Abstract
The brain is frequently confronted with sensory information that elicits conflicting response choices. While much research has addressed the top down control mechanisms associated with detection and resolution of response competition, the effects of response competition on sensory processing in the primary visual cortex remain unclear. To address this question we modified a typical ‘flanker task’ (Eriksen and Eriksen, 1974) so that the effects of response competition on human early retinotopic visual cortex could be assessed. Healthy human participants were scanned using fMRI while making a speeded choice response that classified a target object image into one of two categories (e.g. fruits, animals). An irrelevant distractor image that was either congruent (same image as target), incongruent (image from opposite category as target), or neutral (image from task-irrelevant category, e.g. household items) was also present on each trial, but in a different quadrant of the visual field relative to the target. Retinotopic V1 areas responding to the target stimuli showed increased response to targets in the presence of response-incongruent (compared to response-neutral) distractors. A negative correlation with behavioral response competition effects indicated that an increased primary visual cortical response to targets in the incongruent (vs. neutral) trials is associated with a reduced response competition effect on behavior. These results suggest a novel conflict resolution mechanism in the primary visual cortex.
Keywords: fMRI, Attention, Response competition, Flanker, V1, Retinotopic cortex
Highlights
-
•
fMRI was used to examine effects of distractor congruency on retinotopic cortex.
-
•
V1 showed increased response to targets in the presence of incongruent distractors.
-
•
V1 effect was negatively correlated with distractor congruency effect on behavior.
-
•
A mechanism for conflict resolution involving primary visual cortex is proposed.
Introduction
Most stimuli encountered in the environment elicit some form of response, related either to previous direct experience or to an indirect association. Coherent goal-directed behavior requires the suppression of responses to stimuli that are irrelevant to the current task in order to prevent response conflicts. This is not always successful; people often fail to ignore irrelevant stimuli and the tendency to respond to them elicits response conflicts, which reduce the efficiency of task performance (e.g. by slowing down task responses).
The neural correlates of response conflict include a network of parietal and prefrontal regions responsible for identifying response conflict, resolving it in favor of the goal-relevant ‘target’ in accords with current task goals, and redeploying attention accordingly (e.g., Bunge et al., 2002; Carter et al., 1998; Fan et al., 2003; Hazeltine et al., 2000; Kerns et al., 2004; MacDonald et al., 2000; van Veen et al., 2001). However, typically this ‘resolution’ of conflict does not prevent perception of the distracting stimuli (exceptions are cases of high perceptual load in the task, see Lavie, 2005, 2010; or conflict adaptation through sequential repetition of incongruent stimuli, e.g. Egner, 2007). That task-irrelevant stimuli are perceived even in cases of correct response selection (following resolution of the conflict in response tendencies) is clearly evident from typical findings that response times are slower on incongruent compared to congruent or neutral trials. Thus while it is clear that the fronto-parietal network controls response selection, it remains unclear whether the identification of conflict and its resolution in terms of response selection has any effect on the sensory processing of target and of the distractor stimuli. Specifically, when people encounter a response conflicting distractor stimulus but successfully select the correct target response, are there any effects on sensory visual processing related to the target or distractor perception?
In the present study we used fMRI to elucidate the effects of response competition on the sensory processing of the target and distractor stimuli. To that purpose we modified a well-established response-competition task (Eriksen and Eriksen, 1974) for an experiment that allowed us to investigate the sensory visual correlates of response competition. Using images of common objects presented in separate visual quadrants, we were able to isolate the early visual cortical response to the target and distractor images under varying conditions of response congruency. We also further analyzed the response in retinotopic cortex relative to the magnitude of behavioral congruency effects.
Material and methods
Participants
Seventeen people recruited from the University College London experiment pool participated in this study for monetary compensation. Two people were excluded from the final analysis: one because of excessive head motion during the scanning session, and one because the participant's mean RT and overall accuracy on the behavioral task were more than 2.5 standard deviations away from the group mean. This resulted in a final pool of 15 participants (six females, ages 18–35). All participants provided informed consent in accordance with the UCL ethics committee.
Stimuli
For the main task, the stimuli consisted of 12 gray scale images of objects spanning three different categories: fruits (strawberry, apple, pineapple, banana), household items (desk, sofa, fan, chair), and animals (cat, bird, bear, turtle). These images fit within a square that measured six degrees of visual angle on a side, and were positioned within the middle of each visual quadrant at a center-to-center distance of six degrees from the fixation point in the middle of the display. The stimuli were presented on a gray background; text and the fixation cross were presented in black.
