Abstract
Purpose
To investigate the prognostic value of intratumoral invariant natural killer T (iNKT) cells and interferon-gamma (IFN-γ) in hepatocellular carcinoma (HCC) after curative resection.
Experimental Design
Expression of TRAV10, encoding the Vα24 domain of iNKT cells, and IFN-γ mRNA were assessed by quantitative real-time polymerase chain reaction in tumor from 224 HCC patients undergoing curative resection. The prognostic value of these two and other clinicopathologic factors was evaluated.
Results
Either intratumoral iNKT cells and IFN-γ alone or their combination was an independent prognostic factor for OS (P = 0.001) and RFS (P = 0.001) by multivariate Cox proportional hazards analysis. Patients with concurrent low levels of iNKT cells and IFN-γ had a hazard ratio (HR) of 2.784 for OS and 2.673 for RFS. The areas under the curve of iNKT cells, IFN-γand their combination were 0.618 vs 0.608 vs 0.654 for death and 0.591 vs 0.604 vs 0.633 for recurrence respectively by receiver operating characteristic curve analysis. The prognosis was the worst for HCC patients with concurrent low levels of iNKT cells and IFN-γ, which might be related with more advanced pTNM stage and more vascular invasion.
Conclusions
Combination of intratumoral iNKT cells and IFN-γ is a promising independent predictor for recurrence and survival in HCC, which has a better power to predict HCC patients’ outcome compared with intratumoral iNKT cells or IFN-γ alone.
Introduction
Hepatocellular carcinoma (HCC) ranks fifth in frequency worldwide among all malignancies and is the third leading cause of cancer mortality [1]. Although hepatectomy is the best method to provide long-term survival for HCC patients, high postoperative recurrence rate is still a major problem [2]. Hence, investigation of predictive factors for HCC prognosis will help to screen high-risk factors influencing postoperative recurrence and develop better strategies for HCC management. Recently, it has been reported that biologic behaviors of HCC are associated with a unique immune response signature [3], increased CD4(+)CD25(+)Foxp3(+) regulatory T cells (Treg) may promote hepatitis B virus-related HCC progression [4], and intratumoral balance of regulatory and cytotoxic T cells is a promising independent predictor for recurrence and survival [5], [6]. In addition, adjuvant immunotherapy might have potential to prevent postoperative recurrence [7]. Thus,it is very important to investigate immune response of HCC for better understanding of the mechanism underlying HCC metastasis and for prevention of recurrence after curative resection.
Lymphocytes are important components of liver which is considered as an immunologic organ [8]. Natural killer T (NKT) cells are one of the richest sources for hepatic lymphocytes [8]–[10], however, with the limitations of existing methodology, only a few studies of intrahepatic NKT cells in liver immunity, especially in HCC immune response, have been reported. Invariant NKT (iNKT) cells, which have a highly restricted T-cell receptor (TCR) repertoire encoded by Vα24 Jα18 paired with a set of Vβ chains in human, make up 90%–95% of total NKT cells and have been intensely investigated in most studies about NKT cells [11], [12], [13]. The expression of gene TRAV10, encoding the Vα24 domain of the T-cell receptor that characterizes iNKT cells, can be used as a specific marker of iNKT cells [14]. A hallmark of iNKT cells is the capacity to rapidly produce a mixture of T helper type 1 (Th1,i.e. IFN-γ) and T helper type 2 (Th2, i.e. IL-4) cytokines upon TCR engagement [9], [15]–[18]. Recent studies have proposed iNKT cells as type I NKT cells that enhance antitumor responses, while type II NKT cells suppress these responses [19]. The anti-tumor immunity of iNKT cells depends on the production of IFN-γ from themselves. Consistent with the fact that iNKT cells enhance tumor immunosurveillance, a positive correlation between iNKT cells and tumor prognosis has been observed in head and neck squamous cell carcinoma [20], colorectal cancer [21] and neuroblastoma patients [22]. Although cumulative studies indicate that NKT cells contribute to the pathogenesis of cirrhosis by expressing a set of cytokines involved in the progression of fibrosis, a few studies of NKT cells in HCC have been reported [23], however, intratumoral NKT cells remain potential to kill HCC cells [24]. Adoptive transfer of NKT cells exposed ex vivo to HCC-derived antigens loaded on dendritic cells (DC) can inhibit the growth of mouse HCC in vivo [25]. CD1d-mediated stimulation of NKT cells selectively activates hepatic natural killer cells to eliminate experimentally disseminated hepatoma cells in murine liver [26]. But there is no report about iNKT cells as prognostic factor for HCC recurrence and survival.
The liver, as an immune-privileged organ, has different types and function of lymphocytes compared with that in peripheral circulation, such as CD4+ iNKT cells that secret Th2 cytokines and increase in frequency in HCC patients [27]. The fate and polarization of NKT lymphocyte might be influenced by host microenvironment signaling [28]. The imbalances of proinflammatory Th1 and anti-inflammatory Th2 cytokines in the microenvironment may play a prominent role in modulating HCC tumor progression and metastasis [29]. In the present study, we selected genes TRAV10 and IFN-γ (representative Th1 cytokines gene) as target genes and analyzed these genes’ mRNA expression using quantitative real-time polymerase chain reaction (qRT-PCR). We demonstrated that either intratumoral iNKT cells and IFN-γalone or their combination was a promising independent prognostic factor for HCC recurrence and survival, however, combination of these two parameters has a better power to predict HCC patients’ outcome.
