The juxtaposition of a double-stranded DNA end and a short single-stranded DNA gap triggers robust activation of endogenous ATR and Chk1 mediated by DNA-PKcs.
Abstract
Three phosphatidylinositol-3-kinase–related protein kinases implement cellular responses to DNA damage. DNA-dependent protein kinase catalytic subunit (DNA-PKcs) and ataxia-telangiectasia mutated respond primarily to DNA double-strand breaks (DSBs). Ataxia-telangiectasia and RAD3-related (ATR) signals the accumulation of replication protein A (RPA)–covered single-stranded DNA (ssDNA), which is caused by replication obstacles. Stalled replication intermediates can further degenerate and yield replication-associated DSBs. In this paper, we show that the juxtaposition of a double-stranded DNA end and a short ssDNA gap triggered robust activation of endogenous ATR and Chk1 in human cell-free extracts. This DNA damage signal depended on DNA-PKcs and ATR, which congregated onto gapped linear duplex DNA. DNA-PKcs primed ATR/Chk1 activation through DNA structure-specific phosphorylation of RPA32 and TopBP1. The synergistic activation of DNA-PKcs and ATR suggests that the two kinases combine to mount a prompt and specific response to replication-born DSBs.
Introduction
From the early stages of carcinogenesis, replication-associated lesions trigger DNA damage responses (Bartkova et al., 2005; Gorgoulis et al., 2005), which are mediated by the phosphatidylinositol-3-kinase–like protein kinases (PI3KKs) ataxia-telangiectasia mutated (ATM), ataxia-telangiectasia and RAD3-related (ATR), and DNA-dependent protein kinase (DNA-PK) catalytic subunit (DNA-PKcs). ATM signals DNA double-strand breaks (DSBs), whereas ATR responds to a variety of obstacles that block the progression of replication forks (Jackson and Bartek, 2009). Activated ATM and ATR phosphorylate hundreds of substrate proteins to activate DNA repair mechanisms and adjust ongoing physiological processes (Matsuoka et al., 2007). Two important targets of ATR and ATM are Chk1 and Chk2, which implement cell cycle checkpoints (Abraham, 2001).
ATR activation depends on the nucleation of multiple factors that bind single-stranded DNA (ssDNA) and 5′ double-stranded DNA to single-stranded DNA (ds/ssDNA) junctions (MacDougall et al., 2007; Van et al., 2010). The ATR signal is amplified when either ssDNA or ds/ssDNA junctions accumulate (Byun et al., 2005; MacDougall et al., 2007; Van et al., 2010). The recruitment of ATR to stalled replication forks is mediated by ATRIP, which binds human replication protein A (RPA) bound to ssDNA (Zou and Elledge, 2003). ATRIP also facilitates the recruitment of TopBP1 (Choi et al., 2010), a direct activator of the ATR–ATRIP complex (Kumagai et al., 2006).
DNA-PKcs is recruited to DNA ends by Ku70–Ku80 and activated upon binding to DNA (Dvir et al., 1992; Gottlieb and Jackson, 1993). DNA-PKcs is a central component of the machinery that repairs DSBs by nonhomologous end joining (NHEJ; Smith and Jackson, 1999). DNA-PKcs has additional functions, notably in telomere maintenance and in the response to DNA replication stress (Smith and Jackson, 1999; Allen et al., 2011).
DNA-PKcs and ATR phosphorylate the 32-kD subunit of human RPA (RPA32) on multiple sites and these modifications promote DNA repair (Shao et al., 1999; Block et al., 2004; Sakasai et al., 2006; Anantha et al., 2007; Shi et al., 2010; Liaw et al., 2011). The underlying mechanism of functional cross talk between DNA-PKcs and ATR, however, remains elusive, and intriguing, as DNA-PKcs and ATR are recruited to and activated by distinct DNA structural elements, respectively, by DSBs and by RPA-covered ssDNA.
To gain insights into the mechanisms of replication checkpoint signaling, we designed a DNA substrate that contains dsDNA ends and a short ssDNA gap. In human cell-free extracts, linear gapped DNA (gDNA) promotes the assembly of a potent ATR signaling complex that includes DNA-PKcs, ATR, RPA, and TopBP1. We propose a novel mechanism for the cooperation of DNA-PKcs and ATR at collapsed replication forks.
Results and discussion
Induction of RPA and Chk1 phosphorylation in human cell-free extracts
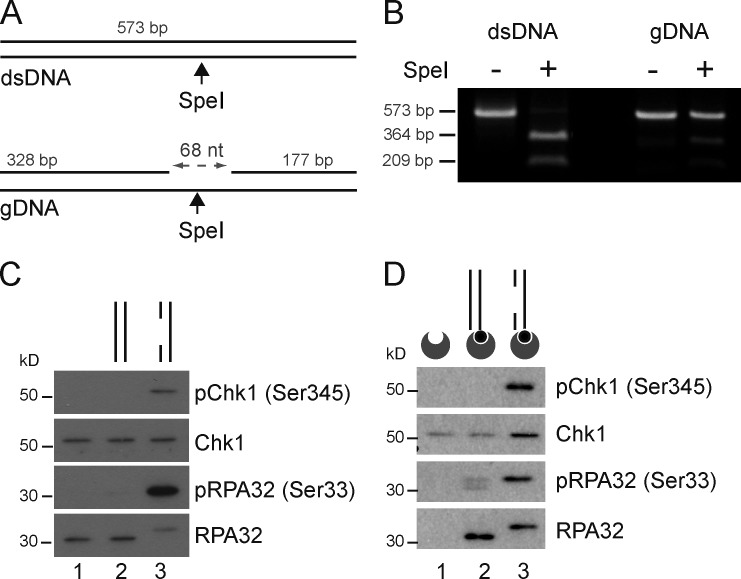
During DNA replication, oncogenes and chemotherapeutic agents induce the accumulation of ssDNA gaps in newly replicated DNA and four-way junctions at replication forks (Fig. S1; Lopes et al., 2006; Ray Chaudhuri et al., 2012; Neelsen et al., 2013). Whereas ssDNA gaps are fragile and prone to breaking (Lopes et al., 2006), overwhelming DNA replication stress or checkpoint defects can lead to the precocious processing of regressed forks by Mus81-Eme1 (Hanada et al., 2007; Neelsen et al., 2013; Szakal and Branzei, 2013). The collapse of these unusual replication intermediates is expected to yield DSBs that can activate DNA-PKcs and ATM in DNA molecules containing ssDNA gaps that can trigger ATR activation (Fig. S1). To study how DNA molecules that mimic broken replication intermediates are detected and signaled, we designed a linear duplex DNA molecule that contains one defined ssDNA gap (gDNA). The 573-bp DNA duplex was generated by PCR amplification of a DNA template (pG68) that comprises closely spaced recognition sites for a nicking endonuclease (Ralf et al., 2006). The nicks yield short oligonucleotides eliminated by heat denaturation. This treatment creates a 68-nt ssDNA gap in the DNA duplex and removes a SpeI restriction site (Fig. 1, A and B).
Figure 1.
DNA damage signal activation in human cell-free extracts. (A) The duplex DNA substrates are blunt ended and 573 bp long. The gDNA contains a 68-nt single-stranded gap. (B) The gDNA is refractory to digestion with SpeI. (C) gDNA-specific phosphorylation of RPA32 and Chk1. Nuclear extracts were incubated without DNA (lane 1), with duplex DNA (lane 2), or with gDNA (lane 3). The indicated proteins were analyzed by Western blotting. (D) Proteins bound to biotinylated DNA were pulled down with streptavidin-coated beads and detected by Western blotting. The dsDNA and gDNA substrates are represented schematically. Biotin (black circles) and streptavidin-coated beads (dented gray circles) are shown.
