Abstract
Fungal entomopathogens rely on cellular heterogeneity during the different stages of insect host infection. Their pathogenicity is exhibited through the secretion of secondary metabolites, which implies that the infection life history of this group of environmentally important fungi can be revealed using metabolomics. Here metabolomic analysis in combination with ex vivo insect tissue culturing shows that two generalist isolates of the genus Metarhizium and Beauveria, commonly used as biological pesticides, employ significantly different arrays of secondary metabolites during infectious and saprophytic growth. It also reveals that both fungi exhibit tissue specific strategies by a distinguishable metabolite secretion on the insect tissues tested in this study. In addition to showing the important heterogeneous nature of these two entomopathogens, this study also resulted in the discovery of several novel destruxins and beauverolides that have not been described before, most likely because previous surveys did not use insect tissues as a culturing system. While Beauveria secreted these cyclic depsipeptides when encountering live insect tissues, Metarhizium employed them primarily on dead tissue. This implies that, while these fungi employ comparable strategies when it comes to entomopathogenesis, there are most certainly significant differences at the molecular level that deserve to be studied.
Introduction
Isogenic cell populations of both prokaryotic and eukaryotic organisms can exhibit a highly dynamic transcriptome, proteome and metabolome. In many cases cellular heterogeneity occurs in response to environmental stresses, functioning as an important mechanism for the survival of cells under adverse conditions [1]–[3]. Entomopathogenic fungi are microorganisms that infect and typically kill their insect hosts as a developmental necessity. Spores must first overcome chemical and physical barriers of the insect cuticle before responding to the heterogeneous nature of the insect haemocoel, that includes the innate immune system, and metabolizing host materials to fuel growth [4]. Finally, the fungus kills the host before utilizing nutrients from the dead tissue for spore formation and transmission. The multiple steps, involving a variety of stages (cuticle penetration, blastospore growth and vegetative growth) and changing environmental conditions as the parasite encounters different parts of the host, implies significant cellular variability during the different stages of the infection process.
The genera Beauveria and Metarhizium are both comprised of anamorph entomopathogenic fungi that infect a wide range of arthropod hosts. Fungal strains for both these genera are frequently isolated from soil and have been the most widely employed as mycoinsecticides for the biological control of insect pests worldwide. In recent years, interest in the nature of the secondary metabolites responsible for their pathogenesis has increased. As a result, toxic cyclic depsipeptides, such as beauverolides in Beauveria sp. [5]–[8] and destruxins in Metarhizium sp. [9]–[11], have been well described, among other secondary metabolites such as beauvericin [12], bassianolide [13] and NG-291 [14]. The similar biological control function of these two entomopathogens against hundreds of species of insects has resulted in a commonly held view that they are ecologically similar species with comparable modes of action leading to similar results [15]–[18]. However, Beauveria and Metarhizium are phylogenetically distinct by at least 150 million years [19], and some of the different groups of metabolites they produce are described implying different modes of action inside the insect host. Therefore, in spite of their similar effects on tested insect pests, they likely possess significant differences in both the range of metabolites and the manner in which they are deployed.
To address this hypothesis, we adopted a global metabolomics approach in combination with a newly developed ex vivo insect culturing assay. As such, we have grown vegetative mycelium of a Beauveria and Metarhizium isolate in the presence of Camponotus pennsylvanicus ant brains and muscles kept alive in an insect cell culture medium. Liquid chromatography coupled with quadrupole time-of-flight mass spectrometry on the medium after prolonged contact between fungus and ant tissue shows that these two fungal isolates indeed secrete a very different array of metabolites (hereafter referred to as their secretome) on both live and dead ant material. In addition, they each exhibit a significantly different secretome when encountering different insect tissues. Furthermore, using this novel culturing approach, we discovered additional cyclic depsipeptides for both species that have not yet been described. These compounds have been reported to have roles in cellular paralysis and cytotoxic effects, which have been shown to have an antitumor, antibiotic, antifungal, immunosuppressant and anti-inflammatory effect [20]–[24]. Finally, we discovered that these insecticidal compounds are heterogeneously secreted depending on the type of tissue: the Beauveria isolate secretes its beauverolides mainly on live tissues, while the Metarhizium isolate adopts a strategy of releasing destruxins predominantly on dead tissue.
Results and Discussion
Analysis of the Heterogeneous Nature of Metarhizium brunneum (F52) and Beauveria bassiana (GHA)
In this study we made use of a fungal isolate from the species Metarhizium brunneum and Beauveria bassiana. Their secondary metabolite secretion was tested when vegetative mycelium encountered C. pennsylvanicus tissues. Carpenter ants of the species C. pennsylvanicus are considered a pest in the United States since they preferably nest in dead wood and cause structural damage to many houses every year [25]. For this experiment we dissected brains and muscles out of heads of C. pennsylvanicus workers, something that is commonly being performed in insect physiology and behavioral studies [26]. We chose brain and muscle tissue as fat, malphigian tubule and gut tissue were liable to break extensively during dissections making it unsuitable. Ant brains and muscles were placed in cell culture inserts (Millipore) in Schneider’s medium (Gibco), designed to resemble hemolymph for insect cell culture when freshly supplemented with fetal bovine serum (PAA laboratories Inc.) [27]. As such, ex vivo tissues stay viable (though we cannot with certainty state they are metabolically similarly active), creating fungal growth conditions that can be used to simulate an ant infection and examine the reaction of entomopathogens to the different tissues it encounters. Tissue viability was checked after a week of incubation using a trypan blue staining (Sigma), which indicated that ant brain and muscle tissue cultured ex vivo were indeed still alive (Figure S1), however similarly tested gut and fat tissue were not. The possibility for using actual hemolymph extracted from live ants as a medium was considered, but rejected because of potential microbial activities. After a 3-day incubation of Schneider’s medium, ant brain and muscle tissue, with and without fungal growth, the supernatant from the different conditions was harvested and prepared for metabolomic analysis.
