Abstract
Background
For late gadolinium enhancement (LGE) cardiovascular magnetic resonance (CMR) assessment of atrial scar to guide management and targeting of ablation in atrial fibrillation (AF), an objective, reproducible method of identifying atrial scar is required.
Objective
To describe an automated method for operator-independent quantification of LGE that correlates with colocated endocardial voltage and clinical outcomes.
Methods
LGE CMR imaging was performed at 2 centers, before and 3 months after pulmonary vein isolation for paroxysmal AF (n = 50). A left atrial (LA) surface scar map was constructed by using automated software, expressing intensity as multiples of standard deviation (SD) above blood pool mean. Twenty-one patients underwent endocardial voltage mapping at the time of pulmonary vein isolation (11 were redo procedures). Scar maps and voltage maps were spatially registered to the same magnetic resonance angiography (MRA) segmentation.
Results
The LGE levels of 3, 4, and 5SDs above blood pool mean were associated with progressively lower bipolar voltages compared to the preceding enhancement level (0.85 ± 0.33, 0.50 ± 0.22, and 0.38 ± 0.28 mV; P = .002, P < .001, and P = .048, respectively). The proportion of atrial surface area classified as scar (ie, >3 SD above blood pool mean) on preablation scans was greater in patients with postablation AF recurrence than those without recurrence (6.6% ± 6.7% vs 3.5% ± 3.0%, P = .032). The LA volume >102 mL was associated with a significantly greater proportion of LA scar (6.4% ± 5.9% vs 3.4% ± 2.2%; P = .007).
Conclusions
LA scar quantified automatically by a simple objective method correlates with colocated endocardial voltage. Greater preablation scar is associated with LA dilatation and AF recurrence.
Abbreviations: 2D, 2-dimensional; AF, atrial fibrillation; CMR, cardiovascular magnetic resonance; ECG, electrocardiogram; LA, left atrial/atrium; LGE, late gadolinium enhancement; MRA, Magnetic resonance angiography; PAF, paroxysmal atrial fibrillation; RF, radiofrequency; SD, standard deviation
Keywords: Atrial fibrillation, Delayed-enhancement magnetic resonance imaging, Radiofrequency ablation
Introduction
Success rates for catheter ablation of atrial fibrillation (AF) are approximately 50%–75%1 and remain largely unchanged despite efforts to improve targeting and delivery of ablation. Improved outcomes require better understanding of the patient’s atrial myocardial substrate for case selection, tailored ablation therapy, and better evaluation of the resulting tissue injury. Several recent studies suggest that high-spatial-resolution late gadolinium enhancement (LGE) cardiovascular magnetic resonance (CMR) imaging can be used to visualize preexisting atrial fibrotic change and evaluate radiofrequency (RF) lesions.2, 3, 4, 5, 6, 7, 8
Current methods to identify atrial scar rely on operator judgement to define the level of enhancement assigned as scar.2, 3, 4, 5, 6, 7, 8 One approach is to look for a bimodal distribution of intensity to define the threshold of scar as the trough between the peaks.3 In many patients, however, the distribution of intensity is not bimodal, preventing this from being a universally applicable technique. An alternative approach is to manually select regions of scar and nonscar tissue in order to define a patient-specific threshold above which enhancement is defined as scar.9 However, in many patients, scar may be patchy and different observers may choose different regions to define scar. Despite the operator dependence of these methods, visually appreciable correlations between regions of scar and low voltage (<0.5 mV) have been demonstrated.10, 11 Furthermore, blinded scoring systems have been used to show an association between total atrial scar and the total burden of low voltage in a given patient, but these measures lack the ability to confirm the colocality of scar and low voltage.11
In this study, we examine the use of the blood pool mean as an intensity reference in an automated process that expresses atrial myocardial intensity as multiples of standard deviation (SD) above blood pool mean. We tested the hypothesis that this method will identify LGE with a level of consistency that will enable both point-by-point correlation with colocalized voltage and correlation with procedural and patient characteristics.
Methods
Patients undergoing first ablation for paroxysmal atrial fibrillation (PAF) were recruited. LGE CMR scan was performed before and 3 months after either cryoballoon or conventional RF ablation. A randomly selected subset of patients underwent endocardial voltage mapping during the ablation procedure. All CMR scans and voltage maps were performed in sinus rhythm. Patients were followed up with electrocardiogram (ECG) and clinical history at 3, 6, and 12 months and with 24-hour Holter monitor at 6 months. The study was approved by the local research ethics committees (UK). Written informed consent was obtained. All patients included in the study had diagnostic quality images and completed 12-month follow-up.
LGE CMR protocol
A Philips Achieva 1.5-T MRI system and a 5- or 32-element phased-array cardiac coil was used for LGE imaging, as described previously.3 Fifty-phase 2-dimensional (2D) cine-determined time delay for ECG gating. The anatomic details of the left atrium (LA) and pulmonary veins (PVs) were obtained by using non-ECG-gated 3D spoiled gradient-echo contrast-enhanced timing robust angiography (CENTRA) during the first pass of 20-mL gadobenatedimeglumine-enhanced contrast. A 3D left ventricular LGE breath-hold sequence, approximately 9-minute postcontrast, was used to identify optimal nulling time for the left ventricular.
