Abstract
Background
The pharmacokinetics (PKs) and pharmacodynamics (PDs) of telmisartan varies among the individuals, and the main causes remain unknown. The aim of this study was to evaluate the impact of ORM1, as well as ABCC2, ABCB1, ABCG2 and SLCO1B3 polymorphisms, on the disposition of the drug and BP change after taking 40 mg telmisartan in 48 healthy Chinese males.
Method
A total of 48 healthy males were included in this trial. Every volunteer ingested a single dose of 40 mg telmisartan, and the plasma drug concentration and blood pressure (BP) were measured up to 48 h.
Result
In this study, the area under the plasma concentration-time curve (AUC) in the heterozygotes of ORM1 113AG was higher than that in the wild-type homozygotes, AUC(0–48) (113AA vs. 113AG, 1,549.18±859.84 ng·h/ml vs. 2,313.54±1,257.71 ng·h/ml, P = 0.033), AUC(0–∞) (113AA vs. 113AG, 1,753.13±1,060.60 ng·h/ml vs. 2,686.90±1,401.87 ng·h/ml, P = 0.016), and the change(%) of the diastolic blood pressure (DBP) from the baseline BP value also showed a significant difference between the ORM1 113AG and 113AA genotypes at 5 h after taking telmisartan (P = 0.026). This study also showed that the allele of ABCC2 C3972T would affected the disposition of telmsiartan and the DBP change significantly after taking the drug. However, the common SNPs of ABCG2 C421, ABCB1 C3435T, and SLCO1B3 T334G showed no impacts on the PKs of telmisartan or BP change(%) in our trial.
Conclusion
The ORM1 A113G polymorphism was associated with the PKs variability after taking telmsiartan, as well as ABCC2 C3972T. The heterozygotes of ORM1 113AG showed a larger AUC and a notable BP change(%) from the baseline compared with the wild-type.
Trial Registration
Chinese Clinical Trial Registry ChiCTR-TNC-10000898
Introduction
There are about 1 billion hypertension patients worldwide and the number is still sharply increasing. Effective use the antihypertensive agents has become a particular challenge for the public health, as the blood pressure (BP)-lowering responses is diverse and hard to predict in individuals [1]. Telmisartan is a highly selective antagonist for the angiotensin II receptor type 1, and has the unique pharmacological properties characterized by its long duration of action and increased the tolerability. Telmisartan is equivalent to ramipril in the patients with vascular disease or high-risk diabetes occurred and associated with less angioedema [2].
In the healthy volunteers and hypertensive patients, a remarkable inter-individual variability had been reported with the peak concentration in plasma (Cmax) and the area under the plasma concentration-time curve (AUC) of telmisartan [3]–[6]. Furthermore, it has been reported that after a high dose of telmisartan, the drug was eliminated faster in Caucasian than in Chinese [4]–[5], suggesting that an “ethnic” difference was existed in the drug’s disposition with unknown mechanism, while another population pharmacokinetics study of telmisartan suggested that the “race difference” was not large [6]. There were a few studies focused on the pharmacogenetics of telmisartan, however, the discoveries contributed little to the variability of pharmacokinetics (PKs). Telmisartan was not metabolized by the cytochrome P450 system, and almost all of the drug was excreted as the unchanged parent drug into the feces [7]. Thus, the variability of the pharmacokinetics (PKs) of telmisartan may occur in the absorption, distribution, or excretion processes.
When administrated orally, telmisartan shows an absolute bioavailability of 43%, a long elimination half-life of almost 24 h, and more than 99.5% of telmisartan binds to the plasma proteins [8]. Telmisartan displays a saturable binding to the alpha-1-acid glycoprotein 1 (AAG1; encoded by ORM1) in addition to the human serum albumin [9]. The AAG is a major binding protein in plasma for various basic drugs, and is encoded by 2 loci, i.e., ORM1 and ORM2, and the content of ORM1 is dominant. Unlike ORM2, ORM1 is highly polymorphic [1]. There are 3 common haplotypes present at the ORM1 locus (ORM1*F1 has 113A/520G; ORM1*F2 has 113A/520A and ORM1*S has 113G/520G) [10], [12]. Recent investigations suggested that the ORM1 *S genotypes had a stronger binding force to the drugs, such as quinidine, disopyramide and nortriptyline, thus the *S alleles may decreased the concentrations of the free drugs in the serum [11], [13]. And it’s reported that the allele of 520A was rare in Asia [10], [11], and the minor allele frequency (MAF) is 0.03% in Asia (data from hapmap), which supported that the haplotype of *F2 may also rare [12],[15]. Meanwhile, our institute previous researches found that the alleles of ORM1 A113G can significantly affect the disposition of the drugs, nortriptyline and warfarin (data wasn’t published).
The multidrug-resistance protein 2 (MRP2, encoded by ABCC2), is a member of the ATP-binding cassette transporters family and plays a dominant role in the transport of various drugs, including telmisartan [6], [17]–[18]. ABCC2 was highly polymorphic, and it was reported that three common SNPs C-24T, G1249A and C3972T [19], [25] were in linkage disequilibrium and likes to decrease the transporter function probably by a posttranscriptional modification on transporter protein expression [19], [20]. It was reported that ABCC2 C-24T altered the pharmacokinetics of various drugs, including irinotecan, SN-38 and mycophenolic acid [21], [22]. However, some other studies found that ABCC2 C-24T polymorphism can’t alter the drug’s transport [26]. Besides MRP2, some other transporters also participated in the disposition of telmisartan, such as the multiple drug resistance-1 protein (MDR1, encoded by ABCB1), including the human breast cancer resistance protein 2 (BCRP2, encoded by ABCG2), and the influx pump of the human organic anion transporting polypeptide 1B3 (OATP1B3, encoded by SLCO1B3) [16], [27]–[30]. For MDR1, the polymorphism C3435T on exon 26 as a silent mutation, would affect the expression level and transfer functions [18], even affect the concentration of the substrates via absorption or excretion. It also has been suggested that the ABCG2 C421A may affect the protein expression, membrane surface translocation, efflux activity, or ATPase activity [24]. It was reported that the patients with C421A mutation achieved a higher plasma drug levels, suggesting a significant reduction in BCRP2 mediated excretion [23],even affected the drug’s response [24]. And the previous reports had shown that the common polymorphisms T334G on SLCO1B3 may alter the transporter’s activity in vitro and vivo [31], then alter the pharmacokinetic profile of the drugs, such as mycophenolic acid, rosuvastatin [31].
In this study, we intend to investigate the impacts of the polymorphisms of ORM1, ABCC2, ABCB1, ABCG2, and SLCO1B3 on the PKs and the BP change after taking the drug.
Methods
The protocol for this trial and supporting TREND checklist are available as supporting information; see Checklist S1 and Protocol S1.
