Abstract
More than 15 human genetic diseases have been associated with the expansion of trinucleotide DNA repeats, which may involve the formation of non-duplex DNA structures. The slipped-strand nucleation of duplex DNA within GC-rich trinucleotide repeats may result in the changes of repeat length; however, such a mechanism seems less likely for the AT-rich (GAA)n·(TTC)n repeats. Using two-dimensional agarose gels, chemical probing and atomic force microscopy, we characterized the formation of non-B-DNA structures in the Friedreich ataxia-associated (GAA)n·(TTC)n repeats from the FRDA gene that were cloned with flanking genomic sequences into plasmids. For the normal genomic repeat length (n = 9) our data are consistent with the formation of a very stable protonated intramolecular triplex (H-DNA). Its stability at pH 7.4 is likely due to the high proportion of the T·A·T triads which form within the repeats as well as in the immediately adjacent AT-rich sequences with a homopurine· homopyrimidine bias. At the long normal repeat length (n = 23), a family of H-DNAs of slightly different sizes has been detected. At the premutation repeat length (n = 42) and higher negative supercoiling, the formation of a single H-DNA structure becomes less favorable and the data are consistent with the formation of a bi-triplex structure.
INTRODUCTION
Friedreich ataxia, an autosomal recessive neurodegenerative disease, is the most common inherited ataxia. Its clinical manifestations include progressive gait and limb ataxia, dysarthria, lower limb areflexia, diminished vibration sense, muscle weakness of the legs and extensor plantar response (1). The gene responsible for Friedreich ataxia, FRDA, in chromosome 9q13–q21.1 contains seven exons and encodes a 210 amino acid protein, frataxin (1–3). Approximately 98% of Friedreich ataxia patients have an expanded (GAA)n·(TTC)n repeat in the first intron of the FRDA gene. Normal alleles have five to 33 repeats of uninterrupted (GAA)n·(TTC)n repeats, premutation alleles have 34–65 repeats, whereas Friedreich ataxia-associated alleles have 66–1700 repeats (3–7). The length of (GAA)n·(TTC)n repeats inversely correlates with the age of onset and directly correlates with the severity of the disease (4,8,9). Friedreich ataxia patients carrying expanded (GAA)n·(TTC)n repeats have low levels of mature frataxin transcript (3,10) and of frataxin (11).
Structural properties of the (GAA)n·(TTC)n repeats affect the stability of the repeat length as well as expression of the FRDA gene. On the one hand, aberrant structures in the repeats may lead to uncontrolled repeat expansion, possibly, during replication and/or recombination. On the other hand, the aberrant structures appear to influence gene expression. The structures are likely asymmetric because they differently alter transcription from the constituent strands: the synthesis of the GAA-rich transcript is impaired to a greater extent that the synthesis of the UUC transcript (12–14). The aberrant structures may potentially form and stably exist between the rounds of DNA replication and transcription. Intrinsic superhelical tension present in eukaryotic DNA (15–17) or a transient wave of supercoiling from a preceding round of transcription (18) may drive the formation of non-B-DNA structures. Aberrant structures may also form during DNA replication, repair, recombination, or possibly during transcription when the complementary strands temporarily separate to provide a template for the synthesis of nascent DNA or RNA.
The structural potential of (GAA)n·(TTC)n repeats may depend on their two different sequence symmetry elements. (i) As direct repeats they may form slipped-strand DNA structures. Misaligned nucleation of the duplex DNA within the GC-rich (CTG)n·(CAG)n or (CCG)n·(CGG)n repeats may lead to the formation of slipped-strand DNA structures (19), for example, during transcription. A slipped misalignment is feasible for the pure polymeric (GAA)n·(TTC)n repeats with the loop-outs possibly forming hairpins (20,21). However, such a slippage seems unlikely when the AT-rich (GAA)n· (TTC)n repeats re-anneal in the genomic context because the correct re-association of separated strands may easily start in the flanking sequences with a higher GC content. Annealing from flanking regions would lead to the re-association of strands into duplex DNA. (ii) In the (GAA)n·(TTC)n repeats, pyrimidine (Py) and purine (Pu) bases are segregated in complementary strands and the resultant homopyrimidine· homopurine (Py·Pu) sequence character is appropriate for the formation of triple-stranded DNA structures (22–24). Additionally, (GAA)n·(TTC)n lengths possess mirror repeat symmetry, which is necessary to form an intramolecular triplex/single strand structure (H-DNA). Early investigations of the pure (GAA)n·(TTC)n repeats (n = 9, 18) cloned in supercoiled plasmids showed the formation of H-DNA that was stable at acidic pH, where cytosines are hemi-protonated, and less stable at pH 7 (25,26). Additionally, 18 repeats formed more complex bi-triplex structures at acidic but not at neutral pH (26). Studies of longer (GAA)n·(TTC)n repeats (n = 38, 58) produced somewhat inconsistent results. Plasmid DNA showed supercoil relaxation at both pH 4.5 and 8.3, as expected for a local non-B-DNA formation; however, the chemical probing experiments confirmed the formation of non-B-DNA structures only at pH 4.5 (27). Long (GAA)n·(TTC)n repeats (n ≥ 75) cloned together with their flanking genomic sequences formed complicated ‘sticky DNA’ structures that were too large for a definitive chemical probe characterization either as bi-triplexes or as one long triplex (28,29). Additional uncertainty about the triplex structures in (GAA)n·(TTC)n repeats relates to the identity of the third strand. Some workers have proposed that it is the Pu DNA strand (28) or the Pu RNA strand (12,27) that binds to the duplex (GAA)n·(TTC)n repeats.
Here we analyzed the local structures formed by the (GAA)n·(TTC)n repeats that fall in the categories of short normal (n = 9), long normal (n = 23) and premutation length (n = 42) that were cloned with their flanking genomic sequences into plasmids. These repeat lengths are short enough for a detailed structural evaluation by chemical probes. We expected that the overall local structures might involve both the (GAA)n·(TTC)n repeats and the immediately adjacent AT-rich sequences that are quasi-mirror-repeated. At the normal genomic length of the repeats (n = 9), a very stable protonated Py·Pu·Py intramolecular triplex (H-DNA) forms at pH 7.4. Formation and stability of this structure are likely due to the high proportion of T·A·T triads which form within the repeats as well as in the immediately adjacent AT-rich flanks. At the long normal repeat length (n = 23), a family of H-DNAs of slightly different sizes has been detected. At the premutation repeat length (n = 42) and higher negative supercoiling, the formation of a single H-DNA structure becomes less favorable and the data are consistent with the formation of a bi-triplex structure.
