Abstract
In the Xenopus oocyte system mitogen treatment triggers the G2/M transition by transiently inhibiting the cAMP-dependent protein kinase (PKA); subsequently, other signal transduction pathways are activated, including the mitogen-activated protein kinase (MAPK) and polo-like kinase pathways. To study the interactions between these pathways, we have utilized a cell-free oocyte extract that carries out the signaling events of oocyte maturation after addition of the heat-stable inhibitor of PKA, PKI. PKI stimulated the synthesis of Mos and activation of both the MAPK pathway and the Plx1/Cdc25C/cyclin B-Cdc2 pathway. Activation of the MAPK pathway alone by glutathione S-transferase (GST)-Mos did not lead to activation of Plx1 or cyclin B-Cdc2. Inhibition of the MAPK pathway in the extract by the MEK1 inhibitor U0126 delayed, but did not prevent, activation of the Plx1 pathway, and inhibition of Mos synthesis by cycloheximide had a similar effect, suggesting that MAPK activation is the only relevant function of Mos. Immunodepletion of Plx1 completely inhibited activation of Cdc25C and cyclin B-Cdc2 by PKI, indicating that Plx1 is necessary for Cdc25C activation. In extracts containing fully activated Plx1 and Cdc25C, inhibition of cyclin B-Cdc2 by p21Cip1 had no significant effect on either the phosphorylation of Cdc25C or the activity of Plx1. These results demonstrate that maintenance of Plx1 and Cdc25C activity during mitosis does not require cyclin B-Cdc2 activity.
INTRODUCTION
Protein phosphorylation plays a key role in controlling cell-cycle progression. Most prominent among the enzymes regulating cell-cycle transitions are the cyclin-dependent kinases (cdks; Norbury and Nurse, 1992; Morgan, 1995). However, protein kinases structurally distinct from cdks also make important contributions to cell-cycle progression. The polo-like kinases (plks) make up an evolutionarily conserved, newly emerging family of essential cell-cycle regulators. Plks regulate the activities of cdks and cooperate with cdks to control particular cell-cycle transitions (Glover et al.,1998; Nigg, 1998). One example of plk regulation of cdk activity has been identified at the G2/M transition. Entry into mitosis depends on phosphorylation and activation of the dual-specificity phosphatase Cdc25C, which dephosphorylates and activates the cyclin B-Cdc2 complex that catalyzes the G2/M transition (Dunphy and Kumagai, 1991; Gautier et al., 1991; Izumi et al., 1992; Kumagai and Dunphy, 1992; Lee et al., 1992; Hoffmann et al., 1993). Cyclin B-Cdc2 itself is able to phosphorylate Cdc25C at the activating sites, forming a positive feedback loop that contributes to the abrupt transition from G2 into M phase (Izumi and Maller, 1993). However, at the G2/M transition initial phosphorylation of Cdc25C occurs before cyclin B-Cdc2 activation, and full phosphorylation and activation of Cdc25C can be obtained in microcystin-treated egg extracts devoid of Cdc2 and Cdk2 (Izumi and Maller, 1995). This has focused attention on the identification of other protein kinases that might function as “trigger” kinases for Cdc25C activation and the G2/M transition. Substantial data indicate that the Xenopus plk homologue Plx1 is such a trigger kinase. First, Plx1 is able to bind, phosphorylate, and activate Cdc25C in vitro (Kumagai and Dunphy, 1996; Qian, Erikson, Taieb, and Maller, unpublished data). Second, Plx1 is activated with the same kinetics as Cdc25C during oocyte maturation (Qian et al., 1998a). Third, in resting oocytes constitutively active Plx1 is sufficient to activate Cdc25C and the G2/M transition (Qian et al., 1999). However, expression of catalytically inactive Plx1 or injection of anti-Plx1 antibodies into oocytes only delayed but did not abrogate the activation of Cdc25C (Qian et al., 1998a). This suggests the possibility that other protein kinases also act as trigger kinases and that Plx1 activation might not be required for Cdc25C activation.
The pathway of Plx1 activation has been characterized in recent years. Substantial evidence indicates that plks in various species are activated by phosphorylation (Hamanaka et al., 1995; Tavares et al., 1996; Kotani et al., 1998; Qian et al., 1998a). Although activation of mitogen-activated protein kinase (MAPK) during oocyte maturation coincides with that of Plx1, Plx1 is activated by progesterone treatment in the presence of U0126, an inhibitor MAPK kinase that potently blocks MAPK activation (Favata et al., 1998; Gross et al., 2000). With the use of an activation assay, Qian et al. (1998b) purified a Plx1-activating kinase to near homogeneity, obtained microsequence data, and cloned the gene encoding the activity. The gene product, termed xPlkk1, is an Ste 20-like kinase, and a related kinase is present in mice (Kuramochi et al., 1997). Activation of xPlkk1 parallels the activation of Plx1, and xPlkk1 is also activated by phosphorylation, indicating that a protein kinase cascade regulates Plx1 activation (Qian et al., 1998b).
A positive feedback loop between cyclin B-Cdc2 and Plx1 was identified initially by the finding that expression of active Cdc25C in a G2 environment leads to activation of both cyclin B-Cdc2 and Plx1 (Qian et al., 1998a). The Cdc2-dependent activation of Plx1 is presumed to be mediated by action on a component of the Plx1 kinase cascade upstream of Plx1 and xPlkk1, because neither Plx1 nor xPlkk1 is a substrate for cyclin B-Cdc2 (Hamanaka et al., 1995; Lee et al., 1995; Qian, Erikson, Taieb, and Maller, unpublished data). In any feedback loop system, signaling depends on both components of the loop having activity, and it can be difficult to establish upstream/downstream relationships. However, inhibition of Cdc2 in metaphase-arrested mammalian cells does not block Plk activity (Smits et al., 2000), and Cdc25C becomes phosphorylated and activated in egg extracts upon microcystin treatment in the absence of Cdc2 and Cdk2 (Izumi and Maller, 1995).