Subjects and Study Design
A total of 48 unrelated Chinese healthy males from Changsha city were enrolled in this clinical trial between May 2010 and Dec 2010. The subjects were excluded if they had a history or evidence of hepatic, renal, gastrointestinal, or hematologic abnormalities; any other acute or chronic disease; or an allergy to any drug. No medications, herbal medicines, alcohol, citrus juices, or beverages containing caffeine were permitted during the study. The mean age of the subjects was 22.83±2.51 y (range 19∼34 y), the mean weight was 63.33±7.75 kg (range 49∼78 kg), and the mean height was 171.57±5.22 cm (range 162∼183 cm) (Table 1, Table S1).
Table 1. The demographics information of the participants.
| Number | Gender | Age(y) | Height (cm) | Weight (kg) | BMI(kg/m2) | Baseline SBP (mmHg) | Baseline DBP (mmHg) |
| 48 | male | 22.83±2.51(19.00, 34.00) | 171.57±5.22(162.00, 183.00) | 63.33±7.75(49.00, 78.00) | 21.73±2.09(17.57, 26.89) | 115.00±11.53(95.00, 145.00) | 77.04±8.39(60.00, 100.00) |
BMI, body mass index.
The study protocol was approved by the Ethical Committee of the Institute of Clinical Pharmacology, Central South University. The registration number (ChiCTR-TNC-10000898) and the trial protocol were validated in the Chinese Clinical Trial Registry. The trial was carried out in the institute of Clinical Pharmacology, Changsha, Hunan, China. Written informed consent and the clinical characteristics were obtained from each subjects before the trial. Each of the volunteers ingested a single tablet of a 40 mg dose of telmisartan (Micardis; Boehringer Ingelheim International GmbH, Ingelheim, Germany) with 200 mL water at 08∶00. The subjects received a standardized breakfast 2 h after the administration of telmisartan, and received a warm meal at 12∶00 and supper at 18∶00. Five milliliters of the venous blood samples were drawn into EDTA tubes at 0 (before administration), 0.25, 0.5, 0.75, 1, 1.25, 1.5, 2, 3, 4, 6, 8, 10, 12, 24, and 48 h after administration. The sitting BP of each participants were measured twice using a mercury manometer at 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 24, 48 h after administration. The plasma was separated by centrifugation at 3000×g for 15 min and stored at −20°C until extraction and analysis. The DNA was extracted through the standard phenol-chloroform extraction method.
PKs and BP Change(%) from the Baseline
The PKs of telmisartan were characterized by Cmax, Tmax, T1/2, AUC(0–48), and AUC(0–∞). The elimination rate constant (ke) was determined by linear regression analysis of the log-linear part of the elimination phase of the concentration-time curve. The T1/2 was calculated by the equation T1/2 = ln2/ke. The AUC(0–∞) was calculated by the combination of the linear and log-linear trapezoidal rules, with extrapolation to infinity by division of the last measured concentration by ke. The CL/F was calculated with the quotation CL/F = Pxo/AUC(0–∞), where Pxo represents the administration dose [15]; [16]. The BP change (%) from the baseline was calculated using the equation (BP - BP0)/BP0×100%, which BP0 represents the BP before the drug administration.
Genotyping
The alleles of ORM1 A113G were determined by a PCR-RFLP procedure as described below. The primers for ORM1 A113G were 5′-GAACTGAATCTATGTTTGTCTTCC-3′ (Forward) and 5′-CGACCACAGCCAGCAGGG-3′ (Reversed) and the endonuclease enzyme was EconI. For there is a high degree of homology between ORM1 and OMR2, then we took the PCR product sequencing, and verified that the PCR products were belong to ORM1 but not ORM2 gene. ABCC2 C-24T SNPs was detected by pyro-sequencing (Pyromark Q96 ID System, Qiagen, Germany). The forward primer was 5′-GGCAAGGTTAACGATTAAATGG-3′; the reverse primer was 5′-GCAGAACTTCTCCAGCATGAT-3′ (with 5′-biotin), and the sequencing primer was 5′-TCATATTAATAGAAGAGTCT-3′. We determined the ABCG2 C421A alleles by direct sequencing. The forward primer was 5′-ACTGCAGGTTCATCATCATTAGCTAGA-3′, and the reverse primer was 5′-CCGTTCGTTTTTTTCATGATTC-3′. Genotyping the ABCB1 C3435T as the method reported [33]. The genotypes of ABCC2 C3972T, ABCC2 G1249A, and, SLCO1B3 T334G were determined by a PCR-RFLP procedure as described in previous reports. The primers for ABCC2 C3972T were 5′-GTGGACTGTTCGGCTGAGTT-3′ (Forward) and 5′-TCACTCCACCTACCTTCTCCATG-3′ (Reversed) and the endonuclease enzyme was Bsh1285I. The primers for ABCC2 G1249A were 5′-GGGCAAAGAAGTGTGTGGAT-3′ (Forward) and 5′-TGGGATTACAAGCACCATCA-3′ (Reversed) and the endonuclease enzyme was NcoI. The primers for SLCO1B3 T334G were 5′-GAAGGTACAATGTCTTGGGC-3′ (Forward) and 5′-CTCTCAAAAGGTAACTGCCC-3′ (Reversed) and the endonuclease enzyme was Alu I.
The Linkage Disequilibrium Analysis
The linkage disequilibrium analysis of the ABCC2 variants (C-24T, G1249A and C3972T) was constructed using the online software SHEsis (http://analysis2.bio-x.cn/my Analysis.php).
Measurement of the Plasma AAG1 level
The concentration of AAG1 was determined using a commercial ELISA kit (ab108852, ABCAM, Cambridge, UK) for the quantitative determination according to the instruction by the manufacturer. The detection linearity of this kit was from 3.13 to 200 ng/ml. The plasma samples were diluted 1∶25000 with the diluted buffer. Absorbance values were read at 450 nm on the multimode ELISA reader (Beckman Coulter, Miami, FL, USA).
Determining the Concentration of Telmisartan in Plasma
A Waters Alliance 2695 liquid chromatographic system (Waters, Milford, MA, USA) equipped with a HyPurity C18 column (2.1 mm×150 mm, 5 µm; Thermo Hypersil-Keystone, America) was used to determine the concentration of telmisartan in the plasma. The column temperature was 40°C. The mobile phase consisted of acetonitrile and 5 mM ammonium acetate (containing 0.1% acetic acid) at a ratio of 45∶55 (v: v), and the flow rate was 0.3 mL/min. Irbesartan (100 ng/mL) was used as the internal standard. Fifty microliters of irbesartan and 400 µL of acetonitrile were added into 180 µL of plasma. After mixing on a vortex mixer for 5 min and waiting for 10 min, the mixture was then centrifuged at 15000 rpm for 5 min. A total of 200 µL of supernatant was put into an injection tube, and an aliquot of 5 µL of supernatant in tubes was injected into the HPLC system each time. Mass spectrum (MS) detection was performed on a Waters Quattro Micro API mass spectrometer (Waters, Milford, MA, USA). For telmisartan and irbesartan, the precursor-to-production reactions monitored were m/z 515 to 429 and m/z 428 to 207, respectively. The range of quantification for telmisartan in the plasma was 5 ng/mL to 1000 ng/mL, and the inter-day and intraday precisions for all analyses were less than 15% (coefficient of variation).