MATERIALS AND METHODS
Plasmids
Plasmids pGAA9, pGAA23 and pGAA42 contain respectively nine, 23 and 42 (GAA)·(TTC) repeats, flanked by 67 and 155 bp of the FRDA gene (Table 1). To obtain these plasmids, genomic DNA sequences originally cloned in pCR3.1 (12) were PCR amplified using a pair of primers containing EcoRI and BamHI sequences, and then recloned between the EcoRI and BamHI sites of pUC8. Subcloned inserts contained repeated sequences of correct lengths as can be seen in Figures 3–5. A control plasmid pGAA9NF (no flanks) contains nine (GAA)·(TTC) repeats cloned between the EcoRI and HindIII sites of pUC8. All plasmids were purified by CsCl/ethidium bromide gradient procedures (30).
Table 1. Sequences of the top strands containing (GAA)n repeats and immediate AT-rich flanks.
| Name | Sequence |
|---|---|
| pGAA9a |
TAAAAAATAC(A)15(GAA)9AATAAAGAAAAGTTA |
| pGAA23a |
TAAAAAATAC(A)15(GAA)23AATAAAGAAAAGTTA |
| pGAA42a |
TAAAAAATAC(A)15(GAA)32GAAA(GAA)2GAG(GAA)6AATAAAGAAAAGTTA |
| pGAA9NFb | (GAA)9 |
aThe EcoRI–BamHI DNA fragments containing 42 and 140 bp of non-repeating human genomic DNA left and right of the repeats, respectively, in addition to the indicated sequences, were cloned in the pUC8 polylinker.
bThe EcoRI–HindIII fragment containing the pure (GAA)9·(TTC)9 repeat was cloned in the pUC8 polylinker.
Figure 3.
H-DNA formation in (GAA)9·(TTC)9 repeats and adjacent sequences mapped by chemical probe analysis. In (A) and (B), DNA sequence lanes are indicated by T, C, G and A. Lanes 0, 4 and 8 correspond to the individual DNA topoisomer population with lane 0 being relaxed DNA, and lanes 4 and 8 containing topoisomers with an average σ = –0.046 and σ = –0.088, respectively. DNA topoisomers were incubated with CAA, KMnO4 and DMS and the modified sites detected by primer extension, as described in Materials and Methods. Bands of radioactivity denote sites of chemical modification that block further DNA polymerization. (A) Chemical modifications in the strand containing (TCC)9 repeats. (B) Chemical modifications in the strand containing (GAA)9 repeats. (C) Summary of chemical reactions and the H-DNA structure consistent with detected modifications.
Figure 5.
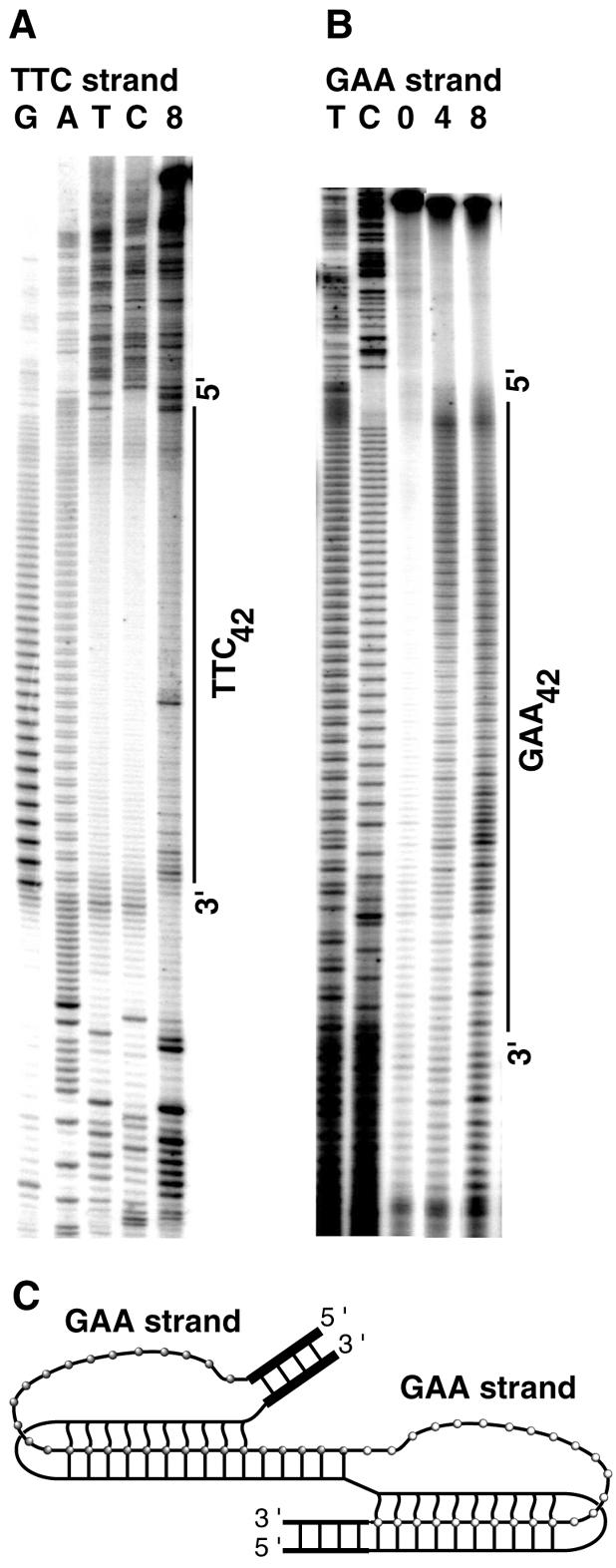
H-DNA formation in (GAA)42·(TTC)42 repeats and adjacent sequences mapped by chemical probe analysis. Lanes 0, 4 and 8 correspond to the individual DNA topoisomer population with lane 0 being relaxed DNA, and lanes 4 and 8 containing topoisomers with an average σ = –0.041 and σ = –0.06, respectively. (A) CAA modifications in the strand containing (TTC)42 repeats. (B) CAA modifications in the strand containing (GAA)42 repeats. (C) At intermediate superhelicity, the 5′ end of the GAA strand in H-DNA is unpaired, whereas at higher superhelicity, the 3′ end of the GAA strand also becomes unpaired, thereby forming a bi-triplex structure.
