Abstract
The role of the mitogen-activated protein kinase kinase (MKK)/extracellular-activated protein kinase (ERK) pathway in mitotic Golgi disassembly is controversial, in part because Golgi-localized targets have not been identified. We observed that Golgi reassembly stacking protein 55 (GRASP55) was phosphorylated in mitotic cells and extracts, generating a mitosis-specific phospho-epitope recognized by the MPM2 mAb. This phosphorylation was prevented by mutation of ERK consensus sites in GRASP55. GRASP55 mitotic phosphorylation was significantly reduced, both in vitro and in vivo, by treatment with U0126, a potent and specific inhibitor of MKK and thus ERK activation. Furthermore, ERK2 directly phosphorylated GRASP55 on the same residues that generated the MPM2 phospho-epitope. These results are the first demonstration of GRASP55 mitotic phosphorylation and indicate that the MKK/ERK pathway directly phosphorylates the Golgi during mitosis.
INTRODUCTION
In preparation for cell division, the highly ordered stacked cisternae of the mammalian Golgi complex undergo mitotic breakdown (Roth, 1999). This breakdown is triggered by protein phosphorylation events (Nelson, 2000) that function, at least in part, to inhibit one or more trafficking steps (Lowe et al., 1998b). The exact nature of the Golgi breakdown product is controversial in that it may comprise dispersed small vesicles (Jesch and Linstedt, 1998), clustered small vesicles (Shima et al., 1998), or membranes that fuse with the ER (Zaal et al., 1999). In any event, the disassembled Golgi is subsequently partitioned into each daughter cell where it reassembles a ribbon of stacked membranes positioned near the microtubule organizing center.
Currently, the identity of the protein kinases that mediate Golgi disassembly is controversial. Both cyclin-dependent kinase 1 (CDK), considered a master regulatory kinase in the G2/M transition (Ohi and Gould, 1999), and a kinase in the mitogen-activated protein kinase (MAPK) signaling pathway known as MAPK kinase (MKK) have been implicated in mitotic Golgi breakdown. In its better characterized role in cell cycle entry from Go, MKK (present as 2 isoforms, MKK1 and MKK2), becomes activated by phosphorylation and then phosphorylates and activates the MAP kinase isoforms known as extracellular signal-regulated protein kinase (ERK) 1 and ERK2. Importantly, the MKK and ERK isoforms are present in their active forms in mitotic extracts (Kuang and Ashorn, 1993) and cells (Shapiro et al., 1998; Zecevic et al., 1998; Tolwinski et al., 1999), and mammalian fibroblasts treated with an MKK inhibitor arrest in G2 failing to enter M-phase (Wright et al., 1999). One of the first observations implicating MKK in mitosis came from an assay that measures mitotic cytosol-dependent Golgi disassembly in permeabilized normal rat kidney cells. In this assay the Golgi breakdown activity is separable from CDK1 and is blocked by MKK inactivation or MKK immunodepletion (Acharya et al., 1998). In contrast, in an assay that measures mitotic cytosol-dependent disassembly of purified rat liver Golgi, breakdown is blocked by CDK inhibition or depletion but not by MKK inhibition or depletion (Lowe et al., 1998a). Recent work with a different permeabilized cell assay for mitotic Golgi breakdown has suggested a possible resolution to these controversial reports in that both CDK1 and MKK were found to be required for disassembly, but each may mediate distinct steps (Kano et al., 2000).
A key point, however, is that because both CDK1 and MKK are required for events in the G2/M transition that are upstream of Golgi breakdown, elucidation of the direct roles of these kinases will ultimately rest on identification of their Golgi-localized substrates and the development of specific inhibitors against these substrates. In the case of CDK1, it directly phosphorylates GM130 (Lowe et al., 1998a), and mitotically phosphorylated GM130 has reduced affinity for the vesicle docking protein p115. Thus, GM130 phosphorylation could, in principle, block docking of transport vesicles and cause Golgi vesiculation (Nakamura et al., 1997). Nevertheless, other events must also take place because inhibition of the GM130/p115 interaction is not sufficient to cause Golgi breakdown (Seemann et al., 2000). In the case of MKK, a Golgi localized substrate for the MKK pathway has not been identified; thus, it remains possible that the role of MKK in Golgi fragmentation is indirect.
A proteomic study to identify novel downstream targets of the MKK/ERK pathway identified Golgi reassembly stacking protein of 55 kDa (GRASP55) as a potential substrate for a MAPK (Lewis et al., 2000). GRASP55 is related to GRASP65, a Golgi-localized protein that participates in the docking of transport vesicles. Inhibition of either GRASP55 or GRASP65 during cell free reassembly of previously disassembled rat liver Golgi prevents stacking (Barr et al., 1997; Shorter et al., 1999). GRASP65 is highly phosphorylated during mitosis (Barr et al., 1997). However, to our knowledge, the phosphorylation state of GRASP55 during mitosis has not been reported. Because of the proposed direct role of MKK in Golgi disassembly during mitosis (Acharya et al., 1998; Colanzi et al., 2000; Kano et al., 2000), we have tested the possibility that GRASP55 is a mitotic substrate of the MKK/ERK pathway.