Statistical Analyses
All results were expressed as mean ± SD in the text and tables and, for clarity, as mean ± SEM in the figures. Statistical significance of the difference was evaluated using SPSS statistical software (SPSS Inc., ver.18.0). A P value of <0.05 was considered to be statistically significant. The contribution of the multiple factors to the variability of the parameters was evaluated using the stepwise linear regression analysis, and those variables with significant P value in the models were shown. The χ2 test was used to test whether the distribution met the Hardy-Weinberg equilibrium. Statistical comparison of the PKs parameters including Cmax, AUC(0–48), and AUC(0–∞) among non-carriers, heterozygous, and homozygous carriers was done by ANOVA after data points were naturally log-transformed, while CL/F was analyzed directly through ANOVA. When the P value was significant, a Tukey HSD test was followed to analysis the distribution between each two groups. The distribution of the Tmax and T1/2, among the different genotypes were analyzed by the Kruskal-Wallis test. Repeated-measure ANOVA (genotypes×time) with Greenhouse-Geisser correction, and the Tukey tests for post-hoc multiple comparisons were used to compare the BP change(%) from the baseline BP value.
Results
Genotyping
Of these 48 subjects, we detected the polymorphisms of ORM1 A113G and the genotypes of ABCC2, ABCB1, ABCG2, and SLCO1B3, and the frequencies of each genotype was shown in Table 2. The distributions of all the alleles in this population met the Hardy-Weinberg equilibrium. The linkage disequilibrium among the ABCC2 single nucleotide polymorphisms (SNPs) was analyzed, and the results showed that the alleles of ABCC2 C3972T was mostly in a linkage with ABCC2 C-24T (D′ = 0.997, r2 = 0.031), and the D′ value was 0.793 and r2 = 0.606 when the analysis tested between the SNPs of ABCC2 G1249A and C-24T.
Table 2. Summary of Genetic Variations of SNPs in this Study.
| Genes | RS No. | Nucleotide substitution | Location | Amino Acids | MAFa (CHB) | MAFa (ASW) | MAFa (CEU) | MAFb (CHB) |
| ORM1 | rs17650 | A113G | Exon | Gln/Arg | 0.275[37] | N/A | N/A | 0.208 |
| ABCC2 | rs717620 | C-24T | 5′UTR | – | 0.201 | 0.035 | 0.181 | 0.281 |
| rs3740066 | C3972T | Exon | Ile/Ile | 0.267 | 0.331 | N/A | 0.250 | |
| rs2273697 | G1249A | Exon | Val/Ile | 0.106 | 0.243 | 0.132 | 0.083 | |
| ABCB1 | rs1045642 | C3435T | Exon | Ile/Ile | 0.374 | 0.205 | 0.571 | 0.375 |
| SLCO1B3 | rs4149117 | T334G | Exon | Ala/Ser | 0.266 | 0.518 | 0.143 | 0.354 |
| ABCG2 | rs2231142 | C421A | Exon | Gln/Lys | 0.292 | 0.044 | 0.111 | 0.219 |
data published on hapmap; b, data calculated in this study, N = 48; N/A, no data found in hapmap.
MAF: Minor Allele frequencies; CHB: Han Chinese in Beijing, China; ASW: African ancestry in Southwest USA; CEU: Utah residents with Northern and Western European ancestry from the CEPH collection ORM1, orosomucoid 1; ABCC2, ATP-binding cassette, sub-family C, member 2; ABCG2, ATP-binding cassette, sub-family G, member 2; ABCB1, ATP-binding cassette, sub-family B, member 1; SLCO1B3, solute carrier organic anion transporter family, member 1B3; SNP, single nucleotide polymorphisms; AUC(0–48) the area under the plasma concentration-time curve (AUC) from 0 to 48 h; AUC(0–∞), AUC from 0 to ∞; Cmax, the peak concentration in plasma; CL/F, clearance; T1/2, elimination half-life; Tmax, the time to Cmax.
Telmisartan PKs and BP Change(%) from the Baseline
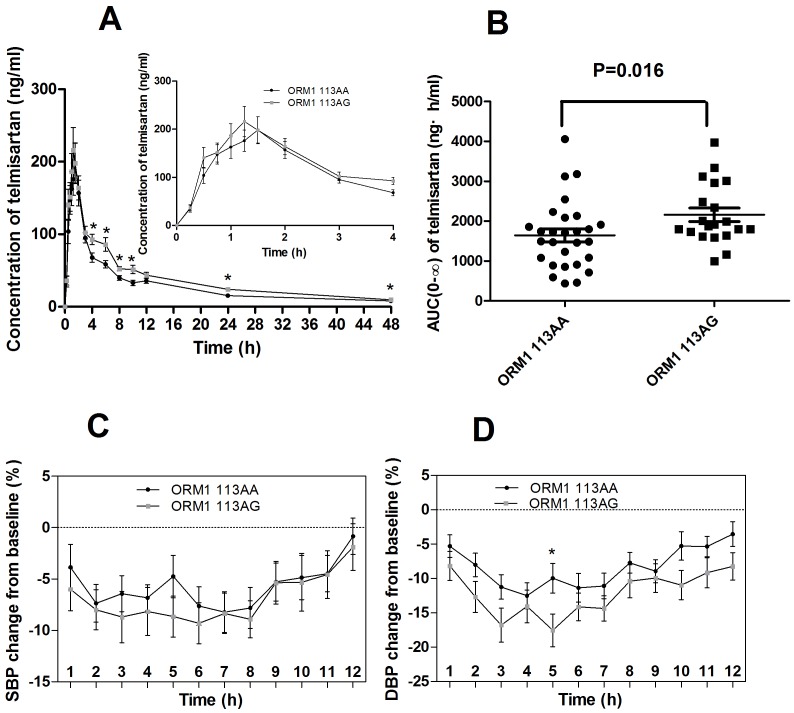
After an oral dose of 40 mg telmisartan, the mean AUC from 0 to 48 h (AUC(0–48)) in 48 healthy males was 1,793.75±1,065.94 ng·h/mL, the AUC from 0 to infinity (AUC(0–∞)) was 2,048.23±1,251.07 ng·h/mL, the Cmax was 2,61.15±165.42 ng/mL, time to Cmax (Tmax) was 1.28±0.54 h, elimination half-life (T1/2) was 17.15±8.83 h, and the clearance (CL/F) was 27.88±19.10 L/h. The PK parameters of telmisartan varied significantly in inter-individuals, for example, the AUC(0–48), AUC(0–∞), Cmax, Tmax, T1/2, and CL/F of telmisartan varied 3.9-, 14.5-, 17.8-, 6.0-, 7.6-, and 14.5-folds, respectively. After taking 40 mg telmisartan, the BP values of the participants declined lasted for more than 12 h, compared with the basal BP values. The decline degree of the BP was more notable in DBP than SBP, and the most significantly declined DBP occurred at 3∼9 h (Table 3, Figure 1, 2) when measured the BP change(%) from the baseline up to 48 h.