Patients and Methods
Patients and Specimens
224 patients receiving hepatectomy in Zhongshan hospital were randomly retrieved from a prospectively collected database as previously described [5]. Meanwhile, a normal liver tissue pool from 10 healthy liver donors was constructed as the calibrator for qRT-PCR reactions. Tumor stage was determined according to the International Union Against Cancer TNM classification system [30]. The histological grade of tumor differentiation was assigned by the Edmondson grading system. Liver function was assessed by Child-Pugh score system. The pathologic features of all the cases were re-reviewed by a skillful pathologist who had no idea about the original pathology reports. The study was approved by the Zhongshan Hospital Research Ethics Committee. Informed consent was obtained according to the committee’s regulations.
Follow-up and Postoperative Treatment
All patients were observed with a median observation time of 28.0 months (range, 1.5 to 83.0 months). Follow-up procedures and treatment modalities after relapse were administered according to a uniform guideline as previously described [5], [31]. Overall survival (OS) time was defined as the interval between the dates of surgery and death; the data were censored at the last follow-up for living patients. Recurrence-free survival (RFS) time was defined as the interval between the dates of surgery and the first confirmed relapse, otherwise the data were censored on the date of death or the last follow-up.
qRT-PCR
Samples were collected and snap-frozen in liquid nitrogen immediately following surgical removal of the tissue in the operating room. Adjacent nontumorous liver tissue was always derived from the same segment of the HCC nodular with a free margin from the tumor tissue. Expression of the genes encoding Vα24 (TRAV10) and IFN-γ were detected by qRT-PCR [32]. Hypoxanthine phosphoribosyltransferase 1 (HPRT1) and TATA box binding protein (TBP) were used as housekeeping genes (HKGs) as described previously [33], [34]. Primers for these genes were listed in Table 1. For data analysis the 2−ΔΔCt method was used [35]. The fold-change in the target genes, normalized to average HKGs and calibrated to the expression of the normal liver pool, was calculated for each sample.
Table 1. Primer sequence for qRT-PCR.
| Target mRNA | Primer sequence | Annealing temperature (°C) | Fragment amplified (bp) |
| TRAV10 | F:5′AGAAAGGACGAATAAGTGCCA3′ R:5′CAGGGTCAGGGTTCTGGATA3′ | 60 | 186 |
| IFN-γ | F:5′GCAGGTCATTCAGATGTAGCGG3′ R:5′TCATGTATTGCTTTGCGTTGGA3′ | 60 | 287 |
| HPRT1 | F:5′CCTGGCGTCGTGATTAGTG3′ R:5′CAGAGGGCTACAATGTGATGG3′ | 60 | 182 |
| TBP | F:5′ACCACTCCACTGTATCCCTCC3′ R:5′CTGTTCTTCACTCTTGGCTCCT3′ | 60 | 285 |
Note. TRAV10, T cell receptor alpha variable 10; IFN-γ, interferon gamma; HPRT1, Hypoxanthine phosphoribosyltransferase 1;TBP, TATA box binding protein.
Statistical Analysis
X-tile software [36] was used to find the optimal cut-off point for the level of TRAV10 and IFN-γ mRNA expression on the basis of overall survival. Statistical analyses were performed with SPSS 15.0 software (SPSS, Chicago, IL). Kaplan-Meier analysis was used to determine the survival. Log-rank test was used to compare patients’ survival between subgroups; the Cox proportional hazards regression model was used to perform univariate and multivariate analysis. Factors showing P<0.05 by univariate analysis were adopted when multivariate analysis was performed. The relationship between survival and each variable was summarized using hazard ratios (HR) and 95% confidence intervals (95% CI). Receiver operating characteristic (ROC) curve analysis was used to determine the predictive value of the parameters.
A secondary analysis was performed to assess the relationship among iNKT cells, IFN-γ and clinicopathologic characteristics. For the comparison of individual variables, χ2 tests, Fisher’s exact tests, and nonparametric tests were carried out as appropriate. All statistical tests were two-sided. A P value of less than 0.05 was considered to indicate statistical significance.
Results
Association of Intratumoral Vα24 (TRAV10) and IFN-γ mRNA with Patients’ Clinicopathological Characteristics
The relative level of iNKT Vα24 (TRAV10) and IFN-γ mRNA expression in intratumoral tissue was significantly lower than that in adjacent nontumorous liver tissue (Table S1). The HCC patients were divided into 2 groups based on the cutoff points of intratumoral Vα24 (TRAV10) and IFN-γ mRNA expression by X-tile software as previously described [36] As shown in Table 2, there were no difference between the two groups in terms of sex, age, hepatitis history, alpha-fetal protein (AFP), alanine transaminase (ALT), Child-Pugh score, liver cirrhosis, tumor size, tumor number, tumor encapsulation or tumor differentiation; only vascular invasion and tumor pTNM stage were strongly significantly associated with the level of intratumoral Vα24 (TRAV10) or IFN-γ mRNA expression, respectively. Low intratumoral Vα24 (TRAV10) or IFN-γ mRNA expression correlated with more advanced pTNM stage and more frequent vascular invasion.
Table 2. Clinico-pathological characteristics based on the level of intratumoral iNKT cells, intratumoral IFN-γ and combination of two factors.