Biological triplicates of the different samples were run in a randomized order using an AB Sciex (Framinhgam, MA) 5600 quadrupole time-of-flight mass spectrometer connected to a Shimadzu (Columbia, MD) UFLC system. Prior to data analysis the raw LC-MS peak data of all 60 samples were aligned together using the parameters described in the material and methods. Subsequently, the dataset was processed to include only monoisotopic ions between 100 and 800 m/z with retention times between 1 and 16 minutes. This resulted in a total of 15000 unique retention time/mass to charge ratio peaks (Data File S1, http://www.metabolomics.psu.edu) on which extensive analyses, using both univariate and multivariate statistics, were performed. Principal component analysis (PCA) showed that despite the complex nature of the medium used, there is a distinguishable clustering of the four major groups of samples: medium controls, ant tissue controls, Beauveria growth and Metarhizium growth (Figure 1a). This clustering allows us to confidently claim that although we are using complex media and tissue conditions, due to the incorporation of controls for the medium, ant tissues and fungal growth in the medium, we can distinguish fungal metabolites from those found in the cell culture media and the insect tissues. In addition to demonstrating the ability to conduct global metabolomics profiling on ex vivo tissues, this approach revealed the differences in secretomes between isolates of these two generalist fungal species since they secreted a significantly different array of metabolites, when experiencing to the same environment. Comparing by t-test all ions secreted by the Beauveria isolate to those of the Metarhizium isolate, 2540 and 1177 ion peaks respectively, were found to be secreted in significantly different amounts (p<0.01) and of those 31.5% and 32.2% were unique to either of the fungal isolates (Data File S2, sheet 1 and 2). To illustrate this the loading data for 10 representative peaks found using this analysis can be found in Figure S2a,b. A similar comparison was done making use of the OPLS-DA (orthogonal projections to latent structures discriminant analysis) and S-plot analysis using SIMCA-P+13. This multivariate analysis facilitates the identification of potentially biochemically significant metabolites since the S-plot reveals which ions are driving the clustering of the groups being compared. Using a cut off of p(corr) [1]>0.8 (Figure 1b) this analysis identified 372 and 328 significantly different retention time/mass to charge ratio signals for the Beauveria and Metarhizium isolates respectively (Data File S3, sheet 1). Given their different and distant position on the phylogenetic tree of the order Hypocreales [28], [29] different secretomes are expected. This expectation is strengthened by a recent comparative genomics study in which B. bassiana was compared to two Metarhizium species as well as Cordyceps militaris [30]. The study revealed the differences and similarities of gene clusters encoding for e.g. toxins, enzymes and secondary metabolites in these fungi and demonstrated that insect pathogenicity in B. bassiana and C. militaris evolved independently from Metarhizium [30]. Our metabolomic analyses shows how this phylogenetic independence results in a significantly different array of secreted secondary metabolites. Since both of these fungi are used in the biological control of insect pests, understanding the nature of their heterogeneous secretomes could help in designing more effective control measures.
Figure 1. Separate clustering of fungal and control samples used in this study.
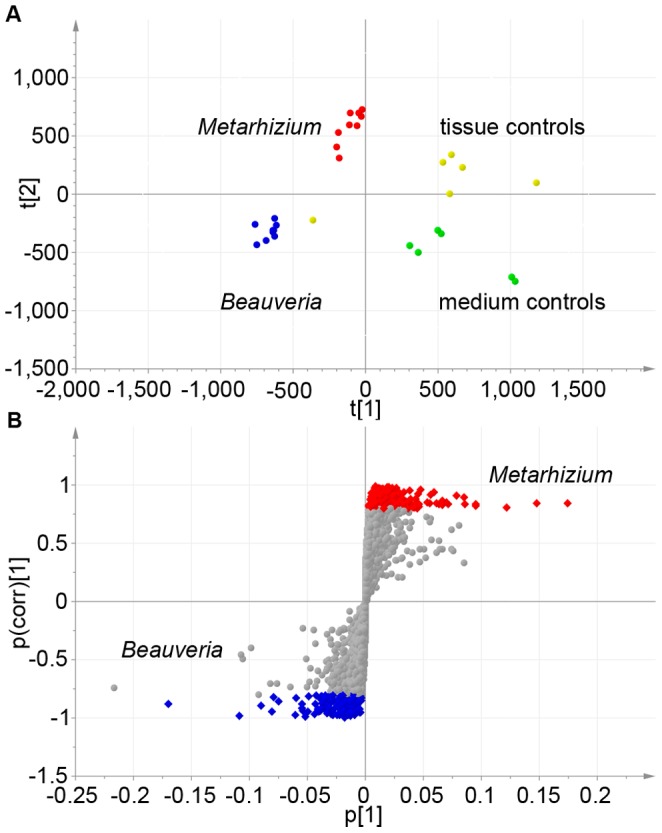
A) Principle Component Analysis (PCA) of samples incubated in Schneider’s medium supplemented with 10% FBS. Clustering of different sample types based on all samples for medium controls (green), ant brain and muscle controls (yellow), Beauveria growth (blue), and Metarhizium growth (red) in a three-dimensional score space. B) S-plot analysis of all Metarhizium samples against all Beauveria samples. All Metarhizium related ions with p(corr) [1]>0.8 are marked red, while all Beauveria related ions with p(corr) [1]>0.8 are marked blue.