To monitor DNA damage signaling in vitro, we used human cell-free extracts and probed by Western blotting the phosphorylation of RPA32 at Ser33 (Olson et al., 2006) and of Chk1 at Ser345 (Guo et al., 2000; Liu et al., 2000). These phosphosignals were induced in cell-free extracts after incubation for 15 min in the presence of gDNA (Fig. 1 C). In contrast, markers of ATR activation were not visible when extracts were incubated without DNA or in the presence of dsDNA (Fig. 1 C). To verify that DNA damage signals emanated from the defined gDNA molecules, we labeled DNA substrates with biotin and then isolated biotin DNA substrates from the reaction mixtures using streptavidin-coated beads. Ser345 pChk1 and Ser33 pRPA32 were pulled down exclusively with gDNA (Fig. 1 D). RPA32 also bound the DNA duplex (Fig. 1 D), most likely as a result of helix destabilization and binding to ssDNA (Lao et al., 1999). Ser33 pRPA32, however, was detected principally in association with the gDNA substrate (Fig. 1 D).
Presumably, DNA damage signaling does not depend on DNA end resection because the DNA ends of duplex DNA and gDNA molecules would be equally susceptible to nucleases, and yet phosphorylation reactions were prompt and gDNA specific (Fig. 1). Consistent with this, we did not detect significant DNA degradation during a 1-h incubation in nuclear extracts (Fig. S2 A). In addition, linear DNA duplexes containing either a 68- or a 160-nt ssDNA gap equally induced Chk1 phosphorylation (Fig. S2 B), suggesting that nucleolytic enlargement of the ssDNA gap is not essential to trigger Chk1 activation. These data indicate that linear duplex DNA with a short ssDNA gap can promote the formation of a singularly active DNA damage signaling complex that phosphorylates endogenous protein substrates in human cell-free extracts.
Juxtaposed DNA gap and DNA ends induce robust phosphorylation of RPA and Chk1
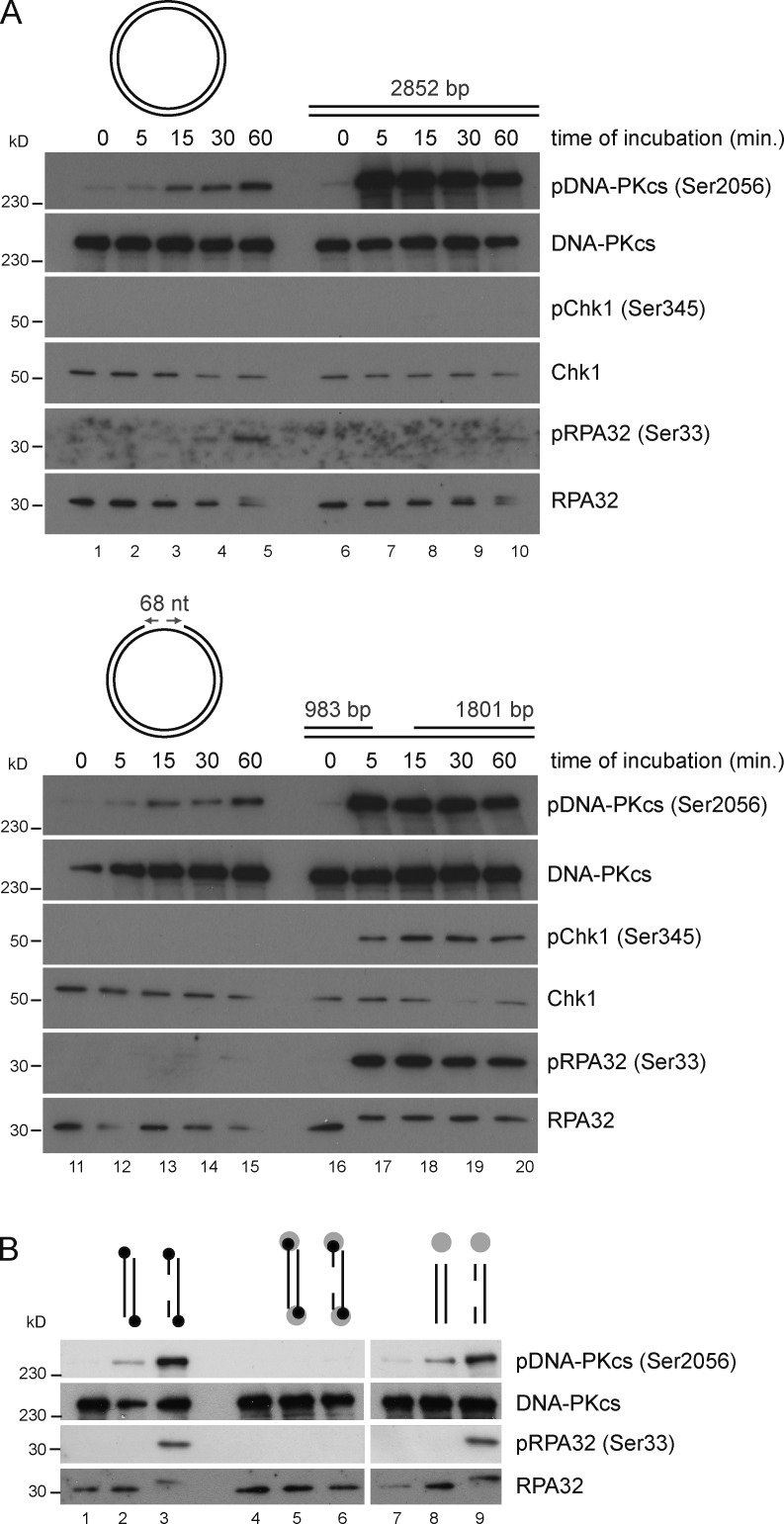
To dissect the structural determinants of DNA that activate DNA damage signaling, we supplemented the human nuclear extract with different forms of plasmid pG68 (Ralf et al., 2006). In addition to Ser33 pRPA32 and Ser345 pChk1, we probed the extracts for DNA-PKcs autophosphorylation on Ser2056, a marker of DNA-PKcs activation that occurs upon binding of DNA-PKcs to DNA ends. In an open circular form, the plasmid induced background phosphorylation of RPA32 on Ser33 and of DNA-PKcs on Ser2056, after 1 h of incubation in cell-free extracts (Fig. 2 A). As expected, linearized pG68 promptly triggered DNA-PKcs autophosphorylation on Ser2056, whereas Chk1 and RPA32 remained unmodified (Fig. 2 A). The gapped circular plasmid DNA did not induce phosphosignals above background levels (Fig. 2 A). In contrast, Ser345 pChk1 and Ser33 pRPA32 were detected already after a 5-min incubation with gapped linear duplex DNA (Fig. 2 A). In a reaction mixture containing a combination of linear duplex DNA and gapped circular DNA molecules, Chk1 phosphorylation was less efficient than in a reaction mixture supplemented with gapped linear duplex DNA (Fig. S2 C). In contrast, the induction of DNA-PKcs autophosphorylation was equivalent in both reactions (Fig. S2 C). Hence, the close juxtaposition of a dsDNA end and an ssDNA gap promotes DNA damage signaling. Although the distance between the DNA ends and the ssDNA gap was increased by more than fivefold in plasmid-based gDNA (Fig. 2 A) compared with PCR-based gDNA (Fig. 1 A), both DNA substrates were potent inducers of DNA damage signaling. This indicates that DNA damage signaling reactions are permissive to variations of the distance between the DNA ends and the ssDNA region.