In this paper, to fully evaluate the role of Plx1 as an upstream Cdc25C-activating trigger kinase as well as to analyze feedback controls on Plx1 and cyclin B-Cdc2, we have utilized depletion/reconstitution approaches with an extract system from G2-arrested prophase oocytes that activates the signaling pathways characteristic of the G2/M transition in response to inhibition of PKA. Moreover, we have used the extract system to evaluate the dependence of different signaling pathways on one another.
MATERIALS AND METHODS
Reagents
The heat-stable inhibitor protein (PKI) of the cAMP-dependent protein kinase (PKA) was expressed in bacteria and purified as described (Thomas et al., 1991). Active glutathione S-transferase (GST)-Mos and His(6)-FLAG-tagged Plx1 were prepared from baculovirus-infected Sf9 cells; GST-Mos was purified with the use of glutathione-agarose beads (Roy et al., 1996), and Plx1 was purified with the use of Talon beads (Clontech, Palo Alto, CA) followed by chromatography on hydroxyapatite and Mono S (Amersham Pharmacia Biotech, Piscataway, NJ) columns (Qian et al., 1998b). Bacterially expressed human GST-p21Cip1 was prepared as described (Frank-Vaillant et al., 1999). Affinity-purified Plx1 antibodies and anti-Plx1-depleted (control) antibodies were each covalently coupled to Affi-Prep protein A support beads (Bio-Rad, Hercules, CA) at a concentration of 5 mg of immunoglobulin/1 ml of beads (Harlow and Lane, 1988).
Preparation and Manipulation of G2-arrested Prophase Extracts
Xenopus laevis females were obtained from Xenopus I (Ann Arbor, Michigan), and the ovary from a large frog was cut into small pieces. The oocytes were released by digestion at ambient temperature in Ca2+-free modified Barth's solution containing dispase (0.5 mg/ml) for 2 h and then by digestion with collagenase type 1A (0.8 mg/ml) for 1 h or longer until the oocytes were freed of blood vessels. The oocytes were washed six times with modified Barth's solution and small oocytes were discarded by decantation. Several thousand G2-arrested stage VI oocytes were manually collected under a dissecting microscope and incubated in medium (25 mM HEPES [pH 7.5], 0.65× DMEM, 50 U of penicillin, and 50 μg of streptomycin/ml) at 18°C overnight. Damaged or morphologically atypical oocytes were removed. The G2-arrested prophase extract was prepared from the healthy oocytes by a crushing method similar to that used previously to make oocyte or egg extracts (Lohka and Maller, 1985; Shibuya et al., 1992). After centrifugation, the extract was supplemented with 100 μM EGTA, 1 mM MgCl2, 100 μM 8-(4-chlorophenylthio)-cAMP, 1 μM caspase-3 inhibitor Ac-DEVD, (Biomol, Plymouth Meeting, PA) creatine phosphate (2 μg/ml), pepstatin, and chymostatin (25 μg of each/ml), and cytochalasin B (10 μg/ml). The addition of the cAMP analogue was found necessary to maintain the G2 arrest of the extract for long periods. Aliquots of 50 μl were distributed to 1.5-ml microcentrifuge tubes and kept on ice. After addition of PKI or other agents, the samples were incubated at 22°C, and aliquots of 2 μl were taken at various times, diluted into 18 μl of cold extraction buffer, frozen on dry ice, and stored at −80°C. Extraction buffer comprises 80 mM β-glycerophosphate (pH 7.4), 20 mM EDTA, 1 mM dithiothreitol, 0.1 mM sodium vanadate, 10 mM NaF, 3 μM microcystin, 1 mM phenylmethylsulfonyl fluoride, and 10 μg each of pepstatin, chymostatin, and leupeptin/ml. The total volume of additions did not exceed 10% of the volume of the extract. For immunodepletion experiments, a 50-μl sample of extract was incubated with 5 μl of antibody-coupled beads for 1 h at 4°C and centrifuged briefly, and the supernatant was treated as described above.
Kinase Assays, Immunoprecipitation, and Immunoblotting
Oocytes were extracted, and histone H1 kinase assays, immunoprecipitations, and immunoblotting were done as described previously (Qian et al., 1998a). For assay of prophase extracts, samples of 1 μl of the diluted extract were used for histone H1 kinase assays as described previously, except that the reaction volumes were 15 μl and the reactions were stopped by the addition of one-half volume of threefold concentrated sample buffer. The products of the reaction were subjected to SDS-PAGE, the histone H1 bands were visualized by staining, and incorporation of radiolabel was quantified by liquid scintillation spectrometry of the excised gel bands. Samples of 4 μl of the diluted extract were used for immunocomplex kinase assays of Plx1 activity, and samples of 2 μl of the diluted extract were used for immunoblotting. Immunoblots were developed with the appropriate peroxidase-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA) and enhanced chemiluminescence (Amersham, Arlington Heights, IL). The MAPK immunoblots were developed with alkaline phosphatase-conjugated secondary antibody and a colorimetric detection system (Bio-Rad). Anti-Mos (C237) rabbit polyclonal antibody was from Santa Cruz Biotechnology (Santa Cruz, CA), and antiphospho-p44/42 MAPK E10 monoclonal antibody was from New England Biolabs (Beverly, MA). Antibodies against Cdc25C, MAPK and Plx1 have been characterized previously (VanRenterghem et al., 1994; Qian et al., 1998a).