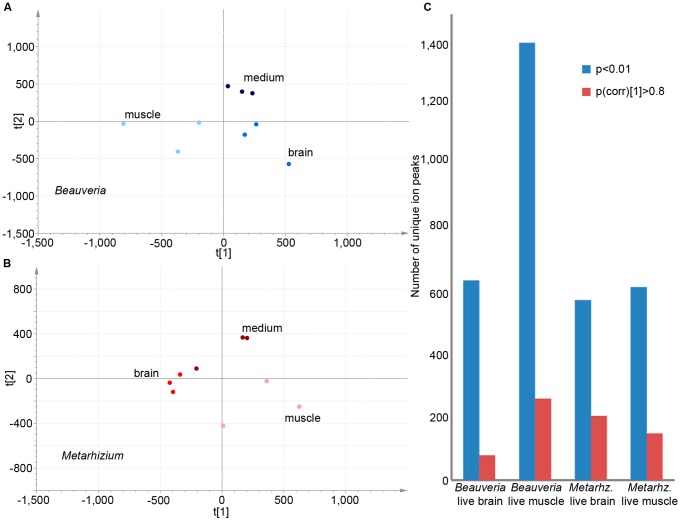
In addition to having different secretomes these two fungi exhibited contrasting secondary metabolite deployment behavior, demonstrating that, when studying these organisms, we should appreciate the differences underlying their pathogenicity. Distribution of ions in a three-dimensional metabolic space of all growth condition samples within a species resulted for both in a separate supervised clustering for growth on brain tissue, muscle tissue and Schneider’s medium (Figure 2a and b). Our interpretation of these results is that both fungi condition a significant amount of their overall metabolite profile to the two ex vivo and control conditions tested, implying they exhibit a heterogeneous approach to encountering different situations and environments within the body of the host. When assessing what signals are significantly different in the various growth conditions, univariate analyses revealed for Beauveria 643 and 1349 significant (p<0.01) retention time/mass to charge ratio peaks for growth on live brain and muscle tissue respectively (Figure 2c; Data File S2, sheet 3 and 4; Figure S2c,d). Focusing only on the peaks best fitting the multivariate model (p(corr) [1]>0.8), 75 and 245 signals were found on live brain and muscle tissue respectively (Figure 2c; Data File S3, sheet 2 and 3). This indicates that the Beauveria isolate specifically increases the secretion of 2.1–3.3 times more compounds when in contact with live ant muscle tissue compared to growth on live ant brain tissue. For Metarhizium, we also found a significant amount of tissue growth dependent ions. However, in contrast to the Beauveria isolate, similar specificity numbers were found for brain and muscle tissue growth (Figure 2c; Data File S2, sheet 5 and 6; Figure S2e,f; Data File S3, sheet 4 and 5).
Figure 2. Overview of analyses to determine the tissue specificity of Beauveria and Metarhizium.
A) Supervised PCA plot showing the clustering of different types of Beauveria growth; growth on Schneider’s medium (dark blue), Schneider’s medium with ant brains (bright blue) and Schneider’s medium with ant muscles (light blue). B) Supervised PCA plot showing the clustering of different types of Metarhizium growth: growth on Schneider’s medium (dark red), Schneider’s medium with ant brains (bright red) and Schneider’s medium with ant muscles (light red). C) Bar graph displaying the number of unique ion peaks found to be significantly specific for growth on either live brain or muscle tissue. Both statistical methods used in this study are represented, p<0.01 being the number of peaks found significant from the t-test and p(corr) [1]>0.8 the ones found significant for the S-plot analysis.
Fungal Metabolome on Dead Compared to Live Insect Tissues
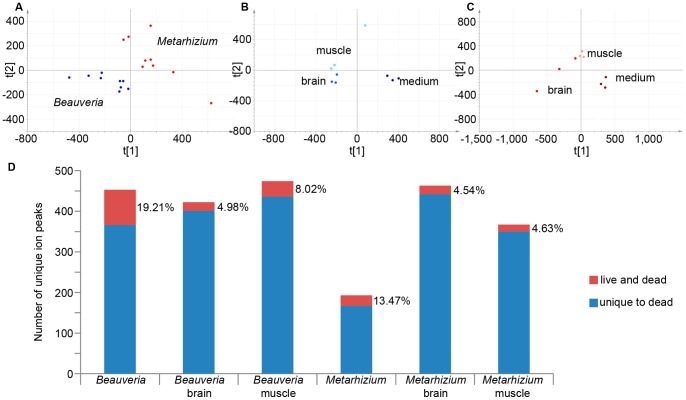
After invasively growing into the insect hemocoel, overcoming the immune system, replicating, and eventually killing the host, entomopathogenic fungi switch their single cell growth to a filamentous one by forming hyphae. The latter uses the insect body as a carbon source for the formation of spore structures for fungal dispersal. Since host death is a developmental necessity for the transmission of this pathogen we can expect that these fungi can respond to cues signaling the death of the host and respond with different secreted compounds. To test this we grew Metarhizium and Beauveria on C. pennsylvanicus tissues kept in PBS. Trypan blue staining 16 hours after dissection showed that ant tissues were dead after a prolonged period in this saline buffer, and fungal hyphae were not able to grow in it unless a carbon source was added (Figure S1). PCA revealed that these PBS grown samples cluster separately from the samples discussed above. This is to be expected since PBS and the Schneider’s medium used above consist of very different compounds. Also, these fungi did not grow in PBS, which resulted in completely different background LC-MS signals and less generated ion peaks overall. A direct comparison between our live tissue data set and the dead tissue dataset is thus not valid. However, we can analyze the dead tissue data set separately, using the same bioinformatics tools we employed above, and make the comparison based on the output afterwards. OPLS-DA is used in all cases for this subset of the data since there were not enough abundant ion peaks that were significantly different to cluster the different sample types using PCA. This again resulted in data suggesting the different metabolic nature of Beauveria versus Metarhizium (Figure 3a) and the heterogeneous reaction to different insect tissues of both fungi (Figure 3b and c). Again a great amount of significantly different signals for the different comparisons made were found, which are all again taken up in Data File S2 (sheets 7–12) and Data File S3 (sheets 6–10). The graph in Figure 3d presents an overview of all the significant (p<0.01) monoisotopic ion peaks found using univariate analysis. Only 4.54–19.21% of these peaks were also found when these fungi were grown on live ant material. Metabolomics on these two experimental set ups thus demonstrate that entomopathogenic fungi can distinguish between dead and live host material and as such exhibit a different metabolic response. This strengthens our point that, in order to study secondary metabolite secretion in entomopathogens, the heterogeneous nature of fungal entomopathogens should be taken into account when designing a culturing method.