For a functional localizer, the stimuli consisted of a disk with alternating black and white quarters, presented at the same size and location as the images in the main task. The contrast of the disks reversed at a rate of 5 Hz. The background, fixation cross and text color were the same as in the main task. For the retinotopic mapping runs, the stimuli consisted of pairs of wedges oriented along either the horizontal or vertical midlines and arranged in a “bow-tie” pattern. The interior of the wedges contained a black and white checkerboard pattern whose luminance oscillated at 8 Hz, and were presented on a gray background. Each wedge constituted an arc of 30 radial degrees.
The experiment was run on an Intel-based computer running Windows XP. The stimuli were generated and presented using MATLAB software (MathWorks, Natick, Massachusetts). This experiment was realized using Cogent 2000 developed by the Cogent 2000 team at the WTCN and the ICN and Cogent Graphics developed by John Romaya at the LON at the WTCN.
Task procedure
Participants were instructed to perform a category judgment task on one member of a pair of briefly presented images. Images were presented in two of the four possible locations (one location in each visual quadrant), both within the same hemifield (upper, lower, left, right). Two of the four possible locations were defined a priori as target locations during the participants' instruction period; these locations were always arranged along the diagonal (i.e. upper-left and lower-right, or lower-left and upper-right, counterbalanced across participants), such that one (and only one) image of each trial pair was presented in a target location. Participants judged which object category the target image belonged to in a 2AFC task; two of the three object categories (fruit, household item, animal) were defined as target categories at the beginning of the session (counterbalanced across subject, crossed with target locations).
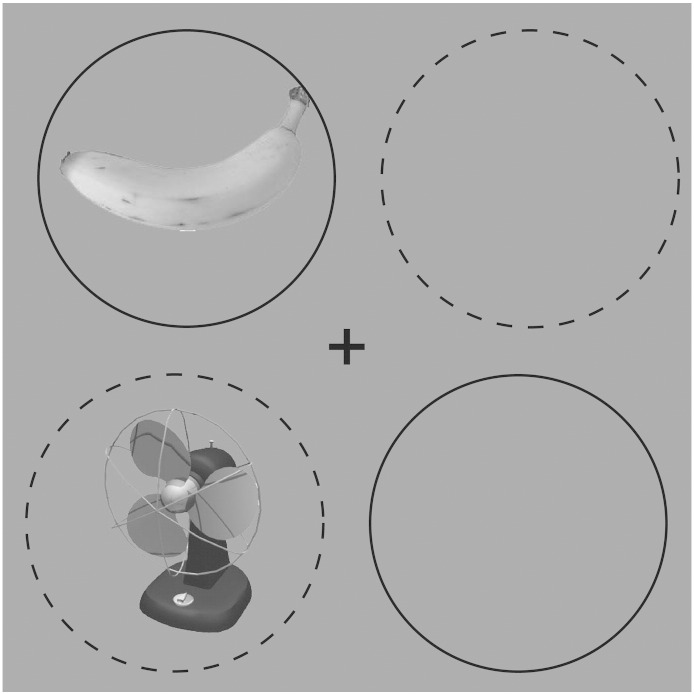
Trials proceeded as follows. The fixation cross appeared in the middle of a blank display. 500 ms later, task images appeared in one of the configurations described above, and were present for 200 ms. Fig. 1 shows the stimuli from an example trial, where the upper-left and lower-right quadrants are defined as target locations. Participants then had 1.8 s to respond; the fixation cross remained visible during this time. Trials fell into one of three types, based on the identity of the non-target image: congruent (same image as the target), incongruent (image from opposite category as the target), or neutral (image from the task irrelevant category). Although our imaging comparisons involved only the incongruent and neutral conditions, the congruent condition was included to drive conflict in the incongruent condition (otherwise if the response-related distractors were always incongruent, the incongruent condition would become predictive of the (opposite) target). Identical images were used for the congruent condition, rather than different images from the same category, so as to avoid inducing conflict due to condition ambivalence (e.g. Santee and Egeth, 1982, Perception & Psychophysics).
Fig. 1.
Display example from a single trial. Solid circles indicate target locations; dashed circles indicate distractor locations. These locations were defined based on instructions to the participant. Circles were not present during the experiment. Target and distractor diagonals varied from participant to participant.
At the beginning of each experiment session, prior to the start of scanning, participants were given verbal and written instructions, followed by a practice block that was identical in all respects to block of trials during the main task. Participants completed four blocks of trials in the scanner. Each block consisted of 60 trials; trial type, target location and target category were all counterbalanced within each block. Blocks also contained 20 null trials, where the fixation cross appeared alone for 2.5 s and no response was required. Trials were separated by a variable interval (measured from the onset of fixation of one trial to the onset of fixation of the subsequent trial) of 3 to 7 s, to facilitate an event-related analysis. Each block began with a fixation period measuring 22.8 s (10 functional volumes) and ended with a fixation period measuring 11.4 s (5 functional volumes), and lasted ~ 428 s.