Each of eight fractions of differently supercoiled DNA (topoisomers) were prepared by incubating 5 µg of plasmid DNA in the presence of appropriate concentrations of ethidium bromide (0–13 µM) with 8 µl of topoisomerase I-containing nuclear extract from HeLa cells in 100 µl of reaction buffer (100 mM NaCl, 10 mM Tris–HCl, 1 mM EDTA, pH 7.6) (31). Average superhelical densities of the topoisomer fractions were calculated as σ = –10.5τ / N, where N is the number of base pairs in the plasmid and τ is the number of negative superhelical turns determined by a band counting method after topoisomer separation in an agarose gel in the presence of chloroquine (31). After removal of ethidium bromide and proteins by two phenol and two chloroform extractions, DNA was ethanol precipitated and dissolved in 10 mM Tris (pH 7.5), 1 mM EDTA. This created topoisomers with superhelical densities from σ = 0 to σ = –0.09.
Two-dimensional agarose gel electrophoresis
A mixture of topoisomers (4 µg) with a broad distribution of superhelical densities was pre-incubated at 37°C in 10 µl of TAE buffer (40 mM Tris-acetate, 5 mM sodium acetate, 1 mM EDTA, pH 8.3) for 1 h and, after addition of 5 µl of dye mixture, the sample was loaded onto a 1.5% agarose gel (15 × 15 cm). Two-dimensional gel electrophoresis was performed at 4 V/cm using TAE buffer for the first dimension and the same buffer containing 3 µg/ml chloroquine for the second dimension. The gel was run at 37°C in a temperature-controlled incubator for the first dimension, and at room temperature for the second dimension. After electrophoresis in the second dimension, the gel was extensively washed to remove the chloroquine, stained with ethidium bromide and photographed.
Chemical probe analysis
Chemical probe analysis under the conditions of one hit per molecule, was performed as previously described (31,32). Unpaired regions in supercoiled plasmids (0.25 µg of DNA) in 40 µl of 0.5× TEN buffer (5 mM Tris–HCl, 25 mM NaCl, 0.5 mM EDTA, pH 7.6) or 0.5× TEN buffer appended with 2 mM MgCl2 were probed with 0.5% chloroacetaldehyde (CAA) for 8 min and 1 mM KMnO4 for 5 min at 37°C. Protection of guanines from dimethylsulfate (DMS) reaction upon triplex formation was probed with 0.1% DMS for 10 min. CAA reactions were terminated by diethyl ether extraction, while KMnO4 and DMS reactions were stopped by addition of β-mercaptoethanol to 400 mM. After ethanol precipitation, 0.1 µg of DNA was used to reveal chemically susceptible bases by the radiolabeled primer extension technique using a Stoffel fragment of Taq polymerase that terminates synthesis at the chemically modified sites. Primer extension products were separated on a 7% sequencing gel alongside a DNA sequence pattern obtained by sequencing unmodified DNA with the same primers and a mixture of KlenTaq1 and Pfu polymerases as described (33). The gel was exposed to a PhosphoImager plate for further analysis of reactive sites by the ImageQuant software (Molecular Dynamics).
AFM imaging
The AFM images were taken with a MultiMode SPM instrument equipped with D-scanner (Digital Instruments, Santa Barbara, CA) operating in a tapping mode as described elsewhere (34). NanoProbe TESP (Digital Instruments) and OTESPA probes (K-TEK International, Portland, OR) were used for imaging in air. The typical tapping frequency was 240–280 kHz for TESP tips and 340–380 kHz for the K-TEK probes, and the nominal scanning rate was 2–3 Hz. DNA samples were deposited from 10 mM HEPES, 1 mM EDTA, pH 7.5, at 20°C.
RESULTS
To study the formation of non-B-DNA structures by the Friedreich ataxia-associated (GAA)n·(TTC)n repeats within their genomic context, we cloned fragments of the FRDA gene containing nine, 23 and 42 (GAA)·(TTC) repeats flanked by 67 and 155 bp of human genomic sequences 5′ and 3′ of the (GAA)n repeat, respectively (Table 1). The sequences immediately flanking the repeats are quasi homopyrimidine· homopurine in nature and have almost perfect mirror symmetry; therefore, they enlarge the region capable of intramolecular triplex formation. Figure 1 shows two- dimensional gels of plasmids containing nine, 23 and 42 repeats separated in TAE buffer, pH 8.3. In all cases, the retardation in the first direction mobility of topoisomers with more than 12 supercoils indicates that local non-duplex structures formed in supercoiled plasmids. The structural transition in plasmid pGAA9 (Fig. 1A) relaxed about three to four supercoils, consistent with a structural rearrangement that may involve unwinding of three to four turns of the double helix. In plasmids pGAA23 and pGAA42 increasing superhelical tension induced gradual unwinding of a longer DNA region that presumably rearranges into a non-B-DNA structure (Fig. 1B and C). This is consistent with the initial formation of a rather small non-B-DNA structure followed by its rearrangement into a larger, perhaps different, structure. The non-B-DNA structures formed may involve the entire repeat tracts. An estimated relaxation of nine supercoils can be seen in the gel for pGAA23, corresponding to an unwound region of ∼94 bp. Since the entire repeated sequence is only 69 bp, it is likely that an unwound region also includes a part of the adjacent AT-rich flanking sequences, similar to the previous observation for (ATTCT)n ·(AGAAT)n repeats flanked by AT-rich sequences (35). In the case of pGAA42 a maximum of approximately 11 supercoils were relaxed, corresponding to unwinding ∼115 bp, which is close to the length of the entire repeated sequence (127 bp).
Figure 1.
