6.83 | ||
| GG | 13 | 5.06 | ||
| Confirmatory study | AA | 122 | 8.00 | 0.033 |
| AG | 107 | 7.05 | ||
| GG | 26 | 6.61 |
p-values were calculated using Kruskal-Wallis test.
Of the 355 Japanese patients who enrolled in the study, 353 completed the study. The data from the preoperative cold pressor-induced pain test or postoperative pain management could not be obtained for two patients. The patients’ clinical data are summarized in Table 3. In the preoperative cold pressor-induced pain test, fentanyl (2 µg/kg) increased pain perception latency (PPLpost vs. PPLpre, p<0.0001, paired t-test; Table 3).
Table 3. Patient demographic and clinical data.
| All patients | Male | Female | |
| Number of subjects | 355 | 125 | 230 |
| Age (years) | 25.9±7.6 (15–52) | 24.5±6.9 | 26.6±7.9 |
| Body weight (kg) | 57.6±10.9 (38–128) | 65.9±11.2 | 53.1±7.5 |
| PPLpre (s) | 14 [9], [23] (2–150) | 15 [10], [26] | 14 [9], [22] |
| PPLpost (s) | 28 [16, 53] (4–150) | 35 [19, 70] | 26 [15, 48]* |
| Analgesic effect (PPLpost-PPLpre) (s) | 12 [4, 35] (−21 – +143) | 15 [5, 40] | 10 [4], [29] ( * ) |
| Intraoperative fentanyl use (µg/kg) | 4.4 [3.2, 5.9] (0–13.6) | 4.0 [2.9, 5.3] | 4.6 [3.4, 6.1]* |
| 24 h postoperative fentanyl use (µg/kg) | 2.3 [1.0, 4.2] (0–13.8) | 1.8 [0.6, 4.0] | 2.4 [1.4, 4.3]( * ) |
| Perioperative fentanyl use (µg/kg) | 6.9 [5.2, 9.1] (0.8–24.2) | 6.4 [4.7, 8.5] | 7.2 [5.8, 9.3]* |
| VAS pain score at 3 h (mm) | 27 [15, 50] (0–90) | 26 [15, 50] | 28 [15, 50] |
| VAS pain score at 24 h (mm) | 25 [10, 42] (0–85) | 25 [10, 40] | 25 [10, 42] |
The data are expressed as numbers, mean ± SD (range), or median [interquartile range].
*) p<0.1,
p<0.05, compared with male subjects.
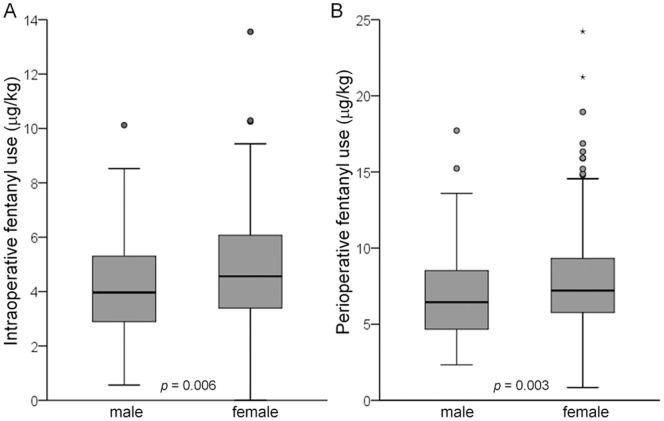
The Mann-Whitney U-test revealed that although sex had no significant association with PPLpre or VAS at 3 or 24 h, the analgesic effect of fentanyl in the cold pressor-induced pain test (PPLpost - PPLpre) tended to be greater in males than in females (p = 0.062; Table 3), and 24 h postoperative fentanyl use tended to be less in males than in females (p = 0.052; Table 3). Furthermore, intraoperative fentanyl use and perioperative fentanyl use were significantly less in males than in females (p = 0.006 and 0.003, respectively; Table 3, Fig. 2). Spearman’s rank correlation test revealed that age had a significant association with 24 h postoperative fentanyl use (p = 0.044; Fig. 3) but not with any other clinical endpoints (data not shown). Older age was associated with less fentanyl use for postoperative pain management.