ECG-triggered, free-breathing navigator-gated whole-heart 3D spoiled gradient-echo acquisition was performed in axial orientation, with resolution approximately 1.5 × 1.5 × 4 mm, reconstructed to 1.25 × 1.25 × 2 mm. Complete LA coverage was obtained with 40–50 slices. Data were acquired within 100–150-ms window for each RR interval, with a low-high k-space ordering and spectral presaturation with inversion recovery for fat suppression. Inversion recovery delay, determined from the Look-Locker sequence,12 was chosen to null the myocardial signal. Navigator inflow artifact was reduced by lowering the navigator rescale factor and positioning the Navigator away from the right-sided PVs. Free-breathing images acquired 12–20-minute postinjection depending on the successful leading navigator placement, aiming for a Navigator efficiency of >30%.
Voltage mapping
Patients underwent voltage mapping either during the initial procedure or the redo procedure for recurrent AF. The LA segmentation was imported into Ensite NavX (St Jude Medical, St. Paul, MN) or CARTO 3 (Biosense Webster, Diamond Bar, CA). The LA geometry was collected by using the duodecapolar AFocus catheter (St Jude Medical) or 20-pole lasso catheter (Biosense Webster). The electroanatomical geometry was registered to the imported LA segmentation using surface registration. Peak-to-peak voltages were collected from the 10 bipoles of the circular mapping catheter. Bipolar electrogram amplitudes can be influenced by catheter orientation and the direction of wave-front propagation.13 We therefore performed additional unipolar recordings in 5 patients using a single reference electrode within the inferior vena cava. Bipolar (16–500 Hz) and unipolar (2–240 Hz) filter settings were used.
Automated method of scar mapping
LA segmentation was performed on a Philips Healthcare workstation by using the maximum intensity projection view to remove structures external to the LA blood pool. This surface was the reference anatomy on which LGE and voltage were compared. Automated software written in C++ was used to perform rigid or nonrigid registration14 (depending on atrial wall overlap) between the segmented magnetic resonance angiography (MRA) and LGE surfaces.
The LA blood pool was used as a nonenhancing region against which the LA wall enhancement could be compared and normalized. The blood pool was identified automatically by shrinking the LA segmentation using mathematical morphology, and mean (MBP) and standard deviation (SDBP) intensity of the blood pool were calculated. The maximum LA wall intensity (ILA) was determined along the normal to the wall at each location, 3 mm inside and outside the LA surface15 to allow for wall thickness and minor registration mismatch. LA wall intensities were expressed as multiples of SDBP above the blood pool mean to provide a normalized LA wall intensity (NLA), such that NLA = (ILA − MBP)/SDBP.
Comparison of LGE and voltage
The registered voltage map was exported for offline comparison with the atrial scar map. Each electrogram was assumed to represent a region of 2-mm radius from the point of endocardial contact, given the electrode spacing of the catheters used. The mean intensity of the surface “cells” within the 2-mm radius was used to compare with colocated voltage. Areas without a voltage point within a 2-mm radius were not included in the analysis. These methodological steps are summarized in Figure 1. For each patient, the mean voltage was calculated at each integer intensity or LGE level and compared for intensity K vs K − 1.
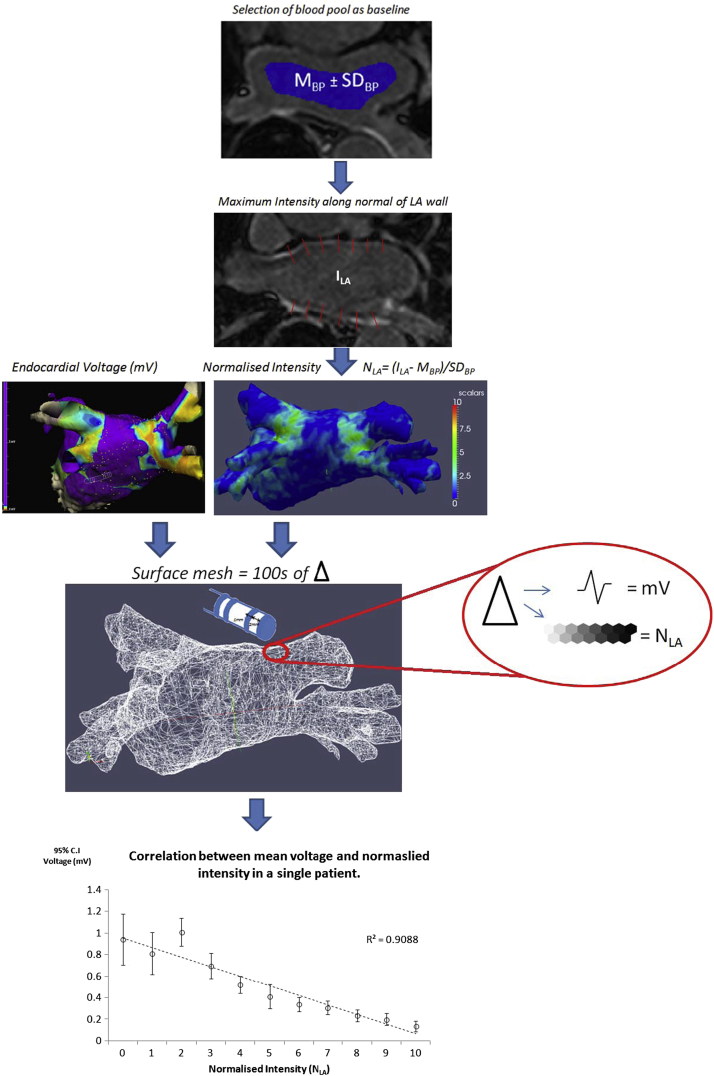
Figure 1.