Table 3. Impacts of different SNPs on BP change (%) from the baseline after an oral administration of 40 mg telmisartan in 48 health males.
| BP | Genes | Genotypes | Basal BPvalue (0 h) | Change (%) from the baseline 3 h | Change(%) from the baseline 4 h | Change(%) from the baseline 5 h | Change (%) from the baseline 6 h | Change(%) from the baseline 7 h | Change(%) from the baseline 8 h | Change(%) from the baseline 9 h | Change(%) from the baseline 10 h |
| DBP | ORM1(A113G) | AA(28) | 75.75±7.20 | 11.23±9.48 | 12.52±9.98 | 9.96±11.37 | 11.35±11.06 | 11.08±9.77 | 7.72±8.08 | 8.94±8.86 | 5.26±10.99 |
| AG(20) | 78.85±9.74 | 16.79±11.06 | 14.07±10.68 | 17.55±10.65 | 14.14±8.94 | 14.36±8.13 | 10.40±10.77 | 9.95±9.29 | 10.96±9.50 | ||
| P value | 0.211 | 0.074 | 0.564 | 0.026* | 0.418 | 0.182 | 0.364 | 0.564 | 0.061 | ||
| SBP | ORM1(A113G) | AA(28) | 113.96±10.76 | 6.42±9.26 | 6.82±6.71 | 4.75±10.84 | 7.63±9.89 | 8.22±10.52 | 7.80±10.57 | 5.28±9.72 | 4.85±11.44 |
| AG(20) | 116.45±12.68 | 8.70±11.11 | 8.16±10.44 | 8.65±8.92 | 9.30±8.88 | 8.34±8.78 | 8.91±8.06 | 5.35±9.31 | 5.32±12.54 | ||
| P value | 0.468 | 0.373 | 0.355 | 0.180 | 0.253 | 0.916 | 0.818 | 0.950 | 0.967 | ||
| DBP | ABCC2(C3972T) | CC (26) | 79.15±8.96 | 15.72±10.55 | 14.79±10.23 | 16.07±9.78 | 14.03±9.90 | 12.95±8.36 | 9.82±8.00 | 11.09±7.98 | 7.64±11.39 |
| CT (20) | 74.00±7.21 | 8.95±7.54 | 9.88±9.46 | 7.98±12.19 | 10.11±10.87 | 11.16±10.49 | 5.64±8.76 | 5.56±8.10 | 6.20±8.85 | ||
| TT (2) | 80.00±0.00 | 31.25±8.84 | 25.00±0.00 | 26.25±1.77 | 16.88±2.65 | 18.75±0.00 | 28.13±4.42 | 25.00±8.84 | 21.88±13.26 | ||
| P value | 0.103 | 0.005* | 0.069 | 0.013* | 0.445 | 0.394 | 0.026* | 0.010* | 0.203 | ||
| SBP | ABCC2(C3972T) | CC (26) | 115.96±11.96 | 7.30±8.47 | 7.04±5.91 | 6.84±7.64 | 8.25±5.93 | 7.83±7.96 | 8.71±6.54 | 6.18±8.69 | 3.99±10.87 |
| CT (20) | 113.75±10.69 | 5.55±10.52 | 6.90±10.69 | 4.75±12.67 | 7.60±12.69 | 7.82±11.49 | 6.67±12.12 | 3.31±10.00 | 5.58±12.64 | ||
| TT (2) | 115.00±21.21 | 26.54±4.90 | 16.54±9.25 | 16.54±9.25 | 16.54±9.25 | 18.46±11.97 | 18.46±11.97 | 14.04±12.78 | 13.46±19.04 | ||
| P value | 0.819 | 0.062 | 0.290 | 0.241 | 0.346 | 0.298 | 0.317 | 0.350 | 0.719 |
Data were shown as mean±SD; *P<0.05; **P<0.01.
ORM1, orosomucoid 1; ABCC2, ATP-binding cassette, sub-family C, member 2; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Figure 1. The impacts of ORM1 genotypes on the PKs and BP change(%) after 40 mg telmisartan.
A, Concentration; B, AUC(0–∞); C, SBP change(%) from the baseline; D, DBP change(%) from the baseline.
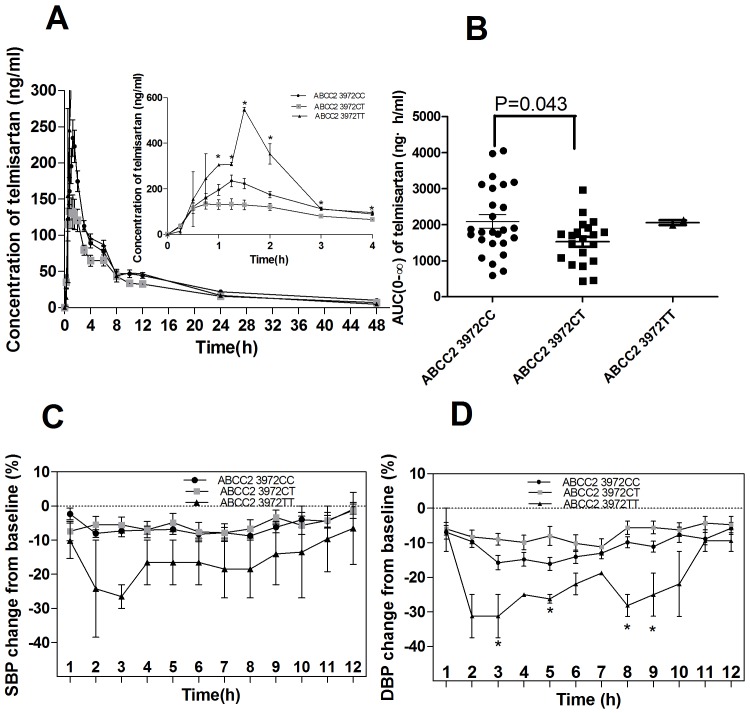
Figure 2. The impacts of ABCC2 genotypes on the PKs and BP change(%) after 40 mg telmisartan.
A, Concentration; B, AUC(0–∞); C, SBP change(%) from the baseline; D, DBP change(%) from the baseline.
The Plasma AAG1 Concentration Level
There was no significant difference of the plasma AAG1 concentration in different ORM1 genotypes (P = 0.801), the concentration of AAG1 in individual with ORM1 113AA was 444.25±112.86 ng/ml (range 264.17∼700.83 ng/ml), and the concentration in ORM1 113AG was 452.92±133.05 ng/ml (range 240.83∼738.33 ng/ml).
Association between the ORM1 Genotypes and Telmisartan PKs and BP Change(%) from the Baseline
As shown in Figure 1 and Table 4, the subjects with ORM1 113AG had a higher AUC compared with the wild-type ORM1 113AA genotype, AUC(0–48) (AA vs. AG, 1,549.18±859.84 vs. 2,313.54±1,257.71 ng·h/ml, P = 0.033), AUC(0–∞) (AA vs. AG, 1,753.13±1,060.60 vs. 2,686.90±1,401.87 ng·h/ml, P = 0.016). The results of the repeated measure ANOVA which corrected by Greenhouse-Geisser showed that the ORM1 A113G alleles had no significant impacts on the BP change(%) from the baseline after taking 40 mg telmisartan. While we found that the subjects with the genotype of ORM1 113AG appeared to have a more apparent BP change compared with the wildtype, although the P value was not significant except the DBP change(%) 5 h (P = 0.026) after administration (Figure 1, Table 3). There was no significant difference in the Cmax, CL/F, and T1/2 between the ORM1 genotypes after taking 40 mg telmisartan (Table 4).