Figure 2. Associations between sex and fentanyl use.
Associations between sex (male, n = 125; female, n = 230) and (A) intraoperative fentanyl use and (B) perioperative fentanyl use. The data are expressed by box and whisker plots. The upper and lower ends of the boxes represent the 75th and 25th percentiles, respectively. Whiskers represent the highest and lowest values that are not outliers or extreme values. Outliers (i.e., values that are between 1.5- and 3-times the interquartile range) and extreme values (i.e., values that are more than 3-times the interquartile range) are represented by circles and starbursts beyond the whiskers, respectively. The median is depicted by a solid line in the box.
Figure 3. Association between age and fentanyl use.

The scatterplot shows the association between age and 24 h postoperative fentanyl use. Each point represents an individual patient.
The Kruskal-Wallis test revealed that intraoperative fentanyl use (p = 0.013), 24 h postoperative fentanyl use (p = 0.042), and perioperative fentanyl use (p = 0.0036) were significantly associated with genotypes of the rs3845446 SNP (Fig. 4). Steel-Dwass multiple comparisons tests also revealed that subjects who carried the minor G allele of the rs3845446 A/G SNP needed significantly less intraoperative fentanyl and perioperative fentanyl (p<0.05) for pain management than those who did not carry this allele (Table 4). The rs3845446 A/G SNP had no significant association with PPLpre, the analgesic effect of fentanyl, or VAS scores at 3 or 24 h (Table 4). The Kruskal-Wallis test revealed no significant differences in age or sex among the subjects who carried different genotypes in the rs3845446 A/G SNP (Table 4). Thus, the differences in fentanyl use among the genotype groups could not be controlled by age and sex. Furthermore, multivariate regression analyses showed that the rs3845446 A/G SNP was retained as an independent predictor of intraoperative fentanyl use (F 2,351 = 6.36, p = 0.002), 24 h postoperative fentanyl use (F 1,352 = 6.25, p = 0.013), and perioperative fentanyl use (F 2,351 = 9.37, p<0.001; significant variables are shown in Table 5).
Figure 4. Associations between genotypes of the rs3845446 A/G SNP and fentanyl use.
Associations between genotypes of the rs3845446 A/G SNP (AA, n = 168; AG, n = 148; GG, n = 39) and (A) intraoperative fentanyl use and (B) perioperative fentanyl use. The data are expressed by box and whisker plots. The upper and lower ends of the boxes represent the 75th and 25th percentiles, respectively. Whiskers represent the highest and lowest values that are not outliers or extreme values. Outliers and extreme values are represented by circles and starbursts beyond the whiskers, respectively. The median is depicted by a solid line in the box.
Table 4. Clinical data of genotypes of the rs3845446 SNP.
| SNP name | rs3845446 | ||
| Genotype | AA | AG | GG |
| Number of subjects(male/female) | 55/113 | 57/91 | 13/26 |
| Age (years) | 25.5±7.3 | 26.2±7.9 | 26.7±8.1 |
| PPLpre (s) | 14 [9], [23] | 15 [10], [24] | 14 [8], [21] |
| PPLpost (s) | 28 [16, 58] | 26 [16, 53] | 27 [16, 43] |
| Analgesic effect (PPLpost-PPLpre) (s) | 13 [5, 38] | 11 [4, 35] | 11 [4], [28] |
| Intraoperative fentanyluse (µg/kg) | 4.5 [3.3, 6.0] | 4.6 [3.3, 6.0] | 3.8 [2.7, 4.8]* |
| 24 h postoperativefentanyl use (µg/kg) | 2.6 [1.2, 4.6] | 2.0 [1.1, 3.6] | 2.1 [0.5, 3.9] |
| Perioperative fentanyluse (µg/kg) | 7.3 [5.8, 9.6] | 7.0 [5.1, 9.0] | 6.2 [4.7, 6.9]* |
| VAS pain score at 3 h (mm) | 26 [15, 50] | 30 [18, 50] | 24 [10, 43] |
| VAS pain score at 24 h (mm) | 25 [12, 40] | 25 [9, 48] | 21 [10, 38] |
The data are expressed as numbers, mean ± SD (range), or median [interquartile range].
p<0.05, compared with subjects who did not carry the minor allele (Steel-Dwass multiple comparisons tests).
