Abstract
Zambia’s estimated incidence of all forms of human tuberculosis (TB) is 707/100,000. High prevalence of bovine tuberculosis (BTB) – infection with Mycobacterium bovis – in cattle and the Kafue lechwe antelopes (Kobus leche Kafuensis) has been reported in the Kafue basin. Consumption of unpasteurised milk and meat products from infected animals poses a risk of transmitting zoonotic tuberculosis to people living at the human-animal interface. Despite the reported high prevalence of BTB in both livestock and wildlife, information on the proportion of human patients infected with M. bovis is unknown in Zambia. This paper reviews the available information in English on human, livestock and wildlife TB in Zambia with the purpose of assessing the burden of animal infections with M. tuberculosis complex and its public health implications.
Keywords: Bovine tuberculosis, Kafue lechwe, Interface, Zoonotic tuberculosis
Multilingual abstracts
Please see Additional file 1 for translations of the abstract into the six official working languages of the United Nations.
Review
Introduction
Human tuberculosis (TB), although an ancient disease, has re-emerged with devastating consequences on global public health and is currently one of the most widespread infectious diseases. In addition, it is the leading cause of death due to a single infectious agent among human adults in the world [1]. Tuberculosis is caused by members of the Mycobacterium tuberculosis complex (MTC), which includes Mycobacterium tuberculosis, Mycobacterium bovis, Mycobacterium africanum, Mycobacterium caprae, Mycobacterium microti, Mycobacterium pinnipedii and Mycobacterium canettii[1]. Approximately one third of the world’s population is infected with bacteria belonging to the MTC complex, with Sub-Saharan Africa having the highest annual incidence since the advent of HIV and AIDS [2]. The TB bacilli are non-motile, non-sporulating, weakly Gram-positive acid-fast bacilli (AFB) that appear microscopically as straight or slightly curved rods [3].
The World Health Organization (WHO) estimates that the incidence of all forms of TB in Zambia stands at 707/100,000 [4]. Mycobacterium tuberculosis is usually transmitted to a human by inhalation of aerosol droplets containing tubercle bacilli which are expectorated from infected individuals with open pulmonary TB [3].
Mycobacterium bovis (M. bovis), the bovine tubercle bacilli, is the cause of bovine tuberculosis (in this paper, it will be referred to as BTB when talking about infection to animals and zoonotic tuberculosis when talking about infection to humans). It has a wide range of host animal species, which includes cattle, goats, bisons, antelopes, humans and non-human primates, and can cause disease in susceptible hosts [5].
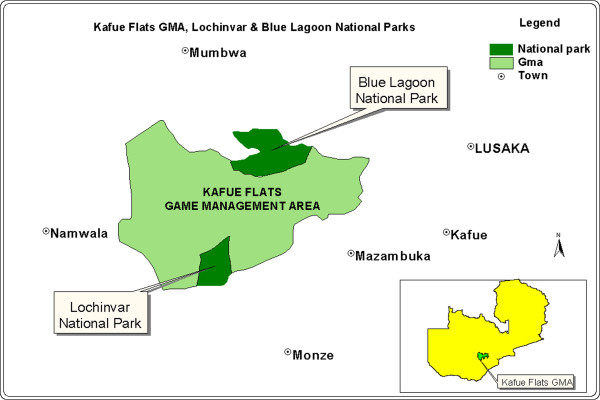
High prevalence of BTB in cattle and the Kafue lechwe antelopes (Kobus leche Kafuensis) has been reported at the wildlife-livestock interface in the Kafue basin [6,7]. Despite the evidence indicating that cultural and socio-economic factors (among others) increase the likelihood of M. bovis transmission between species sharing the same environment (cattle, wildlife and humans), zoonotic tuberculosis remains significantly underrepresented as causal agents of extra pulmonary and pulmonary TB in developing countries, especially in rural regions at the human-animal interface [8]. Humans and animals (both livestock and wildlife) share the same micro-environments and water points within the Kafue basin (see Figure 1), particularly during the dry season, thereby increasing the risk of TB transmission between infected and susceptible hosts [9].
Figure 1.
Showing the interface of Kafue basin
The purpose of this paper is to review the available information on TB in livestock and wildlife in order to identify knowledge gaps, and to assess the burden of animal infections with M. tuberculosis and M. bovis in addition to their public health importance in Zambia.