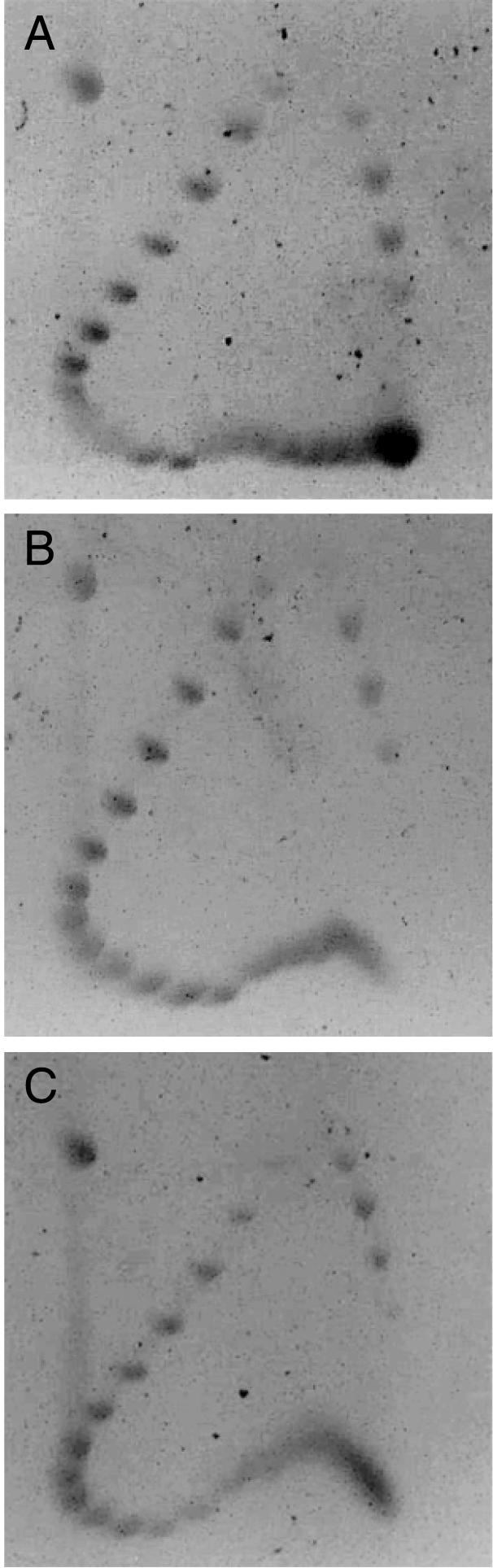
The formation of non-B-DNA in (GAA)n·(TTC)n repeats results in supercoil relaxation in the plasmids. Two-dimensional agarose gel analysis of topoisomer migration for plasmids (A) pGAA9, (B) pGAA23 and (C) pGAA42. Mixtures of DNA topoisomers with different amounts of supercoils corresponding to superhelical densities from σ = 0 to σ = –0.09 were electrophoresed from top to bottom in the first dimension and from left to right in the second dimension.
Atomic force microscopy images of repeat-containing plasmids show that many molecules contain thick protrusions at positions where the DNA duplex makes a sharp U-turn. The visible protrusion size increases with the length of cloned (GAA)n·(TTC)n repeats. Figure 2 presents several images of plasmids containing nine and 42 repeats in which the protrusions have been unambiguously identified. The appearance of the observed protrusions is similar to those seen previously for DNA molecules with a 46 bp mirror-repeated Py·Pu sequence that only had an option to locally unwind and form an H-DNA structure (36).
Figure 2.
Detection of non-B-DNA structures by atomic force microscopy. (A) Representative tapping mode AFM images of supercoiled pGAA9 (σ = – 0.046, left and σ = – 0.067, right). (B) Representative tapping mode AFM images of supercoiled pGAA42 (σ = –0.06). Arrows point to thick protrusions similar in appearance to earlier identified H-DNA structures (35).
To confirm H-DNA formation in the (GAA)n·(TTC)n repeat-containing plasmids and to identify the structural details, we used the chemical probe analysis. CAA is reactive with unpaired adenines and cytosines, potassium permanganate (KMnO4) is reactive with unpaired thymines, and the absence of DMS reactivity with guanines indicates that their N7 positions are involved in hydrogen bonding, as in triplex DNA or the protein–DNA complex. Figure 3 shows chemical modification patterns for nucleobases in the strands containing (TTC)9 (A) and (GAA)9 (B) at pH 7.4. As shown in Figure 3A, upon increasing superhelical tension in the plasmid, cytosines in the middle of the Py-rich strand, mostly the central one, and adenines in the AT-rich 3′ flank (bottom of the gel) become unpaired and reactive with CAA. That the bases in the AT-rich flank unpair is supported by the reactivity of accessible thymines with KMnO4. In the Pu-rich strand, at increasing superhelical tension, adenines in the 5′ half of the (GAA)9 region become accessible to CAA (Fig. 3B). Consistent with the transition superhelical density of σ = –0.046 (13 supercoils), determined from two-dimensional gel analysis, CAA reactivity indicates initial plasmid unwinding in the topoisomer fraction of an average superhelical density of σ = –0.047. At high superhelical tension, DNA unwinding extends into the flanking sequence, as deduced from the CAA reactivity of adenines and the KMnO4 reactivity of thymines 5′ of the (GAA)9 stretch. At the same time, guanines in the 3′ half of the (GAA)9 repeats become partially protected from reaction with DMS. Figure 3C shows a Py·Pu·Py intramolecular triplex that is consistent with the described pattern of chemical reactivity. Several important features of this structure are evident: (i) it is formed by folding the (TTC)9 strand in the middle; (ii) it involves four protonated C·G·C+ triads that are stable at neutral pH, which is unusual as cytosine normally requires acidic conditions for protonation; (iii) there are seven T·A·T triads that extend the intramolecular triplex past the end of the (GAA)9·(TTC)9 repeat; (iv) the triplex is very AT-rich and contains 76.5% T·A·T triads. It is very likely that the high proportion of the pH-independent T·A·T triads and the seven T·A·T triads at the triplex end make the partially protonated triple-stranded structure very stable at pH 7.4. Chemical probing of plasmid pGAA9NF which contains only (GAA)9·(TTC)9 without additional triad- forming nucleobases in the flanks supports this suggestion. Compared with pGAA9, unwinding of pGAA9NF at pH 7.4 requires much higher supercoiling: CAA reactivity was only detected in the topoisomer fractions of average superhelical densities σ ≥ –0.09 (data not shown).