Table 4. Telmisartan PKs of 48 health males with different genotypes.
| Gene | SNPs | Genotype | AUC(0–48)(ng·h/ml) | AUC(0–∞) (ng·h/ml) | Cmax(ng/ml) | CL/F(l/h) | T1/2 (h) |
| ORM1 | A113G | AA (28) | 1,549.18±859.84 | 1,753.13±1,060.60 | 246.87±156.93 | 32.38±21.43 | 15.63±5.09 |
| AG (20) | 2,313.54±1,257.71 | 2,686.90±1,401.87 | 293.00±184.06 | 18.63±8.74 | 20.01±12.81 | ||
| P VALUE | 0.033* | 0.016* | 0.289 | 0.355 | 0.113 | ||
| ABCC2 | C3972T | CC (26) | 1,871.18±817.89 | 2,090.83±952.69 | 313.32±172.05 | 18.80±10.32 | 15.83±4.11 |
| CT (20) | 1,289.89±506.60 | 1,526.47±626.97 | 176.48±106.47 | 10.59±6.39 | 20.09±13.27 | ||
| TT (2) | 1,973.90±135.44 | 2,057.50±97.27 | 548.22±12.93 | 32.89±0.78 | 12.26±3.11 | ||
| P VALUE | 0.031* | 0.105 | 0.001** | 0.001** | 0.321 | ||
| CC vs. CT | 0.035* | 0.105 | 0.005** | 0.008** | 0.476 | ||
| CC vs.TT | 0.896 | 0.964 | 0.243 | 0.083 | 0.237 | ||
| CT vs. TT | 0.311 | 0.526 | 0.014* | 0.004** | 0.147 | ||
| ABCC2 | C-24T | CC (24) | 1,809.42±843.05 | 2,017.04±976.09 | 306.16±182.01 | 18.37±10.92 | 15.61±4.19 |
| CT (21) | 1,348.93±525.05 | 1,588.64±637.52 | 191.52±112.96 | 25.10±10.75 | 19.98±12.95 | ||
| TT (3) | 2,214.17±427.04 | 2,411.88±617.65 | 467.57±139.99 | 17.24±3.89 | 14.62±4.65 | ||
| P VALUE | 0.060 | 0.161 | 0.008** | 0.005** | 0.523 | ||
| ABCC2 | G1249A | GG(41) | 1597.79±667.24 | 1832.01±790.09 | 256.45±156.87 | 15.39±9.41 | 18.01±9.84 |
| GA(6)+AA(1) | 1998.44±1108.08 | 1232.57±992.12 | 355.01±236.12 | 21.30±14.17 | 14.09±4.01 | ||
| P VALUE | 0.285 | 0.417 | 0.296 | 0.296 | 0.614 | ||
| ABCG2 | C421A | CC (30) | 1,579.22±713.71 | 1,800.86±797.05 | 258.14±165.95 | 15.49±9.96 | 17.69±10.97 |
| CA (15) | 1,679.29±719.04 | 1,840.07±798.27 | 297.90±170.29 | 17.87±10.22 | 16.34±11.36 | ||
| AA (3) | 1,943.43±921.94 | 2,459.68±1,394.14 | 186.58±133.13 | 19.57±8.94 | 20.75±5.44 | ||
| P Value | 0.669 | 0.573 | 0.481 | 0.540 | 0.265 | ||
| ABCB1 | C3435T | CC (20) | 1,606.83±646.33 | 1,791.71±814.66 | 237.23±126.55 | 14.23±7.59 | 15.48±5.05 |
| CT (20) | 1,673.53±789.53 | 1,920.07±849.29 | 291.52±206.17 | 17.49±12.37 | 18.95±12.29 | ||
| TT (8) | 1,598.62±913.64 | 1,846.30±1,026.48 | 274.70±165.31 | 16.48±9.92 | 18.67±8.88 | ||
| P VALUE | 0.587 | 0.317 | 0.951 | 0.984 | 0.076 | ||
| SLCO1B3 | T334G | GG (18) | 1,702.25±740.60 | 1,877.33±806.34 | 298.07±204.22 | 17.88±12.25 | 15.90±4.08 |
| GT (26) | 1,574.47±755.06 | 1,829.07±896.92 | 232.81±132.75 | 13.97±7.97 | 18.56±11.78 | ||
| TT (4) | 1,704.89±797.20 | 1,914.58±939.86 | 338.56±196.27 | 25.38±13.32 | 17.31±8.43 | ||
| P VALUE | 0.793 | 0.929 | 0.424 | 0.307 | 0.994 |
Data were shown as mean±SD;
P<0.05; **P<0.01.
PKs, pharmacokinetics; ORM1, orosomucoid 1; ABCC2, ATP-binding cassette, sub-family C, member 2; ABCG2, ATP-binding cassette, sub-family G, member 2; ABCB1, ATP-binding cassette, sub-family B, member 1; SLCO1B3, solute carrier organic anion transporter family, member 1B3; SNP, single nucleotide polymorphisms; AUC(0–48) the area under the plasma concentration-time curve (AUC) from 0 to 48 h; AUC(0–∞), AUC from 0 to ∞; Cmax, the peak concentration in plasma; CL/F, clearance; T1/2, elimination half-life; Tmax, the time to Cmax.
Association between the Transporters Gene Polymorphisms and Telmisartan PKs and BP Change(%) from the Baseline
The parameters of AUC(0–48), Cmax, CL/F in the different ABCC2 C3972T genotypes varied significantly (P = 0.031, 0.001, and 0.001, respectively) (Figure 2, Table 4); The repeated measure ANOVA results showed that ABCC2 C3972T polymorphism would significantly affected the DBP change(%) from the baseline after taking telmisartan(P = 0.018) (Table 5). The DBP change(%) from the baseline varied significantly at 3, 5, 8, 9 h (P = 0.05, 0.013, 0.026 and 0.010 respectively) among the ABCC2 C3972T genotypes(Figure 2, Table 3). The Cmax and CL/F of the different ABCC2 C-24T genotypes also manifested a remarkable difference (P = 0.008 and 0.005, respectively)(Table 4). In this study, the polymorphisms of ABCC2(G1249A), ABCB1 (C3435T), SLCO1B3 (T334G) and ABCG2 (C421A) had no significant influence on the PKs of telmisartan and the BP change(%) from the baseline after taking telmisartan (Table 3, 4, 5).