Human demography in Zambia
Zambia is located in south-central Africa and covers an area of about 752,618 square kilometres. The population of Zambia has increased from 7,759,161 in 1990 to 9,885,591 in 2000 and to 13,046,508 in 2010, resulting in an average annual growth rate of 2.8% between 2000 and 2010 [10]. According to the 2010 population figures, 49% of the population is male and 51% is female. The country’s population is characterised by extreme youth with 49.6% of the population being under 15 years of age [10]. The regional population distribution illustrates that 7,978,274 people (61%) reside in rural areas and 5,068,234 (39%) live in urban areas [10]. The rural population in Zambia have based their culture and livelihood around the collection and utilisation of natural resources from the environment [11], which includes activities as diverse as animal husbandry and crop production [12].
BTB in cattle in the Kafue basin of Zambia
Zambia has approximately three million head of cattle, with an estimated 80% of the national cattle population being held by traditional farmers [13]. The concentration of livestock farming is mainly in three provinces: the Southern, Western and Eastern Provinces. The Kafue Basin area, which is one of the few lacustrine wetlands, supports almost 300,000 cattle [14]. According to Musso et al. (2012), three types of herding systems exist within the Kafue basin. These include village resident herding, with herds kept in the villages; the moving of cattle from villages into the flood plains with regard to the water levels in the plains; and interface herding, where herds are always present within the floodplains and rarely return to the villages, thus being under constant contact with BTB infected wildlife within the floodplains [15].
Bovine tuberculosis (BTB) has been reported to be endemic in the Zambian traditional cattle sector with a high herd prevalence of 49.8% recorded from areas within, and adjacent to, the Kafue basin as far back as 1947 [16,17]. Reports from abattoirs in the Namwala district, located within the Kafue basin, indicate that 16.8% of the cattle slaughtered were infected with BTB based on the presence of typical TB lesions [6].
BTB in the wildlife in the Kafue basin
The Kafue lechwe (Kobus leche Kafuensis) is a medium-sized, semi-aquatic antelope with a population of 44,000 that is endemic to the Kafue flats [18]. The history of BTB in the Kafue lechwe dates as far back as 1954 when it was diagnosed from the Lochinvar National Park, which is located within the Kafue basin [19]. In 1972, Gallagher et al. estimated that BTB was responsible for the deaths of at least 20% of lechwe annually on the southern bank of the Kafue flats [19]. A recent study has shown a magnitude of 27.7% of BTB in the Kafue lechwe [18]. This level of BTB in the Kafue lechwe represents a potential risk of transmission of M. bovis to livestock, wildlife and local communities. A resident population of the African buffalo (Syncerus caffer) is present in the Kafue basin. In 2011, a study reported that no BTB positive reactors were found in the comparative intradermal tuberculin test (CIDT), suggesting an absence of M. bovis infection in buffaloes [20]. In addition, this study reported that the buffaloes do not come into direct contact with cattle like the lechwe antelopes do. All the cattle keepers and herdsmen in the Kafue basin reported the same observation that cattle will never go near buffaloes and vice versa; however, lechwe and cattle are often observed grazing together.
Diagnosis of bovine tuberculosis (BTB) in Zambia
Diagnosis of BTB in cattle and wildlife in Zambia offers numerous challenges and difficulties. The presumptive ante mortem diagnosis of TB is made using the CIDT (see Table 1) [7], as recommended by the World Organisation for Animal Health (OIE). This test is usually performed by the veterinary services. However, given that no compensation scheme is in place, farmers are reluctant to slaughter their animals, resulting in this diagnosis test not being routinely implemented in Zambia. Therefore, BTB is diagnosed post mortem and is based on the presence of gross lesions compatible with BTB in the lungs and/or associated lymph nodes found during meat inspection in the abattoir. Carcasses are declared fit for human consumption once the organs showing gross lesions are removed according to standard regulations [21]. The laboratory procedures (AFB staining, culture and typing, including molecular methods) (see Table 2) [18,22] are only implemented in the Veterinary Research Institutes and the Faculty of Veterinary Science at the University of Zambia in Lusaka. Furthermore, no resources are allocated for BTB testing and control at the national level as it is not considered a disease of national economic importance.
Table 1.