Figure 4 presents the CAA and KMnO4 reactivity of nucleobases in the Py-rich and Pu-rich strands of the plasmid containing (GAA)23·(TTC)23 repeats. In the (TTC)23 sequence, the CAA reactivity is observed at the eleventh cytosine, thus indicating a predominant site of an alternative folding of the Py-rich strand (Fig. 4A). At this repeat length, the 5′ half of the (GAA)23 region is predominantly modified with CAA (Fig. 4B); however, there is no clear-cut boundary of the modified region as typically observed for a defined triplex structure (Fig. 3B) (31,32). The reactivity pattern of Figure 4B is consistent with a set of multiple similarly folded structures. The maximum CAA reactivity in the (GAA)23 sequence is observed at about the ninth to tenth repeats from the 5′ end, which presumably reflects the most abundant folded conformation. A predominant boundary of an unpaired strand in H-DNAs of slightly different sizes would be expected given a variable folding pattern. The triplex structure may extend from the repeats into the flanking AT-rich sequences where several thymines in both strands are reactive with KMnO4 (Fig. 4A and B). Thus, in a family of similar structures formed in plasmid pGAA23, the H-DNA with about 10 GAA trinucleotides involved in the formation of the triple-stranded region is the predominant one.
Figure 4.

H-DNA formation in (GAA)23·(TTC)23 repeats and adjacent sequences mapped by chemical probe analysis. Lanes 0, 4 and 8 correspond to the individual DNA topoisomer population, with lane 0 being relaxed DNA, and lanes 4 and 8 containing topoisomers with an average σ = –0.038 and σ = –0.07, respectively. DNA topoisomers were incubated with CAA and KMnO4. (A) Chemical modifications in the strand containing (TTC)23 repeats. (B) Chemical modifications in the strand containing (GAA)23 repeats.
DNA unwinding and alternative folding within the (GAA)42·(TTC)42 repeats at intermediate superhelical tension are similar to those for (GAA)23·(TTC)23 repeats (Fig. 5A and B). The predominant H-DNA folding involves about 12 GAA trinucleotides in the formation of the triple-stranded region (Fig. 4A, lane 8 and B, lane 4). However, at higher superhelical tension the distribution of CAA-reactive sites in the (GAA)42 strand starts to show a bi-modal character (Fig. 4B, lane 8). In addition to the major single-stranded region at the 5′ region of the (GAA)42 sequence, which is slightly shifted downward, a second CAA-reactive region appears toward the 3′ end of the (GAA)42 sequence. The maximum CAA reactivity maps to the eighth to ninth trinucleotides from the end (Fig. 4B). One model that may explain the bi-modal distribution of CAA reactivity in the (GAA)42 sequence is a bi-triplex presented in Figure 5C.
The chemical probing data presented in Figures 3–5 indicate the formation in TEN buffer of an H-DNA with the Py third strand. One might expect that in the presence of divalent metal cations the H-DNA would isomerize in the H′-DNA with the Pu third strand (37,38). However, at 2 mM MgCl2 the observed patterns of CAA reactivity for nine, 23 and 42 (GAA)·(TTC) repeats flanked by human genomic sequences remained the same as without MgCl2 (data not shown).
DISCUSSION
(GAA)n·(TTC)n repeat length-dependent structure formation at neutral pH
Our results show that under superhelical tension short (GAA)n·(TTC)n repeats form protonated H-DNA which exists at neutral pH. This structure may involve the entire repeated sequence and the quasi mirror-repeated AT-rich flanks. Compared with other mirror-repeated Py·Pu sequences that normally form H-DNA at pH 4.0–5.5, which are close to the pKa of cytosine (pKa = 4.3), the formation of protonated H-DNA in the short (GAA)n·(TTC)n repeats may occur at higher pH (pH 6.0–6.5) (25,26). H-DNA in the Friedreich ataxia (GAA)n·(TTC)n repeats and their genomic flanking sequences is even more stable and exists at pH 7.4. It does not require stabilization beyond an appropriate pH range by the usual stabilizers, magnesium or polyamine cations (31,39,40). H-DNA stability in the Friedreich ataxia (GAA)n·(TTC)n repeats and their genomic flanking sequences is probably due to the high proportion of the pH-independent T·A·T triads, especially the seven T·A·T triad closure at the end. Consistent with this suggestion, H-DNA in (GAA)9·(TTC)9 without additional T·A·T triads requires stabilization by a much higher superhelicity than in (GAA)9·(TTC)9 in the human genomic context. Upon increasing the repeat length, H-DNA in pGAA23 forms closer to one side of the repeats, however, it also involves an adjacent AT-rich flank, which increases the proportion of neutral T·A·T triads and likely stabilizes this H-DNA. Another factor that may account for H-DNA stability at neutral pH is a rather long length of triple-stranded structure, in accord with the previously described trend (41–43). When the Py·Pu sequence becomes sufficiently long, in our case 42 repeats plus the adjacent Py·Pu tract, the formation of a bi-triplex structure becomes possible. This also agrees well with the previously reported preference of long Py·Pu tracts to form two shorter triplexes instead of one long structure (26,40,44). This may be related to accumulation of minor sterical inconsistencies in fitting the third strand into the major groove of an acceptor duplex, which eventually results in destabilization of a long triplex and its rearrangement into two shorter ones (23). In addition to the supercoil-driven triplex formation described above, a stable protonated Py·Pu·Py triplex has been reported for linear (GAA)n·(TTC)n repeats (45,46). For example, during extension of the GAA primer by DNA polymerase on a single-stranded TTC template, half of the (TTC)n tract folds and binds as a third strand to the nascent duplex. This is similar to other Py·Pu sequences where either the Py or Pu template folded to form the triplex during DNA polymerization (47,48).