Table 5. The significance affected by time or genotypes on the BP change(%) from the baseline.
| SBP change (%) from the baseline | DBP change (%) from the baseline | |||||||
| Mauchly’s | Time | Time*genotype | genotype | Mauchly’s | Time | Time*genotype | genotype | |
| ORM1 A113G | 0.004 | 0.000 | 0.748 | 0.770 | 0.000 | 0.000 | 0.258 | 0.096 |
| ABCC2 C3972T | 0.006 | 0.000 | 0.262 | 0.162 | 0.000 | 0.000 | 0.067 | 0.018* |
| ABCC2 G1249A | 0.005 | 0.000 | 0.795 | 0.370 | 0.000 | 0.030 | 0.975 | 0.144 |
| SLCO1B3 T334G | 0.001 | 0.000 | 0.842 | 0.995 | 0.000 | 0.000 | 0.431 | 0.634 |
| ABCG2 C421A | 0.001 | 0.000 | 0.300 | 0.255 | 0.000 | 0.000 | 0.451 | 0.896 |
| ABCB1 C3435T | 0.001 | 0.000 | 0.369 | 0.375 | 0.000 | 0.000 | 0.372 | 0.241 |
P<0.05;
ORM1, orosomucoid 1; ABCC2, ATP-binding cassette, sub-family C, member 2; SBP, systolic blood pressure; DBP, diastolic blood pressure; Time, the time point in this study including 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 24, 48 h after taking telmisartan.
Stepwise Regression Analysis
The stepwise multiple regression analysis results showed that the AUC variability of telmisartan was affected by the polymorphisms of ORM1 A113G (P = 0.028) and ABCC2 C3972T (P = 0.031). In this stepwise multiple regression models, we correct the multiple factors Age, BMI, and basal BP values. The genetic polymorphisms ORM1 A113G*ABCC2 C3972T contributed R2 = 0.150 and 0.153 respectively to AUC(0–48) and AUC(0–∞). The ORM1 A113G*ABCG2 C421A contributed R2 = 0.160 to the DBP 5 h change(%) from the baseline. There was no genetic variants contributed significantly to Cmax, CL/F, T1/2 and SBP 5 h change (%) from the baseline (Table 6).
Table 6. The P valve of variable factors on PK and BP change parameters in the corrected models.
| Independents | AUC(0–48) | AUC(0–∞) | 5 h DBP change(%) from the baseline |
| ORM1 A113G | 0.028* | 0.018* | 0.012* |
| MRP2 C3972T | 0.031* | 0.045* | – |
| ABCG2 C421A | – | – | 0.099 |
| ABCB1 C3435T | – | – | – |
| SLCO1B3 T334G | – | – | – |
| R2 | 0.150 | 0.153 | 0.160 |
| Ajusted R2 | 0.113 | 0.115 | 0.122 |
Data were shown as mean±SD;
P<0.05;
P<0.01.
ORM1, orosomucoid 1; ABCC2, ATP-binding cassette, sub-family C, member 2; ABCG2, ATP-binding cassette, sub-family G, member 2; ABCB1, ATP-binding cassette, sub-family B, member 1; SLCO1B3, solute carrier organic anion transporter family, member 1B3; AUC(0–∞) the area under the plasma concentration-time curve(AUC) from 0 to ∞; Cmax, the peak concentration in plasma; CL/F, clearance; T1/2, elimination half-life; Tmax, the time to Cmax; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Discussion
In this study, we for the first time reported that the polymorphism of ORM1 A113G was associated with the variability of the drug AUC and BP change(%) from the baseline after taking 40 mg telmisartan in Chinese healthy males. The content of the plasma AAG1 had no significant difference between ORM1 genotypes, while the heterozygotes of ORM1 113AG demonstrated a higher AUC of the drug and a apparent BP decline after taking 40 mg telmisartan. This result was consistent with the previous reports that the ORM1 variants can affect the disposition of the drug, alter the concentrations of the free drugs in the serum [11]–[14]. It was reported that there were 7 drug-protein binding sites with different affinities on the AAG surface, and more binding sites on the AAG surface with ORM1 113G than ORM1 113A, thus the affinity was much stronger in the former haplotype [2], [32]. The more drugs bind to plasma proteins, the fewer drugs would be excreted; and more stored bound-drugs would transformed into the plasma free drug. Thus the total plasma drug concentration and AUC in ORM1 113AG heterozygote would be remains in a higher level. We can speculate that when the drug was in a high plasma concentration for a long duration of time, the substrate would manifest as a stronger pharmacodynamics. In our study, when compared with the wild-type ORM1 113AA, the ORM1 113AG genotype decrease the basal BP more markedly at 5 h after taking the drug (SBP, P value = 0.180; DBP, P value = 0.026). The same tendency of BP change(%) from the baseline between 113AA and 113AG genotype was also presented in the other time points, although the P value were not significant (Table 3). The difference of BP change between the ORM1 genotypes was not as significant as the PKs, the reasons may be there were many physical, condition or mental factors would influence the BP. In this research, the significant difference of drug concentration between ORM1 genotypes were in 1∼4 h after the drug administration, while the most significant difference of DBP change(%) from the baseline occurred in 3∼9 h. It seemed that the BP change was lagged behind the PKs several hours. We speculated that because the ORM1 mostly affected the distribution phase of the drugs rather than the other disposition phase. So the significant difference caused by ORM1 polymorphisms may occurred in an earlier period of drug disposition(1–4 h, in Figure 1), and the previous researches found that after administering 3 h later, the BP started to decline, so the BP change seemed to be lagged.
The difference of BP change(%) from the baseline among ABCC2 C3972T three genotypes was manifested in a concentration-dependent fashion. After administering 40 mg telmisartan, the heterozygotes of ABCC2 39732CT showed the lowest AUC, Cmax, and displayed the minimum SBP and DBP change(%) from the baseline, while the genotype TT manifested as the highest AUC, Cmax, and the maximum change of the BP. The significant difference of DBP change among ABCC2 3972 genotypes occurred at 3, 5, 8 and 9 h after taking telmisartan(Table 3). For ABCC2 C3972 and C-24T in a strong linkage disequilibrium, our research was consistent to the previous reports that the Cmax of telmisartan in the −24CT genotype group was significantly greater than that in the −24CC genotype (P = 0.0094) in Japanese renal transplant patients (3). While, the impacts of ABCC2 C3972T polymorphism on PKs and BP chang(%) from the baseline were not consistent with the gene-dose effect, the possible reasons may be two. On the one hand, as ABCC2 C-24T, C3972T, and G1249A SNPs were in a strong linkage disequilibrium, thus the other two SNPs might interfere with C3972T to affect the variability of PKs/PDs. It has been reported that the MRP2 transport function may be affected by the ABCC2 polymorphisms in a haplotype-dependent model(33). And besides this three SNPs, some unknown SNPs may also participated in the disposition of telmisartan and may interacted with ABCC2 C3972T, which need a further research. On the other hand, only 2 subjects carried the genotype of ABCC2 3972TT participated in this study, thus the parameters of this two subjects may won’t reflect the population characteristics of the homozygote group. This results need confirmed in a more large population.