Herd prevalence of BTB in cattle determined by cross-section study around the Kafue basin
| Study site | Prevalence (%) | Method | Reference |
|---|---|---|---|
| Blue lagoon |
48 |
CIDT |
Munyeme et al. 2009 |
| Lochinvar |
43 |
CIDT |
Munyeme et al. 2009 |
| Kazungula |
4 |
CIDT |
Munyeme et al. 2009 |
| Monze |
33 |
CIDT |
Cook et al. 1996 |
| Livingstone |
1 |
Necropsy |
Anonymous, 1957 |
| Mazabuka |
5 |
Necropsy |
Anonymous, 1957 |
| Lusaka |
2 |
Necropsy |
Anonymous, 1957 |
| Namwala | 17 | Necropsy | Anonymous, 1957 |
Table 2.
Prevalence of BTB by area based on gross post-mortem examination, Ziehl-Neelsen and culture results from Kafue lechwe tissue samples (n=119) (Munyeme et al. 2010)
| Study area | Test method | Prevalence (%) |
|---|---|---|
| Lochnivar/Blue lagoon |
Necropsy |
24.34 |
| Lochnivar/Blue lagoon |
Ziehl-Neelsen |
17.6 |
| Lochnivar/Blue lagoon | Culture | 27.7 |
Source: Munyeme et al. /Preventive Medicine 95 (2010) 305–308.
Public health importance of Mycobacterium tuberculosis infections in animals
Mycobacterium tuberculosis has been incidentally reported from cattle and other livestock animals and may have a negative public health impact. For example, in a study conducted by Ameni et al. on grazing cattle in central Ethiopia, 11.5% of the isolated bacteria, M. tuberculosis, was identified [23]. It has also been isolated from livestock and wildlife across the world [1,24-26]. The isolation of M. tuberculosis from livestock raises a number of questions relating to the role of livestock as a source of human infections. Of relevance is a study by Srivastava et al. (2008), where M. tuberculosis was isolated from milk samples obtained from cattle in India, suggesting that infections may spill back to humans through consumption of unpasteurised milk [27].
Public health importance of zoonotic tuberculosis in Zambia
In 1998, the WHO reported that 3.1% of tuberculosis cases in humans worldwide are attributable to M. bovis and that 0.4-10% of sputum isolates from patients in African countries may be M. bovis. This is despite the fact that M. bovis is mainly associated with extra pulmonary disease in humans [28]. Data on the prevalence of human disease due to M. bovis in Zambia and other developing countries is limited, owing to technical problems posed by identification of this species, such as trained personnel and laboratory facilities [16,29]. Indeed, as a general rule, only Ziehl-Neelsen staining is performed on sputum samples to identify AFB. This technique cannot differentiate between the different species from the genus Mycobacterium[30]. However, zoonotic tuberculosis is acquiring increasing recognition in developing countries, including Zambia, as animals and humans share the same environment. This has prompted researchers to evaluate its impact on human health, particularly among pastoral communities. An additional factor that these developing countries are now facing is the HIV/AIDS pandemic, which may favour human-to-human transmission of M. bovis leading rapidly to disease [29].
In a more recent study, Gumi et al. (2012) documented, by using molecular tools, an epidemiological link in the zoonotic transmission between livestock and pastoralists of south-east Ethiopia [31]. Zoonotic tuberculosis is an economical and public health threat in developing countries [32]. However, very few studies quantify its economical and public health burden. Recently, a preliminary study suggested that the cost of controlling BTB always exceed the calculated benefits if considered from a purely monetary viewpoint. However, the benefits are likely to outweigh the costs if wider implications of BTB on humans, e.g. avoiding infirmity-related production losses (indirect costs such as time away from farming and on livestock and wildlife) are taken into account [33]. In the Kafue basin, consumption of raw and soured milk is one of the common practices in the local communities. This, therefore, poses a health risk in the event that the milk is drawn from infected animals [34]. Physical contact with cattle and sharing of shelter/space is another common practice in these communities.
Lastly, the Kafue lechwe is hunted for meat, trophies and hides. It is estimated that approximately 80% of lechwe carcasses hunted for meat may be infected with BTB. However, poaching levels specific to lechwe are speculated at 50% of the official annual hunting quota [35]. This places both the poachers and consumers of the meat at risk of contracting zoonotic tuberculosis. Unfortunately, information on zoonotic tuberculosis in humans is virtually absent from the Kafue basin where there is a high prevalence of BTB in livestock and wildlife.