RESULTS
PKI Stimulates Multiple Signaling Pathways during Oocyte Maturation
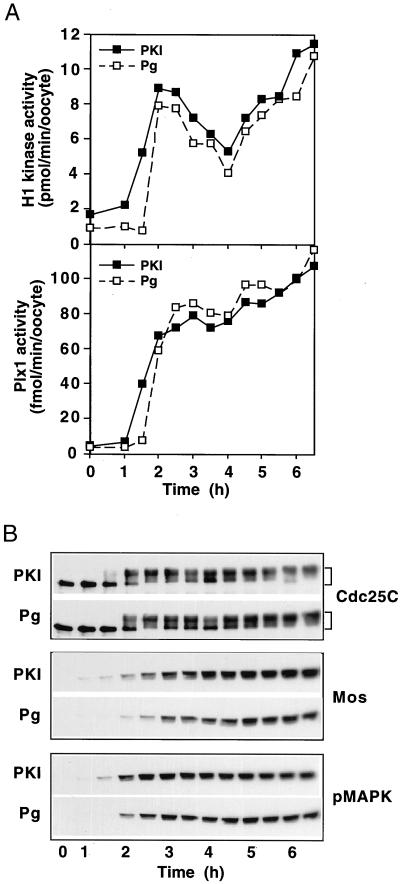
Reduced PKA activity is one of the very early events in progesterone-induced oocyte maturation (Maller and Krebs, 1977; Huchon et al., 1981). Previous studies have shown that inhibition of PKA by microinjection into oocytes of regulatory subunit, or of PKI, is sufficient to induce germinal vesicle (nuclear) breakdown (GVBD), and elevated PKA is able to block GVBD even in the presence of progesterone (Maller and Krebs, 1977; Huchon et al., 1981). Considerably more is known at present about signaling pathways downstream of PKA inhibition than was the case in earlier studies with PKI, which monitored only GVBD. To compare the molecular events that occur during oocyte maturation induced by inhibition of PKA with those induced by progesterone, G2-arrested stage VI oocytes were either microinjected with PKI or exposed to progesterone, and key parameters of the G2/M transition were monitored. As shown in Figure 1, inhibition of PKA by PKI resulted in the synthesis of Mos and the activation of MAPK, Plx1, Cdc25C, and cyclin B-Cdc2, key events that occur during progesterone-induced oocyte maturation (Sagata et al., 1989; Haccard et al., 1995; Roy et al., 1996; Qian et al., 1998a; Sagata, 1998). The PKI-induced events occurred slightly earlier (≈30 min) than those induced by progesterone, consistent with the fact that inhibition of PKA is a downstream event after progesterone stimulation. In both cases cyclin B-Cdc2 activity underwent a transient partial decrease in activity 4 h after progesterone addition (Figure 1A), an effect characteristic of the meiosis I → II transition. Thus, in ooctyes PKI appears to stimulate progression through the events of both meiosis I and meiosis II.
Figure 1.
Comparison of oocyte maturation induced by progesterone or by microinjection of PKI. Oocytes were treated with progesterone (10 μg/ml; Pg) or microinjected with 40 nl of PKI (1.5 mg/ml) and incubated at 22°C. At the indicated times groups of six oocytes were frozen. Extracts were prepared and analyzed for histone H1 kinase and Plx1 activities (A) or immunoblotted for Cdc25C, Mos, and active (phosphorylated) MAPK (pMAPK) as indicated (B). The upper (shifted) form of Cdc25C has previously been demonstrated to reflect phosphorylation and activation of the enzyme (Izumi et al., 1992; Kumagai and Dunphy, 1992).
PKI Stimulates Multiple Signaling Pathways in Prophase Extracts
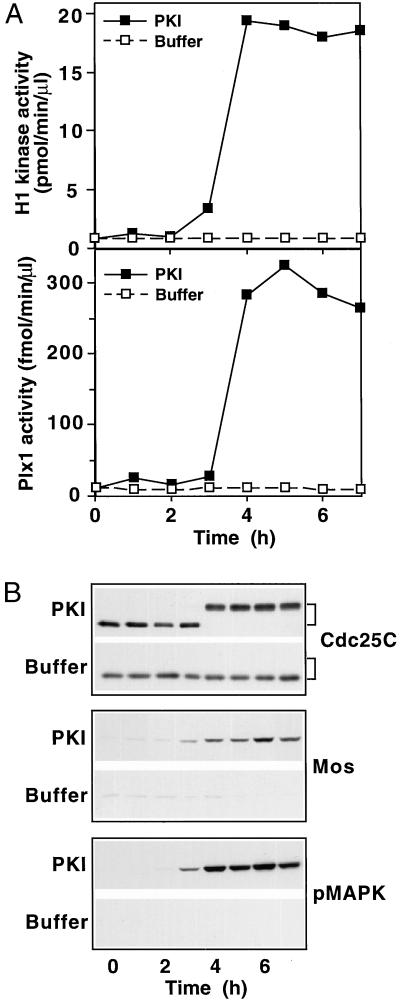
Because PKI mimics the effect of progesterone on maturation of intact G2-arrested stage VI oocytes, it was plausible that PKI could activate extracts prepared by crushing such oocytes by centrifugation. To evaluate this possibility, crushates from stage VI oocytes, termed prophase extracts, were prepared as described in MATERIALS AND METHODS. Addition of PKI to such an extract resulted in the synthesis of Mos and the activation of MAPK, Plx1, Cdc25C, and cyclin B-Cdc2 (Figure 2), events that are diagnostic of oocyte maturation in vivo induced by either progesterone or PKI (Figure 1). Interestingly, in the PKI-activated prophase extract the entire complement of activated Cdc25C exists solely in the high activity, most slowly migrating form, with no intermediate forms detectable. This full shift of Cdc25C may reflect the greater synchrony of the extract compared with a population of oocytes and resembles that seen in oocytes at MII or in unfertilized eggs, which are arrested at metaphase II by cytostatic factor (CSF) (Qian et al., 1998a). However, assay of cyclin B-Cdc2 at further time points showed no evidence of progression to MII. (See for example Figure 4.) Addition of progesterone to a prophase extract had no effect (data not shown), consistent with the fact that progesterone acts on an unidentified receptor associated with the plasma membrane (Maller, 1998), which was removed by centrifugation during preparation of the extract. These results indicate that the prophase extract system can undergo the key molecular events that occur during the G2/M transition of oocyte maturation and make feasible overexpression/depletion approaches to study the signaling pathways.
Figure 2.
Induction of the G2/M transition in prophase extracts by PKI. Prophase extracts were supplemented with PKI (final concentration 15 μg/ml) or buffer and incubated at ambient temperature. At the indicated times samples were taken, diluted, and frozen. Samples were assayed for histone H1 kinase and Plx1 activities (A) or immunoblotted for Cdc25C, Mos, and pMAPK as indicated (B).
