Abstract
Plant cells possess three DNA-containing compartments, the nucleus, the mitochondria and the plastids. Accordingly, plastid gene regulation is fairly complex. Albeit plastids retained their own genome and prokaryotic-type gene expression system by a plastid-encoded RNA polymerase (PEP), they need a second nuclear-encoded plastid transcription activity, NEP. Candidate genes for putative NEP catalytic subunits have been cloned in Arabidopsis thaliana (AtRpoTp) and Nicotiana sylvestris (NsRpoTp). To provide evidence for RpoTp as a gene encoding a NEP catalytic subunit, we introduced the AtRpoTp and NsRpoTp cDNAs into the tobacco nucleus under the control of the strong constitutive CaMV 35S promoter. Analysis of transcription from NEP and PEP promoters in these transgenic plants using primer extension assays revealed enhanced transcription from typical type I NEP promoters as PatpB-289 in comparison with the wild type. These data provide direct evidence that RpoTp is a catalytic subunit of NEP and involved in recognition of a distinct subset of type I NEP promoters.
INTRODUCTION
Plastids evolved from ancestral cyanobacteria by gradual conversion of the endosymbiont to a plant organelle (1). The plastid genome contains functional rpo genes encoding the homologs of the eubacterial RNA polymerase α, β and β′ subunits (2–4), which form the core of the plastid-encoded RNA polymerase (PEP) (5–7). Sigma factors interact with PEP to confer promoter-specific binding and transcription specificity (8,9). Nonetheless, in surprising contrast to their eubacterial ancestors, this RNA polymerase is not sufficient to transcribe the chloroplast genome in higher plants.
The existence of a second, plastid-localized, nuclear-encoded transcription activity [nuclear-encoded plastid RNA polymerase (NEP)] has been established by analyzing mutant and transplastomic plants, respectively, that lack PEP yet transcribe a subset of plastid genes (10–17). It appeared that for tobacco rpoB NEP promoter function, a CRT motif is critical in vitro. Sequence alignments revealed that most NEP promoters contain such a core sequence (YRTA), similar to plant mitochondrial promoters (type Ia) (18–20). A subclass of NEP promoters shares a GAA-box motif upstream of the YRTA motif, which was shown to be important in transcription from the tobacco PatpB-289 NEP promoter (type Ib) (21). The pattern of plastid NEP drug resistance was shown to be different from bacterial-type RNA polymerases and analogous to the phage T7 RNA polymerase, pointing to phage-type RNA polymerases as candidates for NEP activity (22). Genes encoding organellar phage-type RNA polymerases have been found in several higher plant genomes. Aside from mitochondrial targeting (RpoTm), it was shown that a second RpoT enzyme is targeted into plastids both in monocots and in dicots (RpoTp) (23–29). An RNA polymerase activity approximately of the size of RpoTp has previously been enriched from spinach chloroplasts (30). More recently, immunoblot analysis and an antibody-linked polymerase assay indicated that the maize RpoTp indeed specifies a chloroplast-localized enzyme (27). Interestingly, a third RpoT gene found exclusively in dicots (RpoTmp) has been shown to be dually targeted both into mitochondria and plastids (24,31).
Though it is commonly suggested that the phage-type RNA polymerases account for NEP transcription activity, direct evidence for an identity of RpoTp (and/or RpoTmp) with NEP is still lacking (18,19). Also, some other aspects of NEP transcription remain uncertain. Non-consensus NEP promoters, for example, the type II PclpP-53 in tobacco, do not contain a YRTA motif (32). Furthermore, some plastid tRNA genes seem to be transcribed from internal promoters (19). Finally, there is no indication regarding why dicots have two plastid-targeted phage-type RNA polymerases and if they fulfill a distinct role in plastid gene expression.
To investigate the effect of overexpression of the putative NEP catalytic subunit RpoTp on NEP promoter activity, Nicotiana sylvestris and Arabidopsis thaliana RpoTp cDNAs were cloned into a constitutive expression cassette. Analysis of transcription from NEP and PEP promoters in these mutant plants using primer extension assays revealed that transcription from typical type I NEP promoters, such as PatpB-289, is enhanced in comparison with the PEP promoter PatpB-255. These data provide direct evidence that RpoTp is a catalytic subunit of the nuclear-encoded plastid transcription machinery NEP.
MATERIALS AND METHODS
Plasmid construction
Plasmid pKL85 was constructed by PCR amplification of the AtRpoTp sequence (GenBank accession no. Y08463) (23) from an A.thaliana cDNA library (Arabidopsis Biological Resource Center, Columbus, OH; CD4-15/16) using primers 703, ccctctagaATGGCTTCCGTCGCGGCG and 704, ccccccgggctcgagttaGTTGAAGAAGTAcTGGG, digested with XbaI and SmaI, and cloned into the XbaI and SacI-blunt sites of pBI121 (33). Lower case letters indicate non-cDNA sequences. Plasmid pKL194 was constructed by PCR amplification of a 300 bp PCR NsRpoTp fragment (GenBank accession no. AJ416576) (24) from a N.sylvestris cDNA library using primer 56, gggtctagAAAGaTGGCTTCCACAGC (AS 1) and primer 57, gggctgcagATTTGATTCTTTAGTCAAAAC (AS 98), and subsequently cloning it XbaI/PstI into pBSC (Stratagene). A triple FLAG-tag was added into the PstI and ApaI sites (primers 60, GGACTACAAAGACCATGACGGTGATTATAAAGATCATGACATCGATTACAAGGATGACGATGACAAGGGGCC and 61, CCTTGTCATCGTCA TCCTTGTAATCGATGTCATGATCTTTATAATCACCGTCATGGTCTTTGTAGTCCTGCA). Finally, a 2.9 kb NsRpoTp PCR fragment (primer 58, cccgggcccAAGAGAGTTTTTATTCAAGAC, AS 99; primer 59, cccggtaccTCAGTTAAAGAAGTAGGGC, AS 978) was cloned ApaI/Acc65I (KpnI) into the construct resulting in the complete cDNA, which was cloned XbaI/KpnI into pBI121 resulting in pKL197 or cloned XbaI/KpnI-blunted into the XbaI and SmaI sites of the vector pGPTV (34), resulting in pKL199. Plasmid pKL59 was previously described by Liere and Maliga (22).