Figure 2.
The juxtaposition of DNA ends and an ssDNA gap triggers activation of the DNA damage signal. (A) Nuclear extracts were incubated for the indicated time periods with open circular plasmid DNA (lanes 1–5), linear duplex DNA (lanes 6–10), gap circular DNA (lanes 11–15), or gapped linear duplex DNA (lanes 16–20). The indicated proteins were analyzed by Western blotting. (B) ATR activation depends on accessible DNA ends. (lanes 1–3) dsDNA and gDNA were biotinylated at both ends and incubated with nuclear extracts. (lanes 4–6) Biotinylated DNA substrates (5 nM) were preincubated with 30 nM streptavidin for 15 min at 37°C before addition of nuclear extracts. (lanes 7–9) Unmodified DNA substrates were preincubated with streptavidin before addition of nuclear extracts. The DNA substrates are represented schematically. Biotin (black circles) and streptavidin (gray circles) are shown.
To confirm that DNA ends were necessary to potentiate signals emanating from ssDNA, we labeled both 5′ ends of the PCR-amplified gDNA product with biotin. 5′-biotin labels did not impinge on gDNA-induced phosphorylation of DNA-PKcs and RPA32 (Fig. 2 B). When streptavidin was added to the reaction mixture, however, phosphorylation reactions induced by biotin-labeled gDNA were inhibited (Fig. 2 B), most likely as a consequence of steric hindrance caused by streptavidin–biotin complexes at DNA ends. Consistent with this, streptavidin had no impact on phosphorylation reactions induced by gDNA in the absence of 5′-biotin labels (Fig. 2 B). In conclusion, the juxtaposition of an ssDNA gap and DNA ends triggers the phosphorylation of RPA32 and Chk1 in human cell-free extracts.
Linear gDNA promotes the assembly of a potent ATR signaling complex
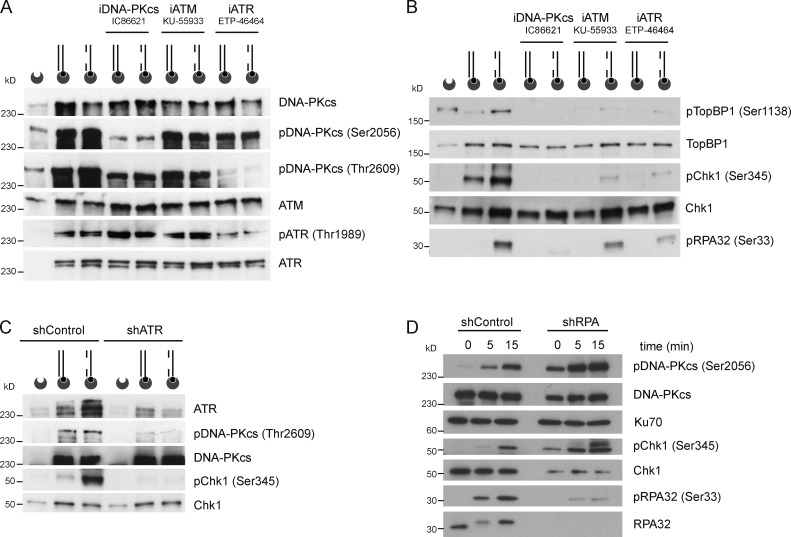
To characterize the composition of the DNA damage signaling complex and mimic single-ended DNA molecules that may arise at collapsed replication forks, we coupled the DNA fragments labeled with biotin at only one end with streptavidin-coated beads and then incubated the beads in nuclear extracts. The beads were isolated, and proteins associated with gDNA were analyzed by Western blotting. ATM, ATR, and DNA-PKcs were all pulled down with biotinylated DNA substrates (Fig. 3 A).
Figure 3.
Concerted activation of DNA damage signaling by DNA-PKcs, ATM, and ATR. (A) DNA-PKcs, ATM, and ATR bind to the biotinylated DNA substrates. DNA structures biotinylated at one DNA end were coupled to streptavidin-coated beads and then incubated with nuclear extracts for 10 min at 20°C, in the absence or presence of the DNA-PKcs inhibitor IC86621 (100 µM), the ATM inhibitor KU-55933 (10 µM), or the ATR inhibitor ETP-46464 (1 µM), as indicated. DNA-bound DNA-PKcs, ATM, and ATR were pulled down with streptavidin-coated beads, resolved by SDS-PAGE, and detected by Western blotting. (B) DNA-bound TopBP1 and Chk1 were isolated and detected as described in A. (C) Nuclear extracts prepared from cells treated with control shRNA or ATR shRNA were incubated with the indicated biotinylated DNA substrates, as described in A. ATR, DNA-PKcs, and Chk1 proteins were pulled down with streptavidin-coated beads, resolved by SDS-PAGE, and detected by Western blotting. (D) Nuclear extracts from cells treated with control shRNA or RPA shRNA were incubated for the indicated time periods with gDNA and probed for the indicated proteins by Western blotting. iDNA-PKcs, inhibitor of DNA-PKcs; iATM, inhibitor of ATM; iATR, inhibitor of ATR.
We then sought evidence of regulatory interplay between PIKKs using ATR-, ATM-, and DNA-PKcs–specific inhibitors. Two major clusters of phosphorylation sites in DNA-PKcs regulate the processing of DNA ends (Meek et al., 2008). DNA-PKcs autophosphorylation within the PQR cluster (Ser2056 cluster), protects DNA ends from nucleolytic resection while allowing access to components of the NHEJ machinery (Meek et al., 2008). In contrast, phosphorylation of DNA-PKcs within the ABCDE cluster (Thr2609 cluster) allows access to enzymes that trim DNA ends to promote homologous recombination (Meek et al., 2008). This modification can be mediated by ATR in response to UV-induced DNA replication stress (Yajima et al., 2006). The ATM-specific inhibitor KU-55933 had no major impact on the phosphorylation of DNA-PKcs and ATR (Fig. 3 A). In contrast, the DNA-PKcs inhibitor IC86621 blocked the phosphorylation of DNA-PKcs on Ser2056 and partially interfered with DNA-PKcs phosphorylation on Thr2609 (Fig. 3 A). Surprisingly, the ATR inhibitor ETP-46464 inhibited the phosphorylation of DNA-PKcs on Thr2609, specifically, along with phosphorylation of ATR on Thr1989 (Fig. 3 A). Thus, gDNA-induced signaling in cell-free extracts recapitulates a critical step in the regulation of DNA-PKcs by ATR. The phosphorylation of Xenopus laevis TopBP1 on Ser1131 augments the capacity of TopBP1 to activate the ATR–ATRIP complex (Yoo et al., 2009). TopBP1 was also retrieved with biotinylated DNA substrates (Fig. 3 B). TopBP1 associated indiscriminately with dsDNA and gDNA, but Ser1138 pTopBP1 was detected preferentially on the gDNA substrate (Fig. 3 B). Hence, gDNA-specific phosphorylation of TopBP1 may account for gDNA-induced ATR activation and Chk1 phosphorylation. Addition of any one of the three PIKKs inhibitors to the reaction mixtures interfered with gDNA-induced phosphorylation of TopBP1 on Ser1138 and of Chk1 on Ser345 (Fig. 3 B). Compared with reaction mixtures containing DNA-PKcs or ATR inhibitors, however, ATM inhibition had a lesser impact on RPA32 phosphorylation (Fig. 3 B). This suggests that ATR and DNA-PKcs can function synergistically to activate DNA damage signaling, in the presence of limited amount of ssDNA.