Scanning sessions also contained two blocks of retinotopic mapping and two blocks of a functional localizer. Retinotopic mapping scans lasted 296 s, and consisted of alternating periods of stimulation along the horizontal and vertical visual meridians, lasting 18 s each. Participants were instructed to maintain fixation, but given no other task. Functional localizer scans lasted 347 s, and consisted of alternating periods of stimulation in the target and non-target locations lasting 22.8 s. Blocks also contained 22.8 s of fixation at the beginning of the block, 11.4 s of fixation at the end of the block, and two 22.8 s of fixation occurring during the middle of the block. Participants were instructed to maintain fixation, and respond with a button press to a luminance increment in the fixation cross lasting 200 ms (this occurred once during each stimulation period).
The stimuli were presented on a projection screen mounted at the end of the scanner bore, and viewed using a mirror mounted on the head coil. Responses were made using MR compatible fiber-optic button boxes.
Imaging data collection and analysis
Imaging data were collected at the Wellcome Trust Centre for Neuroimaging using a 3-T Siemens Allegra Scanner with an 8-channel head coil. Anatomical images were acquired using a 3D MDEFT sequence (Deichmann et al., 2004) with a sagittal partition direction yielding images with 1-mm isovoxel resolution (time repetition (TR) = 7.92 ms, time echo (TE) = 7.92 ms, flip angle = 15°). Whole-brain echoplanar functional images were acquired in 38 transverse slices (TR = 2280 ms, TE = 30 ms, matrix = 64 × 72, field of view = 216 mm, slice thickness = 2mm, 1 mm gap, descending slice acquisition order). Additionally, a double-echo FLASH field mapping sequence was collected to measure the distribution of field inhomogeneities (TE1 = 10 ms, TE2 = 12.46 ms, 3 × 3 × 2 mm resolution with 1 mm gap).
Data were processed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). Functional images collected from the scanner were slice-time corrected, aligned with a representative functional volume and motion corrected using an unwarping procedure that employed field inhomogeneity information from the field mapping scans, and spatially smoothed using a 4-mm full-width at half maximum Gaussian kernel. Structural images were also aligned with the representative functional volume. For the main experiment, separate events representing target position, distractor position and distractor congruency were modeled using a general linear model (GLM). These events were modeled as impulses of activity at the onset of the stimuli and were convolved with the canonical hemodynamic response function (HRF) included with SPM8. For the retinotopic and localizer scans, boxcars representing the duration of each block were convolved with the HRF and similarly modeled using a GLM. Results of the analyses, in the form of statistical maps, were overlaid on the structural images collected for each subject. For group-level analyses, these statistical maps were spatially normalized to the standard Montreal Neurological Institute space and entered into a one-way t-test to produce group average statistical maps, which were then overlaid onto an average structural image. To examine which regions of the cortex showed greater blood oxygen-level dependent (BOLD) responses to the various trial types, the group-level maps were generated using an uncorrected voxel-level threshold of p < 0.005 and a contiguous-voxel threshold producing a corrected threshold of p < 0.05.
Data from the retinotopic mapping scans were used to define the borders between areas V1, V2, V3/Vp, and V3a/V4v in each hemisphere (Engel et al., 1994; Sereno et al., 1995). Data from the localizer scans were then used to define the segments of each of these areas that responded most strongly to stimuli in the location of the targets or distractors. To define regions of interest (ROIs) in the early visual cortex, the functional data were projected onto inflated representations of each participant's brain. Cortical reconstruction and volumetric segmentation were performed with the Freesurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/). These ROIs were imported into SPM8, and the GLM parameter estimates corresponding to signals elicited by the stimuli appearing in the contralateral visual field for each of the previously described events were extracted from each ROI, allowing for analysis of the response to the different distractor types under the different load conditions. The ROI data were extracted and analyzed separately for each participant, then averaged.
Results
Behavioral data
Table 1 presents participants' response times and accuracies on the main behavioral task. Planned comparisons showed that the incongruent distractors produced significant response competition effects compared to both the neutral (t(14) = 3.31, p < 0.01) and congruent conditions (t(14) = 4.62, p < 0.001). In contrast, congruent distractors produced a facilitation effect compared to the neutral condition (t(14) = 2.79, p < 0.02). As can be seen from the table, accuracy was high and the very small number of errors did not vary between the distractor conditions (t ≤ 1.3).