MATERIALS AND METHODS
Cloning and Mutagenesis
Sequence corresponding to the N-terminus of human GRASP55 was cloned from a human HeLa cDNA library by PCR amplification with the use of a primer at the 5′ flanking sequence of the library vector insert site and a primer corresponding to an internal human GRASP55 site as determined from a partial human GRASP55 cDNA clone, DKFZp43D156 (RZPD, Berlin). The PCR products, after generating cohesive termini with the use of T4 DNA polymerase (Stoker, 1990), were cloned into pBluescript KS (Stratagene, La Jolla, CA) and sequenced. To generate the full-length human GRASP55 cDNA, the sequence corresponding to the remaining C-terminal portion of human GRASP55 obtained from DKFZp43D156 was cloned in frame and downstream of the sequence corresponding to the N-terminus, with the use of a shared restriction site. Site-directed mutagenesis to introduce alanine codons at the required positions was carried out as described (Deng and Nickoloff, 1992) and verified by sequencing. For HeLa cell transfection, carboxyl-terminally myc-tagged GRASP55 was generated by cloning the full-length cDNA into pCS2-MT (Turner and Weintraub, 1994). Amino-terminally GST-tagged fusion contructs were generated by cloning GRASP55 cDNA into pGEX-3X (Amersham Pharmacia Biotech, Piscataway, NJ).
HeLa Transfection and Labeling
Subconfluent HeLa cells were transfected with the use of calcium phosphate (Sambrook et al., 1989) and 20 μg of pCS2GRASP55-MYC plasmid DNA per 10-cm plate. The cells were split 1:2, treated with 0.5 μg/ml nocodazole for 18–20 h (for mitotic shake-off), and harvested at 16, 48, and 72 h posttransfection, respectively. Immunofluorescence staining was carried out as described (Jesch and Linstedt, 1998) with the use of anti-myc 9e10 mouse antibody (1:200; Evan et al., 1985) and anti-giantin rabbit antibody (1:1000; Linstedt et al., 1995). For 32P labeling of mitotic cells, nocodazole-blocked transfected HeLa cells (2.7 × 106 cells) were collected by shake-off and replated in a 10-cm dish in phosphate-free DMEM supplemented with 100 IU/ml penicillin-streptomycin and 0.5 μg/ml nocodazole. Mitotic cells were pretreated with 20 μM U0126 or 0.2% DMSO, incubated for 30 min at 37°C, and then subsequently incubated with 0.4 mCi/ml [32P]H3PO4 (Dupont NEN, Boston, MA) for 4 h at 37°C.
Cell Extract Preparation
Extracts were prepared as described (Acharya et al., 1998). Mitotic HeLa cells were isolated by shake-off from subconfluent cultures grown in MEM supplemented with 10% fetal bovine serum and 0.5 μg/ml nocodazole for 18–20 h (Knehr et al., 1995). With the use of Hoechst 33258 staining, we confirmed that chromatin was intact and condensed in the collected cells, and we also confirmed that the arrested cells were viable if reincubated in the absence of nocodazole. To prepare U0126-treated extracts, mitotic shake-off cells were replated for 30 min at 37°C in the same medium supplemented with 20 μM U0126 (Promega, Madison, WI) or 0.2% DMSO (Sigma, St. Louis, MO), the solvent for U0126. Mitotic cells were collected by centrifugation at 2000 rpm in a SA1600 rotor (Sorvall, Newtown, CT) for 5 min at 4°C, washed two times in phosphate-buffered saline (PBS), and then once in mitotic extract buffer (MEB; 15 mM PIPES [pH 7.4], 50 mM KCl, 10 mM MgCl2, 20 mM β-mercaptoethanol, 20 mM β-glycerol phosphate, 15 mM EGTA, 0.5 mM spermidine, 0.2 mM spermine, 1 mM DTT, 0.1 mM PMSF, 0.2 μg/ml aprotinin, 10 μg/ml leupeptin, and 10 μg/ml pepstatin A). The cell pellet was resuspended in two packed cell volumes of MEB, swollen on ice for 10 min, and homogenized by repeated passages through a 25-gauge needle. The homogenized cells were pelleted in a tabletop ultracentrifuge in a TLA 100.2 rotor (Beckman, Fullerton, CA) at 60,000 rpm for 45 min at 4°C. The supernatant was aliquoted, snap-frozen in liquid nitrogen, and stored at −80°C. Interphase extracts were prepared from unsynchronized cells and treated exactly as for mitotic extracts. Measured protein concentration for both mitotic and interphase extracts was between 8 and 10 mg/ml.