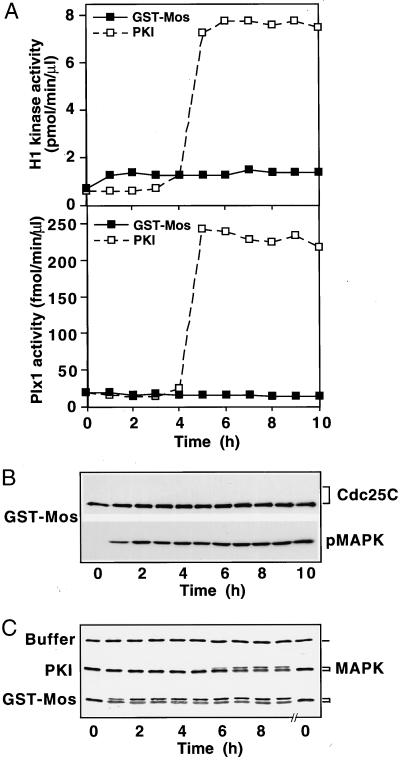
Figure 4.
The Mos-MAPK pathway is not sufficient for induction of the G2/M transition. Prophase extracts were supplemented with either active GST-Mos (50 μg/ml final concentration) or with PKI, and samples were frozen at the indicated times, analyzed for Plx1 and histone H1 kinase activity (A), immunoblotted for Cdc25C and pMAPK (B), and for MAPK (C).
The Mos/MAPK Pathway Is Neither Necessary nor Sufficient for Activation of the Plx1 Pathway in Prophase Extracts
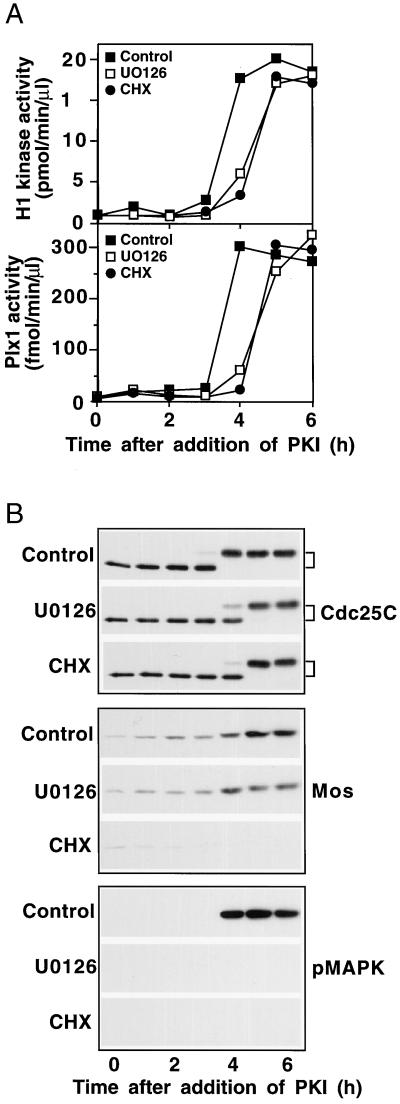
In intact oocytes the MAPK pathway is activated by progesterone treatment because of translational activation of mRNA encoding Mos, a MAPK kinase kinase (for review, see Nebreda and Ferby, 2000). Inhibition of MAPK activation delays entry into meiosis I in Xenopus and completely blocks the onset of meiosis II (Fisher et al., 1999; Gross et al., 2000). To determine the role of the MAPK pathway in the activation of cyclin B-Cdc2 by PKI, prophase extracts were supplemented with U0126, an inhibitor of the MAPK kinase MEK1 (Favata et al., 1998; Gross et al., 2000), and then PKI was added. As shown in Figure 3, inhibition of MEK1 by U0126 completely prevented the activation of MAPK but had no effect on the synthesis of Mos. Accumulation of Mos paralleled the activation of Cdc25C, perhaps reflecting a known feedback loop from cyclin B-Cdc2 to mos mRNA translation (Gotoh et al., 1995). In any case this inhibition of the MAPK pathway delayed, but did not reduce, the activation of Plx1, Cdc25C, and cyclin B-Cdc2 upon PKI addition, indicating that the MAPK pathway is not essential for activation of the Plx1 pathway in G2, similar to the case in intact oocytes (Fisher et al., 1999; Gross et al., 2000).
Figure 3.
The Mos-MAPK pathway is not required for the G2/M transition in response to PKI. U0126 or cycloheximide (CHX) was added to prophase extracts to a final concentration of 50 μM or 0.1 mg/ml, respectively. DMSO (0.5 μl–50 μl of extract) served as a control (control). Then, PKI was added, samples were frozen at the indicated times and assayed for histone H1 kinase and Plx1 activities (A) or immunoblotted for Cdc25C, Mos, and pMAPK as indicated (B).
Because inhibition of MEK1 has no effect on the stimulation of Mos synthesis by PKI, the data do not exclude the possibility that Mos could lead to activation of the Plx1 pathway by a mechanism other than through MAPK activation. Indeed, recent studies in mouse oocytes have suggested that Mos might have other functions besides MEKK activity (Verlhac et al., 2000). To address this possibility, prophase extracts were treated with cycloheximide, a potent inhibitor of protein synthesis, and then PKI was added. As shown in Figure 3, treatment with cycloheximide completely inhibited the synthesis of Mos but only delayed the activation of Plx1, Cdc25C, and cyclin B-Cdc2, indicating that the synthesis of Mos is not essential for activation of the Plx1 pathway. Interestingly, the magnitude of the delay in activation of the Plx1 pathway in the presence of cycloheximide was similar to that in the presence of U0126, suggesting that the only relevant function of Mos is activation of MAPK. Metabolic labeling with [35S]methionine confirmed that cycloheximide totally inhibited synthesis of all cellular proteins. Inasmuch as de novo protein synthesis is not essential for stimulation of the Plx1/Cdc25/cyclin B-Cdc2 pathway by PKI, activation must occur by a purely posttranslational mechanism after PKA inhibition, even though several hours are required (Figure 3).