Table 5. Multivariate regression models of factors that predict interindividual differences in fentanyl sensitivity.
| Predictive variable | Beta | p-value |
| Intraoperative analgesic use (adjusted r2 = 0.029) | ||
| Genotypes of rs3845446 SNP | −0.113 | 0.031 |
| Sex | 0.146 | 0.006 |
| 24 h postoperative fentanyl use (adjusted r2 = 0.015) | ||
| Genotypes of rs3845446 SNP | −0.132 | 0.013 |
| Perioperative fentanyl use (adjusted r2 = 0.045) | ||
| Genotypes of rs3845446 SNP | −0.173 | 0.001 |
| Sex | 0.139 | 0.008 |
Multivariate regression analysis was performed using the Stepwise method (independent variables: Genotypes of rs3845446 SNP, sex, and age). Beta represents the regression coefficient.
Discussion
We studied patients who underwent mandibular sagittal split ramus osteotomy. Subjects who undergo this cosmetic surgery are usually young and healthy. The operation causes considerable perioperative pain that arises from the dissected mandibular bone, and the surgical technique is highly standardized at our institute. We conducted a standardized pain test before the induction of general anesthesia in opioid-naive subjects without pain. Using these ideal subjects and methods, we found that intraoperative fentanyl use, 24 h postoperative fentanyl use, and perioperative fentanyl use decreased in subjects who carried the minor G allele of the rs3845446 A/G SNP compared with subjects who did not carry this allele in the CACNA1E gene. These results suggest that the rs3845446 A/G SNP or other polymorphisms in the same LD11 region could affect the analgesic effects of opioids. Although we analyzed five types of BeadChips merged together in the present study because the sample sizes were small for all the five datasets and they all presumably had Japanese ancestry, performing a meta-analysis is usually better than merging. Moreover, the sample size of the present association study was also quite small. Thus, further studies that have a greater number of samples might be required to reveal the influences of these polymorphisms in the CACNA1E gene. PPLpre and the analgesic effects of fentanyl evaluated with the cold pressor test were not associated with the genotypes of the rs3845446 A/G SNP. Although further validation is needed, cold pain sensitivity and the analgesic effects of opioids for acute cold pain might not be associated with genetic polymorphisms in the CACNA1E gene. Furthermore, no significant association was found between the genotypes of the rs3845446 A/G SNP and VAS scores, indicating that comparable levels of postoperative analgesia were achieved with PCA fentanyl in our patients, regardless of genotype. The analgesic effects of fentanyl tended to decrease, and fentanyl use for pain management increased in females compared with males, consistent with our previous findings [19]. We also found that older age was significantly associated with less 24 h postoperative fentanyl use. The present results of the influence of demographic factors on pain sensitivity and opioid analgesia were nearly consistent with a recent well-controlled twin study [27]. However, because no significant differences in age or sex were found among the subjects who carried the different genotypes in the rs3845446 A/G SNP, the differences in fentanyl use among the genotype groups in the rs3845446 A/G SNP could not be controlled by these covariates. Furthermore, multivariate regression analyses also showed that the rs3845446 A/G SNP was retained as an independent predictor of fentanyl requirements for pain management.