Protein Purification and In Vitro Phosphorylation Assays
GST-GRASP55 fusion proteins were purified after induction with 0.5 mM IPTG from 1 L exponentially growing Escherichia coli cultures. After harvesting by centrifugation, cell pellets were frozen at −80°C, thawed on ice, and resuspended in ice-cold 20 ml TM buffer (50 mM Tris-HCl [pH 8.0], 12.5 mM MgCl2, 1 mM EDTA, 15 mM β-mercaptoethanol) containing protease inhibitors (1 mM PMSF, 10 μg/ml leupeptin, and 10 μg/ml pepstatin A), 200 μg/ml lysozyme, and 1% Triton X-100. Cell lysates were disrupted by sonication and then cleared by centrifugation at 10,000 × g for 30 min. Supernatants were adjusted to 40 ml with TM buffer containing 1% Triton X-100 and bound to 2 ml S-linked glutathione agarose (Sigma) for 2 h at 4°C with mixing. Beads were collected and washed by gravity with 40 ml buffer containing 50 mM Tris-HCl (pH 8.0), 1 M NaCl, then 40 ml PBS containing 1% Triton X-100, and a final wash of 40 ml TM buffer. All wash buffers contained 15 mM β-mercaptoethanol and protease inhibitors. Proteins were left attached to beads or eluted in TM containing 20% glycerol in 15 mM reduced glutathione (Sigma), snap-frozen in liquid nitrogen, and stored in aliquots at −80°C. Phosphorylation reactions with the use of cell extracts contained 25 mM Tris-HCl (pH 8.0), 6.25 mM MgCl2, 0.5 mM EDTA, 15 mM β-mercaptoethanol, 250 μM ATP, 2.25 μg mitotic or interphase extract, and GST or GST-GRASP55 fusion proteins attached to glutathione agarose beads in a final volume of 20 μl. Reaction mixtures were incubated at 30°C for 30 min. Beads were washed in ice-cold TM buffer containing protease inhibitors. In phosphatase-treated reactions, beads were further washed with 40 mM PIPES (pH 6.0) and incubated with 0.3 U potato acid phosphatase (Cooper and King, 1986) in 40 μl 40 mM PIPES (pH 6.0) for 20 min at 30°C. Proteins were resolved by SDS-PAGE and analyzed by immunoblotting for MPM-2 reactivity. Phosphorylation reactions with the use of 250 ng purified recombinant kinases, prepared as described (Shapiro et al., 1998), were performed under identical buffer and incubation conditions, except that soluble GST-GRASP55 fusion proteins (5 μg) were used.
RESULTS
Our first goal was to test if GRASP55 is mitotically phosphorylated. A partial-length human cDNA encoding a protein homologous to rat GRASP55 (Shorter et al., 1999) was obtained from the German sequencing consortium. PCR was used to amplify a cDNA containing the missing 5′end out of a human cDNA library. The full-length human GRASP55 cDNA obtained encodes a 452-residue protein with a high degree of sequence identity to the 454-amino acid rat GRASP55 (Figure 1). With the use of the BLOSUM62 comparison matrix the two proteins showed 89.2% identity.
Figure 1.
Comparison of human and rat GRASP55 sequences. Alignment of the human GRASP55 and rat GRASP55 (AF110267) sequences. Capital letters indicate exact identity. The underlined region designates the amino-terminus that was cloned for this study. Potential sites of proline-directed phosphorylation are boxed. The ideal ERK consensus motif is shown in bold, and residues that were mutated to alanine are indicated with asterisks.
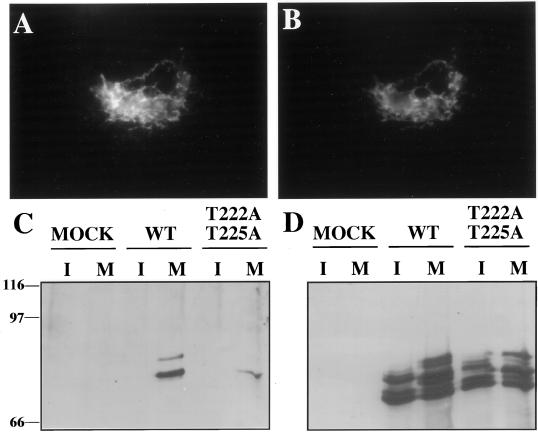
Human GRASP55, tagged at its carboxy terminus with a myc-epitope (GRASP55-MYC), was transiently expressed in HeLa cells. As expected for a properly targeted Golgi protein, the expressed GRASP55-MYC colocalized with giantin (Figure 2, A and B). To analyze GRASP55 phosphorylation in these cells, we used an immunoblotting assay that used the mitotic phosphoprotein monoclonal-2 (MPM2) antibody (Davis et al., 1983). MPM2 recognizes a phosphorylated S/T-P epitope in a subset of mostly unidentified mitotic phosphoproteins (Westendorf et al., 1994). Of the MPM2 antigens that are identified, many play key mitotic roles regulated by phosphorylation (Kumagai and Dunphy, 1996; Renzi et al., 1997). Significantly, GRASP55-MYC immunoprecipitated from nocodazole-arrested mitotic cells was strongly MPM2 reactive (Figure 2C, lane 4). In contrast, GRASP55-MYC immunoprecipitates from nonsynchronized cells or control immunoprecipitates from nonsynchronized or mitotic cells were not detectably MPM2 reactive (Figure 2C, lanes 1–3). The overall recovery of GRASP55-MYC was roughly equal from control and mitotic cells (Figure 2D). For a reason that is currently unknown, transfected GRASP55-MYC was always recovered as multiple bands. In addition, there were noticeable band shifts in mitotic cells that could in principle reflect non-MPM2 reactive mitotic phosphorylation. On the other hand, incorporation of 32P during mitosis indicated that only a single GRASP55-MYC band was abundantly phosphorylated (see below, Figure 4B), suggesting that differential phosphorylation does not account for the other bands. Whether the major phosphorylated band contains phosphorylation sites in addition to the MPM-2 sites was not determined because the MPM-2 sites were the focus of this study. In summary, based on MPM2 reactivity and confirmed by 32P incorporation, GRASP is mitotically phosphorylated in vivo and this generates one or more MPM2 epitopes.