Figure 3. Overview of data obtained from analyses done to determine secretome heterogeneity on dead ant tissues.
A-C depict the supervised PCA plots for A) general Beauveria and Metarhizium growth on dead tissues, B) different types of Beauveria growth, and C) different types of Metarhizium growth. D) Bar graph representing the number of unique ion peaks found in the comparisons of the two fungal species with each other and the comparison of dead tissue growth related ion peaks within those species. The top part of the bars represents the percentage of metabolites in common with fungal growth on live tissues.
Discovery of Novel Cyclic Depsipeptides
Metabolomics coupled with ex vivo organ culturing has allowed us to begin the important process of understanding the key differences between two ecologically and agronomically important fungal isolates. It also allowed us to create an experimental environment in which we simplified host complexity in ways that will bring us closer to the understanding of the heterogeneous mechanisms underlying insect infection compared to other culturing systems reported for metabolite discovery in this important group of fungi [31]. This created a significant opportunity for novel metabolite discovery, which is of general importance given the increasing prominence of these fungi as bio control agents. However, a possible roadblock is that public metabolite databases do not yet hold an extensive amount of information, making it not yet possible to identify metabolites by automated library searching.
For both Beauveria and Metarhizium the cyclic depsipeptides, that are found to play a role in pathogenesis, are well studied. For both cyclic depsipeptides several derivatives have been identified that exhibit different rates of activity in the conditions tested to study their effects. Beauverolides secreted by Beauveria sp. are cyclic tetradepsipeptides containing C9- or C11-β-hydroxy acid residues [32] (Figure 4a). More than twenty different beauverolides have been identified, and sequencing of beauverolides by tandem mass spectrometry has been well described [32], [33]. The product ion mass spectra we obtained in this study were virtually identical to those in the literature. The presence of an immonium ion at m/z 139 or m/z 167 indicates the presence of hydroxy-methyloctanoyl and hydroxy-methyldecanoyl residues respectively at position 1. The presence of additional immonium ions at m/z 72, 86, 104, 120, 139 and 157 indicates the presence of certain amino acids, valine, leucine and isoleucine, methionine, phenylalanine, tyrosine and tryptophan respectively. The presence of prominent b3, b2-H2O, a2-H2O, and 3–4 dipeptide ions allow for the complete sequencing of these compounds. Destruxins secreted by Metarhizium sp. are cyclic hexadepsipeptides consisting of an α-hydroxy acid and 5 amino acid residues (Figure 4b). Thirty-nine different destruxins have been reported [10]. The mass spectral fragmentation patterns of destruxins have also been well characterized [34]–[36], and again the product ion mass spectra we obtained in this study were virtually identical to the in-source CID mass spectra previously reported. The D-alpha-hydroxy acid residue, which is a major site of heterogeneity in the destruxins, gives a prominent fragment ion designated D+ (34), allowing the identification of this residue. The accurate mass capabilities of the mass spectrometer used in this study easily discriminated between the isobaric 2-hydroxy-4-methylpentanoic and 2-hydroxy-4,5-epoxypentanoic side chains. The presence of prominent b5, b4, a4 b3 and b2 ions allows the sequence determination of these compounds. Depending on the amino acids available in the environment, derivatives of these secondary metabolites are being formed that can have different activities and biological functions within metabolic pathways.
Figure 4. Overview cyclic depsipeptides identified in this study.
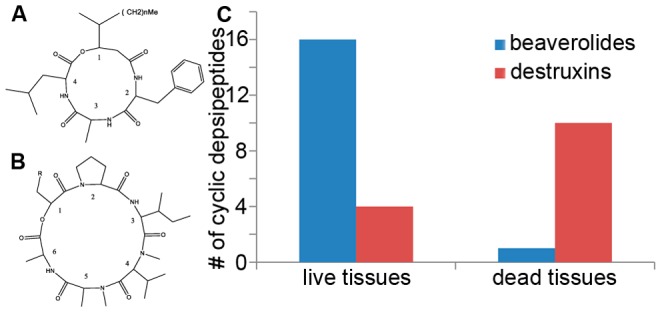
A) General structure of beauverolides. The identities of the amino acids at positions 2–4 and the n descriptor are given in Table 1. B) General structure of destruxins. The identities of the amino acids at positions 2–6 and the R descriptor are given in Table 2. C) Bar chart summarizing the amount of beauverolides (blue) and destruxins (red) found to be secreted on live and dead insect tissues.
Analyzing the metabolite peaks obtained in this study we have identified 20 beauverolides of which 14 have not been described before (Table 1) and 14 destruxins of which three are novel (Table 2). The mass spectra for all these cyclic depsipeptides and their predicted structures can be found in Data File S4. As expected, when comparing the metabolome of these two fungi, these cyclic depsipeptides turn up as being species specific (Data File S2, sheets 1,2 and 8). Next to that they seem to be predominantly secreted (p<0.01) as a result of interacting with one of the two tissues with a fold change on brain or muscle tissue ranging between 4.4 and 128.3 (Tables 1 and 2). Comparing this result again to the multivariate analysis by means of S-plot analysis however, only half of the beauverolides and one of the destruxins are found to be above p(corr) [1]>0.8 when comparing all Beauveria to all Metarhizium samples. Using this statistical analysis to look at tissue specificity, none of them appear to be above the set threshold of 0.8. This implies that although these important secondary metabolites are clearly heterogeneously secreted, they are overall not the main heterogeneous compounds driving PCA clustering of the different sample conditions used in this study. Other, yet unidentified compounds seem to be of more biochemical significance. We therefore hope our supplementary data sets will help researchers interested in the secondary metabolites of these two fungi to identify those compounds eventually.