Summary of the method of automated atrial scar mapping and anatomical registration for correlation with voltage distribution. From top to bottom: Intensity of the blood pool (MBP ± SDBP) determined from the area (blue) automatically selected as 1 cm within the LA wall was used to normalize LA wall intensity (ILA), calculated as the maximum intensity along chords (red lines in the second panel) perpendicular to the LA wall. The normalized intensity (NLA) was mapped onto the segmented 3D surface according to a color lookup table (third panel). Measured endocardial voltage points were registered to the MRA segmentation (left first panel). Each electrogram at the annotated point of endocardial contact was assumed to represent a circular region of 2-mm radius. The segmented MRA was divided into cells from a surface mesh, and cells within a 2-mm radius of a voltage point were combined to provide a single mean intensity value (fourth panel) (see text for discussion). Integer intensity levels were plotted against the mean of all colocated voltage measurements. The correlation between mean bipolar voltage and normalized intensity from a single patient is demonstrated (bottom panel). LA = left atrial; MRA = magnetic resonance angiography.
Ablation procedure
Procedures were performed in the fasted state. A 14-F cryosheath (or channel sheath for RF procedures) was inserted into the right femoral vein. Following transeptal puncture, anatomical and voltage maps were collected. Wide-area circumferential ablation was performed as described elsewhere.16 Lesions were delivered via 3.5-mm thermocool ablation catheter encircling the left and right PVs. Alternatively, cryoballoon ablation was performed, as previously described.17 The cryoballoon was inflated in the antrum of each PV for double 5-minute freezes per vein. Residual PV sleeves were mapped and targeted using a 20-pole circular mapping catheter. No additional ablation was performed following confirmed PV isolation.
Comparison of LGE and clinical outcomes
Paraview software (Kitware Inc, NY) was used by an observer blinded to the location of scar to identify the LA-PV junction on the atrial segmentation. The PV ostial region was defined as extending 1 cm proximal and distal to the PV-LA junction. The lowest LGE level to demonstrate a significantly different voltage from the normal myocardium was defined as the threshold for atrial scar. To identify ablation-related scar, % scar in both the ostia and the LA body was compared in pre- and postablation scans. Total LA scar, in both pre- and postablation scans, was compared between patients with and without AF recurrence at 12 months.
An observer blinded to patient outcome determined whether scar was continuous around the PV circumference in postablation scans. The number of PVs showing circumferential ablation scar was compared for patients with and without AF recurrence, and % ostial scar was compared for veins found to be isolated or reconnected at the redo procedure.
Statistical analysis
IBM SPSS statistics package was used for statistical analysis. Normal variables are expressed as mean ± SD. Paired and unpaired t tests were used when appropriate. Categorical variables were compared between groups by using the χ2 test. The Fisher exact test was used for small sample size. Bonferroni adjustment was applied for multiple tests. The correlation of continuous variables was with Spearman’s correlation coefficient for skewed data. A probability of <.05 was significant.
Results
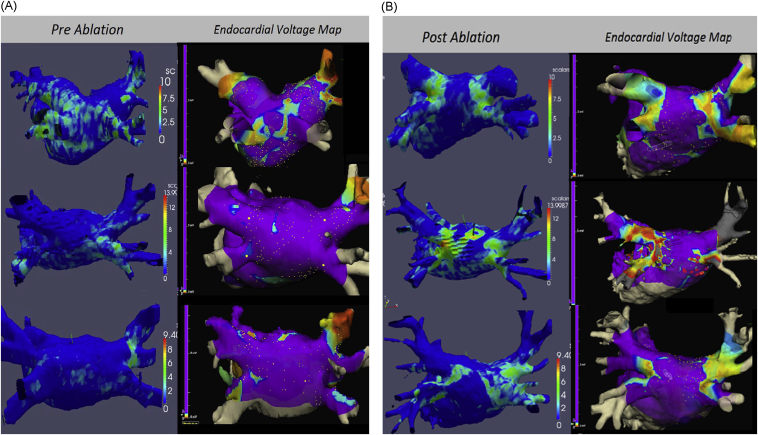
Fifty patients underwent LGE CMR before and 3 months after AF ablation (cryoablation: n = 25; RF ablation: n = 25). Twenty-one had voltage mapping performed during either the initial (n = 10) or the redo (n = 11) procedure. Patient characteristics are listed in Table 1. 3D surface reconstruction and scar maps were created for all patients (see examples in Figures 2A and B).
Table 1.
Patient demographics
| Demographic | All | Cryo | RF | RF vs Cryo P Value |
|---|---|---|---|---|
| Patients(male) | 50 (31) | 25 (16) | 25 (15) | .87 |
| Age (y) | 59.6 ± 13.1 | 58.4 ± 14.2 | 59.9 ± 11.9 | .70 |
| PAF duration, yrs | 4.2 ± 3.2 | 3.5 ± (2.7) | 5.2 ± 3.8 | .09 |
| Hypertension | 40.8% | 38.5% | 38.9% | .83 |
| CVA/TIA | 6.1% | 3.8% | 11.1% | .30 |
| Value disease | 10.2% | 15.4% | 0.0% | .10 |
| CAD | 12.2% | 18.2% | 4.0% | .09 |
| No.of AADs | 1.4 ± 0.7 | 1.4 ± 0.7 | 1.6 ± 0.9 | .64 |
| Diabetes | 4.08% | 0.00% | 5.56% | .22 |
| CHADS2 | 1.0 ± 5.4 | 0.7 ± 0.8 | 1.2 ± 1.3 | .10 |
| LA size | 37.4 ± 5.4 | 37.0 ± 4.7 | 38.2 ± 6.7 | .50 |
AAD = anti-arrhythmic drug; CAD = coronary artery disease; Cryo = cryoballoon; CVA = cerebrovascular accident; PAF = paroxysmal atrial fibrillation; RF = radiofrequency; TIA = transient ischemic attack.