Figure 2.
Human GRASP55 is a Golgi-localized MPM2 antigen. (A) HeLa cells were transfected with human GRASP55-MYC and processed for double-label immunofluorescence microscopy. The GRASP55-MYC localization pattern as indicated by anti-myc staining is shown. (B) The staining pattern of the Golgi protein giantin in the same cell. Bar, 50 μm. (C) Unsynchronized (I) or mitotic (M) HeLa cells that were mock-transfected (lanes 1 and 2), wild-type (wt) GRASP55-MYC transfected (lanes 3 and 4), or T222A T225A GRASP55-MYC transfected (lanes 5 and 6) were subjected to immunoprecipitation with the anti-myc antibody. The resulting precipitates were analyzed for MPM2 reactivity by immunoblotting. (D) The immunoblot was reprobed with anti-myc antibody to determine GRASP55-MYC recovery. The transfected protein was recovered from both mitotic and interphase cells but only mitotic GRASP55-MYC was MPM2 reactive. As determined by densitometry and after normalization to account for the small difference in protein recovery, the MPM2 reactivity of T222A T225A GRASP55-MYC was 9% that of GRASP55-MYC.
Figure 4.
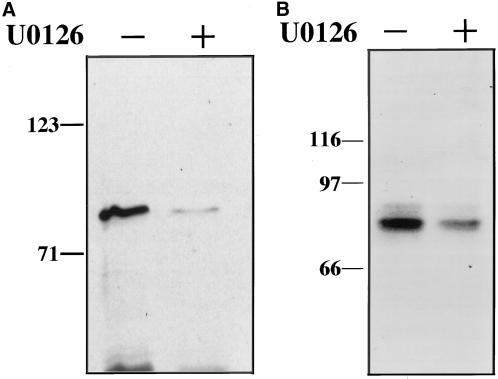
Inhibition of ERK activity by U0126, a specific inhibitor of MKK, inhibits GRASP55 phosphorylation. (A) MPM2 reactivity of GST-GRASP55 was determined after in vitro incubation with added ATP and mitotic cell extracts that had been pretreated with either 0.2% DMSO control or 20 μM U0126 for 30 min at 37°C. (B) 32P incorporation of transfected GRASP55-MYC recovered by immunoprecipitation from mitotic HeLa cells that were pretreated for 30 min at 37°C with either 0.2% DMSO control or 20 μM U0126 and then incubated for 4 h with [32P]H3PO4.
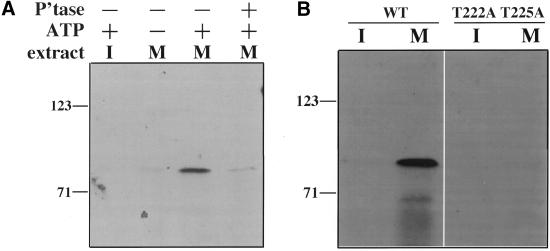
Our next goal was to determine if the MAPK pathway plays a role in GRASP55 mitotic phosphorylation. Toward this end, we first attempted to establish in vitro conditions that would reproduce M-phase specific generation of the MPM2 epitope on GRASP55. Immobilized human GRASP55 fused to glutathione-S-transferase (GST) was incubated with cell extracts prepared from unsynchronized or nocodazole-arrested mitotic HeLa cells. GST-GRASP55 became highly MPM2 reactive after reactions with mitotic extract but not in reactions with interphase extract (Figure 3A, lanes 1 and 3). As expected, MPM2 reactivity was substantially reduced in reactions where ATP was omitted or when GST-GRASP55 was subsequently treated with potato acid phosphatase (Figure 3A, lanes 2 and 4). GST alone showed no MPM2 reactivity showing that the MPM2 reactive epitope is present in GRASP55 (our unpublished results). These results indicate that GRASP55 is phosphorylated by a kinase(s) that is active specifically in mitotic cytosol.