The therapeutic potential of VACCs in pain management has been the subject of intensive recent investigations [28], [29]. Cav2.3 (R-type) VACCs are classified as “resistant” to blockers of L-, N-, P-, and Q-type Ca2+ channels and have been poorly investigated compared with these types of channels. The development of mice that lack Cav2.3 VACCs [6] and the selective Cav2.3 VACC antagonist SNX-482 derived from tarantula venom [30] have made possible the discovery of the direct contribution of Cav2.3 VACCs to pain transmission and opioid analgesia. Cav2.3 VACC knockout mice showed normal pain sensitivity to acute mechanical, thermal, and chemical stimuli but exhibited reduced responsiveness to somatic inflammatory stimulation [6]. Blockade of Cav2.3 VACCs by SNX-482 produced analgesic effects in the second phase of the formalin test, although it increased nociceptive behavior in the first phase [5]. These previous reports suggest that Cav2.3 VACCs play differential roles in pain transmission, depending on the type of pain stimulus. In the present study, PPLpre evaluated with the cold pressor test was not associated with the genotypes of the SNPs in the CACNA1E gene, consistent with previous findings, and might suggest that Cav2.3 VACCs are not involved in cold pain transmission under naive conditions. Furthermore, Cav2.3 VACC knockout mice were reported to display enhanced morphine-induced analgesia in the tail-flick and hot-plate tests [16]. In the same report, intracerebroventricular administration of SNX-482 also enhanced morphine-induced analgesia in wildtype mice [16]. Thus, inhibition of Cav2.3 VACCs may modulate the analgesic effects of opioids. In the present study, we found that fentanyl use for pain management was reduced, but the analgesic effects of fentanyl evaluated with the cold pressor test were not significantly altered in subjects with the minor G allele of the rs3845446 A/G SNP. Altogether, the rs3845446 A/G SNP or other polymorphisms in the same LD11 region may cause reduced expression levels of Cav2.3 VACC mRNA and protein or dysfunctional changes (i.e., amino acid substitutions, splicing variants) and enhance the analgesic effects of opioids. However, the Cav2.3 VACC inhibition-induced enhancement of opioid-induced analgesia may depend on the type of pain stimulus or pain expression (i.e., acute or chronic).
Although many SNPs have been identified in the CACNA1E gene, and some of these SNPs have been reported to be associated with type 2 diabetes [17], [18], we are aware of no association studies of these SNPs and pain sensitivity or opioid analgesia. The rs3845446 A/G SNP in the CACNA1E gene displayed a significant association with opioid analgesia, but still unknown is whether this SNP alters gene function or expression, which may be an important limitation of the present study. The rs3845446 A/G SNP represents LD11 from intron 46 to exon 47 that contains a stop codon of the CACNA1E gene. Interestingly, Cav2.3 VACCs reportedly contain several alternative splicing variants [31], [32], [33]. The Cav2.3d, e, and f isoforms contain a 45-amino-acid insertion between exons 44 and 45 (represented by the gray box in Fig. 1) in the proximal carboxy terminus, and the major Cav2.3c isoform does not contain this insertion [33]. Because this splicing difference occurs between LD10 and LD11 in the CACNA1E gene, polymorphisms in LD10 and LD11 that contain the rs3845446 A/G SNP might affect this splicing mechanism and induce a functional reduction of neuronal transmission via Cav2.3 VACCs. Further studies that focus on gene polymorphisms in LD10 and LD11 in the CACNA1E gene may reveal the functional mechanisms that affect pain sensitivity and the clinical efficacy of opioids.
Conclusions
In Japanese patients who underwent sagittal split ramus osteotomy, the analgesic effects of fentanyl were related to the genotype of the CACNA1E gene. Subjects with the minor G allele of the rs3845446 A/G SNP required less fentanyl for adequate postoperative pain control. Although further validation is needed because the sample size of the present study was quite small, our data might provide valuable information for the appropriate individualization of fentanyl doses to achieve adequate pain control in the future.
Supporting Information
Haploview LD plot of SNPs in and around the CACNA1E gene. D′ values are indicated in the figure.
(TIF)
Acknowledgments
We acknowledge Dr. Wenhua Han for supporting this study and Mr. Michael Arends for his assistance with editing the manuscript. We are grateful to the volunteers for their participation in this study and the anesthesiologists and surgeons at Tokyo Dental College Suidoubashi Hospital for collecting the clinical data.