Plant transformation
Tobacco plants were transformed using Agrobacterium transformation. To introduce the pBI121 derivative pKL85 into Agrobacterium LBA4404, triparental mating was carried out with Escherichia coli cells containing the pKL85 plasmids, and E.coli cells containing helper plasmid pRK2013 resulting in the Agrobacterium strain LKL85. Agrobacterium strain AGL1 (35) was transformed by electroporation with plasmids pKL197 and pKL199, giving rise to strains AKL197 and AKL199. Co-cultivation of tobacco leaf discs with AKL197, AKL199 and LKL85, and subsequent selection on RMOP medium containing 100 mg/l kanamycin and 500 mg/l carbenicillin (36), resulted in green tobacco calli and shoots, which were transferred individually onto selective (50 mg/l kanamycin) RM medium (37) to form roots. Transgenic plants were verified by PCR, Southern- and northern-blot analyses (data not shown).
Primer extension analysis
Primer extension reactions were carried out with 10 µg of total leaf RNA according to standard protocols (38). Briefly, primers PEclpP(206), GGGACTTTTGGAACACCAATAGGCAT (5′ position in the tobacco plastid genome at 74482), PEaccD(204), GAATATCTTATTTCCTATCAGACTAAGC (5′ position 59771) and PEatpB(205), CCCCAGAACCAGAAGTAGTAGGATTGA (5′ position 56744) were end-labeled with [γ-32P]ATP and T4 polynucleotide kinase. Primer extensions were performed using Superscript III MMLV reverse transcriptase (Gibco BRL) at 55°C and the resulting products analyzed on 5% sequencing gels. The transcription initiation sites were previously mapped using the same primers as listed above (16,17). Quantification of obtained signals was done for three independent experiments with a PhosphorImaging system using the complementary software (Bio-Rad).
Primer extension reactions with RNAs from NEP in vitro transcription assays with pKL59 template DNA were carried out as previously described (22).
Northern and western blot analysis
Total leaf RNA was prepared using TRIzol (Gibco BRL) following the manufacturer’s protocol. Fifty micrograms of total RNA was subjected to electrophoresis on 1% agarose–formaldehyde gels, transferred onto nylon membranes and hybridized with a single-stranded DNA probe overnight at 65°C. The NsRpoTp probe was prepared by asymmetric PCR using the C-terminal primer 59 in the presence of [α-32P]dCTP.
For immunoblotting, plastid proteins were isolated as previously described (22). Equal amounts of proteins (10 µg) were separated in 6.5% SDS–polyacrylamide gels and transferred onto nitrocellulose membranes. Immunodetection was carried out using the ECL+-system (Amersham Pharmacia Biotech) according to the manufacturer’s protocol. Concentrations used for the primary monoclonal antibody Anti-FLAG®M2 (Sigma) were 10 µg/ml and for the secondary anti-mouse antibody 1 to 80 000 dilutions.
RESULTS
Expression of AtRpoTp cDNA in tobacco affects transcription from atpB type I NEP promoters
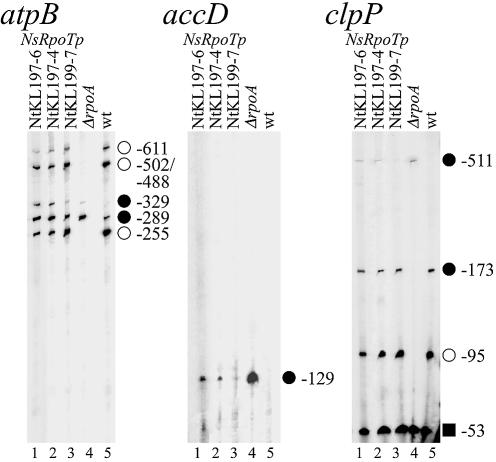
To investigate the effect of overexpressing putative Arabidopsis NEP catalytic subunits on tobacco NEP promoter activity, we constructed transgenic tobacco plants constitutively expressing a AtRpoTp cDNA. Transcript levels from previously characterized NEP and PEP promoters were determined by primer extension analysis mapping transcript 5′ ends in both transgenic and wild-type plants (Fig. 1) (16,17). The atpB gene is transcribed from three PEP promoters (PatpB-255, PatpB-502/488 and PatpB-611), active in the tobacco wild-type but not in ΔrpoB plants (Fig. 1, atpB, lanes 6 and 5; open circles). Additionally, a NEP promoter (PatpB-289) is active in both the leaf of wild-type and ΔrpoB plants (atpB, lanes 6 and 5; filled circles). Analogously, the clpP gene is transcribed from a PEP promoter (PclpP-95; open circles) and three NEP promoters (PclpP-53, PclpP-173, PclpP-511; squares and filled circles), which become principal in the ΔrpoB plants (clpP, lanes 5 and 6).