Figure 3.
In vitro mitotic phosphorylation of GRASP55 on consensus ERK sites. (A) MPM2 reactivity of GST-GRASP55 was determined after an in vitro incubation with either interphase (I) or mitotic (M) HeLa cell extracts in the presence or absence of added ATP. One sample (lane 4) was treated with potato acid phosphatase before MPM2 immunoblotting. MPM2 reactivity required ATP and mitotic extract and was lost with phosphatase treatment. (B) After in vitro incubation with interphase or mitotic extracts in the presence of added ATP, the MPM2 reactivity was determined for wild-type (wt) GST-GRASP55 and a version containing T222A and T225A substitutions. Mutation of consensus ERK sites in GRASP55 blocked MPM2 reactivity.
A common approach to validate an in vitro phosphorylation assay is to demonstrate that the reconstituted system results in phosphorylation of the same sites that are hit in vivo. Therefore, we next attempted to locate the MPM2 reactive phosphorylation site in both in vitro and in vivo phosphorylated GRASP55. Eleven potential mitotic phosphorylation sites were identified in human GRASP55, and all but two of these are also present in rat GRASP55 (Figure 1, boxes). Of these, the site at T255 represented the sole ideal ERK consensus motif (Figure 1, bold). The consensus sequence for ERK is P-X-S/T-P, where P at the +1 position is favored, with secondary preference for P at the −2 position (Lewis et al., 1997). We used site-directed mutagenesis to substitute alanine codons in place of T225 and T222 (a nearby site that matches the minimum ERK consensus sequence). These changes essentially abolished mitotic extract-mediated phosphorylation of GST-GRASP55 as determined by the MPM2 assay (Figure 3B). Importantly, the same substitutions in transfected GRASP55-MYC also significantly reduced its MPM2 reactivity after immunoprecipitation from mitotic cells (Figure 2C, lane 6). This version of GRASP55-MYC was also Golgi localized, and Golgi breakdown appeared normal in the transiently transfected cells suggesting that its presence did not exert any dominant effects (our unpublished results). Although alanine substitutions at T222 and T225 were not tested individually, these results demonstrate that mitotic phosphorylation of GRASP55, both in vitro and in vivo, occurs at one or both these sites. This suggests that the same kinase mediates GRASP55 phosphorylation in both the in vitro and in vivo assays and that the identity of the kinase might be ERK.
Because our anti-ERK antibodies did not deplete ERK from the mitotic extracts, we pretreated the extracts with U0126, an inhibitor of MKK1/2 that shows little or no effect on the activities of other protein kinases (Favata et al., 1998; Tolwinski et al., 1999). After 30 min, active ERK was no longer present in mitotic HeLa cell extracts, whereas the remaining pattern of MPM2 reactivity in these extracts was not altered (our unpublished results). Therefore, U0126 had no detectable effect on other mitotically active kinases, and MKK and ERK activities are not required for the mitotic phosphorylation of most MPM2 reactive proteins. Importantly, however, GRASP55 MPM2 reactivity was significantly reduced after incubation in a U0126-treated extract compared with a nontreated control (Figure 4A). We also tested whether U0126 would inhibit 32P incorporation into GRASP55-MYC present in transiently transfected mitotic HeLa cells. As expected mitotic cells treated with U0126 exhibited a significant reduction in GRASP55-MYC phosphorylation compared with untreated controls (Figure 4B). Equal GRASP55-MYC recovery was confirmed by silver staining (our unpublished results). Thus, as indicated by both in vitro and in vivo assays, MKK and/or ERK play a role in GRASP55 mitotic phosphorylation. Also, turnover of GRASP55 phosphorylation site(s), presumably due to phosphatase activity, takes place in cells arrested in mitosis, and MKK and/or ERK activity is required for full rephosphorylation at these sites.
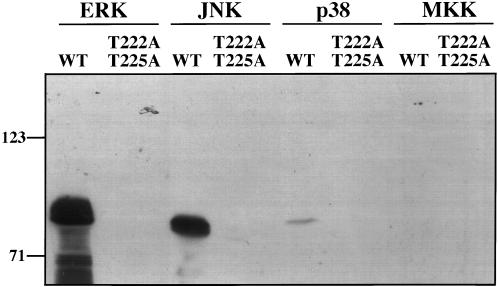
Next we tested whether ERK directly mediates GRASP55 phosphorylation. Therefore, we carried out incubations of purified GST-GRASP55 with purified and activated ERK. Significantly, GST-GRASP55 was strongly MPM2 reactive after incubation with active ERK2 (Figure 5, lane 1). Furthermore, the form of GST-GRASP55 lacking the mapped MPM2 reactive phosphorylation sites was not phosphorylated by ERK2 (Figure 5, lane 2). Two other MAPKs, p38 and much more noticeably JNK, showed some phosphorylation of GST-GRASP55 that was specific to the mapped T222 and/or T225 sites (Figure 5, lanes 3–6). These kinases, involved in the stress response pathway, exhibit overlapping substrate specificity with ERK in vitro. p38 is not activated during mitosis (Shapiro et al., 1998), and JNK is not known to be activated. In contrast to the MAPKs, a constitutively active form of MKK1, G7B:ΔN4/S218D/S222D (Mansour et al., 1996), did not detectably phosphorylate either version of GST-GRASP55 (Figure 5, lanes 7 and 8). In sum, our observations demonstrate that ERK activity is required for and sufficient to mediate mitotic phosphorylation of GRASP55.