Figure 2.
LGE CMR automated atrial scar mapping (left side) obtained (A) before and (B) 3 months after ablation in 6 patients, with corresponding endocardial voltage maps registered to the MRA segmentation of the left atrium (right side). The postablation LGE maps compare well to the corresponding endocardial voltage maps. CMR = cardiovascular magnetic resonance; LGE = late gadolinium enhancement; MRA = magnetic resonance angiography.
Voltage correlation
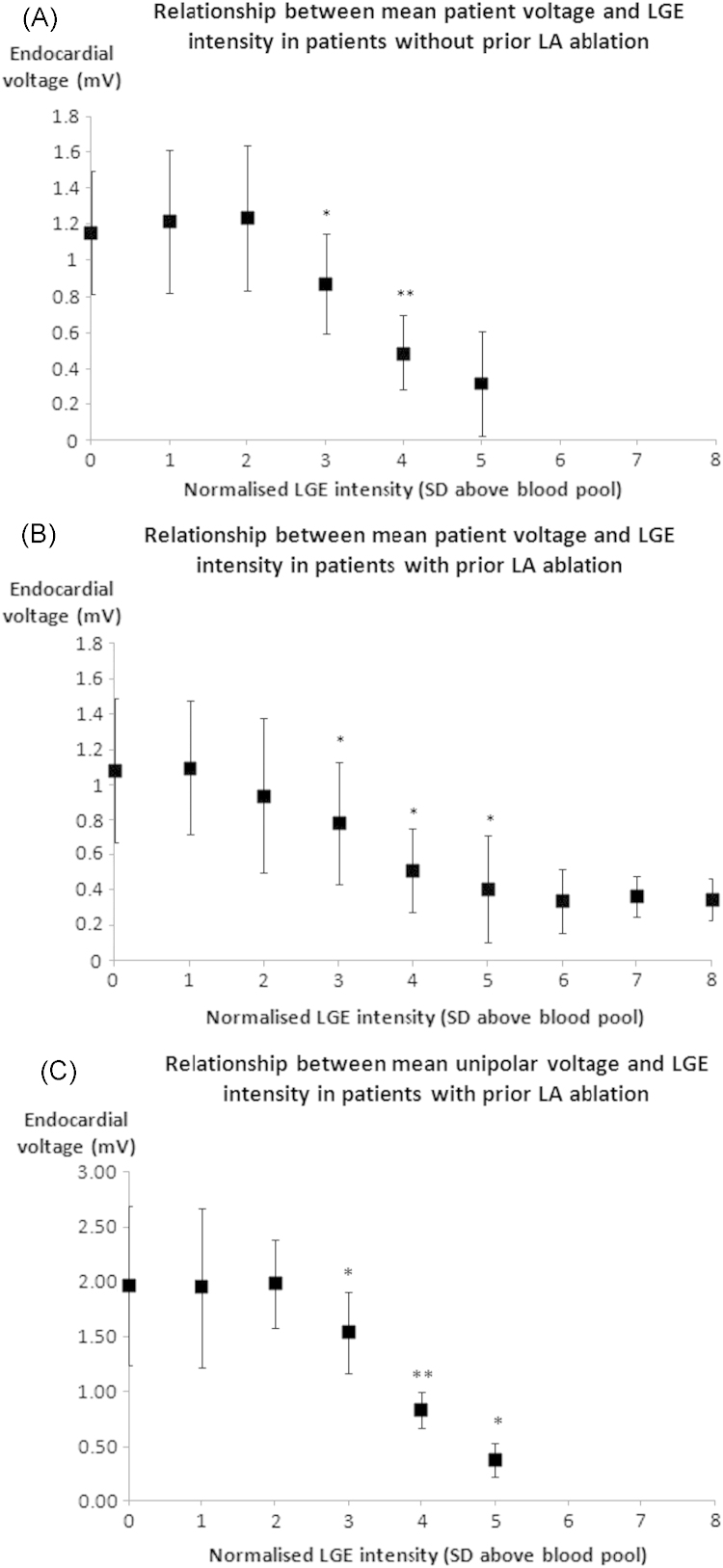
In 21 patients, 4386 bipolar voltage measurements were made (~200 per patient). LGE levels of 3, 4, and 5SD above blood pool mean were associated with progressively lower combined patient mean ± SD bipolar voltages compared to the preceding enhancement level (0.85 ± 0.33, 0.50 ± 0.22, and 0.38 ± 0.28 mV, P = .002, P < .001, and P = .048, respectively). Figure 3 shows the relationship between voltage and integer LGE levels for all patients (Figure 3A) without and (Figure 3B) with prior LA ablation. Figure 3C shows the significantly lower unipolar voltages recorded from the areas of LGE levels of 3SD (P = .04), 4SD (P = .001), and 5SD (P = .02) when compared to the preceding LGE level.
Figure 3.
The mean ± SD voltage is demonstrated for all patients at each intensity level. Comparison is made by using paired t tests between K and K − 1 intensity levels. This was done in (A) 10 patients who had not had any prior ablation, (B) 11 patients who had had prior left atrial ablation, and (C) 5 patients with prior LA ablation in whom additional unipolar voltage mapping was performed. A significant difference in bipolar and unipolar voltages was noted between LGE levels 3, 4, and 5SD in patients both with and without prior LA ablation. LA = left atrial; LGE = late gadolinium enhancement; SD = standard deviation.