Although Mos is not required for activation of Plx1 by PKI treatment, the possibility exists that cross-talk from the MAPK pathway could lead to activation of Plx1. Indeed, numerous studies have shown that overexpression of Mos, MEK1, or MAPK is sufficient to induce GVBD in the absence of progesterone by a mechanism that usually requires protein synthesis (Sagata et al., 1989; Yew et al., 1992; Haccard et al., 1995; Huang et al., 1995; Roy et al., 1996). To address whether Mos is sufficient to activate the Plx1 pathway in prophase extracts, active GST-Mos protein prepared from baculovirus-infected Sf9 cells was added to extracts, and the activities of Plx1, Cdc25C, and cyclin B-Cdc2 were monitored. As shown in Figure 4, addition of Mos protein rapidly activated MAPK; however, this did not lead to the activation of Plx1, Cdc25C, or cyclin B-Cdc2. In a parallel extract, addition of PKI was able to activate the Plx1 pathway. The appearance of the slowly migrating form of MAPK was consistent with the appearance of the active phosphorylated form (Figure 4, B and C). Indeed, the percentage of MAPK in the active form was slightly greater in the sample treated with GST-Mos than in that treated with PKI (Figure 4C). Moreover, the activity of MAPK, as judged by kinase assays with epidermal growth factor receptor peptide as substrate confirmed the results of the immunoblots. Taken together, these results indicate that the Mos/MAPK pathway is neither necessary nor sufficient to activate the Plx1 pathway in the prophase (G2) extract system. Therefore, the Plx1 pathway and the MAPK pathway in the G2/M transition can be studied independently in the PKI-responsive extract. Treatment of a prophase extract with Mos protein will activate only the MAPK pathway, whereas activation of an extract with PKI in the presence of cycloheximide or U0126 will activate only the Plx1 pathway.
Plx1 Is Necessary for Activation of Cdc25C and Initiation of the G2/M Transition
We have shown previously that microinjection of dominant-negative, catalytically inactive Plx1 or anti-Plx1 antibodies into oocytes causes a delay in the activation of Cdc25C and the G2/M transition, but full Cdc25C activation eventually occurs (Qian et al., 1998a). A possible explanation for this result is that a kinase other than Plx1 is also able to act as a trigger kinase and activate Cdc25C. Available evidence does not exclude the possibility that Plx1 activation is not required for Cdc25C activation at the G2/M transition due to other as yet uncharacterized trigger kinases. To evaluate the extent to which Cdc25C activation is dependent on Plx1, we performed immunodepletion-reconstitution experiments. Extracts were treated with either control immunoglobulin G (IgG) or anti-Plx1 IgG coupled to beads, the extracts were then supplemented with PKI, and the activities of Cdc25C and cyclin B-Cdc2 were monitored. Plx1 was completely removed from the extract treated with anti-Plx1 beads and did not reaccumulate because of synthesis during the course of the experiment, whereas treatment with control IgG beads had no effect on the level of Plx1 (Figure 5A). Immunodepletion of Plx1 completely prevented the activation of Cdc25C and cyclin B-Cdc2, whereas mock depletion had no effect (Figure 5, B and C). This indicates that Plx1 is essential for activation of Cdc25C and initiation of the G2/M transition. In addition, immunodepletion of Plx1 also prevented accumulation of Mos protein and activation of the MAPK pathway (Figure 5C), indicating that accumulation of Mos requires activation of the Plx1 pathway and subsequent histone H1 kinase activity, which is known to stimulate Mos synthesis through a feedback loop (Gotoh et al., 1995). To confirm that the effect of the immunodepletion is specifically due to the removal of Plx1, a reconstitution experiment was performed. First, a prophase extract was treated with anti-Plx1 beads, and then recombinant Plx1 was added. After addition of PKI, the activities of Cdc25C, cyclin B-Cdc2, and MAPK and the accumulation of Mos were monitored. As shown in Figure 5, recombinant Plx1 reversed all the effects of the immunodepletion, confirming that Plx1 and not some coprecipitating protein is essential for initiation of the G2/M transition.
Figure 5.
Plx1 is required for activation of Cdc25C and the G2/M transition. Prophase extracts were treated with either control IgG- or anti-Plx1-coupled beads as described in MATERIALS AND METHODS. The extract supernatants were then supplemented with either PKI or PKI plus recombinant Plx1 (25 μg/ml final concentration), and samples were frozen at the indicated times and analyzed. (A) Samples were immunoblotted for Plx1 before immunodepletion (Start) and after treatment with control IgG- or anti-Plx1–coupled beads (IgG and antibody [Ab], respectively) at the indicated times. Samples were also assayed for histone H1 kinase activity (B) or immunoblotted for Cdc25C, Mos, and pMAPK (C). A sample of the Plx1 preparation was subjected to SDS-PAGE and Coomassie blue staining to assess its purity (B, right).