| Characteristic | Intratumoral iNKT cells | Intratumoral IFN-γ | Combination of intratumoral iNKT and intratumoral IFN-γ | |||||||
| Low | High | P | Low | High | P | I | II | III | P | |
| No. of patients | 86 | 138 | 154 | 70 | 79 | 82 | 63 | |||
| No.with post-recurrence | 54 | 60 | 90 | 24 | 51 | 42 | 21 | |||
| No. with death | 55 | 54 | 87 | 22 | 51 | 40 | 18 | |||
| Sex | ||||||||||
| Male | 75 | 117 | 0.697 | 135 | 57 | 0.223 | 70 | 70 | 52 | 0.587 |
| Female | 11 | 21 | 19 | 13 | 9 | 12 | 11 | |||
| Age (y) | ||||||||||
| < = 51 | 47 | 65 | 0.336 | 79 | 33 | 0.666 | 42 | 42 | 28 | 0.565 |
| >51 | 39 | 73 | 75 | 37 | 37 | 40 | 35 | |||
| Hepatitis history | ||||||||||
| No | 5 | 11 | 0.605 | 12 | 4 | 0.781 | 4 | 9 | 3 | 0.238† |
| Yes | 81 | 127 | 142 | 66 | 75 | 73 | 60 | |||
| AFP (µg/L) | ||||||||||
| >20 | 56 | 90 | 1.000 | 104 | 42 | 0.292 | 52 | 56 | 38 | 0.600 |
| < = 20 | 30 | 48 | 50 | 28 | 27 | 26 | 25 | |||
| ALT (U/L) | ||||||||||
| < = 40 | 41 | 77 | 0.272 | 80 | 38 | 0.774 | 37 | 47 | 34 | 0.400 |
| >40 | 45 | 61 | 74 | 32 | 42 | 35 | 29 | |||
| Child-Pugh score | ||||||||||
| A | 85 | 137 | 1.000† | 153 | 69 | 0.528† | 78 | 82 | 62 | 0.547† |
| B | 1 | 1 | 1 | 1 | 1 | 0 | 1 | |||
| Liver cirrhosis | ||||||||||
| Yes | 77 | 120 | 0.675 | 133 | 64 | 0.377 | 71 | 68 | 58 | 0.199 |
| No | 9 | 18 | 21 | 6 | 8 | 14 | 5 | |||
| Tumor size (cm) | ||||||||||
| >5 | 48 | 62 | 0.131 | 82 | 28 | 0.083 | 45 | 40 | 25 | 0.123 |
| < = 5 | 38 | 76 | 72 | 42 | 34 | 42 | 38 | |||
| Tumor number | ||||||||||
| Multiple | 23 | 32 | 0.632 | 41 | 14 | 0.319 | 21 | 22 | 12 | 0.488 |
| Single | 63 | 106 | 113 | 56 | 58 | 60 | 51 | |||
| Tumor encapsulation | ||||||||||
| None | 47 | 71 | 0.681 | 84 | 34 | 0.471 | 44 | 43 | 31 | 0.743 |
| complete | 39 | 67 | 70 | 36 | 35 | 39 | 32 | |||
| Tumor differentiation | ||||||||||
| III-IV | 41 | 60 | 0.582 | 70 | 31 | 0.886 | 38 | 35 | 28 | 0.782 |
| I-II | 45 | 78 | 84 | 39 | 41 | 47 | 35 | |||
| Vascular invasion | ||||||||||
| Yes | 52 | 52 | 0.001* | 81 | 23 | 0.006* | 48 | 37 | 19 | 0.001* |
| No | 34 | 86 | 73 | 47 | 31 | 45 | 44 | |||
| pTNM stage | ||||||||||
| IIIa | 28 | 27 | 0.038* | 47 | 8 | 0.002* | 26 | 23 | 6 | 0.004* |
| I+II | 58 | 111 | 107 | 62 | 53 | 59 | 57 | |||
Pearson χ2 test, †Fisher’s exac tests.
Abbreviations: iNKT cells, invariant natural killer T cells; IFN-γ, interferon gamma; AFP, alpha-fetoprotein; ALT, alanine aminotransferase; TNM, tumor-node-metastasis.
Prognostic Factors
At the time of the last follow-up, 114 patients had tumor recurrence, including 99 intrahepatic recurrences and 15 extrahepatic metastases and 109 patients had died, including 22 patients who died of liver failure without record of tumor recurrence. The 1-, 3-, 5-year OS rates were 75.9%, 52.7% and 45.7%, respectively; and the 1-, 3-, 5-year RFS rates were 62.4%, 46.0% and 42.0%, respectively.
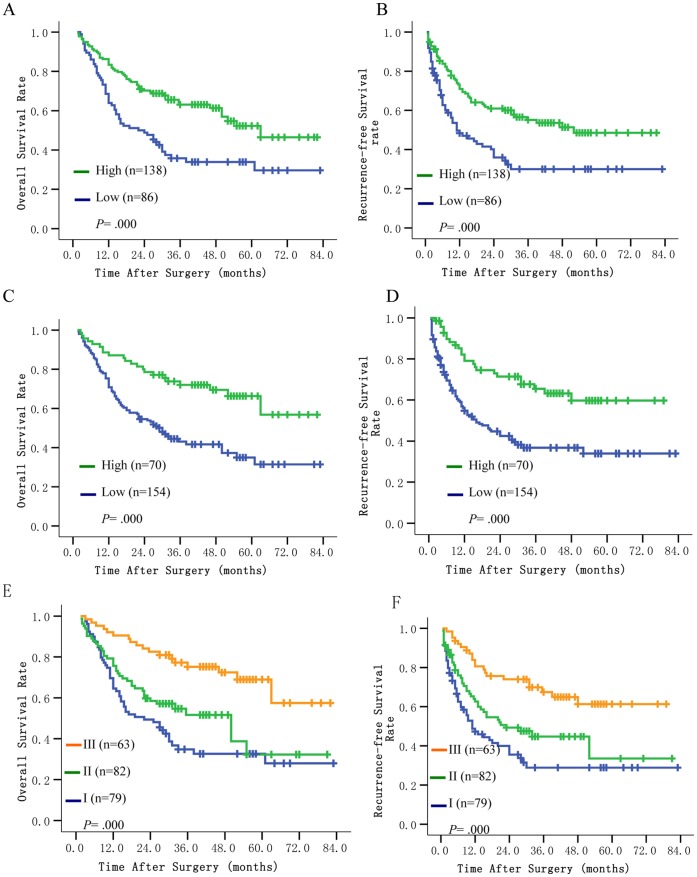
In univariate analysis, AFP, tumor size, tumor number, tumor encapsulation, presence of vascular invasion, pTNM stage, intratumoral iNKT cells, intratumoral IFN-γand combination of intratumoral iNKT cells and IFN-γ were associated with OS and RFS. Liver cirrhosis and tumor differentiation were also associated with OS (Table 3). The median OS and RFS time was prominently shorter for patients with low intratumoral iNKT cells than those with high intratumoral iNKT cells (22.0 months vs. 63.0 months and 11.0 months vs. 52.0 months, respectively; P<0.001 and P<0.001, respectively; Fig. 1A and 1B). The median OS and RFS time was significantly longer for patients with high intratumoral IFN-γ than those with low intratumoral IFN-γ (>82.0 months vs. 30.0 months and >79.0 months vs. 16.0 months, respectively; P<0.001 and P<0.001, respectively; Fig. 1C and 1D).