Figure 5.
GRASP55 is directly phosphorylated by active MAPKs. MPM2 reactivity of equivalent amounts of wild-type (wt) or the alanine-substituted (T222A T225A) GST-GRASP55 was determined after in vitro incubation, 30 min at 37°C, with an ATP containing buffer and purified active ERK2, JNK, p38, or MKK1 (G7B, ΔN4/S218D/S222D). An equivalent amount of each active kinase was used in each assay.
DISCUSSION
MKK activity is implicated in mitotic Golgi fragmentation (Acharya et al., 1998; Colanzi et al., 2000; Kano et al., 2000), but without an identified Golgi substrate it is possible that its role is indirect. Our results indicate that GRASP55 is mitotically phosphorylated and that a MAPK (ERK) mediates its phosphorylation. Mitotic phosphorylation of GRASP55 in both in vitro and in vivo assays generated the MPM2 phospho-epitope. To date, seven protein kinases have been shown to generate this phospho-epitope in vitro including ERK (Kuang and Ashorn, 1993; Taagepera et al., 1994; Kumagai and Dunphy, 1996; Renzi et al., 1997; Escargueil et al., 2000). GRASP55 contains several proline-directed sites for ERK phosphorylation, of which one (T225) matches the P-X-S/T-P consensus sequence and mutation of this, and an adjacent site, significantly blocked GRASP55 MPM2 reactivity. In addition, treatment of mitotic cell extracts with the MKK inhibitor U0126 produced undetectable active ERK and loss of GRASP55 MPM2 reactivity. Also, treatment of intact mitotic cells with U0126 significantly reduced 32P incorporation into GRASP55. This result indicated that non-MPM2 reactive phosphorylation sites, if present in GRASP55, are either minor or also MKK/ERK dependent. Finally, purified active ERK2 directly phosphorylated GRASP55, and this required the same sites that generated MPM2 reactivity in mitotic cells and extracts.
It was previously demonstrated that MKK itself is mitotically phosphorylated, leading to the suggestion that this might generate an altered substrate specificity, allowing MKK to mediate Golgi breakdown in an ERK-independent manner (Colanzi et al., 2000). Our observations not only indicate that the MAPK pathway directly phosphorylates a Golgi-localized substrate but also that the pathway appears to conform to a mechanism in which MKK phosphorylates and activates ERK and ERK phosphorylates the downstream target(s). Interestingly, the relevant ERK population may be Golgi-localized as anti-ERK antibody staining indicates that ERK is partially present at the Golgi (Acharya et al., 1998).
GRASP55, and its homologue GRASP65, are implicated in the process by which Golgi stacks reform after mitotic breakdown (Barr et al., 1997; Shorter et al., 1999). Whether the GRASP proteins directly link membranes to form stacks or whether their role is upstream of the proteins that actually mediate cisternae cross-bridging remains to be determined. Nevertheless, because the Golgi disassembly/reassembly reaction during cell division is governed by reversible phosphorylation, it is reasonable to expect that the activity of proteins required for stack formation, such as the GRASP proteins, is inhibited by mitotic phosphorylation. This would allow Golgi breakdown and subsequent disinhibition by dephosphorylation would promote reassembly. On the other hand there is at present no known example of a specific phosphorylation event that is required for Golgi breakdown. Certainly in the case of GRASP55 the role of its mitotic phosphorylation remains to be determined. It could be that if multiple kinases and multiple substrates are involved, the inhibition of any single substrate will not be sufficient to abrogate breakdown.
It is important to note that the MAPK pathway is not maximally activated at M-phase, but at other times during the cell cycle. Yet the Golgi only disassembles at M-phase, and ERK-dependent GRASP55 phosphorylation was only detected in mitotic cells or extracts. These observations indicate that the MAPK pathway that acts directly on the Golgi is M-phase specific. In this regard it is interesting to note that unlike active ERKs detected in our interphase extracts, which did not detectably phosphorylate GRASP55, purified and activated ERK2 did phosphorylate GRASP55 (Figure 5, lane 1). This suggests that cellular factors present in the extract must act in concert with ERK2 and/or GRASP55 to generate the M-phase specificity. Thus, in addition to its potential usefulness in the study of the MKK/ERK in Golgi breakdown, the work reported here on GRASP55 also provides a key reagent for dissection of the mechanism that underlies MKK/ERK M-phase specificity. The identification of Golgi-localized substrates for both CDK1 and MKK/ERK, i.e., GM130 (Lowe et al., 1998a) and GRASP55, respectively, is consistent with the idea that these kinases act in concert to mediate Golgi inheritance during cell division. The obvious next challenge is to demonstrate the precise role of these phosphorylation events.