Figure 1.
Mapping of transcript 5′ ends in mutant, AtRpoTp expressing (NtKL85) and wild-type tobacco plants. Primer extension data are shown for the atpB (left) and clpP (right) genes. Mapped type I NEP (filled circles), type II NEP (squares) and PEP (open circles) promoters are indicated by their distance between the transcription initiation site and the translation initiation codon in nucleotides (16,17).
Overexpression of AtRpoTp showed no influence on transcript 5′ end levels of the type II NEP promoter PclpP-53 (Fig. 1, clpP). However, the PclpP-173 NEP promoter transcripts in the NtKL85-7, -8 and -9 lines accumulated to rather low ΔrpoB plant levels (clpP, lanes 1–3 and 5), whereas NtKL85-12 showed higher wild-type levels (clpP, lanes 4 and 6). Except for the ΔrpoB plants (lane 5), no transcripts could be detected for the type I NEP promoter PclpP-511. Interestingly, the PEP promoter PclpP-95 RNA 5′ ends accumulated to lower levels in the transgenic than in the wild-type plants (Table 1 and Fig. 1, lanes 1–3 and 6).
Table 1. Comparison of transcript abundance in RpoTp overexpressing NtKL and wild-type plants.
| |
atpB |
accD |
clpP |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
P-255 |
P-289 |
P-329 |
P-502 |
P-611 |
P-129 |
P-53 |
P-95 |
P-173 |
P-511 |
| PEP | NEP-I | NEP-I | PEP | PEP | NEP-I | NEP-II | PEP | NEP-I | NEP-I | |
| AtRpoTp | ||||||||||
| NtKL85-7 |
69 ± 2 |
33 ± 1 |
212 ± 4 |
93 ± 5 |
59 ± 8 |
– |
99 ± 0.5 |
79 ± 18 |
11 ± 4 |
0 |
| NtKL85-8 |
73 ± 3 |
26 ± 5 |
143 ± 30 |
87 ± 3 |
56 ± 7 |
– |
100 ± 3 |
37 ± 3 |
3 ± 1 |
0 |
| NtKL85-9 |
52 ± 6 |
43 ± 9 |
209 ± 2 |
54 ± 17 |
48 ± 10 |
– |
102 ± 4 |
43 ± 5 |
2 ± 1.5 |
0 |
| NtKL85-12 |
108 ± 4 |
12 ± 8 |
0.2 ± 0.1 |
104 ± 8 |
109 ± 8 |
– |
98 ± 2 |
101 ± 3 |
99 ± 2 |
0 |
| ΔrpoB |
0 |
100 |
100 |
0 |
0 |
– |
157 ± 4 |
0 |
2 ± 1 |
100 |
| wt |
100 |
19 ± 4 |
0.5 ± 0.4 |
100 |
100 |
– |
100 |
100 |
100 |
0 |
| NsRpoTp | ||||||||||
| NtKL197-6 |
34 ± 5 |
100 ± 8 |
253 ± 2 |
41 ± 3 |
19 ± 11 |
8 ± 2 |
98 ± 0.5 |
91 ± 3 |
68 ± 1 |
22 ± 4 |
| NtKL197-4 |
31 ± 3 |
103 ± 6 |
248 ± 4 |
36 ± 4 |
38 ± 9 |
5 ± 3 |
97 ± 1 |
92 ± 2 |
63 ± 3 |
27 ± 2 |
| NtKL199-7 |
68 ± 9 |
73 ± 10 |
118 ± 6 |
62 ± 11 |
58 ± 5 |
1 ± 0.4 |
110 ± 5 |
100 ± 1 |
73 ± 4 |
2 ± 2 |
| ΔrpoA |
0 |
100 |
100 |
0 |
0 |
100 |
98 ± 2 |
0 |
1 ± 0.5 |
100 |
| wt | 100 | 50 ± 5 | 0 | 100 | 100 | 0 | 100 | 100 | 100 | 0 |
Summary of results obtained by three independent primer extension analyses. Percentages (%) with standard error values in respect to wild-type (PEP) and ΔrpoB (NEP) plant transcript levels are given. Bold type represents values set to 100% for each promoter. NEP-I, type I NEP promoter; NEP-II, type II NEP promoter.
On the contrary, examination of the atpB transcription initiation sites revealed a significant change in the RNA 5′ end pattern. Three mutant tobacco lines (NtKL85-7, -8 and -9) expressing AtRpoTp were showing a strong band at position –329 in respect to the translation initiation site (Fig. 1, atpB, lanes 1–3). This signal was even more pronounced than in the ΔrpoB plants (lane 5 and Table 1), but not visible in the wild type (lane 6). It is possible that this signal is representing the transcript 5′ end of a novel NEP promoter PatpB-329. The effects of AtRpoTp overexpression on NEP promoter PatpB-289 in these plants were not as prominent as in the ΔrpoB line, but transcript levels were still up to two times higher than in the wild type (Table 1). The transcript levels of PEP promoters (e.g. PatpB-255) in the same mutant plants were up to 40% decreased (Table 1, NtKL85-7, -8, -9; Fig. 1, atpB, lanes 1–3). However, as shown for the clpP transcript 5′ ends, this was not true for NtKL85-12. Although NtKL85-12 was proven to be transgenic for the AtRpoTp cDNA in Southern blot experiments, northern analysis showed no accumulation of transcripts of the transgene (data not shown). Therefore, we used this plant as a control to prove that the expression of the AtRpoTp transgene is causing the effect on accumulation of 5′ transcript ends from the promoters analyzed. The pattern of the atpB transcript 5′ ends in NtKL85-12 showed no difference in comparison with the wild type (Table 1, LKL85-12; Fig. 1, atpB and clpP, lanes 4 and 6). This clearly demonstrates that indeed overexpression of AtRpoTp is responsible for the positive effect on transcription accumulation at positions PatpB-329 and PatpB-289.