Table 3. Univariate analyses of the factors associated with survival and recurrence.
| Factor | OS | RFS | ||||
| Hazard Ratio | 95%CI | P | Hazard Ratio | 95%CI | P | |
| Sex: male v female | 1.125 | 0.652 to 1.940 | 0.673 | 1.449 | 0.813 to 2.583 | 0.209 |
| Age: < = 51 v >51 years | 1.339 | 0.918 to 1.952 | 0.129 | 1.307 | 0.904 to 1.888 | 0.154 |
| Hepatitis history: no v yes | 0.894 | 0.434 to 1.840 | 0.761 | 1.410 | 0.774 to 2.566 | 0.261 |
| Liver cirrhosis: yes v no | 2.578 | 1.198 to 5.549 | 0.015 | 1.646 | 0.883 to 3.066 | 0.117 |
| ALT (U/L): < = 40 v >40 | 0.820 | 0.563 to 1.193 | 0.299 | 0.942 | 0.653 to 1.361 | 0.752 |
| AFP(µg/L) : >20 v < = 20 | 1.976 | 1.282 to 3.046 | 0.002 | 1.907 | 1.260 to 2.887 | 0.002 |
| Child-Pugh score: A v B | 0.779 | 0.108 to 5,597 | 0.804 | 0.667 | 0.093 to 4.787 | 0.687 |
| Tumor size (cm) : >5 v < = 5 | 2.282 | 1.548 to 3.363 | 0.000 | 2.152 | 1.477 to 3.136 | 0.000 |
| Tumor number: multiple v single | 1.555 | 1.028 to 2.353 | 0.037 | 1.737 | 1.162 to 2.597 | 0.007 |
| Tumor encapsulation: none v complete | 1.573 | 1.071 to 2.310 | 0.021 | 2.045 | 1.393 to 3.001 | 0.000 |
| Tumor differentiation: III-IV v I-II | 1.733 | 1.187 to 2.529 | 0.004 | 1.278 | 0.884 to 1.847 | 0.192 |
| Vascular invasion: yes v no | 3.673 | 2.457 to 5.492 | 0.000 | 3.616 | 2.458 to 5.318 | 0.000 |
| pTNM stage: IIIa v I-II | 4.621 | 3.122 to 6.841 | 0.000 | 5.221 | 3.506 to 7.776 | 0.000 |
| iNKT cells: low v high | 2.045 | 1.403 to 2.982 | 0.000 | 1.981 | 1.368 to 2.867 | 0.000 |
| IFN-γ: low v high | 2.496 | 1.557 to 4.001 | 0.000 | 2.444 | 1.553 to 3.847 | 0.000 |
| Combination of iNKT and IFN-γ (I v II v III) | 0.000 | 0.000 | ||||
| I v III | 3.349 | 1.950 to 5.752 | 0.000 | 3.141 | 1.882 to 5.242 | 0.000 |
| II v III | 2.383 | 1.361 to 4.171 | 0.002 | 2.139 | 1.263 to 3.620 | 0.005 |
Abbreviations: OS, overall survival; RFS, recurrence-free survival; ALT, alanine aminotransferase; AFP, alpha-fetoprotein; TNM, tumor-node-metastasis; iNKT cells, invariant natural killer T cells; IFN-γ, interferon gamma.
Figure 1. Survival curve of HCC patients.
Cumulative overall and recurrence-free survival rate of HCC patients with intratumoral iNKT cells (A, B), intratumoral IFN-γ (C, D) and combination of intratumoral iNKT cells and IFN-γ (E, F). High intratumoral iNKT cells, high intratumoral IFN-γ or combination of both high was associated with prolonged overall and recurrence-free survival.
In multivariate analysis, liver cirrhosis, tumor size, tumor differentiation and vascular invasion were independent risk factors for OS and tumor number, tumor encapsulation and vascular invasion were independent risk factors for RFS (Table 4). Low intratumoral iNKT cells was an independent risk factor for both OS (hazard ratio [HR] = 1.603, 95%CI: 1.091 to 2.356, P = 0.016) and RFS (HR = 1.786, 95%CI: 1.223 to 2.607, P = 0.003) (Table S2). Low intratumoral IFN-γ was also an independent risk factor for OS (HR = 2.291, 95%CI: 1.417 to 3.705, P = 0.001) and RFS (HR = 2.134, 95%CI: 1.349 to 3.375, P = 0.001) (Table S3). There was significant correlation between intratumoral iNKT cells and intratumoral IFN-γ (r = 0.634, P<0.001, Spearman’s correlation test).