Conclusion
This review has identified the knowledge gaps in the management of BTB in Zambia. No molecular epidemiological information linking the observed BTB in cattle and wildlife is available. In addition, the review noted that information on the incidences of zoonotic TB is also unavailable despite the reported cultural practices by the people living at the interface. Finally, the review noted that no molecular epidemiological study has been conducted on TB patients living at the interface area and that no information on the presence of M. tuberculosis in animals exists.
The way forward
•There is a need to document epidemiological links of M. bovis infections in humans, cattle and Kafue lechwe in the Kafue basin. This could be done by molecular characterisation of isolates obtained from these three species (human, cattle and Kafue lechwe), as well as from milk and the environment [36,37].
•Although M. tuberculosis infection has not yet been reported from cattle in Zambia, it has been documented elsewhere [38]. This highlights a need to isolate and differentiate the MTC isolates from cattle to ascertain the presence of M. tuberculosis, and to evaluate its epidemiological significance in transmission to humans.
•The interaction of cattle, lechwe and other wildlife, such as buffaloes, at water and grazing points has been documented in the Kafue Basin. Therefore, there is need to conduct BTB field and molecular studies in order to draw epidemiological links between BTB in the different species and to study the risk factors for the transmission of M. bovis.
•The fact that buffaloes are not infected with M. bovis suggests that environmental contamination is not the preferential route by which wildlife gets contaminated [15]. Moreover, infected lechwe primarily show lesions in the lungs suggesting that infection is acquired by the respiratory route, and the chance of infection increases when lechwe share the same pasture with the infected cattle. Studies on the ecology of M. bovis in the Kafue basin are warranted in order to clarify the most important transmission routes at the livestock/wildlife interface.
•Given the high prevalence of BTB in traditional cattle, it is important that surveillance is conducted in communities to ascertain the impact of zoonotic tuberculosis.
Abbreviations
ABF: Acid-fast bacilli; BTB: Bovine tuberculosis; CIDT: Comparative intradermal tuberculin test; MTC: Mycobacterium tuberculosis complex; OIE: World Organisation for Animal Health; TB: Tuberculosis; WHO: World Health Organization.
Competing interests
The authors declared that they have no competing interests.
Authors’ contributions
SM contributed to the conception and drafting of the manuscript, JBM and JG contributed to the conception and writing of the manuscript. All authors have read and approved the final manuscript.
Supplementary Material
Multilingual abstracts in the six official working languages of the United Nations.
Contributor Information
Sydney Malama, Email: malamask@yahoo.com.
John Bwalya Muma, Email: jbwalya@lycos.com.
Jacques Godfroid, Email: Jacques.Godfroid@nvh.no.
Acknowledgements
The authors would like to thank Dr Tone Bjordal Johansen, Dr Francisco Olea-Popelka, Mr Are Berentsen and Dr Melanie Andrews for proofreading the manuscript.
References
- Jenkins AO, Cadmus SIB, Venter EH, Pourcel C, Hauk Y, Vergnaud G. et al. Molecular epidemiology of human and animal tuberculosis in Ibadan, Southwestern Nigeria. Vet Microbiol. 2011;151:139–147. doi: 10.1016/j.vetmic.2011.02.037. [DOI] [PubMed] [Google Scholar]
- Chihota V, Apers L, Mungofa S, Kasongo W, Nyoni IM, Tembwe R. et al. Predominance of a single genotype of Mycobacterium tuberculosis in regions of Southern Africa. Int J Tuberc Lung Dis. 2007;11:311–318. [PubMed] [Google Scholar]
- Sakamoto K. The Pathology of Mycobacterium tuberculosis Infection. Vet Pathol. 2012;49:423–439. doi: 10.1177/0300985811429313. [DOI] [PubMed] [Google Scholar]
- who. World Health Organisation. Geneva, Switzerland: WHO/HTM/TB: Global Tuberculosis Control WHO Report; 2009. p. 411. [Google Scholar]