The underlying mechanisms of telmisartan PKs and PDs highly varied among the individuals still remain unknown. There were a few pharmacogenomics studies on telmisartan’s disposition, while the research results contributed little to the variability [34]. Some other studies reported that genetic factors, such as ABCB1 C3435T, SLCO1B3 T334G and other related polymorphisms, showed no influence on the PKs variability [6],[34]–[36]. In our trial, we confirmed that the polymorphisms of SLCO1B3 T334G, ABCB1 C3435T, ABCG2 G421A had no significant influence on telmisartan disposition. It was reported that the haplotype of UGT1A3*4a can influence the telmisartan’s PKs [35]. The previous reports also showed that CL/F of telmisartan was associated with age, dose, gender, alcohol consumption, race and liver function [6], but in our study the factor of age was nonfunctional. It was also manifested in our study that the DBP decline was stronger than SBP after taking telmisartan (Table 3), which implied that telmisartan would be an ideal choice for the hypertensive with a higher DBP value.
In our study, all the frequencies of the 7 SNPs in the 48 participates was consistent with the reported frequencies in CHB population. As reported in the literatures, Mastana found that there was a significant clinical decrease of the ORM1*S allele frequency from West to East (r = 0.80, P<0.01) in 48 populations, and the frequency of the allele ORM1*S was lowering from 31% to 19% when presented in central Asia towards the Southeast [38]. The ORM1*S allele frequency in the present study was within these ranges among the populations of Asia. Different populations may possess various genetic backgrounds, which would cause the variability of PKs or even PDs of the substrates. As shown in Table 2, the MAF of ABCC2 C3972T and G1249A, ABCB1 C3435T, SLCO1B3 T334G almost be similar in three main populations. While the frequencies of the alleles ABCC2 -24T, ABCG2 421G shown a apparent decrease in ASW population. Thus, we speculated that the PKs of Telmisartan in West may differ from Asia, which was supported the conclusion that after a high dose of telmisartan, the drug was eliminated faster in Caucasian than in Chinese subjects [4]–[5].
However, some shortcomings existed in our study. First, the subjects in our trial were young and healthy males, with well self-regulation ability on the BP fluctuations and their body’s regulation ability may conceal the BP change to some degree. Second, for the BP value was fluctuated in a day, and the time showed a significant impacts on the BP change(%) from the baseline(Table 5). So in this study, there was no enough data to value the actual antihypertensive pharmacodynamics among the genotypes if just compare the BP change via took the pre-dose BP as baseline merely. We should monitor the volunteer’s blood pressure at the same time on another day when taking no telmisartan, in order to remove the diurnal variation within individuals. Third, the number of participants was not large enough to get a convincing conclusion. For example, there was no subject with homozygous ORM1 113GG genotypes enrolled in this trial for us to explore the influence of the genotype 113GG. Hence, this conclusion needs to be identified in a larger population in the further research, especially in the hypertensive.
To explore the possible reasons for the metabolism variation, can benefit us to improve the efficiency of telmisartan. Our study showed that the polymorphisms of ORM1 A113G can absolutely affected the disposition of telmisartan in vivo after administration, as well as the SNPs of ABCC2 C3972T. It is a new discovery would help us to understanding the mechanism of the noteworthy disposition difference after telmisartan taken, however it need further researches to confirm in a large scale population.
Supporting Information
The clinical characteristics data and baseline BP values of the 48 subjects.
(DOC)
The TREND Statement Checklist.
(DOC)
The Clinical Trial Protocol on the pharmacokinetics of telmisartan in Chinese males.
(DOC)
Funding Statement
This work was supported by the National Scientific Foundation of China (No. 81273595, 81001476), the Scientific Foundation of Hunan (No. 11K073, 10JJ4020), the “863” Project (No. 2012AA02A518, No. 2012AA02A517), and NCET-10-0843, NCET-11-0509. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, et al.. (1993) Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med. 328, 914–21. [DOI] [PubMed]
- 2.ONTARGET Investigators, Yusuf S, Teo KK, Pogue J, Dyal L, et al.. (2008) Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 358, 1547–59. [DOI] [PubMed]
- 3.Stangier J, Su CA, Schondorfer G, Roth W (2000) Pharmacokinetics and safety of intravenous and oral telmisartan 20 mg and 120 mg in subjects with hepatic impairment compared with healthy volunteers. J Clin Pharmacol. 40, 1355–64. [PubMed]
- 4.Tatami S, Sarashina A, Yamamura N, Igarashi T, Tanigawara Y (2003) Population pharmacokinetics of an angiotensin II receptor antagonist, telmisartan, in healthy volunteers and hypertensive patients. Drug Metab Pharmacokinet. 18, 203–11. [DOI] [PubMed]
- 5.Zhang P, Zhang Y, Chen X, Li R, Yin J, et al.. (2006) Pharmacokinetics of telmisartan in healthy Chinese subjects after oral administration of two dosage levels. Arzneimittelforschung. 56, 569–73. [DOI] [PubMed]
- 6.Tatami S, Yamamura N, Sarashina A, Yong CL, Igarashi T, et al.. (2004) Pharmacokinetic comparison of an angiotensin II receptor antagonist, telmisartan, in Japanese and western hypertensive patients using population pharmacokinetic method. Drug Metab Pharmacokinet. 19, 15–23. [DOI] [PubMed]
- 7.Israili ZH (2000) Clinical pharmacokinetics of angiotensin II (AT1) receptor blockers in hypertension. J Hum Hypertens. 14, S73–S86. [DOI] [PubMed]
- 8.Stangier J, Schmid J, Türck D, Switek H, Verhagen A, et al.. (2000)Absorption, metabolism, and excretion of intravenously and orally administered[14C]telmisartan in healthy volunteers. J Clin Pharmacol. 40, 1312–22. [PubMed]
- 9.Stangier J, Su CA, Roth W (2000) Pharmacokinetics of orally and intravenously administered telmisartan in healthy young and elderly volunteers and in hypertensive patients. J Int Med Res. 28, 149–67. [DOI] [PubMed]
- 10. Imre T, Schlosser G, Pocsfalvi G, Siciliano R, Molnár-Szöllosi E, et al. (2005) Glycosylation site analysis of human alpha-1-acid glycoprotein (AGP) by capillary liquid chromatography-electrospray mass spectrometry. J Mass Spectrom. 40: 1472–83. [DOI] [PubMed] [Google Scholar]