Table 4. Multivariate analyses of the factors associated with survival and recurrence.
| Factor | OS | RFS | ||||
| Hazard Ratio | 95%CI | P | Hazard Ratio | 95%CI | P | |
| Liver cirrhosis: yes v no | 2.578 | 1.180 to 5.635 | 0.018 | NA | ||
| AFP(µg/L):>20 v < = 20 | NS | NS | ||||
| Tumor size (cm): >5 v < = 5 | 1.562 | 1.025 to 2.379 | 0.038 | NS | ||
| Tumor number: multiple v single | NS | 1.570 | 1.036 to 2.379 | 0.034 | ||
| Tumor encapsulation: none v complete | NS | 1.533 | 1.025 to 2.293 | 0.038 | ||
| Tumor differentiation: III-IV v I-II | 1.631 | 1.110 to 2.395 | 0.013 | NA | ||
| Vascular invasion: yes v no | 2.579 | 1.661 to 4.005 | 0.000 | 2.950 | 1.973 to 4.411 | 0.000 |
| pTNM stage: IIIa v I-II | NA | NA | ||||
| Combination of intratumoral iNKT and IFN-γ (I v II v III) | 0.001 | 0.001 | ||||
| I v III | 2.784 | 1.603 to 4.835 | 0.000 | 2.673 | 1.588 to 4.499 | 0.000 |
| II v III | 2.481 | 1.410 to 4.366 | 0.002 | 1.925 | 1.131 to 3.278 | 0.016 |
Abbreviations: OS, overall survival; RFS, recurrence-free survival; NA, not adopted; NS, not significant; AFP, alpha-fetoprotein; TNM, tumor-node-metastasis; iNKT cells, invariant natural killer T cells; IFN-γ, interferon gamma. NOTE: We evaluated the prognostic factors that affected overall survival and recurrence-free survival using univariate analysis, and entered variables that showed statistical significance in the univariate analysis into multivariate analysis using the Cox proportional hazard regression model.
Combination of Intratumoral iNKT Cells and IFN-γ and ROC Analysis
The combined influence of intratumoral iNKT cells and IFN-γ was evaluated. Based on the cutoff value for intratumoral iNKT cells and IFN-γ mRNA expression, patients were classified into three groups: group I (n = 79), low iNKT and low IFN-γ (neither high); group II (n = 82), high iNKT but low IFN-γ or low iNKT but high IFN-γ (either high); group III (n = 63), high iNKT and high IFN-γ (both high). Differences in both OS (P<0.001) and RFS (P<0.001) were significant among three groups (Fig. 1E and 1F). The 1-, 3-, 5-year OS rates were 69.6%, 34.8%,32.6% in group I, 75.6%, 51.6%, 32.3% in group II and 90.5%, 75.2%, 69.0% in group III. The median OS time is 22.0, 50.0 and >82.0 months in group I, group II and group III, respectively. The 1-, 3-, 5-year RFS rates were 47.3%, 28.9%, 28.9% in group I, 62.6%, 44.7%, 33.6% in group II and 80.6%, 67.5%, 61.3% in group III. The median RFS time is 11.0, 23.0 and >79.0 months in group I, group II and group III, respectively. The prognosis was the worst for HCC patients in group I, which might be related with more advanced pTNM stage and more vascular invasion in HCC (Table 2).
Combination of intratumoral iNKT cells and IFN-γ was also an independent risk factor for OS (group I vs. III, HR = 2.784, 95%CI: 1.603 to 4.835, P = 0.001) and RFS (group I vs. III, HR = 2.673, 95%CI: 1.588 to 4.449, P = 0.001) by multivariate analysis (Table 4). The area under the curve (AUC) of combination of intratumoral iNKT cells and IFN-γ was largest, compared with intratumoral iNKT cells or IFN-γ alone. The area under the curve of this combination was 0.654 (95% CI, 0.582 to 0.725; P<0.001) for death and 0.633 (95% CI, 0.561 to 0.706; P = 0.001) for recurrence (Table 5). The combination of intratumoral iNKT cells and IFN-γ had a better power to predict HCC patients’ outcomes compared with intratumoral iNKT cells or IFN-γ alone on the basis of not only hazard ratio (Table 4, Table S2 and Table S3) but also the area under the ROC curve (Table 5). Although the HR of intratumoral iNKT cells and IFN-γ were less than or comparable with HR of vascular invasion and pTNM stage and the area under ROC of vascular invasion and pTNM stage were more than the area of intratumoral iNKT cells and IFN-γ, the combination of intratumoral iNKT cells and IFN-γ was also an independent prognostic factor of poor prognosis in HCC patients with vascular invasion and pTNM stage II or III (Table S4, Table S5, Table S6 and Table S7).
Table 5. The predictive values of the adopted factors with pTNM stage for OS and RFS by ROC analysis.
| Variables | OS | RFS | ||||
| Area | 95%CI | P | Area | 95%CI | P | |
| Vascular invasion | 0.700 | 0.631 to 0.770 | 0.000 | 0.670 | 0.599 to 0.742 | 0.000 |
| Combination of intratumoral iNKT cells and IFN-γ | 0.654 | 0.582 to 0.725 | 0.000 | 0.633 | 0.561 to 0.706 | 0.001 |
| Tumor encapsulation | NA | 0.616 | 0.542 to 0.689 | 0.003 | ||
| Tumor size | 0.620 | 0.547 to 0.694 | 0.002 | NA | ||
| Tumor number | NA | 0.554 | 0.478 to 0.629 | 0.165 | ||
| Liver cirrhosis | 0.555 | 0.480 to 0.630 | 0.156 | NA | ||
| Tumor differentiation | 0.597 | 0.523 to 0.671 | 0.012 | NA | ||
| pTNM stage | 0.672 | 0.600 to 0.743 | 0.000 | 0.643 | 0.571 to 0.715 | 0.000 |
| intratumoral iNKT cells | 0.618 | 0.544 to 0.691 | 0.002 | 0.591 | 0.517 to 0.666 | 0.018 |
| Intratumoral IFN-γ | 0.608 | 0.534 to 0.682 | 0.005 | 0.604 | 0.530 to 0.678 | 0.007 |
Abbreviations: OS, overall survival; RFS, recurrence-free survival; NA, not adopted; TNM, tumor-node-metastasis; iNKT cells, invariant natural killer T cells; IFN-γ, interferon gamma.