Cyclin B-Cdc2 Activity Is Not Required for Plx1 Activity or Phosphorylation of Cdc25C during M Phase
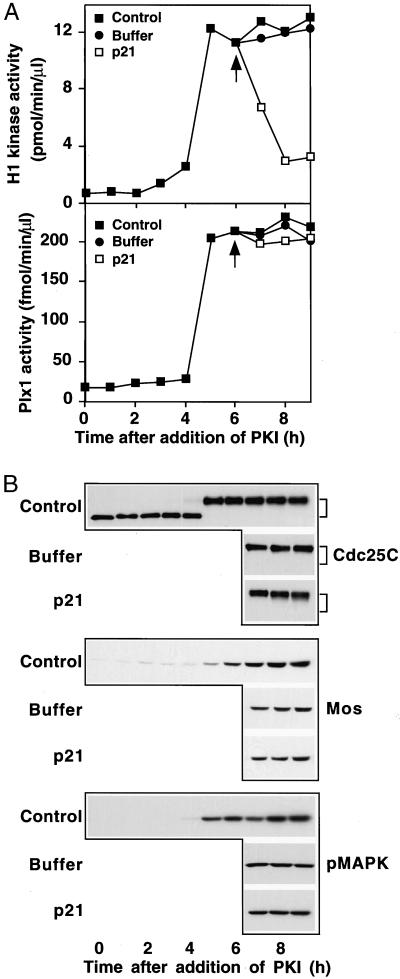
As described in the introduction, cyclin B-Cdc2 itself is able to phosphorylate and activate Cdc25C, forming a positive feedback loop (Izumi and Maller, 1993). Because mutation of Cdc2 consensus sites reduces the ability of Cdc25C to trigger the G2/M transition, it has been suggested that this feedback loop contributes to the abrupt transition from G2 into M phase (Izumi and Maller, 1993). However, the activating sites in Cdc25C are also substrates for Plx1 (Kumagai and Dunphy, 1996), suggesting that active Cdc2 might not be necessary for Cdc25C activity in M phase. Indeed, Cdc25C activation can be obtained in microcystin-treated extracts in which both Cdc2 and Cdk2 have been completely depleted (Izumi and Maller, 1995). To determine whether cyclin B-Cdc2 activity is necessary for Plx1 activity in M phase, the following experiment was performed. First, prophase extracts were activated with PKI, and then after 6 h, when Cdc25C had been fully phosphorylated (Figure 2), the Cdk inhibitor p21Cip1 was added to the extracts. Consistent with previous reports (Frank-Vaillant et al., 1999), p21Cip1 efficiently inhibited cyclin B-Cdc2 (Figure 6). However, this inhibition did not reduce the activity of Plx1 or the phosphorylation of Cdc25C (Figure 6), nor did it affect the feedback loop between MAPK activity and accumulation of Mos (Figure 6B; Matten et al., 1996; Roy et al., 1996). Studies presented here with prophase extracts (Figure 5) and previous studies with constitutively active Plx1 (Qian et al., 1999) indicate that Plx1 is both necessary and sufficient to activate Cdc25C. Moreover, the data in Figure 6 show that activated Plx1 can maintain Cdc25C activity in M phase even in the absence of elevated cyclin B-Cdc2 activity. This result indicates that Plx1 is not only essential for activation of Cdc25C in G2 but also plays an important role in maintaining the activity of Cdc25C during mitosis.
Figure 6.
Cyclin B-Cdc2 activity is not required for Plx1 activity or the phosphorylation of Cdc25C in M phase. Prophase extracts were supplemented with PKI and incubated for 6 h. Then, samples of the extracts were either untreated (control) or further supplemented with either buffer or GST-p21Cip1 (80 μg/ml final concentration; p21). Samples were taken at the indicated times for assay of Plx1 activity and histone H1 kinase activity (A) or immunoblotted for Cdc25C, Mos, and active MAPK (B). The arrows in A depict the time of addition of GST-p21Cip1.
DISCUSSION
The results in this paper show that the activity of Plx1 is absolutely required for the activation of Cdc25C during the G2/M transition (Figure 5). Combined with previous data showing that a constitutively active form of Plx1 is able to cause Cdc25C activation and the G2/M transition in oocytes in the absence of progesterone (Qian et al., 1999), it is evident that Plx1 is an essential trigger kinase for Cdc25C activation at the G2/M transition. No other kinase appears to be able to substitute for this function of Plx1 in G2, although, once activated, cyclin B-Cdc2 is capable of activating Cdc25C in a positive feedback loop (Izumi and Maller, 1993). Previous experiments in vivo with antibody injection or expression of catalytically inactive Plx1 had caused only a delay in Cdc25C activation and the G2/M transition, which increased the possibility that other kinases are also instrumental for Cdc25C activation (Qian et al., 1998a). The present results show that this is not the case. In the earlier experiments, up to 90% of Plx1 activity was inhibited by antibody (Qian et al., 1998a) and yet this caused only a delay in the activation of Cdc25C. One reason it may be necessary to remove all Plx1 for assessing its functional role in Cdc25C activation is that Plx1 and Cdc25C exist in a complex (Kumagai and Dunphy, 1996; Qian, Erikson, Taieb, and Maller, unpublished data), and therefore as little as 10% of normal Plx1 activity may suffice for eventual Cdc25C activation in vivo (Qian et al.,1998a). In contrast, the same inhibitory antibody is able to cause complete disruption of spindle assembly in blastomeres (Qian et al., 1998a), perhaps reflecting a need for higher Plx1 activity for this function or antibody inhibition of Plx1 complex formation with substrate(s) relevant for spindle assembly.
The use of the PKI-stimulated prophase extract in the work reported here has allowed further delineation of the independent mechanisms that control activation of the MAPK and Plx1 kinase cascades. Although both pathways are stimulated solely as a result of PKA inhibition by PKI, the Plx1 pathway can be stimulated in the presence of UO126 and/or cycloheximide, which eliminates Mos and the MAPK pathway (Figure 3; Gross et al., 2000). Conversely, the MAPK pathway can be fully activated by GST-Mos, but this does not lead to Plx1 or Cdc25C activation (Figure 4). The clear delineation of these two kinase cascade pathways in the prophase extract has parallels in previous studies in vivo. For example, UO126 treatment of maturing oocytes to block MAPK activation also delays cyclin B-Cdc2 kinase activation and GVBD without blocking Plx1 activation (Gross et al., 2000). Similarly, active Mos is a poor inducer of GVBD in intact oocytes, generally taking longer to induce GVBD than progesterone and not working effectively in the presence of cycloheximide (Sagata et al., 1989; Yew et al., 1992; Roy et al., 1996). The discrete regulation of these two pathways by PKI in the extract with the feasibility of additional depletion/reconstruction approaches should make this system valuable in further work relating to the role that these two pathways play in the G2/M transition in Xenopus oocytes.