Several variants of the neutral triplex structures in the (GAA)n·(TTC)n repeats have been proposed in which the Pu DNA or a nascent Pu RNA serve as third strands (12–14,27–29). An all-DNA intermolecular triplex with the GAA third strand may stably exist at temperatures, pH and divalent salt concentrations close to the physiological ones (14,24). Such a triplex potentially forms during transcription and impairs the synthesis of the GAA transcript (14). The transcription-driven triplex formation with a Pu RNA third strand (12,13,27) may warrant further investigation since previous attempts to find the stability conditions for such triplexes were unsuccessful (49). Structural models for long (GAA)n·(TTC)n repeats (n > 75) may need additional experimental support. For example, two different ‘sticky DNA’ structures have been proposed for long (GAA)n·(TTC)n repeats in supercoiled DNA: either a bi-triplex or one long triplex of the Py·Pu·Pu type (28,29). For shorter repeat lengths (n = 9, 23, 42) amenable to structural analysis at a nucleotide resolution, we were only able to observe intramolecular triplexes of the Py·Pu·Py type (H-DNA). Even Mg2+ addition could not induce isomerization of the structure into the Py·Pu·Pu type (H′-DNA). The propensity of (GAA)n·(TTC)n repeats in supercoiled DNA to form H-DNA may be related to the high AT-content of the repeats. Previously, the (A)n·(T)n sequences were shown to form only H-DNA but not H′-DNA (50,51). The (AG)n·(TC)n repeats were capable of forming the H′-DNA but only in the presence of strong triplex stabilizers such as transition metal cations that may not be physiologically available (40,52). Magnesium cations at cellular abundance could stabilize H′-DNA only in the (G)n·(C)n sequences or when most of the adenines in the Py·Pu sequences were replaced with thymines (37,38).
Biological relevance of non-B-DNA structures within the (GAA)n·(TTC)n repeats
Several models of triplex formation (14,28,29,45 and this work) together with the proposal of hairpin formation (21) demonstrate the structural versatility of the (GAA)n·(TTC)n repeats. Different aberrant structures in the (GAA)n·(TTC)n repeats may form during several different biological functions. Transcription through the repeats apparently results in the formation of inappropriate DNA or DNA/RNA structures that result in the decreased amounts of nascent RNA strands (12–14). Several relevant models of transcription-dependent triplex formation have been proposed. One of them suggests the formation of H-DNA by wrapping part of the GAA tract, which became single-stranded during transcription, as the third strand, whereas the unwound part of the TTC template and nascent RNA separate and remain single-stranded (13,14). A variant of this structure includes the preservation of the double-stranded hybrid between the TTC template and nascent RNA formed during transcription (13). One more structure presumably forms between the double-stranded (GAA)n·(TTC)n repeats and a nascent GAA RNA strand (12,13). All of these structures may trap RNA polymerase between the duplex and triplex DNA behind and in front of the moving enzyme, respectively, thereby inhibiting RNA synthesis and the subsequent synthesis of the protein frataxin. Such an inhibitory effect of the (GAA)n·(TTC)n repeats is greater at longer repeat lengths.
Before the repeats may elicit their deleterious effect on gene expression, they need to expand long enough to form sufficiently stable aberrant structures. That is where the structural effects in the repeats during replication become relevant. From our data and the results of Gacy et al. (45) one may suggest at least two possible mechanisms. One is the DNA polymerase blockage by a pre-existing H-DNA that may easily form, according to our results. An immediate effect can be a stalled replication (38). Upon the DNA polymerase dissociation from a stalled replication fork and its possible rebinding in an attempt to overcome an obstacle (53), the nascent DNA strand may slip on a repeating template and form a loop of several extra trinucleotides. If the rebound polymerase successfully continues DNA replication, then several extra trinucleotides are included in the replicated strand which results in the expansion of the (GAA)n·(TTC)n repeats in the progeny cells. In the worst-case scenario, such DNA polymerase dissociation/binding may repeat, thereby leading to the so-called reiterative DNA synthesis and larger expansions (54). It is interesting to note that at the premutation repeat length associated with the propensity to hyperexpand the structure formed is a bi-triplex, which may be a stronger replication block than a simple H-DNA. Somewhat similarly, DNA polymerase may be blocked by template folding (45). It is possible that DNA polymerase dissociation/rebinding may be accompanied by looping-out of the slipped nascent strand and, eventually, by an addition of extra trinucleotides in the replicated DNA (45).
Acknowledgments
ACKNOWLEDGEMENTS
This work was partially supported by the NIH grants ES05508 (R.R.S.), NS41547 (T.A.), GM62235 (Y.L.L.), and grants from Muscular Dystrophy Association and American Heart Association (S.I.B.).
REFERENCES
- 1.Pandolfo M. and Koenig,M. (1998) Friedreich’s ataxia. In Wells,R.D. and Warren,S.T. (eds), Genetic Instabilities and Hereditary Neurological Diseases. Academic Press, San Diego, pp. 373–398. [Google Scholar]
- 2.Hanauer A., Chery,M., Fujita,R., Driesel,A., Gigenkrantz,S. and Mandel,J.L. (1990) The Friedreich ataxia gene is assigned to chromosome 9q13-q21 by mapping of tightly linked markers and shows linkage disequilibrium with D9S15. Am. J. Hum. Genet., 46, 133–137. [PMC free article] [PubMed] [Google Scholar]