Pre- and postablation LGE
The LGE level of >3SD above blood pool mean was defined as atrial scar. In preablation scans, there was no difference in % scar between ostial and LA body regions (P = .183). In postablation scans, % scar in the ostia was greater than in the LA body (P < .001); % scar in the ostia was greater in post- vs preablation scans (P < .001).
LGE and patient risk factors for stroke
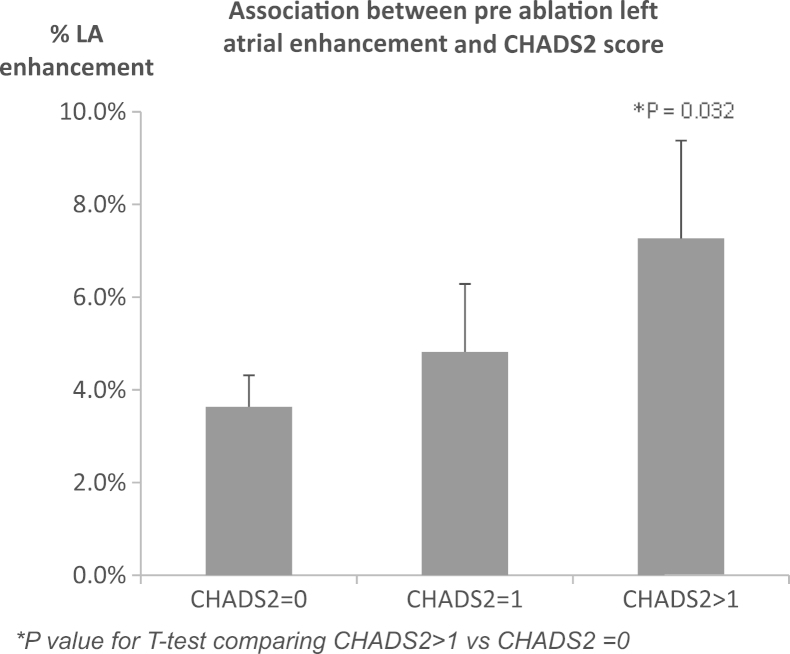
Patients with low, moderate, and high CHADS2 scores had 3.2% ± 3.2%, 4.4% ± 3.4%, and 7.1% ± 7.4% LA scar, respectively (low vs high; P = .035; Figure 4), demonstrating higher amounts of scars in patients with a high risk of stroke; % LA scar was greater in patients with hypertension than in patients without hypertension (10.4% ± 9.9% vs 5.8% ± 5.4%; P = .04), and it correlated with patient age (R2 = .363; P = .009).
Figure 4.
Total LA scar (%) LGE CMR performed preablation is higher in patients at high risk of stroke (CHADS2 score > 2) than in patients at low risk of stroke (CHADS2 score = 0). CMR = cardiovascular magnetic resonance; LA = left atrial; LGE = late gadolinium enhancement.
LGE and outcome from AF ablation
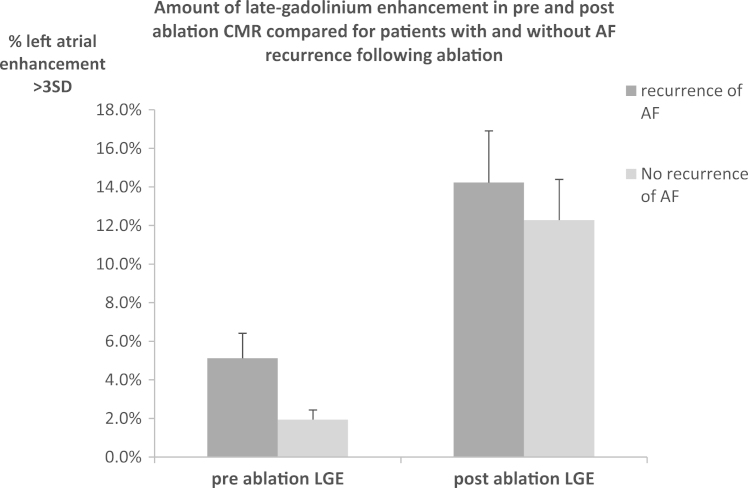
Twenty-five of 50 patients had recurrent AF. As shown in Figure 5, there was less preablation scar in patients without vs with AF recurrence (1.9% ± 1.7% vs 5.1% ± 4.3%; P = .033). This was not due to the increased LA size, since no significant difference was found in LA diameter between patients with and without AF recurrence (37 ± 6 mm vs 36 ± 9 mm; P = .58).
Figure 5.
Total LA scar (%) on preablation LGE CMR in 50 patients: 25 with and 25 without AF recurrence following AF ablation. Greater amounts of scar were seen in the preablation scans of patients with AF recurrence following ablation compared to those who remained free from AF. No difference was seen between the amounts of scar seen in postablation scans between patients with and without recurrent AF. AF = atrial fibrillation; CMR = cardiovascular magnetic resonance; LA = left atrial; LGE = late gadolinium enhancement.