ACKNOWLEDGMENTS
We thank Dr. Jian Kuang for generously providing the MPM2 antibody, Dr. Tina Lee and Dr. Vivek Malhotra for critical reading of the manuscript, and Amy Mehta for useful discussions. This work was supported by National Institutes of Health grant GM-56779–02 to A.D.L.
Abbreviations used:
- CDK
cyclin-dependent kinase
- MAPK
mitogen-activated protein kinase
- MKK
MAPK kinase
- ERK
extracellular signal regulated protein kinase
- GRASP
Golgi reassembly stacking protein
- MPM2
mitotic phosphoprotein monoclonal-2
- MYC
myc tag
- GST
glutathione-S-transferase
REFERENCES
- Acharya U, Mallabiabarrena A, Acharya JK, Malhotra V. Signaling via mitogen-activated protein kinase kinase (MEK1) is required for Golgi fragmentation during mitosis. Cell. 1998;92:183–192. doi: 10.1016/s0092-8674(00)80913-7. [DOI] [PubMed] [Google Scholar]
- Barr FA, Puype M, Vandekerckhove J, Warren G. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 1997;91:253–262. doi: 10.1016/s0092-8674(00)80407-9. [DOI] [PubMed] [Google Scholar]
- Colanzi A, Deerinck TJ, Ellisman MH, Malhotra V. A specific activation of the mitogen-activated protein kinase kinase 1 (MEK1) is required for Golgi fragmentation during mitosis. J Cell Biol. 2000;149:331–339. doi: 10.1083/jcb.149.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA, King CS. Dephosphorylation or antibody binding to the carboxy terminus stimulates pp60c-src. Mol Cell Biol. 1986;6:4467–4477. doi: 10.1128/mcb.6.12.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FM, Tsao TY, Fowler SK, Rao PN. Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci USA. 1983;80:2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng WP, Nickoloff JA. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal Biochem. 1992;200:81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- Escargueil AE, Plisov SY, Filhol O, Cochet C, Larsen AK. Mitotic phosphorylation of DNA topoisomerase II alpha by protein kinase CK2 creates the MPM-2 phosphoepitope on Ser-1469. J Biol Chem. 2000;275:34710–34718. doi: 10.1074/jbc.M005179200. [DOI] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Jesch SA, Linstedt AD. The Golgi and endoplasmic reticulum remain independent during mitosis in HeLa cells. Mol Biol Cell. 1998;9:623–635. doi: 10.1091/mbc.9.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano F, Takenaka K, Yamamoto A, Nagayama K, Nishida E, Murata M. MEK and Cdc2 kinase are sequentially required for Golgi disassembly in MDCK cells by the mitotic Xenopus extracts. J Cell Biol. 2000;149:357–368. doi: 10.1083/jcb.149.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knehr M, Poope M, Enulescu M, Eickelbaum W, Stoehr M, Schroeter D, Paweletz N. A critical appraisal of synchronization methods applied to achieve maximal enrichment of HeLa cells in specific cell cycle states. Exp Cell Res. 1995;217:546–553. doi: 10.1006/excr.1995.1121. [DOI] [PubMed] [Google Scholar]
- Kuang J, Ashorn CL. At least two kinases phosphorylate the MPM-2 epitope during Xenopus oocyte maturation. J Cell Biol. 1993;123:859–868. doi: 10.1083/jcb.123.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- Lewis TS, Hunt J, Aveline LD, Jonscher KR, Louie DF, Yeh J, Nahreini TS, Resing KA, Ahn NG. Identification of novel MAP kinase pathway signaling targets by functional proteomics and mass spectrometry. Mol Cell. 2000;6:1343–1354. doi: 10.1016/s1097-2765(00)00132-5. [DOI] [PubMed] [Google Scholar]
- Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1997;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- Linstedt AD, Foguet M, Renz M, Seelig HP, Glick BS, Hauri H-P. A C-terminally-anchored Golgi protein is inserted into the endoplasmic reticulum and then transported to the Golgi apparatus. Proc Natl Acad Sci USA. 1995;92:5102–5105. doi: 10.1073/pnas.92.11.5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M, Rabouille C, Nakamura N, Watson R, Jackman M, Jamsa E, Rahman D, Pappin DJ, Warren G. Cdc2 kinase directly phosphorylates the cis-Golgi matrix protein GM130 and is required for Golgi fragmentation in mitosis. Cell. 1998a;94:783–793. doi: 10.1016/s0092-8674(00)81737-7. [DOI] [PubMed] [Google Scholar]
- Lowe M, Nakamura N, Warren G. Golgi division and membrane traffic. Trends Cell Biol. 1998b;8:40–44. doi: 10.1016/s0962-8924(97)01189-6. [DOI] [PubMed] [Google Scholar]