Discussion
The tumor-related factors, conventionally considered to be of prognostic value in HCC, are reasonably predictive of survival in our patient population as a whole. Our results show that either intratumoral iNKT cells or intratumoral IFN-γ is a promising factor highly predictive of OS and RFS for HCC. It also indicates that HCC patients with concurrent high intratumoral iNKT cells and IFN-γ showed significantly longer OS and RFS compared with concurrent low group, probably associated with less vascular invasion and earlier pTNM stage in this group. Vascular invasion and pTNM stage were relatively putative clinicopathologic markers of HCC invasiveness and predictive factors for recurrence in literature [37]–[40]. The combination of intratumoral iNKT cells and IFN-γ had a better power to predict HCC patients’ outcome compared with intratumoral iNKT cells or IFN-γ alone. The present results suggest that iNKT cells, which show regulatory activity on tumor, display inhibiting tumor growth and metastasis in local Th1 microenvironment. But there was different opinion about iNKT and IFN-γ as prognostic factor for HCC prognosis. Cariant et al [41] have previously reported that the higher prevalence of intratumoral iNKT cells and increased IFN-γ in adjacent nontumorous liver were associated with shorter OS and RFS, respectively. To explain difference between two studies, one of the reasons was that the related background of hepatocarcinogenesis was significantly different, more than 80% of the patients were combined with hepatitis B virus infection in this study, while the majority of the patients were hepatitis C virus infected and the hepatitis B virus infection rate was less than 10% in Carian’s study [41]. Another reason was that iNKT cells had a dual immune regulatory function, as Carian et al [41] had shown, iNKT cells producing Th2 cytokines might inhibit anti-tumor immunity through inhibiting expansion of tumor antigen-specific CD8 T cells, but iNKT cells could promote successful tumor immunosurveillance through secreting IFN-γ or under the Th1 microenvironment, which was consistent with the present study. Besides, much more cases would be investigated to further assess the prognostic value of intratumoral iNKT cells for HCC patients in Carian’s study.
IFN-γ is crucial for tumor control, and is important part of innate and adoptive immune response. This cytokine is produced by NK cells as well as CD4+ Th1 cells and CD8+ cytotoxic T lymphocyte (CTL) [42]. Locally low IFN-γ level may result from iNKT cells’ impaired function or decreased adoptive immune response. We assumed that the influence of high intratumoral iNKT cells on prognosis might probably be counteracted by simultaneously low intratumoral IFN-γ, and vice versa. In the present study, 1-, 3-, 5-year OS and RFS rates of HCC patients were significantly lower in group II than that in group III, which supports our hypothesis. Although the mechanism of interaction between intratumoral iNKT cells and IFN-γ is poorly understood, anti-metastasis effect of iNKT cells might surpass potential of pro-metastasis only under local Th1 cytokine milieu since iNKT cells secrete Th1 and Th2 cytokines at the same time.
Animal studies indicate that adoptive transfer of NKT cells exposed ex vivo to HCC-derived antigens loaded on dendritic cells (DC) can inhibit the growth of mouse HCC in vivo [25]. In some clinical trials, immunotherapy with alpha-galactosylceramide (α-GalCer) or α-GalCer–pulsed DCs can induce the activation of Vα24 NKT cells in situ [43]–[46]. A phase I study using in vitro expanded Vα24 NKT cells in patients with recurrent or advanced non-small cell lung cancer confirms that immunotherapy of activated Vα24 NKT cells administration is well tolerated with minor adverse events and in vivo immunologic responses [47]. HCC is difficult to cure due to high recurrence rate. Adjuvant immunotherapy might be a promising treatment since HCC is resistant to conventional chemotherapy. From this point of view, HCC patients undergoing curative resection may thus be candidates for immunotherapy aimed at iNKT cells activation and microenvironment IFN-γ up-regulation.
By reviewing literatures, Tachibana et al [21] have reported that intratumoral NKT cell infiltration was an independent prognostic factor for the overall and disease-free survival rate by immunohistochemistry in formalin-fixed and paraffin-embedded colorectal carcinomas tissues, but iNKT cell infiltration immunohistochemistry in HCC was not successfully carried out according to the manufacturer’s instructions with modifications [21]. Moreover approach to analysis intratumoral iNKT cell by flow cytometry might not be suitable in large sample research for the clinical outcome of HCC. Furthermore, TRAV10 gene encoding Vα24 domain of iNKT cells and its mRNA expression level has significant correlation with the number of iNKT cells [14], [22], so we measured the expression of TRAV10 gene mRNA by qRT-PCR to evaluate the prognostic value of iNKT cells for HCC patients on the basis of long-term extremely low temperature preserved specimens. In this study, we selected our institute’s cultured cells such as hepatic stellate cells (LX2), hepatocellular carcinoma cells (MHCC97L, MHCC97H, HepG2) as a negative control, the qRT-PCR did not amplify expected PCR products from these cells. In addition, real time PCR was performed using detection kits for the TRBV25-1 gene encoding the Vβ11 domain of iNKT cells as the following primer [14], F-GCACAGTTTGGGCTTTTATTTT,R-GAGAACATTCCAGAGTGATCTTC, the results was similar with the present study (data was not shown). With the improvement of iNKT cells isolation in the tissue [48], further perspective investigation is to directly detect intratumoral iNKT cells frequency or to obtain intratumoral iNKT cells. Further investigation of interaction between iNKT cells from HCC patients with different metastatic potential and IFN-γ ex vivo would give us more understanding of microenvironment influence on activity of iNKT cells.