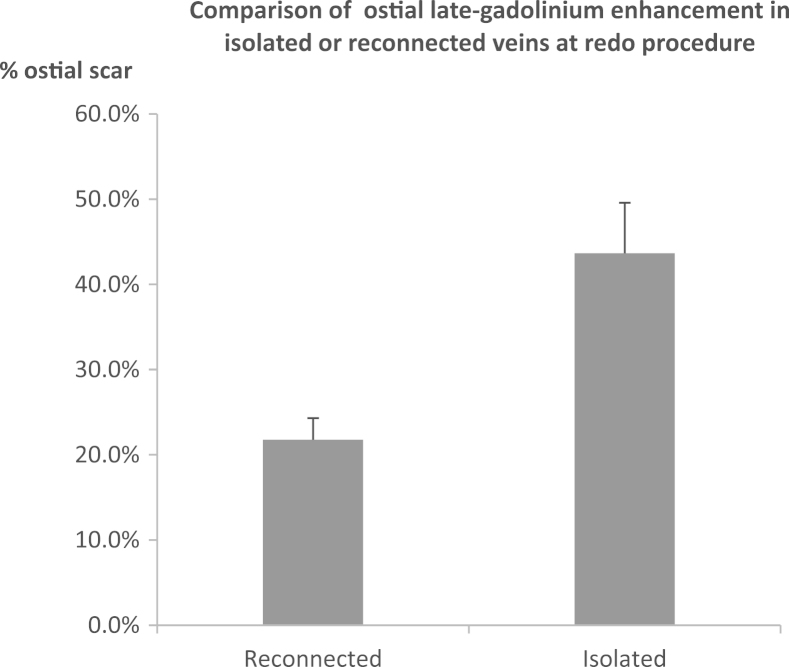
In postablation scans, there was no difference in the amount of atrial scar between patients with and without AF recurrence (14.2 ± 8.9 vs 12.3 ± 7.0; P = .675), nor was there a difference in the number of veins with circumferential scar. However, as demonstrated in Figure 6, in 21 patients who underwent redo procedures, 63 of 84 PVs had reconnected and a significantly higher amount of ostial scar was seen in veins, which remained isolated compared to those that had reconnected (43.5% ± 20.7% vs 21.8% ± 19.5%; P < .001).
Figure 6.
Ostial scar (%) on postablation LGE CMR compared for veins that were isolated and veins that were reconnected at the redo AF ablation procedure (n = 21). AF = atrial fibrillation; CMR = cardiovascular magnetic resonance; LGE = late gadolinium enhancement.
LGE and LA size
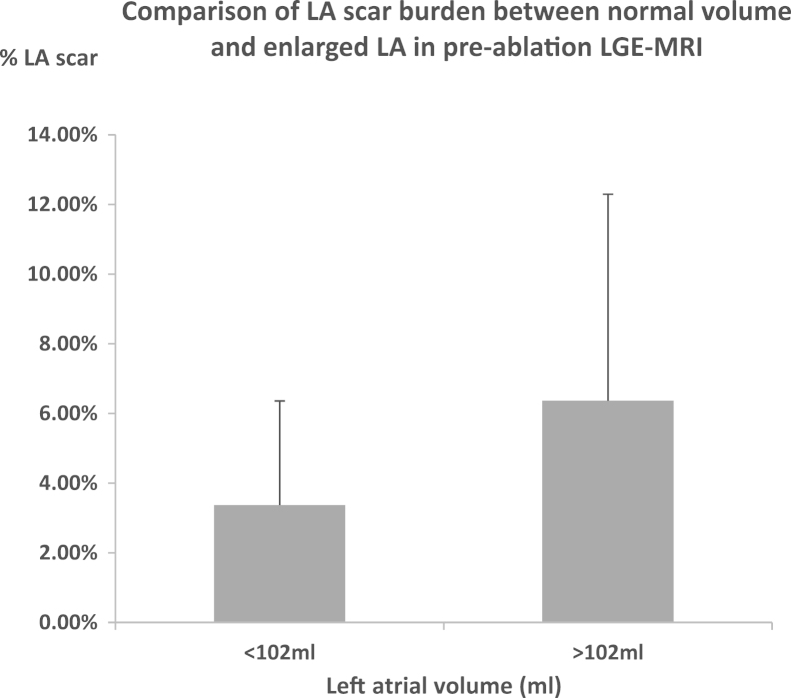
Patients were divided into those with normal LA volume (<102 mL)18 and enlarged LA. Patients with enlarged LA had a greater amount of atrial scar compared to those with normal LA size (7.5% ± 7.2% vs 3.5% ± 2.9%; P = .018; Figure 7).
Figure 7.
Comparison of % total LA scar on preablation LGE CMR between atria volume <102 mL and atria volume >102 mL (n = 50). CMR = cardiovascular magnetic resonance; LA = left atrial; LGE = late gadolinium enhancement.
LGE following cryoballoon vs RF ablation
There was no difference in the amount of atrial scar following cryoballoon or RF ablation (total LA enhancement: 16.5% ± 14.9% vs 16.1% ± 11.0%; P = 0.93; ostial enhancement: 23.5% ± 19.9% vs 25.8% ± 12.9%; P = .81).
Discussion
We report the first point-by-point comparison of LA LGE and colocated endocardial voltage by using an objective, operator-independent method of identifying atrial enhancement. Increasing levels of LGE correlated with lower bipolar and unipolar voltages. Significant associations were found between the amount of preablation LA enhancement and patient risk factors for stroke. We also identified increased amounts of preablation atrial scar in patients with LA dilatation and recurrent AF following ablation. These clinical correlates, however, are based on a limited population of 50 patients with PAF. Larger studies will subsequently be required to confirm these results in a much broader population of patients, both with and without AF.