Table 1. Secreted beauverolides in the ex vivo culturing system.
| [M+H]+ | RT/min | n | AA2 | AA3 | AA4 | beauverolide | fold change on brain | fold change on muscle |
| 482.3261 | 13.99 | 3 | Val | Lxx | Lxx | this study | L 4.6D ∞ | |
| 488.2894 | 13.54 | 3 | Ala | Phe | Lxx | this study | L 5.9 | |
| 500.3652 | 13.62 | 3 | Val | Met | Lxx | this study | L 4.4 | |
| 510.3574 | 15.07 | 5 | Val | Lxx | Lxx | this study | L 6.3 | |
| 514.3808 | 13.93 | 3 | Lxx | Met | Lxx | this study | L 6.2 | |
| 516.3432 | 13.98 | 3 | Val | Phe | Lxx | E | L 6.1 | |
| 528.3965 | 14.5 | 5 | Val | Met | Lxx | this study | L 6.2 | |
| 530.3588 | 14.3 | 3 | Lxx | Phe | Lxx | this study | L 6.2 | |
| 532.3426 | 13.3 | 3 | Val | Tyr | Lxx | this study | L 4.7 | |
| 544.3745 | 15.01 | 5 | Val | Phe | Lxx | B | L 7.7 | |
| 558.3902 | 15.5 | 5 | Lxx | Phe | Lxx | this study | L 8.8 | |
| 560.3739 | 14 | 5 | Val | Tyr | Lxx | this study | ||
| 564.3872 | 14.24 | 3 | Phe | Phe | Lxx | F | ||
| 576.4405 | 14.74 | 5 | Phe | Met | Lxx | this study | ||
| 580.3866 | 13.54 | 3 | Tyr | Phe | Lxx | this study | L 4.4 | |
| 592.4073 | 15.4 | 5 | Phe | Phe | Lxx | C | L 9.9 | |
| 603.4239 | 14.02 | 3 | Trp | Phe | Lxx | J | L 6.8 | |
| 608.4067 | 14.28 | 5 | Tyr | Phe | Lxx | this study | ||
| 615.466 | 14.58 | 5 | Trp | Met | Lxx | this study | L 8.2 | |
| 631.444 | 14.99 | 5 | Trp | Phe | Lxx | K | L 8.7 |
For each identified beauverolide the accurate mass of the protonated molecular ion, retention time, n, and the identities of the amino acids at positions 2–4 are given (Figure 4a). The ones discovered in other studies are indicated by the letter assigned to them. The fold changes on brain and muscle indicate the tissue specificity of beauverolides. All of them are heterogeneously secreted on live tissues (indicated by L), while one is uniquely secreted on dead muscle tissue as well (indicated by D). The beauverolides mentioned in gray were identified in our raw data set but were not of enough abundance to be used in the further data analysis.
Table 2. Secreted destruxins in the ex vivo culturing system.
| [M+H]+ | RT/min | R | AA2 | AA3 | AA4 | AA5 | AA6 | destruxin | fold change on brain | fold change on muscle |
| 564.3392 | 11.53 | CH = CH2 | Pro | Val | mVal | mAla | Ala | A2 | D 9.7 | |
| 564.3392 | 10.92 | CH = CH2 | Pro | Lxx | Val | mAla | Ala | DesmA | L 5.4 | |
| 566.3392 | 11.64 | CH-(CH3)2 | Pro | Val | Val | mAla | Ala | this study | ||
| 578.3548 | 11.7 | CH = CH2 | Pro | Lxx | mVal | mAla | Ala | A | D 24.7 | |
| 580.3340 | 9.34 | CH(O)CH2 | Pro | Lxx | Val | mAla | Ala | this study | D 128.3 | |
| 580.3704 | 12.22 | CH-(CH3)2 | Pro | Val | mVal | mAla | Ala | B2 | D 13.5 | |
| 580.3704 | 12.5 | CH-(CH3)2 | Pro | Lxx | Val | mAla | Ala | DesmB | D 9.0 | |
| 594.3861 | 12.73 | CH-(CH3)2 | Pro | Lxx | mVal | mAla | Ala | B | D 36.2 | L 23.1 |
| 594.3497 | 10.17 | CH(O)CH2 | Pro | Lxx | mVal | mAla | Ala | E | ||
| 596.3654 | 9.55 | COH-(CH3)2 | Pro | Lxx | Val | mAla | Ala | DesmC | ||
| 610.3810 | 10.39 | COH-(CH3)2 | Pro | Lxx | mVal | mAla | Ala | C | D 16.3 | |
| 610.3446 | 9.53 | CH2-CH2COOH | Pro | Lxx | Val | mAla | Ala | this study | ||
| 612.3603 | 8.69 | CH2OH-CH2OH | Pro | Lxx | mVal | mAla | Ala | Ed | ||
| 624.3603 | 10.34 | CH2-CH2COOH | Pro | Lxx | mVal | mAla | Ala | D |
For each identified destruxin the accurate mass of the protonated molecular ion, retention time, R and the identities of the amino acids in positions 2–6 are given (Figure 4b). The ones discovered in other studies are indicated by the letter assigned to them. The fold changes found for the majority of them on brain and muscle indicate the tissue specificity of these destruxins. Most of them are heterogeneously secreted on dead tissues (indicated by D), while two are secreted on live tissue (indicated by L). The destruxin mentioned in gray was identified in our raw data set but not of enough abundance to be used in the further analysis of the data.
Lastly, we found that while Beauveria secreted its beauverolides mainly in the presence of live muscle tissue, Metarhizium secreted its destruxins mostly when in contact with dead brain tissue (Figure 4c, Tables 1 and 2). The latter was unexpected since cyclic depsipeptides are functionally described as “killing” compounds, which is reflected in their name, destruxins. However, our experiment shows that while the Beauveria isolate likely uses beauverolides to attack living tissues, the Metarhizium isolate secretes destruxins mainly when tissues are already dead. This is not only surprising, but again suggests that the different metabolic approaches these fungi deploy in different environmental circumstances within the host deserve to be studied in more detail.