- 11.Li JH, Xu JQ, Cao XM, Ni L, Li Y, et al. (2002) Influence of the ORM1 phenotypes on serum unbound concentration and protein binding of quinidine. Clin Chim Acta. 317, 85–92. [DOI] [PubMed]
- 12.Yuasa I, Umetsu K, Vogt U, Nakamura H, Nanba E, et al. (1997) Human orosomucoid polymorphism: molecular basis of the three common ORM1 alleles, ORM1*F1, ORM1*F2, and ORM1*S. Hum Genet. 99, 393. [DOI] [PubMed]
- 13.Kishino S, Nomura A, Di ZS, Sugawara M, Iseki K, et al.. (1995) Alpha-1-acid glycoprotein concentration and the protein binding of disopyramide in healthy subjects. J Clin Pharmacol. 35, 510–4. [DOI] [PubMed]
- 14.Zhang C, Tu ZL, Wang QB, Cheng XL, Zhang PH (2007) Influence of ORM1 polymorphism on serum concentration of free nortriptyline. Yao Xue Xue Bao. 42, 843–8. [PubMed]
- 15.Katori N, Sai K, Saito Y, Fukushima-Uesaka H, Kurose K, et al.. (2011) Genetic variations of orosomucoid genes associated with serum alpha-1-acid glycoprotein level and the pharmacokinetics of paclitaxel in Japanese cancer patients. J Pharm Sci. doi: 10.1002/jps.22648. [DOI] [PubMed]
- 16.Schinkel AH, Jonker JW (2003) Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 55, 3–29. [DOI] [PubMed]
- 17.Nishino A, Kato Y, Igarashi T, Sugiyama Y (2000) Both cMOAT/MRP2 and another unknown transporter(s) are responsible for the biliary excretion of glucuronide conjugate of the nonpeptide angiotensin II antagonist, telmisaltan. Drug Metab Dispos. 28, 1146–8. [PubMed]
- 18.Suzuki H, Sugiyama Y (2002) Single nucleotide polymorphisms in multidrug resistance associated protein 2 (MRP2/ABCC2): its impact on drug disposition. Adv Drug Deliv Rev. 54, 1311–31. [DOI] [PubMed]
- 19.Haenisch S, Zimmermann U, Dazert E, Wruck CJ, Dazert P, et al. (2007) Influence of polymorphisms of ABCB1 and ABCC2 on mRNA and protein expression in normal and cancerous kidney cortex. Pharmacogenomics J. 7, 56–65. [DOI] [PubMed]
- 20.Choudhuri S, Klaassen CD (2006) Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int J Toxicol. 25, 231–59. [DOI] [PubMed]
- 21.de Jong FA, Scott-Horton TJ, Kroetz DL, McLeod HL, Friberg LE, et al. (2007) Irinotecan-induced diarrhea: functional significance of the polymorphic ABCC2 transporter protein. Clin Pharmacol Ther. 81, 42–9. [DOI] [PubMed]
- 22.Naesens M, Kuypers DR, Verbeke K, Vanrenterghem Y (2006) Multidrug resistance protein 2 genetic polymorphisms influence mycophenolic acid exposure in renal allograft recipients. Transplantation. 82, 1074–84. [DOI] [PubMed]
- 23.Deo AK, Prasad B, Balogh L, Lai Y, Unadkat JD (2012) Interindividual Variability in Hepatic Expression of the Multidrug Resistance-associated Protein 2 (MRP2/ABCC2): Quantification by liquid chromatography/tandem mass spectrometry. Drug Metab Dispos. 40, 852–5. [DOI] [PMC free article] [PubMed]
- 24.Moriya Y, Nakamura T, Horinouchi M, Sakaeda T, Tamura T, et al.. (2002) Effects of polymorphisms of MDR1, MRP1, and MRP2 genes on their mRNA expression levels in duodenal enterocytes of healthy Japanese subjects. Biol Pharm Bull. 25, 1356–9. [DOI] [PubMed]
- 25.Choi JH, Ahn BM, Yi J, Lee JH, Lee JH, et al.. (2007) MRP2 haplotypes confer differential susceptibility to toxic liver injury. Pharmacogenet Genomics. 17, 403–15. [DOI] [PubMed]
- 26.Seo T, Ishitsu T, Oniki K, Abe T, Shuto T, et al. (2008) ABCC2 haplotype is not associated with drug-resistant epilepsy. J Pharm Pharmacol. 60, 631–5. [DOI] [PubMed]
- 27.Ishiguro N, Maeda K, Kishimoto W, Saito A, Harada A, et al.. (2006) Predominant contribution of OATP1B3 to the hepatic uptake of telmisartan, an angiotensin II receptor antagonist, in humans. Drug Metab Dispos. 34, 1109–15. [DOI] [PubMed]
- 28.Ishiguro N, Maeda K, Saito A, Kishimoto W, Matsushima S, et al.. (2008) Establishment of a set of double transfectants coexpressing organic anion transporting polypeptide 1B3 and hepatic efflux transporters for the characterization of the hepatobiliary transport of telmisartan acylglucuronide. Drug Metab Dispos. 36, 796–805. [DOI] [PubMed]
- 29.Chang C, Bahadduri PM, Polli JE, Swaan PW, Ekins S (2006) Rapid identification of P-glycoprotein substrates and inhibitors. Drug Metab Dispos. 34, 1976–84. [DOI] [PubMed]
- 30.Weiss J, Sauer A, Divac N, Herzog M, Schwedhelm E, et al.. (2010) Interaction of angiotensin receptor type 1 blockers with ATP-binding cassette transporters. Biopharm Drug Dispos. 31, 150–61. [DOI] [PubMed]
- 31.Schwarz UI, Meyer zu, Schwabedissen HE, Tirona RG, Suzuki A, et al.. (2011) Identification of novel functional organic anion-transporting polypeptide 1B3 polymorphisms and assessment of substrate specificity. Pharmacogenet Genomics. 21, 103–14. [DOI] [PMC free article] [PubMed]
- 32.Nakagawa T, Kishino S, Itoh S, Sugawara M, Miyazaki K (2003) Differential binding of disopyramide and warfarin enantiomers to human alpha(1)-acid glycoprotein variants. Br J Clin Pharmacol. 56, 664–9. [DOI] [PMC free article] [PubMed]
- 33.Laechelt S, Turrini E, Ruehmkorf A, Siegmund W, Cascorbi I, et al. (2011) Impact of ABCC2 haplotypes on transcriptional and posttranscriptional gene regulation and function. Pharmacogenomics J. 11, 25–34. [DOI] [PubMed]
- 34.Deppe S, Boger RH, Weiss J, Benndorf RA (2010) Telmisartan: a review of its pharmacodynamic and pharmacokinetic properties. Expert Opin Drug Metab Toxicol. 6, 863–71. [DOI] [PubMed]
- 35.Ieiri I, Nishimura C, Maeda K, Sasaki T, Kimura M, et al.. (2011) Pharmacokinetic and pharmacogenomic profiles of telmisartan after the oral microdose and therapeutic dose. Pharmacogenet Genomics. 21, 495–505. [DOI] [PubMed]
- 36.Guo X, Chen XP, Cheng ZN, Luo X, Guo R, et al.. (2009) No effect of MDR1 C3435T polymorphism on oral pharmacokinetics of telmisartan in 19 healthy Chinese male subjects. Clin Chem Lab Med. 47, 38–43. [DOI] [PubMed]
- 37.Li JH, Xu JQ, Li Y, Zhuang YY, Gong JB (1999) Genetic polymorphisms of orosomucoid on the Han population in Nanjing of China. Clin Chim Acta. 288, 161–8. [DOI] [PubMed]
- 38.Mastana SS, Jayasekara R, Fisher P, Sokol RJ, Papiha SS (1993) Genetic polymorphism of orosomucoid (ORM) in populations of the United Kingdom, Indian subcontinent, and Cambodia. Jpn J Human Genet. 38, 289–96. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The clinical characteristics data and baseline BP values of the 48 subjects.
(DOC)
The TREND Statement Checklist.
(DOC)
The Clinical Trial Protocol on the pharmacokinetics of telmisartan in Chinese males.
(DOC)