PatpB-329 is accurately recognized in vitro
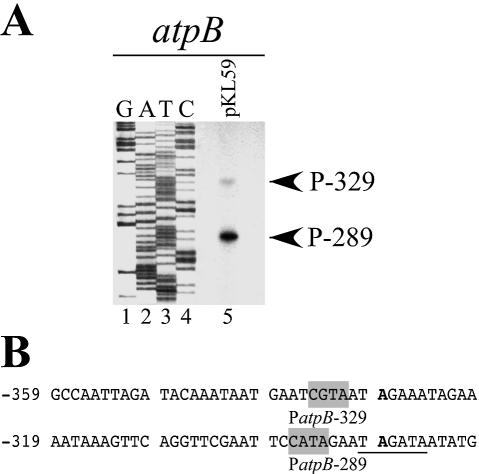
Although the atpB 5′ end originating at –329 was present in a control using a PEP-deficient tobacco mutant, it was not certain whether this 5′ end was a transcription initiation site promoted by the overexpression of the AtRpoTp cDNA or a processing site of a transcript originating further upstream. Therefore, we used an atpB promoter fragment with its 5′ end at position –361, just 32 bp upstream of the putative PatpB-329 initiation site (Fig. 2B), in a NEP in vitro transcription assay (22). Primer extension analysis carried out with derived in vitro transcripts indeed mapped, apart from the the PatpB-289 initiation site, an atpB RNA 5′ end at position –329 (Fig. 2A). Analysis of the surrounding region revealed a CGTA sequence matching the YRTA consensus motif of NEP promoters (Fig. 2B) (18–20), further substantiating that PatpB-329 is a NEP promoter in vivo and in vitro.
Figure 2.
An additional PatpB-329 NEP promoter is accurately recognized in vitro. (A) Primer extension analysis to map the 5′ end of atpB in vitro transcripts from ΔrpoA plastid extracts. For reference, the same end-labeled primer was used to generate a DNA sequence ladder. (B) DNA sequences surrounding the atpB NEP transcription initiation sites. Bold nucleotides denote transcription initiation sites (+1). Putative YRTA core motifs are shaded with gray boxes and the –35 PEP promoter motif is underlined.
Expression of NsRpoTp cDNA in tobacco affects transcription from type I NEP promoters
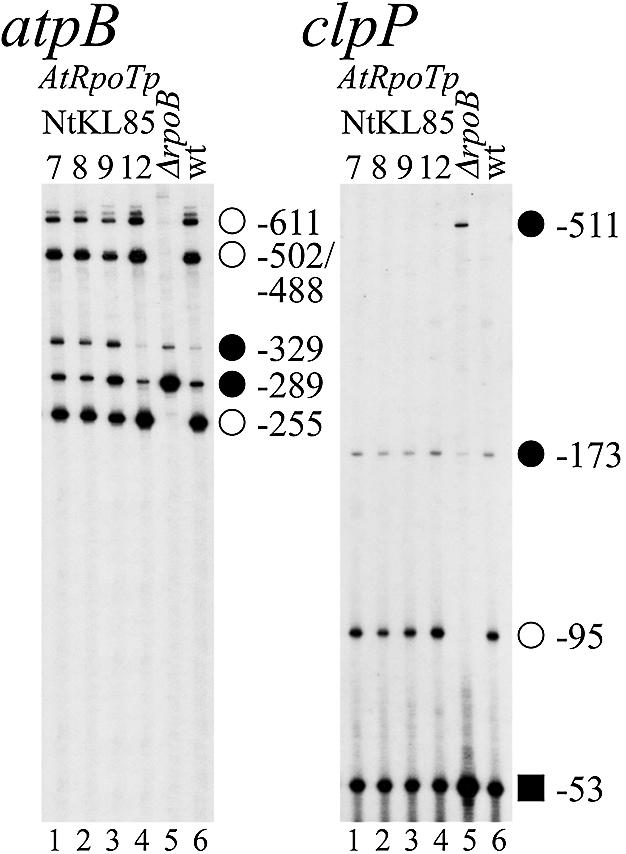
Since a positive effect of overexpressing AtRpoTp on tobacco NEP promoter usage was only apparent for atpB but not clpP promoters, we could not rule out that the heterologous AtRpoTp enzyme was missing distinct factors involved in clpP NEP promoter recognition in tobacco (32,39). Therefore, we constructed transgenic tobacco plants constitutively expressing a N.sylvestris RpoTp cDNA. Transcript levels from NEP and PEP promoters of the previously analyzed atpB and clpP genes were determined by primer extension analysis mapping transcript 5′ ends in both transgenic and wild-type plants (Fig. 3, atpB and clpP). In addition, transcript levels of the accD gene were tested, which is transcribed by a sole type I NEP promoter PaccD-129 usually not detectable in wild-type but in PEP-deficient ΔrpoA plants (Fig. 3, accD, lane 4) (17).