Table 1.
Behavioral performance as a function of distractor congruency. SEM listed in parentheses.
| Reaction time (ms) | Accuracy (% correct) | |
|---|---|---|
| Incongruent | 716 (32.1) | 98.0 (1.99) |
| Neutral | 701 (34.3) | 97.3 (2.49) |
| Congruent | 674 (30.4) | 98.0 (2.30) |
Functional data: retinotopic cortices
Retinotopic analyses focused on the comparison of the incongruent and neutral distractor conditions, because these were visually comparable (in both of these conditions the target and the distractor were different to each other, whereas in the congruent condition the target and distractor stimuli were the same). This removed perceptual conflict as a potential confounding factor.
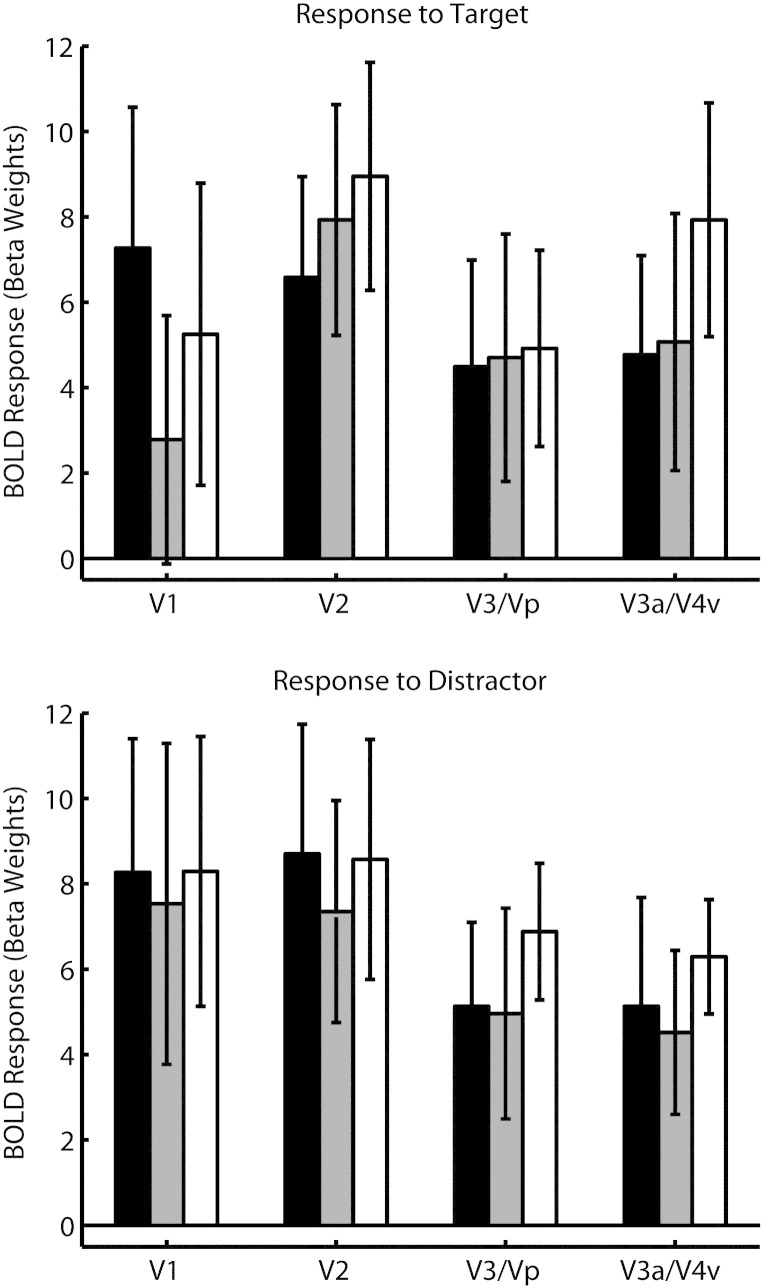
The retinotopic V1 region that responded to the target stimuli showed a significantly greater response to the targets on incongruent compared to neutral distractor trials (t(14) = 2.67, p < .02, see Fig. 2). This difference in V1 BOLD response was also negatively correlated with the difference in behavioral RTs: specifically, participants who had a larger difference between the BOLD response to targets on incongruent vs. neutral trials showed less slowing in RT for incongruent (compared to neutral) trials (r = − 0.71, p < 0.001).
Fig. 2.