To verify the involvement of ATR in gDNA-induced phosphorylation events, we prepared nuclear extracts from HeLa cells treated either with a control shRNA or with an shRNA against ATR. Biotinylated DNA substrates coupled to streptavidin-coated beads were incubated with extracts from shRNA-treated cells, and then, protein–DNA complexes were purified and analyzed by Western blotting. Fig. 3 C shows that partial depletion of ATR was sufficient to completely abolish the phosphorylation of Chk1 on Ser345 and of DNA-PKcs on Thr2609.
Because the recruitment of ATR–ATRIP to ssDNA in vitro is stimulated by RPA (Zou and Elledge, 2003), we knocked down RPA to evaluate its role in gDNA-induced signaling (Fig. 3 D). The amount of total Chk1 protein was significantly reduced in extract prepared from RPA-depleted cells, most likely as a consequence of Chk1 activation and degradation in response to stress induced by RPA depletion. Chk1, however, was more readily phosphorylated in RPA-depleted nuclear extracts than in control nuclear extracts (Fig. 3 D). Thus, RPA is not essential for Chk1 phosphorylation in this experimental setting.
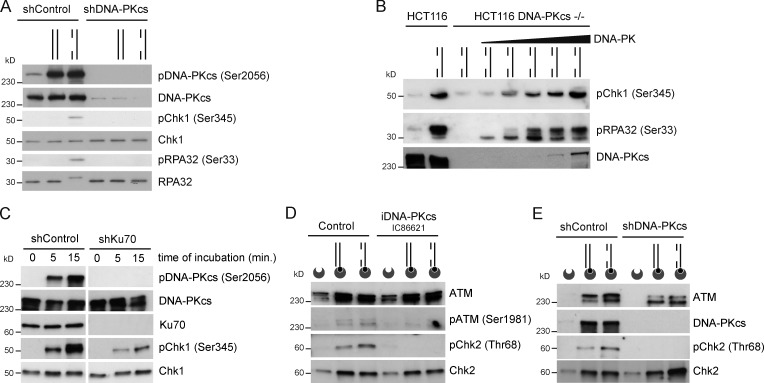
In response to DNA replication stress, DNA-PKcs and ATR phosphorylate RPA32 on multiple sites, and these modifications promote DNA repair (Shao et al., 1999; Block et al., 2004; Sakasai et al., 2006; Anantha et al., 2007; Shi et al., 2010; Liaw et al., 2011). To determine whether DNA-PKcs was necessary for gDNA-induced ATR signaling, we knocked down DNA-PKcs in HeLa cells and then prepared nuclear extracts from these cells. The knockdown of DNA-PKcs fully compromised the phosphorylation of RPA32 and Chk1 induced by linear gDNA (Fig. 4 A). Consistent with this, gDNA-induced Ser33 pRPA32 and Ser345 pChk1 signals were detected in HCT116 cell-free extracts, absent in extracts prepared from isogenic HCT116 homozygous knockout of DNA-PKcs, and detected again in extracts from HCT116 DNA-PKcs−/− cells complemented with recombinant DNA-PK (Fig. 4 B). In addition, ablation of Ku70 by shRNA significantly interfered with gDNA-induced phosphorylation of Chk1 (Fig. 4 C). These data indicate that DNA-PK is necessary to prime ATR activation upon assembly onto gDNA.
Figure 4.
DNA-PKcs is essential for DNA damage signal activation. (A) Nuclear extracts prepared from cells treated with control shRNA (shControl) or DNA-PKcs shRNA were incubated without DNA, with dsDNA, or with gDNA, and the indicated proteins were detected by Western blotting. (B) Nuclear extracts from HCT116 and HCT116 DNA-PKcs−/− cells were incubated with gDNA, and HCT116 DNA-PKcs−/− nuclear extracts were complemented with increasing amounts of DNA-PK purified from HeLa cells (0.025, 0.05, 0.1, 0.2, and 1 U/µl). (C) Nuclear extracts from cells treated with control shRNA or Ku70 shRNA were incubated for the indicated time periods with gDNA and probed for the indicated proteins by Western blotting. (D) Biotinylated DNA substrates were incubated with nuclear extracts in the absence or presence of IC86621 and pulled down, and the indicated DNA-bound proteins were analyzed by Western blotting. (E) Biotinylated DNA substrates were incubated with nuclear extracts from cells treated with control shRNA or ATR shRNA, isolated, and probed for the indicated proteins.
To further investigate the intricate links between PI3KKs, we monitored the activation of ATM/Chk2 signaling. Chemical inhibition of DNA-PKcs interfered with ATM activation and Chk2 phosphorylation without preventing ATM and Chk2 binding to DNA (Fig. 4 D). Consistent with this, we did not observe gDNA-induced Chk2 phosphorylation in nuclear extracts from DNA-PKcs–depleted cells (Fig. 4 E). These observations indicate that DNA-PKcs also connects with ATM signaling induced by gDNA in cell-free extracts.
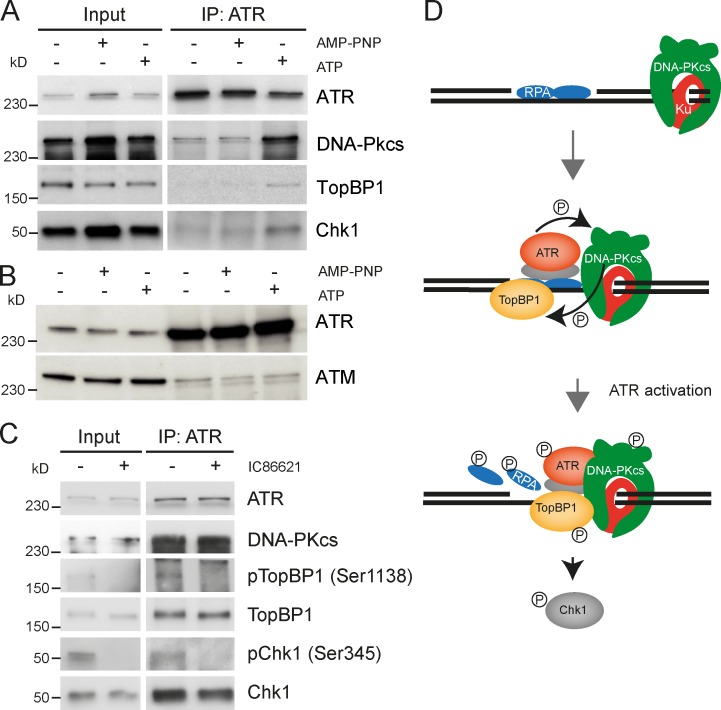
To verify that DNA-PKcs and ATR signaling proteins associate upon gDNA binding and activation, we immunoprecipitated endogenous ATR from reaction mixtures supplemented with gDNA. The pull-down of DNA-PKcs, TopBP1, and Chk1 with an anti-ATR antibody was strictly dependent on the presence of ATP (Fig. 5 A). DNA-PKcs, TopBP1, and Chk1 did not associate with ATR when ATP was omitted or replaced by the nonhydrolyzable analogue AMP-PNP (Fig. 5 A). This indicates that the assembly of the ATR signaling complex on gDNA is regulated by phosphorylation events. In contrast, we did not detect ATM in the ATR immune precipitate (Fig. 5 B), suggesting that ATM is not tightly associated with this ATR signaling complex. Ser1138 pTopBP1 and Ser345 pChk1 signals were noticeable in the ATR immune precipitate (Fig. 5 C) but not detected in extracts containing the DNA-PKcs inhibitor IC86621 (Fig. 5 C), consistent with the data presented in Fig. 3 B. In conclusion, the enzymatic activity of DNA-PKcs is required for TopBP1 phosphorylation and ATR activation but is dispensable for the congregation of DNA-PKcs, ATR, TopBP1, and Chk1 onto gDNA.