The results in this paper also provide new insight into feedback relationships in M phase. Our results confirm previous suggestions that Mos synthesis can be stimulated not only by progesterone/PKA inhibition but also by independent feedback loops from cyclin B-Cdc2 and active MAPK to either the complex machinery that regulates mos mRNA translation or to direct or indirect effects on Mos stability (Nishizawa et al., 1993; Gotoh et al., 1995; Matten et al., 1996; Roy et al., 1996; Frank-Vaillant et al., 1999; Mendez et al., 2000). In particular, Mos accumulation in the extract could be observed in the absence of MAPK activity (Figure 3), presumably reflecting feedback from cyclin B-Cdc2. However, Mos accumulation was not perturbed when cyclin B-Cdc2 was inhibited in M phase (Figure 6), perhaps reflecting stabilization of Mos and/or translational stimulation by MAPK (Nishizawa et al., 1993; Roy et al., 1996; Frank-Vaillant et al., 1999). In any case, the fact that inhibition of cyclin B-Cdc2 by p21Cip1 had no effect on the activation state of Plx1 or Cdc25C (Figure 6) indicates that in M phase a feedback loop from cyclin B-Cdc2 to Plx1 (Qian et al., 1998a) is not necessary for maintaining Plx1 or Cdc25C activity. Similarly, it was recently reported that in mammalian cells inhibition of cyclin B-Cdc2 activity in M phase with specific inhibitors of Cdc2 does not inhibit Plk1 activity (Smits et al., 2000). Moreover, γ-irradiation inhibits Plk activity without inhibiting cyclin B-Cdc2 activity, suggesting that the DNA damage checkpoint in mitosis impacts an upstream step in Plk activation (Smits et al., 2000). The ability to perform inhibition/reconstruction/depletion experiments in the extract system should help provide further insight into the complex feedback loops that operate in mitosis to regulate Plx1 activity.
ACKNOWLEDGMENTS
We thank Brad G. Lattes for the purified GST-p21Cip1 and GST-Mos preparations. The baculovirus-infected Sf9 cells were produced in the tissue culture-monclonal antibody core facility at the University of Colorado Cancer Center (CA46934). This work was supported by an institutional American Cancer Society grant (IRG57-001-41) to Y.-W.Q. and National Institutes of Health grants (GM26743-21 and DK28353-19) to J.L.M. F.E.T. is an Associate and J.L.M. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- Dunphy WG, Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991;67:189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- Favata F, Horiuchi YY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Fisher DL, Brassac T, Galas S, Doree M. Dissociation of MAP kinase activation and MPF activation in hormone-stimulated maturation of Xenopus oocytes. Development. 1999;126:4537–4546. doi: 10.1242/dev.126.20.4537. [DOI] [PubMed] [Google Scholar]
- Frank-Vaillant M, Jessus C, Ozon R, Maller JL, Haccard O. Two distinct mechanisms control the accumulation of cyclin B1 and Mos in Xenopus oocytes in response to progesterone. Mol Biol Cell. 1999;10:3279–3288. doi: 10.1091/mbc.10.10.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Glover DM, Hagan IM, Tavares AA. Polo-like kinases: a team that plays throughout mitosis. Genes Dev. 1998;12:3777–3787. doi: 10.1101/gad.12.24.3777. [DOI] [PubMed] [Google Scholar]
- Gotoh Y, Masuyama N, Dell K, Shirakabe K, Nishida E. Initiation of Xenopus oocyte maturation by activation of the mitogen-activated protein kinase cascade. J Biol Chem. 1995;270:25898–25904. doi: 10.1074/jbc.270.43.25898. [DOI] [PubMed] [Google Scholar]
- Gross SD, Schwab MS, Taieb FE, Lewellyn AL, Qian YW, Maller JL. The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90(Rsk) Curr Biol. 2000;10:430–438. doi: 10.1016/s0960-9822(00)00425-5. [DOI] [PubMed] [Google Scholar]
- Haccard O, Lewellyn AL, Hartley RS, Erikson E, Maller JL. Induction of Xenopus oocyte meiotic maturation by MAP kinase. Dev Biol. 1995;168:677–682. doi: 10.1006/dbio.1995.1112. [DOI] [PubMed] [Google Scholar]
- Hamanaka R, Smith MR, O'Connor PM, Maloid S, Mihalic K, Spivak JL, Longo DL, Ferris DK. Polo-like kinase is a cell cycle-regulated kinase activated during mitosis. J Biol Chem. 1995;270:21086–21091. doi: 10.1074/jbc.270.36.21086. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- Hoffmann I, Clarke PR, Marcote MJ, Karsenti E, Draetta G. Phosphorylation and activation of human cdc25-C by cdc2–cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J. 1993;12:53–63. doi: 10.1002/j.1460-2075.1993.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Kessler DS, Erikson RL. Biochemical and biological analysis of Mek1 phosphorylation site mutants. Mol Biol Cell. 1995;6:237–245. doi: 10.1091/mbc.6.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huchon D, Ozon R, Fischer EH, Demaille JG. The pure inhibitor of cAMP-dependent protein kinase initiates Xenopus laevis meiotic maturation: a 4-step scheme for meiotic maturation. Mol Cell Endocrinol. 1981;22:211–222. doi: 10.1016/0303-7207(81)90092-7. [DOI] [PubMed] [Google Scholar]
- Izumi T, Maller JL. Elimination of cdc2 phosphorylation sites in the cdc25 phosphatase blocks initiation of M-phase. Mol Biol Cell. 1993;4:1337–1350. doi: 10.1091/mbc.4.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Maller JL. Phosphorylation and activation of the Xenopus Cdc25 phosphatase in the absence of Cdc2 and Cdk2 kinase activity. Mol Biol Cell. 1995;6:215–226. doi: 10.1091/mbc.6.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Walker DH, Maller JL. Periodic changes in phosphorylation of the Xenopus cdc25 phosphatase regulate its activity. Mol Biol Cell. 1992;3:927–939. doi: 10.1091/mbc.3.8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S, Tugendreich S, Fujii M, Jorgensen PM, Watanabe N, Hoog C, Hieter P, Todokoro K. PKA and MPF-activated polo-like kinase regulate anaphase-promoting complex activity and mitosis progression. Mol Cell. 1998;1:371–380. doi: 10.1016/s1097-2765(00)80037-4. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992;70:139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- Kuramochi S, Moriguchi T, Kuida K, Endo J, Semba K, Nishida E, Karasuyama H. LOK is a novel mouse STE20-like protein kinase that is expressed predominantly in lymphocytes. J Biol Chem. 1997;272:22679–22684. doi: 10.1074/jbc.272.36.22679. [DOI] [PubMed] [Google Scholar]