BOLD responses to targets (top panel) and distractors (bottom panel) (measured as difference in GLM parameter estimates comparing stated trial type and null trials) as a function of trial type and region of cortex. Error bars represent SEM. Black bars: incongruent trials; gray bars: neutral trials; white bars: congruent trials.
There were no other effects of distractor congruency on target responses in areas V2 to V4 (all other t values ≤ 1.4). There were also no effects of distractor congruency on the retinotopic V1–V4 areas that responded to distractors (all ts ≤ 1.2) but V1 and V4 showed a trend for a positive correlation between BOLD response to the incongruent (vs. congruent) distractor and the magnitude of this congruency effect on RTs (V1: r = 0.548, p < 0.05; V4: r = 0.545, p < 0.05; both p-values uncorrected).
Functional data: full-brain contrasts
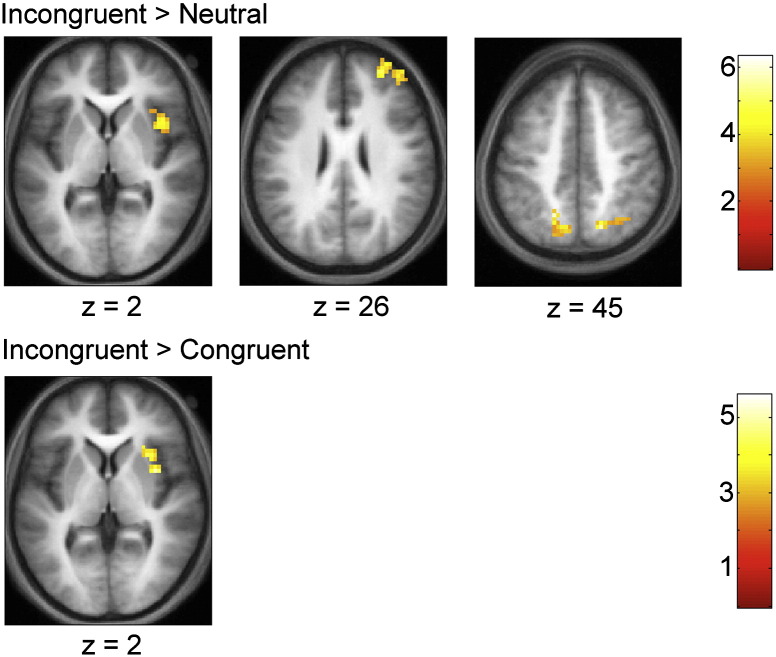
Statistical maps were calculated that examined the differences in the BOLD for the different trial types across all subjects; axial slices showing active regions for various contrasts are presented in Fig. 3 and summary statistics are shown in Table 2. The contrast of incongruent > congruent revealed a difference in BOLD signals in the right anterior insula. The contrast incongruent > neutral revealed clusters in the bilateral posterior parietal cortex, right superior/middle frontal gyrus, and the right anterior insula.
Fig. 3.
Statistical maps for random effects analysis, showing results of contrasts incongruent > neutral (top row) and incongruent > neutral (bottom row). The maps show clusters of increased BOLD response in the anterior insula (left column, z = 2), superior/middle frontal gyrus (middle column, z = 26), and intraparietal sulcus/precuneus (right column, z = 45). Voxel-level threshold is p < 0.005, uncorrected, with a cluster level threshold of p < 0.05, corrected. Results are overlaid on a mean structural image created by averaging the spatially-normalized brains of all subjects. Slice depth is MNI coordinates. Right side of the image corresponds to right side of the brain.
Table 2.
Regions showing greater BOLD signals for various full-brain contrasts.
| Cluster | BA | Peak voxel coordinates (x, y, z) | Volume (mL) | T |
|---|---|---|---|---|
| Incongruent > neutral | ||||
| L. precuneus | 7/19 | 18, − 64, 43 | 1.269 | 6.33 |
| R. precuneus | 7 | − 18, − 58, 46 | 1.431 | 5.72 |
| R. anterior insula | 13 | 39, 11, 4 | 2.889 | 5.73 |
| R. middle frontal gyrus | 10 | 30, 50, 25 | 1.890 | 4.87 |
| Neutral > incongruent | ||||
| L. middle temporal gyrus | 22 | − 51, − 37, 1 | 1.242 | 5.95 |
| Incongruent > congruent | ||||
| R. anterior insula | 13 | 33, 11, − 2 | 1.512 | 5.59 |
To determine whether the current task modulated activity in the anterior cingulate cortex (ACC), as shown in previous studies, a region-of-interest analysis was conducted using small volume correction. Based on the results reported in Botvinick et al. (1999) and Carter et al. (1998), we first defined a sphere with radius of 30 mm and centered at the coordinates (0, 23, 34). We then constrained this volume to not extend past x = − 15 or x = 15, to exclude white matter tracks. Finally, we masked the ROI to exclude corpus callosum. Using an uncorrected voxel-level threshold of p < 0.01 and a corrected cluster size threshold of p < 0.05, the contrast incongruent > congruent revealed a cluster in the right cingulate gyrus (x = 9, y = 14, z = 46; BA 32; t = 4.18).