Taken together, our results showed that combination of intratumoral iNKT cells and IFN-γ is a promising independent predictor for recurrence and survival in HCC. This combination had a better power to predict HCC patients’ outcome compared with intratumoral iNKT cells or IFN-γ alone. Adoptive transfer of iNKT cells and supply of Th1 microenvironment by IFN-γ subcutaneous injection may provide a promising adjuvant immunotherapeutic strategy for prevention of HCC after curative resection.
Supporting Information
The relative level of iNKT Vα24 (TRAV10) and IFN-γ mRNA expression.
(DOC)
Multivariate analyses of the factors associated with survival and recurrence.
(DOC)
Multivariate analyses of the factors associated with survival and recurrence.
(DOC)
Recurrence-free survival time among different groups.
(DOC)
Recurrence-free survival time among different groups.
(DOC)
Overall survival time among different groups.
(DOC)
Overall survivor time among different groups.
(DOC)
Funding Statement
Supported by grants from the National Key Sci-Tech Special Project of China (No. 2012ZX10002010-001/002, 2012ZX10002011-002, 2012ZX10002013-005 and 2012ZX10002-016), the Major Program of the National Nature Science Foundation of China (No. 81030038), and the National Natural Science Foundation of China (No. 81141087 and 81071707). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jemal A, Bray F Center MM, Ferlay J, Ward E, et al (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Bruix J, Sherman M (2010) Management of hepatocellular carcinoma: an update. Hepatology 53: 1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Budhu A, Forgues M, Ye QH, Jia HL, He P, et al. (2006) Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell 10: 99–111. [DOI] [PubMed] [Google Scholar]
- 4. Fu J, Xu D, Liu Z, Shi M, Zhao P, et al. (2007) Increased regulatory T cells correlated with CD8 T-cell impaired and poor survival in hepatocellular carcinoma. Gastroenterology 132: 2328–2339. [DOI] [PubMed] [Google Scholar]
- 5. Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, et al. (2007) Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 25: 2586–2593. [DOI] [PubMed] [Google Scholar]
- 6. Sasaki A, Tanaka F, Mimori K, Inoue H, Kai S, et al. (2008) Prognostic value of tumor-infiltrating FOXP3+ regulatory T cells in patients with hepatocellular carcinoma. Eur J Surg Oncol 34: 173–179. [DOI] [PubMed] [Google Scholar]
- 7. Butterfield LH (2007) Recent advances in immunotherapy for hepatocellular cancer. Swiss Med Wkly 137: 83–90. [DOI] [PubMed] [Google Scholar]
- 8. Racanelli V, Rehermann B (2006) The liver as an immunological organ. Hepatology 43: S54–S62. [DOI] [PubMed] [Google Scholar]
- 9. Gao B, Jeong W, Tian Z (2008) Liver: an organ with predominant innate immunity. Hepatology 47: 729–736. [DOI] [PubMed] [Google Scholar]
- 10. Exley MA, Koziel MJ (2004) To be or not to be NKT: natural killer T cells in the liver. Hepatology 40: 1033–1040. [DOI] [PubMed] [Google Scholar]
- 11. Kronenberg M (2005) Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol 26: 877–900. [DOI] [PubMed] [Google Scholar]
- 12. Seino K, Taniguchi M (2005) Functionally distinct NKT cell subsets and subtypes. J Exp Med 202: 1623–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L (2004) NKT cells: what’s in a name? Nat Rev Immunol 4: 231–237. [DOI] [PubMed] [Google Scholar]
- 14. Vijayanand P, Seumois G, Pickard C, Powell RM, Anqco G, et al. (2007) Invariant natural killer T cells in asthma and chronic obstructive pulmonary. N Engl J Med 356: 1410–1422. [DOI] [PubMed] [Google Scholar]
- 15. Bendelac A, Savage PB, Teyton L (2007) The biology of NKT cells. Annu Rev Immunol 25: 297–336. [DOI] [PubMed] [Google Scholar]
- 16. Van Kaer L (2007) NKT cells: T lymphocytes with innate effector functions. Curr Opin Immunol 19: 1–11. [DOI] [PubMed] [Google Scholar]
- 17. Brigl M, Brenner MB (2004) CD1: antigen presentation and T cell function. Annu Rev Immunol 22: 817–890. [DOI] [PubMed] [Google Scholar]
- 18. Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H (2003) The regulatory role of Va14 NKT cells in innate and acquired immune response. Annu Rev Immunol 21: 483–513. [DOI] [PubMed] [Google Scholar]
- 19. Terabe M, Berzofsky JA (2007) NKT cells in immunoregulation of tumor immunity: a new immunoregulatory axis. Trends Immunol 28: 491–496. [DOI] [PubMed] [Google Scholar]
- 20. Molling JW, Langius JA, Langendijk JA, Leemans CR, Bontkes HJ, et al. (2007) Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol 25: 862–868. [DOI] [PubMed] [Google Scholar]
- 21. Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, et al. (2005) Increased intratumor Valpha24-positive natural killer T cells: A prognostic factor for primary colorectal carcinomas. Clin Cancer Res 11: 7322–7327. [DOI] [PubMed] [Google Scholar]
- 22.Metelitsa LS, Wu HW, Wang H, Yang Y, Warsi Z, et al.. (2004) Natural killer T cells infiltrate neutoblastomas expressing the chemokine CCL2. J Exp Med 199; 1213–1221. [DOI] [PMC free article] [PubMed]
- 23. Notas G, Kisseleva T, Brenner D (2009) NK and NKT cells in liver injury and fibrosis. Clin Immunol 130: 16–26. [DOI] [PubMed] [Google Scholar]
- 24. Shibolet O, Alper R, Zlotogarov L, Thalenfeld B, Engelhardt D, et al. (2003) NKT and CD8 lymphocytes mediated suppression of hepatocellular carcinoma growth via tumor antigen-pulsed dendritic cells. Int J Cancer 106: 236–243. [DOI] [PubMed] [Google Scholar]