- Lee KS, Yuan YL, Kuriyama R, Erikson RL. Plk is an M-phase-specific protein kinase and interacts with a kinesin-like protein, CHO1/MKLP-1. Mol Cell Biol. 1995;15:7143–7151. doi: 10.1128/mcb.15.12.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Ogg S, Xu M, Parker LL, Donoghue DJ, Maller JL, Piwnica-Worms H. cdc25+ encodes a protein phosphatase that dephosphorylates p34cdc2. Mol Biol Cell. 1992;3:73–84. doi: 10.1091/mbc.3.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohka MJ, Maller JL. Induction of nuclear envelope breakdown, chromosome condensation, and spindle formation in cell-free extracts. J Cell Biol. 1985;101:518–523. doi: 10.1083/jcb.101.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller JL. Recurring themes in oocyte maturation. Biol Cell. 1998;90:453–460. [PubMed] [Google Scholar]
- Maller JL, Krebs EG. Progesterone-stimulated meiotic cell division in Xenopus oocytes: induction by regulatory subunit and inhibition by catalytic subunit of adenosine 3′:5′-monophosphate-dependent protein kinase. J Biol Chem. 1977;252:1712–1718. [PubMed] [Google Scholar]
- Matten WT, Copeland TD, Ahn NG, Vande Woude GF. Positive feedback between MAP kinase and Mos during Xenopus oocyte maturation. Dev Biol. 1996;179:485–492. doi: 10.1006/dbio.1996.0277. [DOI] [PubMed] [Google Scholar]
- Mendez R, Hake LE, Andresson T, Littlepage LE, Ruderman JV, Richter JD. Phosphorylation of CPE binding factor by Eg2 regulates translation of c-mos mRNA. Nature. 2000;404:302–307. doi: 10.1038/35005126. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- Nebreda AR, Ferby I. Regulation of the meiotic cell cycle in oocytes. Curr Opin Cell Biol. 2000;12:666–675. doi: 10.1016/s0955-0674(00)00150-2. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Polo-like kinases: positive regulators of cell division from start to finish. Curr Opin Cell Biol. 1998;10:776–783. doi: 10.1016/s0955-0674(98)80121-x. [DOI] [PubMed] [Google Scholar]
- Nishizawa M, Furuno N, Okazaki K, Tanaka H, Ogawa Y, Sagata N. Degradation of Mos by the N-terminal proline (Pro2)-dependent ubiquitin pathway on fertilization of Xenopus eggs: possible significance of natural selection for Pro2 in Mos. EMBO J. 1993;12:4021–4027. doi: 10.1002/j.1460-2075.1993.tb06080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury C, Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- Qian YW, Erikson E, Li C, Maller JL. Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol Cell Biol. 1998a;18:4262–4271. doi: 10.1128/mcb.18.7.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian YW, Erikson E, Maller JL. Purification and cloning of a protein kinase that phosphorylates and activates the polo-like kinase Plx1. Science. 1998b;282:1701–1704. doi: 10.1126/science.282.5394.1701. [DOI] [PubMed] [Google Scholar]
- Qian YW, Erikson E, Maller JL. Mitotic effects of a constitutively active mutant of the Xenopus polo-like kinase Plx1. Mol Cell Biol. 1999;19:8625–8632. doi: 10.1128/mcb.19.12.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy LM, Haccard O, Izumi T, Lattes BG, Lewellyn AL, Maller JL. Mos proto-oncogene function during oocyte maturation in Xenopus. Oncogene. 1996;12:2203–2211. [PubMed] [Google Scholar]
- Sagata N. Introduction: meiotic maturation and arrest in animal oocytes. Semin Cell Dev Biol. 1998;9:535–537. doi: 10.1006/scdb.1998.0247. [DOI] [PubMed] [Google Scholar]
- Sagata N, Daar I, Oskarsson M, Showalter SD, Vande Woude GF. The product of the mos proto-oncogene as a candidate 'initiator' for oocyte maturation. Science. 1989;245:643–646. doi: 10.1126/science.2474853. [DOI] [PubMed] [Google Scholar]
- Shibuya EK, Polverino AJ, Chang E, Wigler M, Ruderman JV. Oncogenic ras triggers the activation of 42-kDa mitogen-activated protein kinase in extracts of quiescent Xenopus oocytes. Proc Natl Acad Sci USA. 1992;89:9831–9835. doi: 10.1073/pnas.89.20.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits VA, Klompmaker R, Arnaud L, Rijksen G, Nigg EA, Medema RH. Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat Cell Biol. 2000;2:672–676. doi: 10.1038/35023629. [DOI] [PubMed] [Google Scholar]
- Tavares AA, Glover DM, Sunkel CE. The conserved mitotic kinase polo is regulated by phosphorylation and has preferred microtubule-associated substrates in Drosophila embryo extracts. EMBO J. 1996;15:4873–4883. [PMC free article] [PubMed] [Google Scholar]
- Thomas J, Van Patten SM, Howard P, Day KH, Mitchell RD, Sosnick T, Trewhella J, Walsh DA, Maurer RA. Expression in Escherichia coli and characterization of the heat-stable inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1991;266:10906–10911. [PubMed] [Google Scholar]
- VanRenterghem B, Browning MD, Maller JL. Regulation of mitogen-activated protein kinase activation by protein kinases A and C in a cell-free system. J Biol Chem. 1994;269:24666–24672. [PubMed] [Google Scholar]
- Verlhac MH, Lefebvre C, Kubiak JZ, Umbhauer M, Rassinier P, Colledge W, Maro B. Mos activates MAP kinase in mouse oocytes through two opposite pathways. EMBO J. 2000;19:6065–6074. doi: 10.1093/emboj/19.22.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew N, Mellini ML, Vande Woude GF. Meiotic initiation by the mos protein in Xenopus. Nature. 1992;355:649–652. doi: 10.1038/355649a0. [DOI] [PubMed] [Google Scholar]