Figure 3.
Mapping of transcript 5′ ends in mutant, NsRpoTp expressing (NtKL197 and NtKL199) and wild-type tobacco plants. Primer extension data are shown for the atpB (left), accD (middle) and clpP (right) genes. Type I NEP (filled circles), type II NEP (squares) and PEP (open circles) promoters are indicated.
As shown for the transgenic NtKL85 plants expressing the AtRpoTp enzyme (Fig. 1), primer extension analysis of the tobacco plants expressing NsRpoTp (NtKL197-6, -4 and NtKL199-7; Fig. 3, lanes 1–3) revealed in comparison with the wild type, significant differences in atpB RNA 5′ end accumulation (Fig. 3, atpB). Though weaker for NtKL199-7, the mutant plants showed in comparison with the PEP-deficient ΔrpoA plant (Fig. 3, lane 4), up to two times enhanced transcript levels from PatpB-329 (Table 1). While almost reaching ΔrpoA levels, transcript abundance from PatpB-329 was up to two times higher as found in the wild type (Table 1). However, the usage of PEP promoters (PatpB-255, PatpB-502/488 and PatpB-611) in the transgenic plants was concurrently decreased with their transcript 5′ ends in NtKL197-6 and NtKL197-4 being clearly less abundant than in NtKL199-7 or wild-type plants (Table 1 and Fig. 3, atpB, lanes 1–3 and 5; open circles).
In the case of the PaccD-129 NEP promoter, an even more pronounced effect was detected in the transgenic NtKL197 and NtKL199 plants in comparison with the wild type (Fig. 3, accD). Whereas no signal was visible in the wild type (lane 5), a distinct transcript 5′ end of PaccD-129 was detectable in NtKL197-6 (lane 1) and NtKL197-4 (lane 2); however, this was barely visible in NtKL199-7 (lane 3). These data suggest that indeed, as shown for AtRpoTp, overexpression of NsRpoTp has a positive effect on transcription from type I NEP promoters as PatpB-329, PatpB-289 and PaccD-129.
Analysis of clpP transcription initiation sites revealed no influence on transcript 5′ end levels of the type II NEP promoter PclpP-53 in the NsRpoTp overexpressing tobacco plants (Fig. 3, clpP). However, the PclpP-173 type I NEP promoter transcripts accumulated in NtKL197-4 and NtKL199-7 to only 70% of the wild-type levels (Table 1 and Fig. 3, clpP, lanes 1–3 and 5). On the contrary, the PclpP-511 type I NEP promoter transcripts not detectable in the wild type accumulated in the transgenic NtKL plants to up to 27% of the ΔrpoA plant levels (Table 1 and Fig. 3, clpP), showing an analogous positive effect as on the atpB and accD type I NEP promoters.
These results strongly suggest that RpoTp is a genuine part of the NEP transcription machinery involved in transcription from type I but not from type II NEP promoters. With one exception, however, RpoTp overexpression rather negatively influenced PclpP-173, which is atypical for type I NEP promoters up-regulated in green tissue.
Changes in RNA levels of NEP-dependent genes correlate with NsRpoTp expression levels
Interestingly, the primer extension results obtained with NtKL199-7 (approximately two transgenes) were less evident than with NtKL197-4 and NtKL197-6 (approximately five transgenes), suggesting differences in the expression of NsRpoTp in these plants. Northern blot analysis of NsRpoTp transcript levels revealed that indeed the transgene-RNA in NtKL199-7 accumulated to lower levels than in NtKL197-4 and NtKL197-6 (Fig. 4). To investigate to what extent the transgene-RNA translates into NsRpoTp protein, we took advantage of a FLAG-tag engineered into the NtKL transgene constructs and performed western blot analyses of plastid extracts from mutant and wild-type plants. Using monoclonal Anti-FLAG antibodies a strong signal with an apparent molecular mass of 120 kDa was detected in both mutant and wild-type plants. However, we detected a second, weaker signal with the expected molecular mass of ∼110 kDa which was absent in the wild type. Therefore, we concluded that this signal represents the FLAG-tagged NsRpoTp protein expressed and imported into plastids in the mutant NtKL lines. The results shown in Figure 4 indicate that NsRpoTp expression is highest in the NtKL197-4 and -6 lines, which reflects the effect observed on transcription from type I NEP promoters in the mutant plants (Fig. 3).
Figure 4.