In conclusion, our data suggests a wealth of difference between two important fungal isolates that are commercially being used in a battle with pest insect species. Although long known to be phylogenetically different the very similar outcomes in tests for biological pest control using these fungi has resulted in them having been considered largely as one tool. What we have shown, using a novel culturing assay with bioinformatics sorting, is that metabolically these fungi behave very differently on different insect tissues. We suggest that knowledge about these differences and the heterogeneous approach when encountering different insect tissues could be important in designing better control measures. Our evidence of different secretomes in these two fungal isolates from two different genera is in line with a recent study resulting in similar conclusions by looking at the genome level [30]. The more artificial culturing methods generally used to study the production of secondary fungal metabolites are not necessarily leading to a good representation of what is happening in nature and might lead to the misinterpretation of metabolic pathways and functions. Furthermore, it might not allow us to identify the derivatives with the highest specific anti-insecticidal activity. Culturing assays, such as the one we developed, would thus aid in understanding the molecular mechanisms underlying pathogenicity of these fungi in their different insect hosts [31].
Materials and Methods
Fungal Strains
Experiments were done with strain F52 of Metarhizium bunneum, the fungal isolate used in the commercial product Met52® and Beauveria bassiana GHA from Botanigard®. Pure axenic cultures were obtained from Nina Jenkins, The Thomas Lab, Dept of Entomology, PSU, and maintained on PDA plates. Inoculations were done using 1.5 µl of a fresh 108 spores/ml saline tween solution (0.8% NaCl, 0.005% Tween-80). Colonies were grown in a two-dimensional way (sandwich culture) using two perforated polycarbonate membranes [37], only allowing the formation of a vegetative mycelium.
Ex vivo Culturing
Ant tissues (brains and muscles) were dissected in PBS from Camponotus pennsylvanicus worker’s heads using a stereomicroscope (Nikon SMZ645) holding C-W10× eyepieces and a zoom range of 0.5× to 5×. Prior to dissection, whole ants were surface sterilized in 100% ethanol on ice for 5 minutes and subsequently rinsed in PBS, again on ice. After dissection, tissues were briefly surface sterilized (5–10 seconds) in 100% ethanol and rinsed in Schneider’s media (Gibco) freshly supplemented with 10% fetal bovine serum (FBS) (PAA Laboratories Inc.), 100 µg/mL kanamycin (Invitrogen), 100 units/mL penicillin and 100 µg streptomycin/mL (Invitrogen) [27]. Brains or muscle tissue from the heads of three ants were placed within a 12 µm pore size cell culture insert (Millipore). Using a laminar flow, the tissues were washed three times and finally placed in 1 mL Schneider’s containing FBS and antibiotics as described above for incubation during 3 days at 28°C. To measure metabolites secreted by the strains used in this study, as a reaction to the ant tissues, a 3×3 mm square cut out of a freshly grown sandwich culture was added per single cell culture insert. The proper controls were incorporated by incubating fungal strains without ant tissues, ant tissues without fungal strains, and the medium (with and without antibiotics) without fungal material or ant tissues. Three biological replicates were done for each condition. A similar set up was used for tissues incubated in PBS. Again, brains or muscle tissue from the heads of three ants were placed within a 12 µm pore size cell culture insert (Millipore). Using a laminar flow, the tissues were washed three times and finally placed in 1 mL PBS containing antibiotics as described above for incubation during 3 days at 28°C. To measure metabolites secreted by the strains used in this study, as a reaction to the dead ant tissues, again a 3×3 mm square cut out of a freshly grown sandwich culture was added per single cell culture insert. The proper controls were incorporated by incubating fungal strains without dead ant tissues in PBS, dead ant tissues without fungal strains, and just PBS (with and without antibiotics) without fungal material or ant tissues. While in the live ant tissue set up both fungal strains managed to grow across the diameter of the well, growth stayed local around the tissues in the set up with PBS. In both set-ups fungal growth was not found to be invasive.
Dead Live Staining
Viability of the ex vivo ant tissues in the experimental set up was tested using Trypan blue (Sigma). Medium was removed by draining the cell culture insert and placing it in a clean well. Subsequently the tissues were washed three times using 1 ml PBS. After washing, tissues were stained with 1 ml of a 10-fold dilution of 0.4% Trypan blue (Sigma). The stain was removed by draining and the tissues were again washed 3 times in PBS. Subsequently, fresh medium was added and the tissues were examined using a dissecting scope (Nikon). When tissues failed to absorb the stain they were considered to be viable [38].
Metabolomics
In order to measure secreted metabolites, tissues were separated from the medium after incubation by draining the cell culture inserts. The medium was harvested, snap frozen using liquid nitrogen, and stored at −80°C upon processing. Subsequently, 100 µl of the medium was mixed with 1 volume of acetonitrile for protein precipitation prior to LC-MS/MS analysis. Samples were randomized and run using the HPLC-QTOFMS (Shimadzu Prominence UFLC XR and AB Sciex 5600 quadrupole time-of-flight mass spectrometry) platform. Samples (5 ul) were separated on a C18 Column (100×2.1 mm 1.7 um, Waters Acquity BEH) using a gradient elution program with aqueous acetonitrile (3–90%) at a flow rate of 250 ul/min. Positive ion electrospray ionization mass spectra were acquired over the mass range 50–1250 Da in IDA (Information Dependent Acquisition) mode with one 100 ms survey scan and up to twenty 100 ms MS/MS product ion scans per duty cycle. The survey scan LC-MS datasets were aligned together using MarkerView (AB Sciex) software and the following parameters were used to extract the peaks from the raw data: Retention times between 0.00 and 16.00 min.; Subtraction Offset of 10 scans; Subtraction Mult. Factor of 1.3; Noise Treshold of 50; Min. Spectral Peak Width of 15 ppm; Min. RT Peak Width of 3 scans; Retention Time Tolerance of 0.10 min.; Mass Tolerance of 10.0 ppm; 3 required samples; Maximum of 100000 peaks. Analyses were done looking at the monoisotopic ions with an m/z between 100 and 800 and a retention time between 1 and 16 minutes, and making use of the multivariate analysis software package SIMCA-P+13 (Umetrics).