Figure 5.
ATP-dependent assembly of a DNA damage signaling complex. (A) Nuclear extracts were incubated for 10 min at 20°C with gDNA with or without 1 mM AMP-PNP or 1 mM ATP as indicated. Next, the reaction mixture was incubated overnight with an anti-ATR antibody, and ATR-associated proteins were pulled down with protein G–coupled magnetic beads, resolved by SDS-PAGE, and revealed by Western blotting with the indicated antibodies. (B) ATR immunoprecipitations were conducted as described in A and probed for ATM by Western blotting. (C) Reactions mixtures were assembled as described in A in the presence of 1 mM ATP, with or without 100 µM IC86621, as indicated. (D) Model for the concerted activation of DNA-PKcs and ATR. RPA binds to the ssDNA gap and promotes the recruitment of ATRIP–ATR. Ku binds the dsDNA end, may translocate up to dsDNA to ssDNA junction, and recruits DNA-PKcs. When amounts of RPA-covered ssDNA are limited, the concerted phosphorylation of RPA32 and TopBP1 by DNA-PKcs and ATR promotes signal amplification and assembly of a potent ATR signaling complex. IP, immunoprecipitation; P, phosphorylated.
To verify the contribution of DNA-PKcs to ATR activation in a cellular context, we subjected U2OS cells to a high dose of camptothecin (CPT) that induces replication-born DSBs (Shao et al., 1999). We detected reduced levels of Ser345 pChk1 in cells treated with shRNAs against DNA-PKcs (Fig. S3 A) or Ku70 (Fig. S3 B), as well as in cells treated with a small molecule inhibitor of DNA-PKcs (Fig. S3 C). These results indicate that DNA-PKcs contributes to sustain ATR signaling upon the collapse of replication intermediates.
Here, we have shown that DNA-PKcs and ATR can combine to form a potent DNA damage signaling complex that activates endogenous mediator and effector proteins in the ATR signal transduction pathway. A recent study has shown that the phosphorylation of RPA32 on Ser4 and Ser8 plays a significant role in ATR-mediated checkpoint activation and that the phosphorylation of RPA32 on multiple residues is amplified by a reciprocal priming effect (Liu et al., 2012). PI3KKs target a subset of amino acids, and each phosphorylated amino acid primes the phosphorylation of a subset of N-terminal residues (Liu et al., 2012). Consistent with this, our observation that inhibition of DNA-PKcs or ATR abolishes RPA32, TopBP1, and Chk1 phosphorylation is best explained by an amplification mechanism dependent on the synergistic action of DNA-PKcs and ATR. At replication-born DSBs, as in cell-free extracts, DNA-PKcs would amplify ATR signals in the presence of a limited amount of RPA-covered ssDNA, through priming of RPA32 and TopBP1 phosphorylation. Previous studies have shown that RPA is necessary to localize ATR–ATRIP at stalled forks but dispensable for Chk1 phosphorylation per se (Ball et al., 2005; Kim et al., 2005). Here, we observed that removal of RPA stimulated gDNA-induced Chk1 phosphorylation in cell-free extracts. These observations suggest that although RPA directs ATR–ATRIP to stalled forks, the ssDNA binding protein is not required for Chk1 phosphorylation per se and that the hyperphosphorylation of RPA bound to ssDNA may overcome a barrier to Chk1 activation.
Our data are reminiscent of the findings by Kumagai and Dunphy (2000) who showed that the DNA substrate poly(dA)70-poly(dT)70 can trigger the phosphorylation of xChk1 in X. laevis egg extracts. In this system, ATR activation occurs independently of RPA, through phosphorylation of TopBP1 by ATM (Kim et al., 2005; Yoo et al., 2007). It would be interesting to define the key molecular features of poly(dA)70-poly(dT)70 that promote xChk1 phosphorylation and to examine whether xDNA-PKcs is also necessary for xChk1 phosphorylation in X. laevis egg extracts.
Pioneering studies have demonstrated that the Ku70/80 heterodimer first binds DNA ends independently of their exact structure (Paillard and Strauss, 1991; Falzon et al., 1993) and then translocates along duplex DNA to form a beads-on-a-string structure (de Vries et al., 1989; Paillard and Strauss, 1991; Zhang and Yaneva, 1992). Ku70/80 also exhibits specific affinity for ssDNA to dsDNA transitions, including nicks and small ssDNA gaps (Blier et al., 1993; Falzon et al., 1993), and DNA-PKcs interacts directly with RPA (Shao et al., 1999). The biochemical properties of DNA-PK components suggest how DNA-PK may engage in ATR activation at replication-born DSBs. In the model presented in Fig. 5 C, we propose that Ku70/80 may first bind DNA ends, translocates up to internal nicks and ssDNA gaps, and then recruits DNA-PKcs, which phosphorylates RPA and primes ATR activation.
DNA-PKcs is extremely abundant. It represents 0.1–1% of soluble nuclear proteins in HeLa cells (Carter et al., 1990) and, therefore, is very likely to first bind replication-born DSBs. It is tempting to speculate that upon encountering DNA-bound RPA at broken replication intermediates, DNA-PKcs would be channeled to the ATR signaling pathway at the expense of NHEJ, thereby contributing to DNA repair pathway choice (Allen et al., 2011).
Materials and methods
Cells, proteins, and chemicals
U2OS and HeLa S3 cells were grown under standard conditions in DMEM (Invitrogen) supplemented with 10% FBS and 1% penicillin-streptomycin. HCT116 (cell line and genotype: HCT116 Hendrickson’s Parental; Horizon Discovery Ltd.) and HCT116 DNA-PKcs−/− cells (clone: w87; cell line and genotype: HCT116 DNA-PKcs−/−; Horizon Discovery Ltd.) were cultured in McCoy’s 5A modified medium (Sigma-Aldrich) supplemented with 10% FBS and 1% penicillin-streptomycin. DNA-PK purified from HeLa cells was purchased from Promega. Streptavidin and CPT were purchased from Sigma-Aldrich. KU-55933 was obtained from Tocris Bioscience. IC86621 was obtained from Merck. ETP-46464 was a gift from O. Fernandez-Capetillo (Spanish National Cancer Research Centre, Madrid, Spain; Toledo et al., 2011).