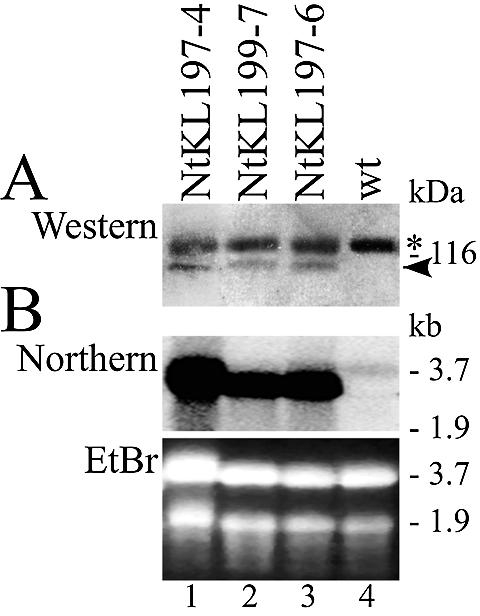
Expression analysis of NsRpoTp in mutant NtKL plants. (A) Western blot analysis of 10 µg of plastidial proteins separated in 6.5% SDS–polyacrylamide gels. Monoclonal Anti-FLAG antibody detected 120 (asterisk) and 110 kDa (arrowhead) proteins. The 120 kDa protein is probably non-specific because it was also detected in wild-type extracts. (B) Northern blot analysis of 50 µg of total RNA separated in 1% agarose–formaldehyde gels. Ethidium bromide stained gel image (EtBr) is shown as a loading control (bottom). The RNA blot was hybridized with a single-stranded full-length NsRpoTp antisense DNA probe. Note the weak 3.7 kb signal detected in the wild-type (lane 4) which most likely represents endogenous NtRpoTp transcripts.
DISCUSSION
We report here the first functional analysis of a nucleus-encoded, phage-type RNA polymerase (RpoTp) in plastids. Through analysis of transcript 5′ ends from various NEP and PEP promoters of the atpB, clpP and accD genes in transgenic plants overexpressing A.thaliana and N.sylvestris RpoTp cDNAs, we have provided evidence that RpoTp is a catalytic subunit of the nuclear-encoded plastid transcription machinery NEP involved in recognition of a distinct subset of type I NEP promoters.
It has been shown that an RpoTp antibody from maize repressed a biochemically purified NEP transcription activity from the unique rrn16 PC promoter in spinach in vitro (NEP-1) (40). Further biochemical analyses in the same study revealed a second NEP transcription activity, NEP-2, as well as recognizing the PC promoter in vitro. However, the maize RpoTp antibody did not inhibit NEP-2 transcription. Although both enzymes seemed to recognize the T7 promoter in vitro, it remained uncertain whether NEP-1 or NEP-2 are phage-type RNA polymerases or even that they share the same core enzyme (i.e. RpoTp). To specifically elucidate the role of RpoTp in plastid NEP transcription in tobacco, we constructed transgenic plants overexpressing the Arabidopsis (AtRpoTp) and tobacco (NsRpoTp) cDNAs for plastid phage-type RNA polymerases and examined the RNA levels of 5′ ends from known NEP and PEP promoters by primer extension analysis.
Analysis of the atpB RNA 5′ ends revealed in RpoTp overexpressing plants, i.e. NtKL85-7, -8, -9, NtKL197-4, -6 and to some lesser extent NtKL199-7 (Figs 1 and 3), a novel type I NEP promoter PatpB-329 not detectable in wild-type plants. A second atpB type I NEP promoter, PatpB-289, also showed, in comparison with the wild-type, up to 2-fold higher transcript levels (Table 1). This was also true for PaccD-129. This type I NEP promoter is usually not detectable in green wild-type tissue but is prominent in PEP-deficient tobacco mutants (Fig. 3) (16,17). However, we observed a signal at this position in green tissue of NtKL197-4, -6 and NtKL199-7 (Table 1), indicating that the improved amounts of RpoTp in these mutants yielded stronger transcription, hence, detectable amounts of accD RNA 5′ ends. When we looked at another, even in PEP-deficient plants, weak type I NEP promoter, PclpP-511, detectable transcript amounts were only found in NsRpoTp- but not in AtRpoTp-expressing mutants (Table 1). It might well be that AtRpoTp, which is expressed in tobacco and interacts with the heterologous transcription factor(s) is not as efficiently recognizing this type I NEP promoter as the homologous enzyme.
Interestingly, a further clpP type I NEP promoter, PclpP-173, is negatively affected by the expression of both AtRpoTp and NsRpoTp. Since PclpP-173 is unusually highly expressed in green wild-type tissue, it is conceivable that its expression is regulated by distinct transcription factors. This is echoed in the PEP-deficient plants, which in fact show in comparison with the wild type a similar effect on PclpP-173 transcription (Figs 1 and 3 and Table 1) (16,17). It is possible that by overexpression or up-regulation of RpoTp these probably limited factors become largely depleted, resulting in generally less transcript levels from this promoter. On the other hand, it is also possible that up-regulation of PclpP-173 in green wild-type tissue is due to a distinct regulatory network, which becomes unbalanced by RpoTp overexpression.
Surprisingly, PEP transcription from PatpB-255, PatpB-502/488, PatpB-611 and PclpP-95 was decreased in the mutant plants (Figs 1 and 3 and Table 1). In the case of PatpB-255 the explanation is fairly easy. The promoters PatpB-255 (PEP) and PatpB-289 (NEP) are both in close proximity. The transcription initiation site of PatpB-289 is located within the –35 consensus box of PatpB-255 (Fig. 2B). Therefore, in the case of higher RpoTp amounts, the binding equilibrium in this region is rather in favor of the NEP promoter than the PEP promoter. However, a similar, but not as eminent effect appears to affect the other atpB and clpP PEP promoters. Since other tested PEP promoters from rbcL and psbA were not affected in the mutant plants (data not shown), it seems that the higher transcription rates of clpP and atpB by the overexpressed RpoTp are paralleled by decreased transcription from the clpP and atpB PEP promoters.