Discussion
The present findings demonstrate for the first time the effects of response competition on visual cortical responses to the target stimuli. Our study revealed two novel key findings. First, the presence of response competing distractors was associated with a greater V1 response to targets. Note that this comprised a selective increase in the V1 response to targets during incongruent trials compared to response-neutral trials, rather than a general boost in the response to targets in the presence of any distractor. Second, this effect on V1 responses to targets was negatively correlated with the magnitude of response competition effects: an increased V1 response to targets in the incongruent (vs. neutral) trials was associated with a reduced congruency cost to behavioral RT.
These findings complement the previous literature, which has focused on the role of parietal and frontal cortex networks in the effects of response competition and their resolution (e.g. Bunge et al., 2001, 2002; Carter and Van Veen, 2007; Carter et al., 1998; Eckert et al., 2009; Fan et al., 2003, 2005; Hazeltine et al., 2000; Kerns et al., 2004; MacDonald et al., 2000; Milham et al., 2001; Roberts and Hall, 2008; van Veen et al., 2001). These studies have identified increased activity in the superior parietal cortex, anterior cingulate cortex (ACC), right anterior insula, and the dorsolateral prefrontal cortex (DLPFC) when the distractor stimuli elicit competing responses. Major parts of this frontoparietal top–down network, including the right anterior insula, ACC, right superior/middle frontal gyrus and bilateral posterior parietal cortex, were also associated with response congruency in the present study, in replication of these previous results. Importantly, our critical new findings of a larger V1 response to targets in the presence of an incongruent distractor that was negatively correlated with the magnitude of distractor response competition effects on behavior offer a new mechanism of competition resolution that operates in the primary visual cortex.
Note that whereas the previous research established a conflict resolution mechanism that is manifested in reduced interference following high conflict trials (e.g. Botvinick et al., 1999, 2001; Egner, 2008; Gratton et al., 1992), our effects are found during the presence of the potential response conflict (i.e. in the comparison of incongruent and neutral trials, rather than on trials following incongruent trials as in the conflict resolution literature e.g. Botvinick et al., 2001). The associated reduction in response competition effects on behavior suggests that the increased V1 response to targets in the face of conflicting distractors reflects an early sensory mechanism that protects the target stimulus from a potential response conflict by boosting its sensory signal in the presence of a response incongruent stimulus. An alternate potential explanation for the difference in activity between neutral and incongruent trials is the presence of one vs. two task-relevant stimuli. However, this is not supported by the data given the lack of difference between the neutral and congruent conditions, as the congruent condition also had two task-relevant stimuli.
Taken together with the findings that response competition resulted in fronto-parietal activation that is associated with top–down control, our research suggests the operation of a rapid top–down control mechanism that serves to control V1 responses to stimuli in accordance with the response-relevance of the context. Given the small receptive fields in V1, and the restricted information coding for visual properties, such a context-based mechanism must result from feedback connections to V1. Indeed, this is suggested by previous demonstrations of rapid response to visual stimuli as early as 45 ms in the parietal cortex and 90 ms in the prefrontal cortex (Foxe and Simpson, 2002; Juan and Walsh, 2003), coupled with demonstrations that V1 has both early and late response periods with the later responses reflecting feedback activation (see also Clavagnier et al., 2004). Our study thus adds to an increasing body of work that demonstrates a role for the primary visual cortex in attention (Allman et al., 1985; Ekstrom et al., 2008; Greenberg et al., 2012; Kastner and Ungerleider, 2000; Mehta et al., 2000a,b; Somers et al., 1999).
The present research also expands on recent work by Kelley and Lavie (2011) and Appelbaum et al. (2011), who show modulation of visual cortex activity related to distractor congruency. Kelley and Lavie (2011) used fMRI to examine how working memory load alters the visual response to incongruent distracting images. They found that load interacted with distractor congruency, such that BOLD signals in area V1 were greater to incongruent than congruent distractors under conditions of high (versus low) working memory load. Appelbaum et al. (2011) used EEG recording to examine the event-related potentials (ERPs) evoked by lateralized letter flankers. They found a lateralized change in voltage over the occipital cortex in the presence of incongruent flankers, which co-occurred with fronto-parietal voltage changes typically observed in studies of response conflict. These voltages were shown to positively correlate with levels of distractor interference across subjects. The present study fills an important gap in these previous findings by examining target-related (rather than distractor-related) activity in the early visual cortex, specifically retinotopic area V1. Our correlation with response competition effects on behavior also provides insight into how the target related V1 effect may play a functional role on an early sensory resolution of any potential response conflict.