- 25. Margalit M, Shibolet O, Klein A, Elinav E, Alper R, et al. (2005) Suppression of hepatocellular carcinoma by transplantation of ex-vivo immune-modulated NKT lymphocytes. Int J Cancer 115: 443–449. [DOI] [PubMed] [Google Scholar]
- 26. Miyagi T, Takehara T, Tatsumi T, Kanto T, Suzuki T, et al. (2003) CD1d-mediated stimulation of natural killer T cells selectively activates hepatic natural killer cells to eliminate experimentally disseminated hepatoma cells in murine liver. Int J Cancer 106: 81–89. [DOI] [PubMed] [Google Scholar]
- 27. Bricard G, Cesson V, Devevre E, Bouzourene H, Barbey C, et al. (2009) Enrichment of human CD4+ V(alpha)24/Vbeta11 invariant NKT cells in intrahepatic malignant tumors. J Immunol 182: 5140–5151. [DOI] [PubMed] [Google Scholar]
- 28. Zigmond E, Shalev Z, Pappo O, Alper R, Zolotarov L, et al. (2008) NKT lymphocyte polarization determined by microenvironment signaling: a role for CD8+ lymphocytes and beta-glycosphingolipids. J Autoimmun 31: 188–195. [DOI] [PubMed] [Google Scholar]
- 29. Budhu A, Wang XW (2006) The role of cytokines in hepatocellular carcinoma. J Leukoc Biol 80: 1197–1213. [DOI] [PubMed] [Google Scholar]
- 30.Sobin LH, Wittekind C (2002) TNM classification of malignant tumors (ed 6). New York, NY, Wiley-Liss, PP 81–83.
- 31. Sun HC, Zhuang PY, Qin LX, Ye QH, Wang L, et al. (2007) Incidence and prognostic values of lymph node metastasis in operable hepatocellular carcinoma and evaluation of routine complete lymphadenectomy. J Surg Oncol 96: 37–45. [DOI] [PubMed] [Google Scholar]
- 32. Ju MJ, Qiu SJ, Fan J, Xiao YS, Gao Q, et al. (2009) Peritumoral activated hepatic stellate cells predict poor clinical outcome in hepatocellular carcinoma after curative resection. Am J Clin Pathol 131: 498–510. [DOI] [PubMed] [Google Scholar]
- 33. Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, et al. (2009) Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 15: 971–979. [DOI] [PubMed] [Google Scholar]
- 34. Gao Q, Wang XY, Fan J, Qiu SJ, Zhou J, et al. (2008) Selection of reference genes for real-time PCR in human hepatocellular carcinoma tissues. J Cancer Res Clin Oncol 134: 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 36. Camp RL, Dolled-Filhart M, Rimm DL (2004) X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10: 7252–7259. [DOI] [PubMed] [Google Scholar]
- 37. Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, et al. (2005) Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg 242: 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, et al. (2006) Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg 243: 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poon RT, Fan ST, Lo CM, Liu CL, Wong J (2007) Difference in tumor invasiveness in cirrhotic patients with hepatocellular carcinoma fulfilling the Milan criteria treated by resection and transplantation: impact on long-term survival. Ann Surg 245: 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shah SA, Cleary SP, Wei AC, Yang J, Taylor BR, et al. (2007) Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 141: 330–339. [DOI] [PubMed] [Google Scholar]
- 41. Cariani E, Pilli M, Zerbini A, Rota C, Olivani A, et al. (2012) Immunological and molecular correlates of disease recurrence after liver resection for hepatocellular carcinoma. PLoS One 7: e32493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schoenborn JR, Wilson CB (2007) Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol 96: 41–101. [DOI] [PubMed] [Google Scholar]
- 43. Giaccone G, Punt CJ, Ando Y, Ruiiter R, Nishi N, et al. (2002) A phase I study of the natural killer T-cell ligand a-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res 8: 3702–3709. [PubMed] [Google Scholar]
- 44. Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, et al. (2004) Therapeutic activation of Vα24+Vβ11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood 103: 383–389. [DOI] [PubMed] [Google Scholar]
- 45. Chang DH, Osman K, Connolly J, Kukreia A, Krasovsky J, et al. (2005) Sustained expansion of NKT cells and antigen-specificT cells after injection of a-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med 201: 1503–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, et al. (2005) A phase I study of a-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res 11: 1910–1917. [DOI] [PubMed] [Google Scholar]
- 47. Motohashi S, Ishikawa A, Ishikawa E, Otsuji M, Izasa T, et al. (2006) A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res 12: 6079–6086. [DOI] [PubMed] [Google Scholar]
- 48. Watarai H, Nakagawa R, Omori-Miyake M, Dashtsoodol N, Taniguchi M (2008) Methods for detection, isolation and culture of mouse and human invariant NKT cells. Nat Protoc 3: 70–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The relative level of iNKT Vα24 (TRAV10) and IFN-γ mRNA expression.
(DOC)
Multivariate analyses of the factors associated with survival and recurrence.
(DOC)
Multivariate analyses of the factors associated with survival and recurrence.
(DOC)
Recurrence-free survival time among different groups.
(DOC)
Recurrence-free survival time among different groups.
(DOC)
Overall survival time among different groups.
(DOC)
Overall survivor time among different groups.
(DOC)