- O'Reilly LM, Daborn CJ. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tuber Lung Dis. 1995;76(Supplement 1):1–46. doi: 10.1016/0962-8479(95)90591-x. [DOI] [PubMed] [Google Scholar]
- Munyeme M, Munang'andu HM. A review of Bovine tuberculosis in the Kafue Basin Ecosystem. Veterinary Medicine International. 2011;2011:1–9. doi: 10.4061/2011/918743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyeme M, Muma JB, Samui KL, Skjerve E, Nambota AM, Phiri IGK. et al. Prevalence of bovine tuberculosis and animal level risk factors for indigenous cattle under different grazing strategies in the livestock/wildlife interface areas of Zambia. Trop Anim Health Prod. 2009;41:345–352. doi: 10.1007/s11250-008-9195-5. [DOI] [PubMed] [Google Scholar]
- Thoen C, Lobue PA, de Kantor I. Why has zoonotic tuberculosis not received much attention? Int J Tuberc Lung Dis. 2010;14:1073–1074. [PubMed] [Google Scholar]
- Phiri AM. Common conditions leading to cattle carcass and offal condemnations at 3 abattoirs in the Western Province of Zambia and their zoonotic implications to consumers. Journal of the South African Veterinary Association-Tydskrif Van Die Suid-Afrikaanse Veterinere Vereniging. 2006;77:28–32. doi: 10.4102/jsava.v77i1.336. [DOI] [PubMed] [Google Scholar]
- CSO. Zambia 2010 census of population and housing 2011. Lusaka, Zambia: Central Statistical Office (CSO; 2011. [Google Scholar]
- Carney D. Implementing the sustainable livelihoods approach: what contribution can we make? London, UK: Department for International Development; 1998. [Google Scholar]
- Garrett Kenneth O. (Master of Science thesis) Forests and Farming: an analysis of rural livelihood programs for poverty reduction in Eastern Zambia. Montana, USA: The university of Montana Missoula; 2007. [Google Scholar]
- UKaid. What would it take for Zambias beef and daily industry to achieve their potential? The world Bank: UKaid; 2011. http://www.worldbank.org/Zambia. [Google Scholar]
- Munyeme M, Muma JB, Munang'andu HM, Kankya C, Skjerve E, Tryland M. Cattle owners' awareness of bovine tuberculosis in high and low prevalence settings of the wildlife-livestock interface areas in Zambia. BMC Vet Res. 2010. p. 6. [DOI] [PMC free article] [PubMed]
- Munyeme M, Muma JB, Skjerve E, Nambota AM, Phiri IGK, Samui KL. et al. Risk factors associated with bovine tuberculosis in traditional cattle of the livestock/wildlife interface areas in the Kafue basin of Zambia. Prev Vet Med. 2008;85:317–328. doi: 10.1016/j.prevetmed.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Cook AJC, Tuchili LM, Buve A, Foster SD, GodfreyFaussett P, Pandey GS. et al. Human and bovine tuberculosis in the Monze District of Zambia - a cross-sectional study. Br Vet J. 1996;152:37–46. doi: 10.1016/s0007-1935(96)80084-4. [DOI] [PubMed] [Google Scholar]
- Munyeme M, Rigouts L, Shamputa IC, Muma JB, Tryland M, Skjerve E, Isolation and characterization of Mycobacterium bovis strains from indigenous Zambian cattle using Spacer oligonucleotide typing technique. BMC Microbiol. 2009. p. 9. [DOI] [PMC free article] [PubMed]
- Munyeme M, Muma JB, Siamudaala VM, Skjerve E, Munangandu HM, Tryland M. Tuberculosis in Kafue lechwe antelopes (Kobus leche Kafuensis) of the Kafue Basin in Zambia. Prev Vet Med. 2010;95:305–308. doi: 10.1016/j.prevetmed.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Gallagher J, Macadam I, Sayer J, Van Lavieren L. Pulmonary tuberculosis in free-living lechwe antelope in Zambia. Trop Anim Health Prod. 1972;4:204–213. doi: 10.1007/BF02360112. [DOI] [PubMed] [Google Scholar]
- Munang'andu HM, Siamudaala VM, Matandiko W, Nambota AM, Muma J, Mweene A. et al. Comparative intradermal tuberculin testing of free-ranging African Buffaloes (Syncerus caffer) captured for Ex Situ conservation in the Kafue Ecosystem in Zambia. Veterinary Medicine International. 2011;2011:1–5. doi: 10.4061/2011/385091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracey JF, Collins DS, Huey RJ, In Meat Hygiene. Animal slaughter: meat inspection statistics. London, New York: W.B.Saunders & Company Toronto: Edited by Gracey JF; 1999. pp. 190–202. [Google Scholar]