Supporting Information
Dead/live staining on ex vivo cultured ant tissues. Result of the trypan blue staining on ant brains (A) and ant muscle (B) kept in Schneider’s insect resembling medium, Grace’s insect resembling medium and PBS for 3 days at 28°C. Tissues failing to absorb the stain are considered viable. Tissues appear to be most viable in Schneider’s medium, hence our choice to use this medium to perform our live tissue experiments in.
(TIF)
Loading plots of representative peaks found to be significantly different across sample types. Each panel in this figure shows an example of what the loading plots across sample types of 10 representative peaks found to be significant in the univariate analyses done in this study, looked like. This figure illustrates the striking differences between and the reproducibility within sample types when comparing A) all Beauveria samples to all other samples, B) all Metarhizium samples to all other samples, C) Beauveria grown on live brain tissue versus other types of Beauveria growth, D) Beauveria grown on live muscle tissue versus other types of Beauveria growth, E) Metarhizium grown on live brain tissue versus other types of Metarhizium growth, and F) Metarhizium grown on live muscle tissue versus other types of Metarhizium growth.
(TIF)
All unique retention time/mass to charge ratio peaks that are monoisotopic and found between 100 and 800 m/z with retention times between 1 and 16 minutes.
(XLSX)
(XLSX)
(XLSX)
(PDF)
Acknowledgments
We would like to thank Dr Nina Jenkins at the Pennsylvania State University for providing us with the fungal isolates used in this study, and her very valuable input on earlier draft of this manuscript.
Funding Statement
This work was supported by National Science Foundation MRI 1126373, Marie Curie Actions IOF Project 299501 and Funds to DPH and ADP from the Core Metabolomic Facility at the Huck Institute of the Life Sciences and from the Pennsylvania State University. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Nobile CJ, Mitchell AP (2007) Microbial biofilms: e pluribus unum. Curr Biol 17: 349–353. [DOI] [PubMed] [Google Scholar]
- 2. Veening JW, Smits WK, Kuipers OP (2008) Bistability, epigenetics, and bet-hedging in bacteria. Annu Rev Microbiol 62: 193–210. [DOI] [PubMed] [Google Scholar]
- 3. Veening JW, Igoshin OA, Eijlander RT, Nijland R, Hamoen LW, et al. (2008) Transient heterogeneity in extracellular protease production by Bacillus subtilis . Mol Syst Biol 4: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hajek AE, St Leger RJ (1994) Interactions between fungal pathogens and insect hosts. Annu Rev Entomol 39: 293–322. [Google Scholar]
- 5. Elsworth JF, Grove JF (1974) Search for biologically active cyclodepsipeptides from Beauveria bassiana . S Afr J Sci 70: 379–379. [Google Scholar]
- 6. Elsworth JF, Grove JF (1977) Cyclodepsipeptides from Beauveria bassiana Bals. Part 1. Beauverolides H and I. J Chem Soc Perkin 1: 270–273. [PubMed] [Google Scholar]
- 7. Elsworth JF, Grove JF (1980) Cyclodepsipeptides from Beauveria bassiana. Part 2. Beauverolides A to F and their relationship to isarolide. J Chem Soc Perkin 1: 1795–1799. [PubMed] [Google Scholar]
- 8. Grove JF (1980) Cyclodepsipeptides from Beauveria bassiana. Part 3. The isolation of beauverolides Ba, Ca, Ja, and Ka. J Chem Soc Perkin 1: 2878–2880. [PubMed] [Google Scholar]
- 9. Donzelli BG, Krasnoff SB, Moon YS, Churchill AC, Gibson DM (2012) Genetic basis of destruxin production in the entomopathogen Metarhizium robertsii . Curr Genet 58: 105–116. [DOI] [PubMed] [Google Scholar]
- 10. Liu BL, Tzeng YM (2012) Development and applications of destruxins: A review. Biotechnol Adv 30: 1242–1254. [DOI] [PubMed] [Google Scholar]
- 11. Wang B, Kang QJ, Lu YZ, Bai LQ, Wang CS (2012) Unveiling the biosynthetic puzzle of destruxins in Metarhizium species. Proc Natl Acad Sci U S A 109: 1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu Y, Orozco R, Wijeratne EM, Gunatilaka AA, Stock SP, et al. (2008) Biosynthesis of the cyclooligomer depsipeptide beauvericin, a virulence factor of the entomopathogenic fungus Beauveria bassiana . Chem Biol 15: 898–907. [DOI] [PubMed] [Google Scholar]
- 13. Xu Y, Orozco R, Wijeratne EMK, Espinosa-Artiles P, Gunatilaka AAL, et al. (2009) Biosynthesis of the cyclooligomer depsipeptide bassianolide, an insecticidal virulence factor of Beauveria bassiana . Fungal Genet Biol 46: 353–364. [DOI] [PubMed] [Google Scholar]
- 14. Donzelli BG, Krasnoff SB, Churchill AC, Vandenberg JD, Gibson DM (2010) Identification of a hybrid PKS-NRPS required for the biosynthesis of NG-391 in Metarhizium robertsii . Curr Genet 56: 151–162. [DOI] [PubMed] [Google Scholar]
- 15. Butt TM, Ibrahim L, Ball BV, Clark SJ (1994) Pathogenicity of the entomogenous fungi Metarhizium anisopliae and Beauveria bassiana against crucifer pests and the honey bee. Biocontrol Sci Technol 4: 207–214. [Google Scholar]
- 16. Bukhari T, Takken W, Koenraadt CJ (2011) Development of Metarhizium anisopliae and Beauveria bassiana formulations for control of malaria mosquito larvae. Parasit Vectors 4: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akbarian J, Ghosta Y, Shayesteh N, Safavi SA (2012) Pathogenicity of some isolates of Beauveria bassiana (Bals.) Vuill. and Metarhizium anisopliae (Metsch.) Sorokin on 2nd and 4th larval instars of Colorado potato beetle, Leptinotarsa decemlineata (Say) (Col.: Chrysomelidae), under laboratory conditions. Afr J Microbiol Res 6: 6407–6413. [Google Scholar]
- 18. Mnyone LL, Ng’habi KR, Mazigo HD, Katakweba AA, Lyimo IN (2012) Entomopathogenic fungi, Metarhizium anisopliae and Beauveria bassiana reduce the survival of Xenopsylla brasiliensis larvae (Siphonaptera: Pulicidae). Parasit Vectors 5: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sung GH, Poinar GO, Spatafora JW (2008) The oldest fossil evidence of animal parasitism by fungi supports a Cretaceous diversification of fungal–arthropod symbioses. Mol Phylogenet Evol 49: 495–502. [DOI] [PubMed] [Google Scholar]
- 20. Sarabia F, Chammaa S, Ruiz AS, Ortiz LM, Herrera FJL (2004) Chemistry and biology of cyclic depsipeptides of medicinal and biological interest. Cur Med Chem 11: 1309–1332. [DOI] [PubMed] [Google Scholar]
- 21. Zimmermann G (2007) Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii . Biocontrol Sci Technol 17: 553–596. [Google Scholar]
- 22. Zimmermann G (2007) Review on safety of the entomopathogenic fungus Metarhizium anisopliae . Biocontrol Sci Technol 17: 879–920. [Google Scholar]
- 23.Anke H, Antelo L (2009) Cyclic peptides and depsipeptides from fungi. In: Anke T, Weber D, editors. The mycota volume 15: Physiology and genetics. Springer Berlin Heidelberg. 273–296.