We have observed that the amount of transcript 5′ ends from the type II PclpP-53 promoter was not affected by RpoTp overexpression (Figs 1 and 3 and Table 1). This was not necessarily expected, even though PclpP-53 is remarkable in both promoter architecture and expression characteristics (high transcript levels in wild-type and PEP-deficient plants). Therefore, we conclude that in tobacco, RpoTp is most likely involved in transcription from type I NEP promoters, but not from the type II PclpP-53. The question remains, which RNA polymerase is responsible for recognition and transcription from this ‘non-consensus’ promoter, active in dicot but not monocot plants (32). A second phage-type RNA polymerase, RpoTmp, is present in plastids of dicot plants (24,31). Furthermore, the existence of additional nuclear-encoded transcription activities was reported for the spinach (NEP-2) (40) and Arabidopsis (39) rrn operon, and for the internal promoters of certain tRNAs (19,41,42). One may speculate that one of these plastid transcription activities is responsible for recognition and transcription from PclpP-53. Further experiments, for example, overexpression of RpoTmp in tobacco, should provide more clues to understand PclpP-53 transcription in dicot plants.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Zora Svab for sharing her skills on plant nuclear transformation and primer extension analysis. K.L. was a recipient of a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft. This work was supported by grants from the National Science Foundation MCB 96-30763, MCB 99-05043 and Monsanto Co. to P.M. and the Deutsche Forschungsgemeinschaft (SFB 429) to K.L. and T.B.
REFERENCES
- 1.Gray M.W. (1993) Origin and evolution of organelle genomes. Curr. Opin. Genet. Dev., 3, 884–890. [DOI] [PubMed] [Google Scholar]
- 2.Bogorad L. (1991) Replication and transcription of plastid DNA. In Bogorad,L. and Vasil,I.K. (eds), The Molecular Biology of Plastids. Academic Press, San Diego, CA, pp. 93–124. [Google Scholar]
- 3.Igloi G.L. and Kössel,H. (1992) The transcriptional apparatus of chloroplasts. Crit. Rev. Plant Sci., 10, 525–558. [Google Scholar]
- 4.Sugiura M. (1992) The chloroplast genome. Plant Mol. Biol., 19, 149–168. [DOI] [PubMed] [Google Scholar]
- 5.Hu J. and Bogorad,L. (1990) Maize chloroplast RNA polymerase: the 180-, 120- and 38-kilodalton polypeptides are encoded in chloroplast genes. Proc. Natl Acad. Sci. USA, 87, 1531–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu J., Troxler,R.F. and Bogorad,L. (1991) Maize chloroplast RNA polymerase: the 78-kilodalton polypeptide is encoded by the plastid rpoC1 gene. Nucleic Acids Res., 19, 3431–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfannschmidt T. and Link,G. (1994) Separation of two classes of plastid DNA-dependent RNA polymerases that are differentially expressed in mustard (Sinapis alba L.) seedlings. Plant Mol. Biol., 25, 69–81. [DOI] [PubMed] [Google Scholar]
- 8.Allison L.A. (2000) The role of sigma factors in plastid transcription. Biochimie, 82, 537–548. [DOI] [PubMed] [Google Scholar]
- 9.Homann A. and Link,G. (2003) DNA-binding and transcription characteristics of three cloned sigma factors from mustard (Sinapis alba L.) suggest overlapping and distinct roles in plastid gene expression. Eur. J. Biochem., 270, 1288–1300. [DOI] [PubMed] [Google Scholar]
- 10.Bünger W. and Feierabend,J. (1980) Capacity for RNA synthesis in 70S ribosome-deficient plastids of heat-bleached rye leaves. Planta, 149, 163–169. [DOI] [PubMed] [Google Scholar]
- 11.Siemenroth A., Wollgiehn,R., Neumann,D. and Börner,T. (1981) Synthesis of ribosomal RNA in ribosome-deficient plastids of the mutant ‘albostrians’ of Hordeum vulgare L. Planta, 153, 547–555. [DOI] [PubMed] [Google Scholar]
- 12.Morden C.W., Wolfe,K.H., dePamphilis,C.W. and Palmer,J.D. (1991) Plastid translation and transcription genes in a non-photosynthetic plant: intact, missing and pseudo genes. EMBO J., 10, 3281–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hess W.R., Prombona,A., Fieder,B., Subramanian,A.R. and Börner,T. (1993) Chloroplast rps15 and the rpoB/C1/C2 gene cluster are strongly transcribed in ribosome-deficient plastids: evidence for a functioning non-chloroplast-encoded RNA polymerase. EMBO J., 12, 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falk J., Schmidt,A. and Krupinska,K. (1993) Characterization of plastid DNA transcription in ribosome deficient plastids of heat-bleached barley leaves. J. Plant Physiol., 141, 176–181. [Google Scholar]
- 15.Allison L.A., Simon,L.D. and Maliga,P. (1996) Deletion of rpoB reveals a second distinct transcription system in plastids of higher plants. EMBO J., 15, 2802–2809. [PMC free article] [PubMed] [Google Scholar]
- 16.Serino G. and Maliga,P. (1998) RNA polymerase subunits encoded by the plastid rpo genes are not shared with the nucleus-encoded plastid enzyme. Plant Physiol., 117, 1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajdukiewicz P.T.J., Allison,L.A. and Maliga,P. (1997) The two RNA polymerases encoded by the nuclear and the plastid compartments transcribe distinct groups of genes in tobacco plastids. EMBO J., 16, 4041–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hess W.R. and Börner,T. (1999) Organellar RNA polymerases of higher plants. Int. Rev. Cytol., 190, 1–59. [DOI] [PubMed] [Google Scholar]