- Hang'ombe M, Munyeme M, Fukushima Y, Suzuki H, Matandiko W, Suzuki Y, Mycobacterium bovis infection at the interface between domestic and wild animals in Zambia. BMC Vet Res. 2012. p. 8. [DOI] [PMC free article] [PubMed]
- Ameni G, Vordermeier M, Firdessa R, Aseffa A, Hewinson G, Gordon SV. et al. Mycobacterium tuberculosis infection in grazing cattle in central Ethiopia. Vet J. 2011;188:359–361. doi: 10.1016/j.tvjl.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocepek M, Pate M, Zolnir-Dovc M, Poljak M. Transmission of Mycobacterium tuberculosis from human to cattle. J Clin Microbiol. 2005;43:3555–3557. doi: 10.1128/JCM.43.7.3555-3557.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obanda V, Poghon J, Yongo M, Mulei I, Ngotho M, Waititu K. et al. First reported case of fatal tuberculosis in a wild African elephant with past human - wildlife contact. Epidemiol Infect. 2013;244:1–5. doi: 10.1017/S0950268813000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angkawanish T. Mycobacterium tuberculosis Infection of Domesticated Asian Elephants, Thailand (vol 16, pg 1949, 2010) Emerg Infect Dis. 2011;17:758. doi: 10.3201/eid1612.100862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava K, Chauhan DS, Gupta P, Singh HB, Sharma VD, Yadav VS. et al. Isolation of Mycobacterium bovis &M. tuberculosis from cattle of some farms in north India - possible relevance in human health. Indian J Med Res. 2008;128:26–31. [PubMed] [Google Scholar]
- Michel AL, Muller B, van Helden PD. Mycobacterium bovis at the animal-human interface: a problem, or not? Vet Microbiol. 2010;140:371–381. doi: 10.1016/j.vetmic.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Moda G, Daborn CJ, Grange JM, Cosivi O. The zoonotic importance of Mycobacterium bovis. Tuber Lung Dis. 1996;77:103–108. doi: 10.1016/s0962-8479(96)90022-2. [DOI] [PubMed] [Google Scholar]
- Grange JM, Yates DM, De Kantor NI. Guidelines for speciation within the Mycobacterium tuberculosis complex. Geneva, Switzerland: WHO/EMC/ZOO/96; 1996. p. 4. [Google Scholar]
- Gumi B, Schelling E, Berg S, Firdessa R, Erenso G, Mekonnen W. et al. Zoonotic transmission of tuberculosis between pastoralists and their livestock in South-East Ethiopia. EcoHealth. 2012;9:139–149. doi: 10.1007/s10393-012-0754-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayele WY, Neill SD, Zinsstag J, Weiss MG, Pavlik I. Bovine tuberculosis: an old disease but a new threat to Africa. Int J Tuberc Lung Dis. 2004;8:924–937. [PubMed] [Google Scholar]
- Mwachalimba KK, Mumba C, Munyeme M. Cost benefit analysis of tuberculosis control in wildlife-livestock interface areas of Southern Zambia. Prev Vet Med. 2012;110:274–279. doi: 10.1016/j.prevetmed.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Grange JM, Yates MD. Zoonotic aspects of Mycobacterium bovis infection. Vet Microbiol. 1994;40:137–151. doi: 10.1016/0378-1135(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Siamudaala VM, Muma J, Munang'andu HM, Mulumbu M, In Conservation and Development Interventions at the Wildlife/Livestock Interface; Implication for Wildlife, Livestock and Human Health. Disease challenges concerning the utilization of the Kafue lechwe(Kobus leche kafuensis) in Zambia. Switzerland and Cambridge, UK: Cambridge: IUCN, Gland: edition. Edited by Osofsky AS; 2005. pp. 75–80. [Google Scholar]
- Fine EA, Bolin AC, Gardiner CJ, Kaneene BJ. A study of the persistence of Mycobacterium bovis in the Environment under Natural Weather Conditions in Michigan. USA. Veterinary Medicine International. 2011;2011:1–12. doi: 10.4061/2011/765430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Kahla I, Boschiroli ML, Souissi F, Cherif N, Benzarti M, Boukadida J. et al. Isolation and molecular characterisation of Mycobacterium bovis from raw milk in Tunisia. Afr Health Sci. 2011;11:S2–S5. doi: 10.4314/ahs.v11i3.70032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicic S, Pate M, Duvnjak S, Katalinic-Jankovic V, Obrovac M, Dezdek D. et al. Molecular epidemiology of Mycobacterium tuberculosis transmission between cattle and man - a case report. Veterinarski Arhiv. 2012;82:303–310. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multilingual abstracts in the six official working languages of the United Nations.