- 3.Campuzano V., Montermini,L., Molto,M.D., Pianese,L., Cossee,M., Cavalcanti,F., Monros,E., Rodius,F., Duclos,F., Monticelli,A. et al. (1996) Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science, 271, 1423–1427. [DOI] [PubMed] [Google Scholar]
- 4.Durr A., Cossee,M., Agid,Y., Campuzano,V., Mignard,C., Penet,C., Mandel,J.L., Brice,A. and Koenig,M. (1996) Clinical and genetic abnormalities in patients with Friedreich’s ataxia. N. Engl. J. Med., 335, 1169–1175. [DOI] [PubMed] [Google Scholar]
- 5.Epplen C., Epplen,J.T., Frank,G., Miterski,B., Santos,E.J. and Schols,L. (1997) Differential stability of the (GAA)n tract in the Friedreich ataxia (STM7) gene. Hum. Genet., 99, 834–836. [DOI] [PubMed] [Google Scholar]
- 6.Montermini L., Andermann,E., Labuda,M., Richter,A., Pandolfo,M., Cavalcanti,F., Pianese,L., Iodice,L., Farina,G., Monticelli,A. et al. (1997) The Friedreich ataxia GAA triplet repeat: premutation and normal alleles. Hum. Mol. Genet., 6, 1261–1266. [DOI] [PubMed] [Google Scholar]
- 7.Sharma R., Bhatti,S., Gomez,M., Clark,R.M., Murray,C., Ashizawa,T. and Bidichandani,S.I. (2002) The GAA triplet-repeat sequence in Friedreich ataxia shows a high level of somatic instability in vivo, with a significant predilection for large contractions. Hum. Mol. Genet., 11, 2175–2187. [DOI] [PubMed] [Google Scholar]
- 8.Filla A., De Michele,G., Cavalcanti,F., Pianese,L., Monticelli,A., Campanella,G. and Cocozza,S. (1996) The relationship between trinucleotide (GAA) repeat length and clinical features in Friedreich ataxia. Am. J. Hum. Genet., 59, 554–660. [PMC free article] [PubMed] [Google Scholar]
- 9.Montermini L., Richter,A., Morgan,K., Justice,C.M., Julien,D., Castellotti,B., Mercier,J., Poirier,J., Capozzoli,F., Bouchard,J.P. et al. (1997) Phenotypic variability in Friedreich ataxia: role of the associated GAA triplet repeat expansion. Ann. Neurol., 41, 675–682. [DOI] [PubMed] [Google Scholar]
- 10.Bidichandani S.I., Ashizawa,T. and Patel,P.I. (1997) Atypical Friedreich ataxia caused by compound heterozygosity for a novel missense mutation and the GAA triplet-repeat expansion. Am. J. Hum. Genet., 60, 1251–1256. [PMC free article] [PubMed] [Google Scholar]
- 11.Campuzano V., Montermini,L., Lutz,Y., Cova,L., Hindelang,C., Jiralerspong,S., Trottier,Y., Kish,S.J., Faucheux,B., Trouillas,P. et al. (1997) Frataxin is reduced in Friedreich ataxia patients and is associated with mitochondrial membranes. Hum. Mol. Genet., 6, 1771–1780. [DOI] [PubMed] [Google Scholar]
- 12.Bidichandani S.I., Ashizawa,T. and Patel,P.I. (1998) The GAA triplet-repeat expansion in Friedreich ataxia interferes with transcription and may be associated with an unusual DNA structure. Am. J. Hum. Genet., 62, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohshima K., Montermini,L., Wells,R.D. and Pandolfo,M. (1998) Inhibitory effects of expanded GAA·TTC triplet repeats from intron I of the Friedreich ataxia gene on transcription and replication in vivo. J. Biol. Chem., 273, 14588–14595. [DOI] [PubMed] [Google Scholar]
- 14.Grabczyk E. and Usdin,K. (2000) The GAA·TTC triplet repeat expanded in Friedreich’s ataxia impedes transcription elongation by T7 RNA polymerase in a length and supercoil dependent manner. Nucleic Acids Res., 28, 2815–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ljungman M. and Hanawalt,P.C. (1992) Localized torsional tension in the DNA of human cells. Proc. Natl Acad. Sci. USA, 89, 6055–6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jupe E.R., Sinden,R.R. and Cartwright,I.L. (1993) Stably maintained microdomain of localized unrestrained supercoiling at a Drosophila heat shock gene locus. EMBO J., 12, 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer P.R. and Sinden,R.R. (1997) Measurement of unrestrained negative supercoiling and topological domain size in living human cells. Biochemistry, 36, 3151–3158. [DOI] [PubMed] [Google Scholar]
- 18.Liu L.F. and Wang,J.C. (1987) Supercoiling of the DNA template during transcription. Proc. Natl Acad. Sci. USA, 84, 7024–7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearson C.E. and Sinden,R.R. (1998) Trinucleotide repeat DNA structures: dynamic mutations from dynamic DNA. Curr. Opin. Struct. Biol., 8, 321–330. [DOI] [PubMed] [Google Scholar]
- 20.Schlotterer C. and Tautz,D. (1992) Slippage synthesis of simple sequence DNA. Nucleic Acids Res., 20, 211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heidenfelder B.L., Makhov,A.M. and Topal,M.D. (2003) Hairpin formation in Friedreich’s ataxia triplet repeat expansion. J. Biol. Chem., 278, 2425–2431. [DOI] [PubMed] [Google Scholar]
- 22.Frank-Kamenetskii M.D. and Mirkin,S.M. (1995) Triplex DNA structures. Annu. Rev. Biochem., 64, 65–95. [DOI] [PubMed] [Google Scholar]
- 23.Soyfer V.N. and Potaman,V.N. (1996) Triple-Helical Nucleic Acids. Springer, New York. [Google Scholar]
- 24.Jain A., Rajeswari,M.R. and Ahmed,F. (2002) Formation and thermodynamic stability of intermolecular (R*R*Y) DNA triplex in GAA/TTC repeats associated with Friedreich’s ataxia. J. Biomol. Struct. Dyn., 19, 61–699. [DOI] [PubMed] [Google Scholar]
- 25.Hanvey J.C., Shimizu,M. and Wells,R.D. (1988) Intramolecular DNA triplexes in supercoiled plasmids. Proc. Natl Acad. Sci. USA, 85, 6292–6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu M., Hanvey,J.C. and Wells,R.D. (1990) Multiple non-B-DNA conformations of polypurine·polypyrimidine sequences in plasmids. Biochemistry, 29, 4704–4713. [DOI] [PubMed] [Google Scholar]
- 27.Ohshima K., Kang,S., Larson,J.E. and Wells,R.D. (1996) Cloning, characterization and properties of seven triplet repeat DNA sequences. J. Biol. Chem., 271, 16773–16783. [DOI] [PubMed] [Google Scholar]