The present findings have implications for how cognitive control is examined in other paradigms and populations. For example, one might predict that in Stroop-type tasks (MacLeod, 1991) top–down feedback from control regions would lead to increase activity in visual area V4 to improve color discrimination. Indeed evidence for occipital cortex activity during the Stroop-task being related to compensatory strategies in aging populations (Zysset et al., 2007) suggests that this is a promising future research avenue.
One question raised by our study is how feedback to V1 (or input from V1) that allows for resolution of distractor congruency relates to connectivity between ACC, DLPFC and motor control regions. It is possible that, in the presence of an incongruent distractor, visual activity could be modulated in advance of, in concert with, or following modulation of motor cortex activity. One might even predict that two individuals that show the same level of behavioral interference rely differentially on sensory or motor resolution. While the limited temporal resolution of fMRI prevents a close examination of the temporal dynamics of the cortical regions in question, it is possible that an EEG study (investigating, for example, the timing of the lateralized readiness potential, see for example Taylor et al., 2007) could address this issue. Such a study would provide a more complete picture of how a variety of sensory, motor, attentional and cognitive control regions are recruited to prevent and resolve distractor-induced response competition.
Acknowledgments
This work was supported by the Wellcome Trust (WT080568MA to N.L., Wellcome Senior Fellowship to G.R.). The Wellcome Trust Centre for Neuroimaging is supported by core funding from the Wellcome Trust 091593/Z/10/Z.
T.A.K. now works for Microsoft Corp.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Allman J., Miezin F., McGuinness E. Stimulus specific responses from beyond the classical receptive field: neurophysiological mechanisms for local–global comparisons in visual neurons. Annu. Rev. Neurosci. 1985;8:407–430. doi: 10.1146/annurev.ne.08.030185.002203. [DOI] [PubMed] [Google Scholar]
- Appelbaum L.G., Smith D.V., Boehler C.N., Chen W.D., Woldorff M.G. Rapid modulation of sensory processing induced by stimulus conflict. J. Cogn. Neurosci. 2011;23:2620–2628. doi: 10.1162/jocn.2010.21575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M., Nystrom L.E., Fissell K., Carter C.S., Cohen J.D. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Ochsner K.N., Desmond J.E., Glover G.H., Gabrieli J.D.E. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Hazeltine E., Scanlon M.D., Rosen A.C., Gabrieli J.D.E. Dissociable contributions of prefrontal and parietal cortices to response selection. NeuroImage. 2002;17:1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- Carter C.S., van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn. Affect. Behav. Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Braver T.S., Barch D.M., Botvinick M.M., Noll D., Cohen J.D. Anterior cingulated cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Clavagnier S., Falchier A., Kennedy H. Long-distance feedback projections to area V1: implications for multisensory integration, spatial awareness, and visual consciousness. Cogn. Affect. Behav. Neurosci. 2004;4:117–126. doi: 10.3758/cabn.4.2.117. [DOI] [PubMed] [Google Scholar]
- Deichmann R., Schwarzbauer C., Turner R. Optimization of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. NeuroImage. 2004;21:757–767. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Eckert M.A., Menon V., Walczak A., Ahlstrom J., Denslow S., Horwitz A., Dubno J.R. At the heart of the ventral attention system: the right anterior insula. Hum. Brain Mapp. 2009;30:2530–2541. doi: 10.1002/hbm.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T. Congruency sequence effects and cognitive control. Cogn. Affect. Behav. Neurosci. 2007;7:380–390. doi: 10.3758/cabn.7.4.380. [DOI] [PubMed] [Google Scholar]
- Egner T. Multiple conflict-driven control mechanisms in the human brain. Trends Cogn. Sci. 2008;12:374–380. doi: 10.1016/j.tics.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Ekstrom L.B., Roelfsema P.R., Arsenault J.T., Bonmassar G., Vanduffel W. Bottom–up dependent gating of frontal signals in early visual cortex. Science. 2008;321:414–417. doi: 10.1126/science.1153276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel S.A., Rumelhart D.E., Wandell B.A., Lee A.T., Glover G.H., Chichilnisky E.J., Shadlen M.N. fMRI of human visual cortex. Nature. 1994;369:525. doi: 10.1038/369525a0. [DOI] [PubMed] [Google Scholar]