- 19.Liere K. and Maliga,P. (2001) Plastid RNA polymerases. In Andersson,B. and Aro,E.-M. (eds), Regulation of Photosynthesis. Kluwer Academic Publishers, Dordrecht, Netherlands, pp. 29–49. [Google Scholar]
- 20.Weihe A. and Börner,T. (1999) Transcription and the architecture of promoters in chloroplasts. Trends Plant Sci., 4, 169–170. [DOI] [PubMed] [Google Scholar]
- 21.Kapoor S. and Sugiura,M. (1999) Identification of two essential sequence elements in the nonconsensus Type II PatpB-290 plastid promoter by using plastid transcription extracts from cultured tobacco BY-2 cells. Plant Cell, 11, 1799–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liere K. and Maliga,P. (1999) In vitro characterization of the tobacco rpoB promoter reveals a core sequence motif conserved between phage-type plastid and plant mitochondrial promoters. EMBO J., 18, 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hedtke B., Börner,T. and Weihe,A. (1997) Mitochondrial and chloroplast phage-type RNA polymerases in Arabidopsis. Science, 277, 809–811. [DOI] [PubMed] [Google Scholar]
- 24.Hedtke B., Legen,J., Weihe,A., Herrmann,R.G. and Börner,T. (2002) Six active phage-type RNA polymerase genes in Nicotiana tabacum. Plant J., 30, 625–637. [DOI] [PubMed] [Google Scholar]
- 25.Weihe A., Hedtke,B. and Börner,T. (1997) Cloning and characterization of a cDNA encoding a bacteriophage-type RNA polymerase from the higher plant Chenopodium album. Nucleic Acids Res., 25, 2319–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young D.A., Allen,R.L., Harvey,A.J. and Lonsdale,D.M. (1998) Characterization of a gene encoding a single-subunit bacteriophage-type RNA polymerase from maize which is alternatively spliced. Mol. Gen. Genet., 260, 30–37. [DOI] [PubMed] [Google Scholar]
- 27.Chang C.-C., Sheen,J., Bligny,M., Niwa,Y., Lerbs-Mache,S. and Stern,D.B. (1999) Functional analysis of two maize cDNAs encoding T7-like RNA polymerases. Plant Cell, 11, 911–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikeda T.M. and Gray,M.W. (1999) Identification and characterization of T7/T3 bacteriophage-like RNA polymerase sequences in wheat. Plant Mol. Biol., 40, 567–578. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi Y., Dokiya,Y. and Sugita,M. (2001) Dual targeting of phage-type RNA polymerase to both mitochondria and plastids is due to alternative translation initiation in single transcripts. Biochem. Biophys. Res. Commun., 289, 1106–1113. [DOI] [PubMed] [Google Scholar]
- 30.Lerbs-Mache S. (1993) The 110-kDa polypeptide of spinach plastid DNA-dependent RNA polymerase: single-subunit enzyme or catalytic core of multimeric enzyme complexes? Proc. Natl Acad. Sci. USA, 90, 5509–5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hedtke B., Börner,T. and Weihe,A. (2000) One RNA polymerase serving two genomes. EMBO Rep., 1, 435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sriraman P., Silhavy,D. and Maliga,P. (1998) The phage-type PclpP-53 plastid promoter comprises sequences downstream of the transcription initiation site. Nucleic Acids Res., 26, 4874–4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jefferson R.A., Kavanagh,T.A. and Bevan,M.W. (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J., 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker D., Kemper,E., Schell,J. and Masterson,R. (1992) New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Mol. Biol., 20, 1195–1197. [DOI] [PubMed] [Google Scholar]
- 35.Lazo G.R., Stein,P.A. and Ludwig,R.A. (1991) A DNA transformation-competent Arabidopsis genomic library in Agrobacterium. Biotechnology (NY), 9, 963–967. [DOI] [PubMed] [Google Scholar]
- 36.Svab Z., Hajdukiewicz,P.T. and Maliga,P. (1990) Stable transformation of plastids in higher plants. Proc. Natl Acad. Sci. USA, 90, 913–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murashige T. and Skoog,F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant, 15, 493–497. [Google Scholar]
- 38.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning. A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 39.Sriraman P., Silhavy,D. and Maliga,P. (1998) Transcription from heterologous rRNA operon promoters in chloroplasts reveals requirement for specific activating factors. Plant Physiol., 117, 1495–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bligny M., Courtois,F., Thaminy,S., Chang,C.-C., Lagrange,T., Baruah-Wolff,J., Stern,D. and Lerbs-Mache,S. (2000) Regulation of plastid rDNA transcription by interaction of CDF2 with two different RNA polymerases. EMBO J., 19, 1851–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gruissem W., Elsner-Menzel,C., Latshaw,S., Narita,J.O., Schaffer,M.A. and Zurawski,G. (1986) A subpopulation of spinach chloroplast tRNA genes does not require upstream promoter elements for transcription. Nucleic Acids Res., 14, 7541–7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu C.Y., Lin,C.H. and Chen,L.J. (1997) Identification of the transcription start site for the spinach chloroplast serine tRNA gene. FEBS Lett., 418, 157–161. [DOI] [PubMed] [Google Scholar]