Antibodies
Primary antibodies were purchased from Abcam (DNA-PKcs and DNA-PKcs-S2056), Bethyl Laboratories, Inc. (ATM, ATR, TopBP1, RPA32-Ser33, and RPA32-Ser4/S8), EMD Millipore (RPA32 and Chk2), Cell Signaling Technology (Chk1-Ser345 and Chk2-Thr68), Rockland (ATM-Ser1981), Thermo Fisher Scientific (DNA-PKcs-Thr2609), and Santa Cruz Biotechnology, Inc. (Chk1). Antibodies against Ser1989 pATR (rabbit polyclonal antibody raised against the peptide NH2-Cys-FPENE(pT)PPEGK-COOH) and Ser1138 pTopBP1 (rabbit polyclonal antibody raised against the peptide NH2-Cys-LNTEP(pS)QNEQI-COOH) were gifts from L. Zou (Massachusetts General Hospital and Harvard Medical School, Boston, MA) and W.G. Dunphy (California Institute of Technology, Pasadena, CA), respectively. Secondary antibodies (anti–rabbit-HRP and anti–mouse-HRP) were obtained from Promega.
RNA interference
shRNA vectors were prepared by cloning dsDNA oligonucleotides into pSUPER-Puro (a gift from J. Lingner, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland) as previously described (Azzalin and Lingner, 2006). The 19-nt target sequences were as follows: for DNA-PKcs, 5′-GATCGCACCTTACTCTGTT-3′; for ATR, 5′-GGAGATTTCCTGAGCATGT-3′; for RPA70, 5′-CTGGTTGACGAAAGTGGTG-3′; and for KU70, 5′-GGAAGAGATAGTTTGATTT-3′. shRNA transfections were performed using Lipofectamine 2000 reagent (Invitrogen).
DNA substrates
Plasmid pG68 (Ralf et al., 2006) was generated by cloning an array of BbvCI restriction sites (insert A) into pUC18 using EcoRI and HindIII. Insert A is as follows: 5′-GAATTCGCCTCATCTCTGGCTTCCCGGATCCCAGGATGCGCAGGCAGCCGCCTAGGGTGACAGGCTCATGGATATGCTGAGGAATCGCTGAGGCGTAGCTGAGGACTAGTGCTGAGGGATCGCTGAGGTGTAGCTGAGGACGTGCTGAGGTGCGTCAGCTACTTGTGAACTCGAGAGGCTCAGTGAGTGAAGCTCCATGGCCTAAGGGCAGCAGACTAAGCTT-3′. Plasmid pG160 (Gari et al., 2008) was generated by cloning an array of BbvCI and BsmI restriction sites (insert B) into pUC18 using EcoRI and HindIII. Insert B is as follows: 5′-GAATTCGCTCCATCTCTGGCTTCCCGCTAGCCATTATGCGCAGGCAGCCGCCTAGGGTGACAGGCTCATGGATATGAATGCACTCGAGGAATGCACGGTAGAATGCAAAGAATGCAGGTTGAATGCATTAGAATGCCCCATGGGAATGCACAGAGAATGCAGTATCGAATGCAAATCGAATGCACGTACCTCAGCGATCCCTCAGCACTAGTCCTCAGCTGTACCTCAGCACGTCCTCAGCAAGCTT-3′.
Biotinylated duplex DNA was generated by PCR amplification of plasmid pG68 or of plasmid pG160 using primer 1 (5′-biotin-TGCGGCATCAGAGCAGATTG-3′) and primer 2 (5′-GCACCCCAGGCTTTACACTTTATG-3′). To produce gDNA molecules, the duplex amplified from pG68 (68-nt gap) was nicked with NbBbvC1 (New England Biolabs, Inc.), and the duplex amplified from pGAP160 (160-nt gap) was nicked with BsmI and NtBbvC1 (New England Biolabs, Inc.), resulting in the formation of short oligonucleotides (11–13 nt), which were melted by heat denaturation at 80°C and removed using a gel extraction spin column (QIAquick Gel Extraction Kit; QIAGEN) as previously described (Ralf et al., 2006). gDNA fragments were control digested with SpeI and purified by 0.8% agarose gel electrophoresis and electroelution using an Elutrap device (Schleicher & Schuell BioScience). Open circular pG68 was generated by treatment of supercoiled pG68 with topoisomerase I. pG68 was gapped with NbBbvC1 as described in this paragraph. Plasmid pG68 was linearized with ScaI.
Nuclear extracts
Nuclear extracts were prepared using Dignam’s method as previously described (Shiotani and Zou, 2009). HeLa S3 or HCT116 cells were grown to ≤80% confluence, collected by scrapping and centrifugation (200 g for 3 min at 4°C), and washed twice in PBS. The cell pellet was suspended in 5× packed cell volume of hypotonic buffer A (10 mM Hepes-KOH, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.5 mM DTT, and 0.5 mM PMSF) supplemented with a cocktail of protease inhibitors (cOmplete, EDTA free; Roche) and phosphatase inhibitors (Thermo Fisher Scientific) and incubated on ice for 5 min. Next, the cells were spun down at 500 g for 5 min, suspended in 2× packed cell volume of buffer A and lysed by dounce homogenization using a tight-fitting pestle. Nuclei were collected by centrifugation at 4,000 g for 5 min at 4°C and extracted in one nuclei pellet volume of buffer C (20 mM Hepes-KOH, pH 7.9, 600 mM KCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol, 0.5 mM DTT, and 0.5 mM PMSF) supplemented with cocktails of protease and phosphatase inhibitors, and mixed on a rotating wheel at 4°C for 30 min. Nuclear extracts (supernatants) were recovered by centrifugation (16,000 g for 15 min at 4°C) and dialyzed using Slide-A-Lyzer Dialysis Cassettes (3,500-D protein molecular weight cutoff; Thermo Fisher Scientific) against buffer D (20 mM Hepes-KOH, pH 7.9, 100 mM KCl, 0.2 mM EDTA, 20% glycerol, 0.5 mM DTT, and 0.5 mM PMSF). Dialyzed nuclear extracts were centrifuged (100,000 g for 30 min at 4°C) to eliminate residual precipitates. The protein concentration of the clear supernatant was determined using Bradford’s estimation method, and aliquots were snap frozen and stored at −80°C.
ATR activation assay
10 µl reaction mixtures contained 5 µg nuclear extracts, 5 nM of the indicated DNA substrates, 10 mM Hepes-KOH, pH 7.6, 50 mM KCl, 0.1 mM MgCl2, 1 mM PMSF, 0.5 mM DTT, 1 mM ATP, 10 µg/ml creatine kinase, and 5 mM phosphocreatine. AMP-PNP, used at 1 mM when indicated, was obtained from Roche. Phosphorylation reactions were conducted at 37°C for 15 min, unless otherwise indicated, and stopped with 10 µl of 2× protein sample buffer (125 mM Tris, pH 6.8, 2% SDS, 10% glycerol, 100 mM DTT, 2% β-mercaptoethanol, and 0.004% bromophenol blue) for 3 min at 37°C. For ATR pull-down experiments, reaction mixtures were supplemented with 1 µg anti-ATR antibody and incubated overnight on a rotated wheel at 4°C. Antibody–protein complexes were immunoprecipitated for 1 h at 4°C with Dynabeads coupled to protein G (Invitrogen), washed, and resuspended in 2× protein sample buffer for Western blot analysis.
DNA pull-down assay
Biotinylated DNA duplexes (500 ng) were attached to 5 µl streptavidin-coated magnetic beads (Bio-Adembeads; ADEMTECH) in binding and washing buffer (100 mM Tris, pH 7.5, and 1 M NaCl) and incubated for 15 min at room temperature. Then, the beads were washed with 10 mM Hepes, pH 7.6, 100 mM KOAc, and 0.1 mM MgOAc and resuspended in reaction buffer (10 mM Hepes, pH 7.6, 50 mM KOAc, 0.1 mM MgOAC, 1 mM PMSF, 0.5 mM DTT, 1 mM ATP, pH 7.0, 10 µg/ml creatine phosphate, 5 mM phosphocreatine, and 0.5 mg/ml BSA). 50 µl reaction mixtures containing 20 µg nuclear extracts and 500 ng DNA duplexes coupled to magnetic beads were incubated for 10 min at 37°C in reaction buffer.