LGE CMR and voltage
In patients both with and without prior LA ablation, increasing enhancement levels of 3, 4, and 5SD correlated with significant reductions in both bipolar and unipolar voltages. There was no further reduction in measured voltage seen in regions with enhancement >5SD, indicating that the enhancement of 5SD or greater may represent fully scarred atrial myocardium. These objective findings from colocated data are broadly consistent with those reported in studies by using blinded scoring systems to correlate the number of regions containing atrial enhancement and low voltage.6, 11 Furthermore, a similar association has previously been demonstrated between overall ventricular LGE-MRI scar mass (>2SD) and endocardial voltage <1.5 mV.19
LGE CMR and stroke risk
We found significantly higher levels of scar in patients with high CHADS2 scores, in keeping with the findings of Daccarett et al,20 who found LGE to be an independent predictor of cerebrovascular events. Patients with higher CHADS2 scores have also been found to have lower atrial voltages, which further supports our findings.21 Of note, Daccarett found higher levels of fibrosis, which may be due to different methods of threshold selection, or the population studied may have had greater levels of atrial fibrosis than our PAF population.
It remains unclear whether preexisting atrial fibrosis is attributable to a single factor, such as AF burden, or multiple patient factors. LGE CMR using automated atrial scar detection may help to investigate the disparate pathological processes, such as hypertension and age, which result in increased stroke risk. Further studies in larger and wider populations are required to ascertain the benefits of LGE CMR to guide anticoagulation strategies.
LGE CMR and procedural outcome
Persistent AF and increased atrial size are the only current predictors of reduced procedural success from AF ablation in the literature.1, 22 In our PAF population with relatively normal LA size, preexisting atrial scarring detected by LGE CMR identified patients who were more likely to suffer from AF recurrence following ablation. Interestingly, in our study population there was no significant difference in atrial size noted between patients with and without AF recurrence following ablation (38 ± 5 mm vs 37 ± 6 mm; P = .536). Although this finding requires further study in larger populations, it is consistent with those of Akoum et al,23 who found that patients with increasing levels of preablation fibrosis, as denoted by Utah levels 1–4, had a higher chance of developing recurrent AF postablation. Similarly, patients with preprocedural atrial scarring found on endocardial voltage mapping had a significantly higher rate of procedural failure.24 LGE CMR may noninvasively identify patients with extensive atrial scarring, unlikely to benefit from PV isolation.
LGE CMR and PV reconnection
Current techniques for AF ablation may achieve the procedural end point of PV conduction block, without creating permanent circumferential full thickness lesions. A method allowing visualization of atrial scar may facilitate the development of improved ablation techniques. Previous studies have shown that the extent of PV antral LGE postprocedure correlates well with lower rates of AF recurrence.11, 25 Our study did not demonstrate an association between postablation LGE and AF recurrence. All patients requiring a redo procedure had LGE and electrical evidence of gaps in ablation lines leading to PV reconnection. However, not all patients with gaps had AF recurrence. This is in keeping with several clinical studies that have identified the presence of reconnected veins in patients free from AF who volunteered for a restudy following AF ablation.26
Objective scar identification methods may help to standardize definitions of atrial ablation scar and explore in a multicenter study how the extent and location of permanent scar translates into longer term freedom from AF.
Study limitations
While experienced operators considered each data point to represent good myocardial contact, we were unable to confirm contact during all voltage measurements. Poor catheter contact could lead to erroneous recordings of low voltage at the locations of healthy myocardium. Future studies using pressure-sensing catheters will be more accurate in comparing voltage and atrial LGE. It should be noted that our conclusion that lower endocardial voltages are associated with higher levels of LGE is based on the 21 patients who underwent voltage mapping and not the full cohort of 50 patients who were included in the study for comparison with clinical outcomes.
Acknowledging the limited spatial resolution of LGE CMR, we elected to assign enhancement levels on the basis of the highest signal intensity on a cord through the atrial wall, with no attempt to differentiate transmural and partial thickness LGE.
The manual selection of regions of interest around the PV ostia used to calculate ostial scar adds an element of subjectivity to our methods. However, the identical region is used to compare pre- and postablation scans, which limits the influence of the selection on the results.
A degree of spatial error is inherent in the process of registering a nongated MRA sequence with an ECG and respiratory motion gated, free-breathing LGE sequence. Similarly, a degree of registration error is present when registering the MRA sequence with the gated electroanatomic map collected during the AF ablation procedure. However, despite these limitations, these methods represent a technique that may be readily implementable in most hospitals and provide objective data on which further developments in the field can be based.
Conclusions
We have described a novel operator-independent technique of atrial LGE CMR analysis identifying atrial scar that correlates with colocalized low-voltage measurements. The associations described between atrial LGE and clinical factors including CHADS2 score, LA size, and AF recurrence highlight the potential clinical value for risk stratification and patient selection for ablation; however, clinical applicability of the technique requires further validation and investigation.
Footnotes
This work was supported by the British Heart Foundation PG/10/37/28347, RG/10/11/28457, NIHR Biomedical Research Centre funding, and the ElectroCardioMaths Programme of the Imperial BHF Centre of Research Excellence.