- Mansour SJ, Candia JM, Matsuura JE, Manning MC, Ahn NG. Interdependent domains controlling the enzymatic activity of mitogen-activated protein kinase kinase 1. Biochemistry. 1996;35:15529–15536. doi: 10.1021/bi961854s. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Lowe M, Levine TP, Rabouille C, Warren G. The vesicle docking protein p115 binds GM130, a cis-Golgi matrix protein, in a mitotically regulated manner. Cell. 1997;89:445–455. doi: 10.1016/s0092-8674(00)80225-1. [DOI] [PubMed] [Google Scholar]
- Nelson WJ. W(h)ither the Golgi during mitosis? J Cell Biol. 2000;149:243–248. doi: 10.1083/jcb.149.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi R, Gould KL. Regulating the onset of mitosis. Curr Opin Cell Biol. 1999;11:267–273. doi: 10.1016/s0955-0674(99)80036-2. [DOI] [PubMed] [Google Scholar]
- Renzi L, Gersch MS, Campbell MS, Wu L, Osmani SA, Gorbsky GJ. M.P.M-2 antibody-reactive phosphorylations can be created in detergent-extracted cells by kinetochore-bound and soluble kinases. J Cell Sci. 1997;110:2013–2025. doi: 10.1242/jcs.110.17.2013. [DOI] [PubMed] [Google Scholar]
- Roth MG. Inheriting the Golgi. Cell. 1999;99:559–562. doi: 10.1016/s0092-8674(00)81544-5. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Seemann J, Jokitalo EJ, Warren G. The role of the tethering proteins p115 and GM130 in transport through the Golgi apparatus in vivo. Mol Biol Cell. 2000;11:635–645. doi: 10.1091/mbc.11.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro PS, Vaisberg E, Hunt AJ, Tolwinski NS, Whalen AM, McIntosh JR, Ahn NG. Activation of the MKK/ERK pathway during somatic cell mitosis: direct interactions of active ERK with kinetochores and regulation of the mitotic 3F3/2 phosphoantigen. J Cell Biol. 1998;412:1533–1545. doi: 10.1083/jcb.142.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima DT, Cabrera-Poch N, Pepperkok R, Warren G. An ordered inheritance strategy for the Golgi apparatus: visualization of mitotic disassembly reveals a role for the mitotic spindle. J Cell Biol. 1998;141:955–966. doi: 10.1083/jcb.141.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter J, Watwson R, Giannakou M-E, Clarke M, Warren G, Barr FA. GRASP55, a second mammalian GRASP protein involved in the stacking of Golgi cisternae in a cell-free system. EMBO J. 1999;18:4949–4960. doi: 10.1093/emboj/18.18.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AW. Cloning of PCR products after defined cohesive termini are created with T4 DNA polymerase. Nucleic Acids Res. 1990;18:4290. doi: 10.1093/nar/18.14.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taagepera S, Dent P, Her JH, Sturgill TW, Gorbsky GJ. The MPM-2 antibody inhibits mitogen-activated protein kinase activity by binding to an epitope containing phosphothreonine-183. Mol Biol Cell. 1994;5:1243–1251. doi: 10.1091/mbc.5.11.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolwinski NS, Shapiro PS, Goueli S, Ahn NG. Nuclear localization of mitogen-activated protein kinase kinase 1 (MKK1) is promoted by serum stimulation and G2-M progression. J Biol Chem. 1999;274:6168–6174. doi: 10.1074/jbc.274.10.6168. [DOI] [PubMed] [Google Scholar]
- Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- Westendorf JM, Rao PN, Gerace L. Cloning of cDNAs for M-phase phosphoproteins recognized by the MPM2 monoclonal antibody and determination of the phosphorylated epitope. Proc Natl Acad Sci USA. 1994;91:714–718. doi: 10.1073/pnas.91.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JH, Munar E, Jameson DR, Andreassen PR, Margolis RL, Seger R, Krebs EG. Mitogen-activated protein kinase kinase activity is required for the G2/M transition of the cell cycle in mammalian fibroblasts. Proc Natl Acad Sci USA. 1999;96:11335–11340. doi: 10.1073/pnas.96.20.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaal KJ, Smith CL, Polishchuk RS, Altan N, Cole NB, Ellenberg J, Hirschberg K, Presley JF, Roberts TH, Siggia E, Phair RD, Lippincott-Schwartz J. Golgi membranes are absorbed into and reemerge from the ER during mitosis. Cell. 1999;99:589–601. doi: 10.1016/s0092-8674(00)81548-2. [DOI] [PubMed] [Google Scholar]
- Zecevic M, Catling AD, Eblen ST, Renzi L, Hittle JC, Yen TJ, Gorbsky GJ, Weber MJ. Active MAP kinase in mitosis: localization at kietochores and association with the motor protein CENP-E. J Cell Biol. 1998;412:1547–1558. doi: 10.1083/jcb.142.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]