Online supplemental material
Fig. S1 summarizes schematically how broken replication intermediates containing DNA ends and ssDNA gaps are generated under stressful conditions of DNA replication. Fig. S2 provides further information on the stability of DNA substrates in cell-free extracts and on key DNA structural determinants in ATR signaling. Fig. S3 shows that DNA-PKcs contributes to ATR signaling in U2OS cells exposed to the topoisomerase I poison CPT. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201304139/DC1.
Supplementary Material
Acknowledgments
We thank William G. Dunphy, Oscar Fernandez-Capetillo, and Joachim Lingner for sharing reagents, Lee Zou for reagents and for helpful comments, and Philippe Pasero, Kerstin Gari, and all of the laboratory members for critical reading of the manuscript.
This work was supported by the Fondation ARC pour la recherche sur le cancer, by Agence Nationale de la Recherche, and by the Swiss National Science Foundation.
Footnotes
Abbreviations used in this paper:
- ATM
- ataxia-telangiectasia mutated
- ATR
- ataxia-telangiectasia and RAD3 related
- CPT
- camptothecin
- DNA-PK
- DNA-dependent protein kinase
- DNA-PKcs
- DNA-PK catalytic subunit
- DSB
- double-strand break
- dsDNA
- double-stranded DNA
- gDNA
- gapped DNA
- NHEJ
- nonhomologous end joining
- PI3KK
- phosphatidylinositol-3-kinase–like protein kinase
- RPA
- replication protein A
- ssDNA
- single-stranded DNA
References
- Abraham R.T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177–2196 10.1101/gad.914401 [DOI] [PubMed] [Google Scholar]
- Allen C., Ashley A.K., Hromas R., Nickoloff J.A. 2011. More forks on the road to replication stress recovery. J. Mol. Cell Biol. 3:4–12 10.1093/jmcb/mjq049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantha R.W., Vassin V.M., Borowiec J.A. 2007. Sequential and synergistic modification of human RPA stimulates chromosomal DNA repair. J. Biol. Chem. 282:35910–35923 10.1074/jbc.M704645200 [DOI] [PubMed] [Google Scholar]
- Azzalin C.M., Lingner J. 2006. The human RNA surveillance factor UPF1 is required for S phase progression and genome stability. Curr. Biol. 16:433–439 10.1016/j.cub.2006.01.018 [DOI] [PubMed] [Google Scholar]
- Ball H.L., Myers J.S., Cortez D. 2005. ATRIP binding to replication protein A-single-stranded DNA promotes ATR-ATRIP localization but is dispensable for Chk1 phosphorylation. Mol. Biol. Cell. 16:2372–2381 10.1091/mbc.E04-11-1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkova J., Horejsí Z., Koed K., Krämer A., Tort F., Zieger K., Guldberg P., Sehested M., Nesland J.M., Lukas C., et al. 2005. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 434:864–870 10.1038/nature03482 [DOI] [PubMed] [Google Scholar]
- Blier P.R., Griffith A.J., Craft J., Hardin J.A. 1993. Binding of Ku protein to DNA. Measurement of affinity for ends and demonstration of binding to nicks. J. Biol. Chem. 268:7594–7601 [PubMed] [Google Scholar]
- Block W.D., Yu Y., Lees-Miller S.P. 2004. Phosphatidyl inositol 3-kinase-like serine/threonine protein kinases (PIKKs) are required for DNA damage-induced phosphorylation of the 32 kDa subunit of replication protein A at threonine 21. Nucleic Acids Res. 32:997–1005 10.1093/nar/gkh265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun T.S., Pacek M., Yee M.C., Walter J.C., Cimprich K.A. 2005. Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev. 19:1040–1052 10.1101/gad.1301205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T., Vancurová I., Sun I., Lou W., DeLeon S. 1990. A DNA-activated protein kinase from HeLa cell nuclei. Mol. Cell. Biol. 10:6460–6471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.H., Lindsey-Boltz L.A., Kemp M., Mason A.C., Wold M.S., Sancar A. 2010. Reconstitution of RPA-covered single-stranded DNA-activated ATR-Chk1 signaling. Proc. Natl. Acad. Sci. USA. 107:13660–13665 10.1073/pnas.1007856107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries E., van Driel W., Bergsma W.G., Arnberg A.C., van der Vliet P.C. 1989. HeLa nuclear protein recognizing DNA termini and translocating on DNA forming a regular DNA-multimeric protein complex. J. Mol. Biol. 208:65–78 10.1016/0022-2836(89)90088-0 [DOI] [PubMed] [Google Scholar]
- Dvir A., Peterson S.R., Knuth M.W., Lu H., Dynan W.S. 1992. Ku autoantigen is the regulatory component of a template-associated protein kinase that phosphorylates RNA polymerase II. Proc. Natl. Acad. Sci. USA. 89:11920–11924 10.1073/pnas.89.24.11920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzon M., Fewell J.W., Kuff E.L. 1993. EBP-80, a transcription factor closely resembling the human autoantigen Ku, recognizes single- to double-strand transitions in DNA. J. Biol. Chem. 268:10546–10552 [PubMed] [Google Scholar]
- Gari K., Décaillet C., Delannoy M., Wu L., Constantinou A. 2008. Remodeling of DNA replication structures by the branch point translocase FANCM. Proc. Natl. Acad. Sci. USA. 105:16107–16112 10.1073/pnas.0804777105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoulis V.G., Vassiliou L.V., Karakaidos P., Zacharatos P., Kotsinas A., Liloglou T., Venere M., Ditullio R.A., Jr, Kastrinakis N.G., Levy B., et al. 2005. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 434:907–913 10.1038/nature03485 [DOI] [PubMed] [Google Scholar]
- Gottlieb T.M., Jackson S.P. 1993. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 72:131–142 10.1016/0092-8674(93)90057-W [DOI] [PubMed] [Google Scholar]
- Guo Z., Kumagai A., Wang S.X., Dunphy W.G. 2000. Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev. 14:2745–2756 10.1101/gad.842500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K., Budzowska M., Davies S.L., van Drunen E., Onizawa H., Beverloo H.B., Maas A., Essers J., Hickson I.D., Kanaar R. 2007. The structure-specific endonuclease Mus81 contributes to replication restart by generating double-strand DNA breaks. Nat. Struct. Mol. Biol. 14:1096–1104 10.1038/nsmb1313 [DOI] [PubMed] [Google Scholar]
- Jackson S.P., Bartek J. 2009. The DNA-damage response in human biology and disease. Nature. 461:1071–1078 10.1038/nature08467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.M., Kumagai A., Lee J., Dunphy W.G. 2005. Phosphorylation of Chk1 by ATM- and Rad3-related (ATR) in Xenopus egg extracts requires binding of ATRIP to ATR but not the stable DNA-binding or coiled-coil domains of ATRIP. J. Biol. Chem. 280:38355–38364 10.1074/jbc.M508673200 [DOI] [PubMed] [Google Scholar]
- Kumagai A., Dunphy W.G. 2000. Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts. Mol. Cell. 6:839–849 10.1016/S1097-2765(05)00092-4 [DOI] [PubMed] [Google Scholar]