Funding Statement
This research was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (20390162, 22790518, 23390377, 25116532), Japanese Ministry of Health, Labour and Welfare (H21-3jigan-ippan-011, H22-Iyaku-015), Smoking Research Foundation, and Astellas Foundation for Research on Metabolic Disorders. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Catterall WA (2000) Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol 16: 521–555. [DOI] [PubMed] [Google Scholar]
- 2. Zhang JF, Randall AD, Ellinor PT, Horne WA, Sather WA, et al. (1993) Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology 32: 1075–1088. [DOI] [PubMed] [Google Scholar]
- 3. Yokoyama CT, Westenbroek RE, Hell JW, Soong TW, Snutch TP, et al. (1995) Biochemical properties and subcellular distribution of the neuronal class E calcium channel α1 subunit. J Neurosci 15: 6419–6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Westenbroek RE, Hoskins L, Catterall WA (1998) Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. J Neurosci 18: 6319–6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murakami M, Nakagawasai O, Suzuki T, Mobarakeh, II, Sakurada Y, et al. (2004) Antinociceptive effect of different types of calcium channel inhibitors and the distribution of various calcium channel α1 subunits in the dorsal horn of spinal cord in mice. Brain Res 1024: 122–129. [DOI] [PubMed] [Google Scholar]
- 6. Saegusa H, Kurihara T, Zong S, Minowa O, Kazuno A, et al. (2000) Altered pain responses in mice lacking α1E subunit of the voltage-dependent Ca2+ channel. Proc Natl Acad Sci U S A 97: 6132–6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ikeda K, Ide S, Han W, Hayashida M, Uhl GR, et al. (2005) How individual sensitivity to opiates can be predicted by gene analyses. Trends Pharmacol Sci 26: 311–317. [DOI] [PubMed] [Google Scholar]
- 8. Coulbault L, Beaussier M, Verstuyft C, Weickmans H, Dubert L, et al. (2006) Environmental and genetic factors associated with morphine response in the postoperative period. Clin Pharmacol Ther 79: 316–324. [DOI] [PubMed] [Google Scholar]
- 9. Loh HH, Liu HC, Cavalli A, Yang W, Chen YF, et al. (1998) μ Opioid receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Brain Res Mol Brain Res 54: 321–326. [DOI] [PubMed] [Google Scholar]
- 10. Sora I, Elmer G, Funada M, Pieper J, Li XF, et al. (2001) μ Opiate receptor gene dose effects on different morphine actions: evidence for differential in vivo μ receptor reserve. Neuropsychopharmacology 25: 41–54. [DOI] [PubMed] [Google Scholar]
- 11. Sora I, Takahashi N, Funada M, Ujike H, Revay RS, et al. (1997) Opiate receptor knockout mice define μ receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci U S A 94: 1544–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kasai S, Ikeda K (2011) Pharmacogenomics of the human μ-opioid receptor. Pharmacogenomics 12: 1305–1320. [DOI] [PubMed] [Google Scholar]
- 13. Dogrul A, Zagli U, Tulunay FC (2002) The role of T-type calcium channels in morphine analgesia, development of antinociceptive tolerance and dependence to morphine, and morphine abstinence syndrome. Life Sci 71: 725–734. [DOI] [PubMed] [Google Scholar]
- 14. Contreras E, Tamayo L, Amigo M (1988) Calcium channel antagonists increase morphine-induced analgesia and antagonize morphine tolerance. Eur J Pharmacol 148: 463–466. [DOI] [PubMed] [Google Scholar]
- 15. Michaluk J, Karolewicz B, Antkiewicz-Michaluk L, Vetulani J (1998) Effects of various Ca2+ channel antagonists on morphine analgesia, tolerance and dependence, and on blood pressure in the rat. Eur J Pharmacol 352: 189–197. [DOI] [PubMed] [Google Scholar]