- 28.Sakamoto N., Chastain,P.D., Parniewski,P., Ohshima,K., Pandolfo,M., Griffith,J.D. and Wells,R.D. (1999) Sticky DNA: self-association properties of long GAA·TTC repeats in R·R·Y triplex structures from Friedreich’s ataxia. Mol. Cell, 3, 465–475. [DOI] [PubMed] [Google Scholar]
- 29.Vetcher A.A., Napierala,M., Iyer,R.R., Chastain,P.D., Griffith,J.D. and Wells,R.D. (2002) Sticky DNA, a long GAA·GAA·TTC triplex that is formed intramolecularly, in the sequence of intron 1 of the frataxin gene. J. Biol. Chem., 277, 39217–39227. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 31.Potaman V.N. and Sinden,R.R. (1998) Stabilization of triple/single strand (H-DNA) structure by cationic peptides. Biochemistry, 37, 12952–12961. [DOI] [PubMed] [Google Scholar]
- 32.Potaman V.N., Ussery,D.W. and Sinden,R.R. (1996) The formation of a combined H-DNA/open TATA box structure in the promoter sequence of the human Na,K-ATPase α2 gene. J. Biol. Chem., 271, 13441–13447. [DOI] [PubMed] [Google Scholar]
- 33.Oussatcheva E.A., Shlyakhtenko,L.S., Glass,R., Sinden,R.R., Lyubchenko,Y.L. and Potaman,V.N. (1999) Structure of branched molecules. Gel retardation and atomic force microscopy studies. J. Mol. Biol., 292, 75–86. [DOI] [PubMed] [Google Scholar]
- 34.Shlyakhtenko L.S., Potaman,V.N., Sinden,R.R. and Lyubchenko,Y.L. (1998) Structure and dynamics of supercoil-stabilized DNA cruciforms. J. Mol. Biol., 280, 61–72. [DOI] [PubMed] [Google Scholar]
- 35.Potaman V.N., Bissler,J.J., Hashem,V.I., Oussatcheva,E.A., Lu,L., Shlyakhtenko,L.S., Lyubchenko,Y.L., Matsuura,T., Ashizawa,T., Leffak,M. et al. (2003) Unpaired structures in SCA10 (ATTCT)n·(AGAAT)n repeats. J. Mol. Biol., 326, 1095–1111. [DOI] [PubMed] [Google Scholar]
- 36.Tiner W.J., Potaman,V.N., Sinden,R.R. and Lyubchenko,Y.L. (2001) The structure of intramolecular triplex DNA: atomic force microscopy study. J. Mol. Biol., 314, 353–357. [DOI] [PubMed] [Google Scholar]
- 37.Kohwi Y. and Kohwi-Shigematsu,T. (1988) Magnesium ion-dependent triple-helix structure formed by homopurine-homopyrimidine sequences in supercoiled plasmid DNA. Proc. Natl Acad. Sci. USA, 85, 3781–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dayn A., Samadashwily,G.M. and Mirkin,S.M. (1992) Intramolecular DNA triplexes: unusual sequence requirements and influence on DNA polymerization. Proc. Natl Acad. Sci. USA, 89, 11406–11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyamichev V.I., Voloshin,O.N., Frank-Kamenetskii,M.D. and Soyfer,V.N. (1991) Photofootprinting of DNA triplexes. Nucleic Acids Res., 19, 1633–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panyutin I.G. and Wells,R.D. (1992) Nodule DNA in the (GA)37·(CT)37 insert in superhelical plasmids. J. Biol. Chem., 267, 5495–5501. [PubMed] [Google Scholar]
- 41.Htun H. and Dahlberg,J.E. (1988) Single strands, triple strands and kinks in H-DNA. Science, 241, 1791–1796. [DOI] [PubMed] [Google Scholar]
- 42.Lyamichev V.I., Mirkin,S.M., Kumarev,V.P., Baranova,L.V., Vologodskii,A.V. and Frank-Kamenetskii,M.D. (1989) Energetics of the B-H transition in supercoiled DNA carrying d(CT)x·d(AG)x and d(C)n·d(G)n inserts. Nucleic Acids Res., 17, 9417–9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collier D.A. and Wells,R.D. (1990) Effect of length, supercoiling and pH on intramolecular triplex formation. Multiple conformers at pur·pyr mirror repeats. J. Biol. Chem., 265, 10652–10658. [PubMed] [Google Scholar]
- 44.Kohwi-Shigematsu T. and Kohwi,Y. (1991) Detection of triple-helix related structures adopted by poly(dG)-poly(dC) sequences in supercoiled plasmid DNA. Nucleic Acids Res., 19, 4267–4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gacy A.M., Goellner,G.M., Spiro,C., Chen,X., Gupta,G., Bradbury,E.M., Dyer,R.B., Mikesell,M.J., Yao,J.Z., Johnson,A.J. et al. (1998) GAA instability in Friedreich’s Ataxia shares a common, DNA-directed and intraallelic mechanism with other trinucleotide diseases. Mol. Cell, 1, 583–593. [DOI] [PubMed] [Google Scholar]
- 46.Mariappan S.V., Catasti,P., Silks,L.A., Bradbury,E.M. and Gupta,G. (1999) The high-resolution structure associated with Friedreich’s ataxia. J. Mol. Biol., 285, 2035–2052. [DOI] [PubMed] [Google Scholar]
- 47.Baran N., Lapidot,A. and Manor,H. (1991) Formation of DNA triplexes accounts for arrests of DNA synthesis at d(TC)n and d(GA)n tracts. Proc. Natl Acad. Sci. USA, 88, 507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Potaman V.N. and Bissler,J.J. (1999) Overcoming a barrier for DNA polymerization in triplex-forming sequences. Nucleic Acids Res., 27, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Semerad C.L. and Maher,L.J.,III (1994) Exclusion of RNA strands from a purine motif triple helix. Nucleic Acids Res., 22, 5321–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fox K.R. (1990) Long (dA)n·(dT)n tracts can form intramolecular triplexes under superhelical stress. Nucleic Acids Res., 18, 5387–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pecinka P., Huertas,D., Azorin,F. and Palecek,E. (1995) Intramolecular TAT triplex in (dA)58·(dT)58. Influence of ions. J. Biomol. Struct. Dyn., 13, 29–46. [DOI] [PubMed] [Google Scholar]
- 52.Bernues J., Beltran,R., Casasnovas,J.M. and Azorin,F. (1990) DNA-sequence and metal-ion specificity of the formation of *H-DNA. Nucleic Acids Res., 18, 4067–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trinh T.Q. and Sinden,R.R. (1991) Preferential DNA secondary structure mutagenesis in the lagging strand of replication in E. coli. Nature, 352, 544–547. [DOI] [PubMed] [Google Scholar]
- 54.Sinden R.R. and Wells,R.D. (1992) DNA structure, mutations and human genetic disease. Curr. Opin. Biotechnol., 3, 612–622. [DOI] [PubMed] [Google Scholar]