- Kumagai A., Lee J., Yoo H.Y., Dunphy W.G. 2006. TopBP1 activates the ATR-ATRIP complex. Cell. 124:943–955 10.1016/j.cell.2005.12.041 [DOI] [PubMed] [Google Scholar]
- Lao Y., Lee C.G., Wold M.S. 1999. Replication protein A interactions with DNA. 2. Characterization of double-stranded DNA-binding/helix-destabilization activities and the role of the zinc-finger domain in DNA interactions. Biochemistry. 38:3974–3984 10.1021/bi982371m [DOI] [PubMed] [Google Scholar]
- Liaw H., Lee D., Myung K. 2011. DNA-PK-dependent RPA2 hyperphosphorylation facilitates DNA repair and suppresses sister chromatid exchange. PLoS ONE. 6:e21424 10.1371/journal.pone.0021424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Guntuku S., Cui X.S., Matsuoka S., Cortez D., Tamai K., Luo G., Carattini-Rivera S., DeMayo F., Bradley A., et al. 2000. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 14:1448–1459 10.1101/gad.14.12.1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Opiyo S.O., Manthey K., Glanzer J.G., Ashley A.K., Amerin C., Troksa K., Shrivastav M., Nickoloff J.A., Oakley G.G. 2012. Distinct roles for DNA-PK, ATM and ATR in RPA phosphorylation and checkpoint activation in response to replication stress. Nucleic Acids Res. 40:10780–10794 10.1093/nar/gks849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes M., Foiani M., Sogo J.M. 2006. Multiple mechanisms control chromosome integrity after replication fork uncoupling and restart at irreparable UV lesions. Mol. Cell. 21:15–27 10.1016/j.molcel.2005.11.015 [DOI] [PubMed] [Google Scholar]
- MacDougall C.A., Byun T.S., Van C., Yee M.C., Cimprich K.A. 2007. The structural determinants of checkpoint activation. Genes Dev. 21:898–903 10.1101/gad.1522607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S., Ballif B.A., Smogorzewska A., McDonald E.R., III, Hurov K.E., Luo J., Bakalarski C.E., Zhao Z., Solimini N., Lerenthal Y., et al. 2007. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 316:1160–1166 10.1126/science.1140321 [DOI] [PubMed] [Google Scholar]
- Meek K., Dang V., Lees-Miller S.P. 2008. DNA-PK: the means to justify the ends? Adv. Immunol. 99:33–58 10.1016/S0065-2776(08)00602-0 [DOI] [PubMed] [Google Scholar]
- Neelsen K.J., Zanini I.M., Herrador R., Lopes M. 2013. Oncogenes induce genotoxic stress by mitotic processing of unusual replication intermediates. J. Cell Biol. 200:699–708 10.1083/jcb.201212058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E., Nievera C.J., Klimovich V., Fanning E., Wu X. 2006. RPA2 is a direct downstream target for ATR to regulate the S-phase checkpoint. J. Biol. Chem. 281:39517–39533 10.1074/jbc.M605121200 [DOI] [PubMed] [Google Scholar]
- Paillard S., Strauss F. 1991. Analysis of the mechanism of interaction of simian Ku protein with DNA. Nucleic Acids Res. 19:5619–5624 10.1093/nar/19.20.5619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralf C., Hickson I.D., Wu L. 2006. The Bloom’s syndrome helicase can promote the regression of a model replication fork. J. Biol. Chem. 281:22839–22846 10.1074/jbc.M604268200 [DOI] [PubMed] [Google Scholar]
- Ray Chaudhuri A., Hashimoto Y., Herrador R., Neelsen K.J., Fachinetti D., Bermejo R., Cocito A., Costanzo V., Lopes M. 2012. Topoisomerase I poisoning results in PARP-mediated replication fork reversal. Nat. Struct. Mol. Biol. 19:417–423 10.1038/nsmb.2258 [DOI] [PubMed] [Google Scholar]
- Sakasai R., Shinohe K., Ichijima Y., Okita N., Shibata A., Asahina K., Teraoka H. 2006. Differential involvement of phosphatidylinositol 3-kinase-related protein kinases in hyperphosphorylation of replication protein A2 in response to replication-mediated DNA double-strand breaks. Genes Cells. 11:237–246 10.1111/j.1365-2443.2006.00942.x [DOI] [PubMed] [Google Scholar]
- Shao R.G., Cao C.X., Zhang H., Kohn K.W., Wold M.S., Pommier Y. 1999. Replication-mediated DNA damage by camptothecin induces phosphorylation of RPA by DNA-dependent protein kinase and dissociates RPA:DNA-PK complexes. EMBO J. 18:1397–1406 10.1093/emboj/18.5.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W., Feng Z., Zhang J., Gonzalez-Suarez I., Vanderwaal R.P., Wu X., Powell S.N., Roti Roti J.L., Gonzalo S., Zhang J. 2010. The role of RPA2 phosphorylation in homologous recombination in response to replication arrest. Carcinogenesis. 31:994–1002 10.1093/carcin/bgq035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiotani B., Zou L. 2009. Single-stranded DNA orchestrates an ATM-to-ATR switch at DNA breaks. Mol. Cell. 33:547–558 10.1016/j.molcel.2009.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G.C., Jackson S.P. 1999. The DNA-dependent protein kinase. Genes Dev. 13:916–934 10.1101/gad.13.8.916 [DOI] [PubMed] [Google Scholar]
- Szakal B., Branzei D. 2013. Premature Cdk1/Cdc5/Mus81 pathway activation induces aberrant replication and deleterious crossover. EMBO J. 32:1155–1167 10.1038/emboj.2013.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo L.I., Murga M., Zur R., Soria R., Rodriguez A., Martinez S., Oyarzabal J., Pastor J., Bischoff J.R., Fernandez-Capetillo O. 2011. A cell-based screen identifies ATR inhibitors with synthetic lethal properties for cancer-associated mutations. Nat. Struct. Mol. Biol. 18:721–727 10.1038/nsmb.2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van C., Yan S., Michael W.M., Waga S., Cimprich K.A. 2010. Continued primer synthesis at stalled replication forks contributes to checkpoint activation. J. Cell Biol. 189:233–246 10.1083/jcb.200909105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima H., Lee K.J., Chen B.P. 2006. ATR-dependent phosphorylation of DNA-dependent protein kinase catalytic subunit in response to UV-induced replication stress. Mol. Cell. Biol. 26:7520–7528 10.1128/MCB.00048-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H.Y., Kumagai A., Shevchenko A., Shevchenko A., Dunphy W.G. 2007. Ataxia-telangiectasia mutated (ATM)-dependent activation of ATR occurs through phosphorylation of TopBP1 by ATM. J. Biol. Chem. 282:17501–17506 10.1074/jbc.M701770200 [DOI] [PubMed] [Google Scholar]
- Yoo H.Y., Kumagai A., Shevchenko A., Shevchenko A., Dunphy W.G. 2009. The Mre11-Rad50-Nbs1 complex mediates activation of TopBP1 by ATM. Mol. Biol. Cell. 20:2351–2360 10.1091/mbc.E08-12-1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.W., Yaneva M. 1992. On the mechanisms of Ku protein binding to DNA. Biochem. Biophys. Res. Commun. 186:574–579 10.1016/S0006-291X(05)80847-2 [DOI] [PubMed] [Google Scholar]
- Zou L., Elledge S.J. 2003. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 300:1542–1548 10.1126/science.1083430 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.