- 24.Anke H (2010) Insecticidal and nematicidal metabolites from fungi. In: Hofrichter M, editor. The mycota volume 10: Industrial applications. Springer Berlin Heidelberg. 151–163.
- 25.Hansen L, Klotz J (2005) Carpenter ants of the United States and Canada: Comstock Publishing Associates. 224 p.
- 26. Truman JW, Riddiford LM (1970) Neuroendocrine control of ecdysis in silkmoths. Science 167: 1624–1626. [DOI] [PubMed] [Google Scholar]
- 27. Hughes GL, Pike AD, Xue P, Rasgon JL (2012) Invasion of Wolbachia into Anopheles and other insect germlines in an ex vivo organ culture system. PloS One 7: e36277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sung GH, Hywel-Jones NL, Sung JM, Luangsa-ard JJ, Shrestha B, et al. (2007) Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol 57: 5–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sung GH, Sung JM, Hywel-Jones NL, Spatafora JW (2007) A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Mol Phylogenet Evol 44: 1204–1223. [DOI] [PubMed] [Google Scholar]
- 30. Xiao G, Ying SH, Zheng P, Wang ZL, Zhang S, et al. (2012) Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana . Sci Rep 2: 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Molnár I, Gibson DM, Krasnoff SB (2010) Secondary metabolites from entomopathogenic Hypocrealean fungi. Nat Prod Rep 27: 1241–1275. [DOI] [PubMed] [Google Scholar]
- 32. Kuzma M, Jegorov A, Kacer P, Havlicek V (2001) Sequencing of new beauverolides by high-performance liquid chromatography and mass spectrometry. J Mass Spectrom 36: 1108–1115. [DOI] [PubMed] [Google Scholar]
- 33. Jegorov A, Paizs B, Kuzma M, Zabka M, Landa Z, et al. (2004) Extraribosomal cyclic tetradepsipeptides beauverolides: profiling and modeling the fragmentation pathways. J Mass Spectrom 39: 949–960. [DOI] [PubMed] [Google Scholar]
- 34. Lange C, Mulheim C, Vey A, Pais M (1992) Sequencing of cyclodepsipeptides (destruxins) using positive fast atom bombardment desorption tandem mass spectrometry. Biol Mass Spectrom 21: 33–42. [DOI] [PubMed] [Google Scholar]
- 35. Jegorov A, Havlicek V, Sedmera P (1998) Rapid screening of destruxins by liquid chromatography/mass spectrometry. J Mass Spectrom 33: 274–280. [DOI] [PubMed] [Google Scholar]
- 36. Pedras MS, Irina Zaharia L, Ward DE (2002) The destruxins: synthesis, biosynthesis, biotransformation, and biological activity. Phytochemistry 59: 579–596. [DOI] [PubMed] [Google Scholar]
- 37. Wösten HA, Moukha SM, Sietsma JH, Wessels JG (1991) Localization of growth and secretion of proteins in Aspergillus niger . J Gen Microbiol 137: 2017–2023. [DOI] [PubMed] [Google Scholar]
- 38. Paul J (1968) Cell and tissue culture–the art and the science. Lab Pract 17: 570–572. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dead/live staining on ex vivo cultured ant tissues. Result of the trypan blue staining on ant brains (A) and ant muscle (B) kept in Schneider’s insect resembling medium, Grace’s insect resembling medium and PBS for 3 days at 28°C. Tissues failing to absorb the stain are considered viable. Tissues appear to be most viable in Schneider’s medium, hence our choice to use this medium to perform our live tissue experiments in.
(TIF)
Loading plots of representative peaks found to be significantly different across sample types. Each panel in this figure shows an example of what the loading plots across sample types of 10 representative peaks found to be significant in the univariate analyses done in this study, looked like. This figure illustrates the striking differences between and the reproducibility within sample types when comparing A) all Beauveria samples to all other samples, B) all Metarhizium samples to all other samples, C) Beauveria grown on live brain tissue versus other types of Beauveria growth, D) Beauveria grown on live muscle tissue versus other types of Beauveria growth, E) Metarhizium grown on live brain tissue versus other types of Metarhizium growth, and F) Metarhizium grown on live muscle tissue versus other types of Metarhizium growth.
(TIF)
All unique retention time/mass to charge ratio peaks that are monoisotopic and found between 100 and 800 m/z with retention times between 1 and 16 minutes.
(XLSX)
(XLSX)
(XLSX)
(PDF)