References
- 1.Cappato R., Calkins H., Chen S.A. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–38. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 2.Peters D.C., Wylie J.V., Hauser T.H. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: initial experience. Radiology. 2007;243:690–695. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 3.McGann C.J., Kholmovski E.G., Oakes R.S. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol. 2008;52:1263–1271. doi: 10.1016/j.jacc.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 4.Badger T.J., Adjei-Poku Y.A., Marrouche N.F. MRI in cardiac electrophysiology: the emerging role of delayed-enhancement MRI in atrial fibrillation ablation. Future Cardiol. 2009;5:63–70. doi: 10.2217/14796678.5.1.63. [DOI] [PubMed] [Google Scholar]
- 5.Badger T.J., Oakes R.S., Daccarett M. Temporal left atrial lesion formation after ablation of atrial fibrillation. Heart Rhythm. 2009;6:161–168. doi: 10.1016/j.hrthm.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 6.Oakes R.S., Badger T.J., Kholmovski E.G. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim R.J., Wu E., Rafael A. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 8.Kim R.J., Hillenbrand H.B., Judd R.M. Evaluation of myocardial viability by MRI. Herz. 2000;25:417–430. doi: 10.1007/s000590050034. [DOI] [PubMed] [Google Scholar]
- 9.Wylie J.V., Jr, Peters D.C., Essebag V., Manning W.J., Josephson M.E., Hauser T.H. Left atrial function and scar after catheter ablation of atrial fibrillation. Heart Rhythm. 2008;5:656–662. doi: 10.1016/j.hrthm.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Taclas J.E., Nezafat R., Wylie J.V. Relationship between intended sites of RF ablation and post-procedural scar in AF patients, using late gadolinium enhancement cardiovascular magnetic resonance. Heart Rhythm. 2010;7:489–496. doi: 10.1016/j.hrthm.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badger T.J., Daccarett M., Akoum N.W. Evaluation of left atrial lesions after initial and repeat atrial fibrillation ablation: lessons learned from delayed-enhancement MRI in repeat ablation procedures. Circ Arrhythm Electrophysiol. 2010;3:249–259. doi: 10.1161/CIRCEP.109.868356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Look D., Locker D. Time saving in measurement of NMR and EPR relaxation times. Rev Sci Instrum. 1970;41:250–251. [Google Scholar]
- 13.Tedrow U.B., Stevenson W.G. Recording and interpreting unipolar electrograms to guide catheter ablation. Heart Rhythm. 2011;8:791–796. doi: 10.1016/j.hrthm.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 14.Rueckert D., Sonoda L.I., Hayes C., Hill D.L., Leach M.O., Hawkes D.J. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- 15.Knowles B.R., Caulfield D., Cooklin M. 3-D visualization of acute RF ablation lesions using MRI for the simultaneous determination of the patterns of necrosis and edema. IEEE Trans Biomed Eng. 2010;57:1467–1475. doi: 10.1109/TBME.2009.2038791. [DOI] [PubMed] [Google Scholar]
- 16.Wnuk-Wojnar A.M., Trusz-Gluza M., Czerwinski C. Circumferential pulmonary vein RF ablation in the treatment of atrial fibrillation: 3-year experience of one centre. Kardiol Pol. 2005;63:362–370. discussion 371–372. [PubMed] [Google Scholar]
- 17.Kojodjojo P., O’Neill M.D., Lim P.B. Pulmonary venous isolation by antral ablation with a large cryoballoon for treatment of paroxysmal and persistent atrial fibrillation: medium-term outcomes and non-randomised comparison with pulmonary venous isolation by radiofrequency ablation. Heart. 2010;96:1379–1384. doi: 10.1136/hrt.2009.192419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maceira A.M., Cosin-Sales J., Roughton M., Prasad S.K., Pennell D.J. Reference left atrial dimensions and volumes by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12:65. doi: 10.1186/1532-429X-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-David E., Arenal A., Rubio-Guivernau J.L. Noninvasive identification of ventricular tachycardia-related conducting channels using contrast-enhanced magnetic resonance imaging in patients with chronic myocardial infarction: comparison of signal intensity scar mapping and endocardial voltage mapping. J Am Coll Cardiol. 2011;57:184–194. doi: 10.1016/j.jacc.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 20.Daccarett M., Badger T.J., Akoum N. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol. 2011;57:831–838. doi: 10.1016/j.jacc.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao T.F., Cheng C.C., Lin W.S. Associations among the CHADS(2) score, atrial substrate properties, and outcome of catheter ablation in patients with paroxysmal atrial fibrillation. Heart Rhythm. 2011;8:1155–1159. doi: 10.1016/j.hrthm.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Arya A., Hindricks G., Sommer P. Long-term results and the predictors of outcome of catheter ablation of atrial fibrillation using steerable sheath catheter navigation after single procedure in 674 patients. Europace. 2010;12:173–180. doi: 10.1093/europace/eup331. [DOI] [PubMed] [Google Scholar]
- 23.Akoum N., Daccarett M., McGann C. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: a DE-MRI guided approach. J Cardiovasc Electrophysiol. 2011;22:16–22. doi: 10.1111/j.1540-8167.2010.01876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verma A., Wazni O.M., Marrouche N.F. Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. J Am Coll Cardiol. 2005;45:285–292. doi: 10.1016/j.jacc.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 25.Peters D.C., Wylie J.V., Hauser T.H. Recurrence of atrial fibrillation correlates with the extent of post-procedural late gadolinium enhancement: a pilot study. JACC Cardiovasc Imaging. 2009;2:308–316. doi: 10.1016/j.jcmg.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verma A., Kilicaslan F., Pisano E. Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction. Circulation. 2005;112:627–635. doi: 10.1161/CIRCULATIONAHA.104.533190. [DOI] [PubMed] [Google Scholar]