- 16. Yokoyama K, Kurihara T, Saegusa H, Zong S, Makita K, et al. (2004) Blocking the R-type (Cav2.3) Ca2+ channel enhanced morphine analgesia and reduced morphine tolerance. Eur J Neurosci 20: 3516–3519. [DOI] [PubMed] [Google Scholar]
- 17. Holmkvist J, Tojjar D, Almgren P, Lyssenko V, Lindgren CM, et al. (2007) Polymorphisms in the gene encoding the voltage-dependent Ca2+ channel Cav2.3 (CACNA1E) are associated with type 2 diabetes and impaired insulin secretion. Diabetologia 50: 2467–2475. [DOI] [PubMed] [Google Scholar]
- 18. Muller YL, Hanson RL, Zimmerman C, Harper I, Sutherland J, et al. (2007) Variants in the Cav2.3 (α1E) subunit of voltage-activated Ca2+ channels are associated with insulin resistance and type 2 diabetes in Pima Indians. Diabetes 56: 3089–3094. [DOI] [PubMed] [Google Scholar]
- 19. Fukuda K, Hayashida M, Ide S, Saita N, Kokita Y, et al. (2009) Association between OPRM1 gene polymorphisms and fentanyl sensitivity in patients undergoing painful cosmetic surgery. Pain 147: 194–201. [DOI] [PubMed] [Google Scholar]
- 20. Bisgaard T, Klarskov B, Rosenberg J, Kehlet H (2001) Characteristics and prediction of early pain after laparoscopic cholecystectomy. Pain 90: 261–269. [DOI] [PubMed] [Google Scholar]
- 21. Carlson CS, Eberle MA, Rieder MJ, Yi Q, Kruglyak L, et al. (2004) Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am J Hum Genet 74: 106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carlson CS, Eberle MA, Rieder MJ, Smith JD, Kruglyak L, et al. (2003) Additional SNPs and linkage-disequilibrium analyses are necessary for whole-genome association studies in humans. Nat Genet 33: 518–521. [DOI] [PubMed] [Google Scholar]
- 23. de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, et al. (2005) Efficiency and power in genetic association studies. Nat Genet 37: 1217–1223. [DOI] [PubMed] [Google Scholar]
- 24. Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21: 263–265. [DOI] [PubMed] [Google Scholar]
- 25. Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, et al. (2002) The structure of haplotype blocks in the human genome. Science 296: 2225–2229. [DOI] [PubMed] [Google Scholar]
- 26. Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191. [DOI] [PubMed] [Google Scholar]
- 27. Angst MS, Phillips NG, Drover DR, Tingle M, Ray A, et al. (2012) Pain sensitivity and opioid analgesia: a pharmacogenomic twin study. Pain 153: 1397–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perret D, Luo ZD (2009) Targeting voltage-gated calcium channels for neuropathic pain management. Neurotherapeutics 6: 679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park J, Luo ZD (2010) Calcium channel functions in pain processing. Channels (Austin) 4: 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Newcomb R, Szoke B, Palma A, Wang G, Chen X, et al. (1998) Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas . Biochemistry 37: 15353–15362. [DOI] [PubMed] [Google Scholar]
- 31. Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, et al. (2000) Nomenclature of voltage-gated calcium channels. Neuron 25: 533–535. [DOI] [PubMed] [Google Scholar]
- 32. Marubio LM, Roenfeld M, Dasgupta S, Miller RJ, Philipson LH (1996) Isoform expression of the voltage-dependent calcium channel α1E. Receptors Channels 4: 243–251. [PubMed] [Google Scholar]
- 33. Pereverzev A, Leroy J, Krieger A, Malecot CO, Hescheler J, et al. (2002) Alternate splicing in the cytosolic II–III loop and the carboxy terminus of human E-type voltage-gated Ca2+ channels: electrophysiological characterization of isoforms. Mol Cell Neurosci 21: 352–365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Haploview LD plot of SNPs in and around the CACNA1E gene. D′ values are indicated in the figure.
(TIF)