- Eriksen B.A., Eriksen C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974;16:143–149. [Google Scholar]
- Fan J., Flombaum J.I., McCandliss B.D., Thomas K.M., Posner M.I. Cognitive and brain consequences of conflict. NeuroImage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Fan J., McCandliss B.D., Fossella J., Flombaum J.I., Posner M.I. The activation of attentional networks. NeuroImage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Foxe J.J., Simpson G.V. Flow of activation from V1 to frontal cortex in humans. A framework for defining “early” visual processing. Exp. Brain Res. 2002;142:139–150. doi: 10.1007/s00221-001-0906-7. [DOI] [PubMed] [Google Scholar]
- Gratton G., Coles M.G., Donchin E. Optimizing the use of information: strategic control of activation of responses. J. Exp. Psychol. Gen. 1992;121:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Greenberg A.S., Verstynen T., Chiu Y.C., Yantis S., Schneider W., Behrmann M. Visuotopic cortical connectivity underlying attention revealed with white-matter tractography. J. Neurosci. 2012;32:2773–2782. doi: 10.1523/JNEUROSCI.5419-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeltine E., Poldrack E., Gabrieli J.D.E. Neural activation during response competition. J. Cogn. Neurosci. 2000;12(Suppl. 2):118–129. doi: 10.1162/089892900563984. [DOI] [PubMed] [Google Scholar]
- Juan C.H., Walsh V. Feedback to V1: a reverse hierarchy in vision. Exp. Brain Res. 2003;150:259–263. doi: 10.1007/s00221-003-1478-5. [DOI] [PubMed] [Google Scholar]
- Kastner S., Ungerleider L.G. Mechanisms of visual attention in the human cortex. Annu. Rev. Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kelley T.A., Lavie N. Working memory load modulates distractor competition in primary visual cortex. Cereb. Cortex. 2011;21:659–665. doi: 10.1093/cercor/bhq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns J.G., Cohen J.D., MacDonald A.W., III, Cho R.Y., Stenger V.A., Carter C.S. Anterior cingulated conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused?: selective attention under load. Trends Cogn. Sci. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Lavie N. Attention, distraction and cognitive control under load. Curr. Dir. Psychol. Sci. 2010;19:143–148. [Google Scholar]
- MacDonald A.W., III, Cohen J.D., Stenger V.A., Carter C.S. Dissociating the role of the dorsolateral prefrontal and anterior cingulated cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- MacLeod C.M. Half a century of research on the Stroop effect: an integrative review. Psychol. Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Mehta A.D., Ulbert I., Schroeder C.E. Intermodal selective attention in monkeys. I: distribution and timing of effects across visual areas. Cereb. Cortex. 2000;10:343–358. doi: 10.1093/cercor/10.4.343. [DOI] [PubMed] [Google Scholar]
- Mehta A.D., Ulbert I., Schroeder C.E. Intermodal selective attention in monkeys. II: physiological mechanisms of modulation. Cereb. Cortex. 2000;10:359–370. doi: 10.1093/cercor/10.4.359. [DOI] [PubMed] [Google Scholar]
- Milham M.P., Banich M.T., Webb A., Barad V., Cohen N.J., Wszalek T., Kramer A.F. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cogn. Brain Res. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Roberts K.L., Hall D.A. Examining a supramodal network for conflict processing: a systematic review and novel functional magnetic resonance imaging data for related visual and auditory Stroop tasks. J. Cogn. Neurosci. 2008;20:1063–1078. doi: 10.1162/jocn.2008.20074. [DOI] [PubMed] [Google Scholar]
- Santee J.L., Egeth H.E. Independence versus interference in the perceptual processing of letters. Percept. Psychophys. 1982;31:101–116. doi: 10.3758/bf03206210. [DOI] [PubMed] [Google Scholar]
- Sereno M.I., Dale A.M., Reppas J.B., Kwong K.K., Belliveau J.W., Brady T.J., Rosen B.R., Tootell R.B. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Somers D.C., Dale A.M., Seiffert A.E., Tootell R.B. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proc. Natl. Acad. Sci. U. S. A. 1999;96:1663–1668. doi: 10.1073/pnas.96.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P.C., Nobre A.C., Rushworth M.F. Subsecond changes in top down control exerted by human medial frontal cortex during conflict and action selection: a combined transcranial magnetic stimulation electroencephalography study. J. Neurosci. 2007;27:11343–11353. doi: 10.1523/JNEUROSCI.2877-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V., Cohen J.D., Botvinick M.M., Stenger V.A., Carter C.S. Anterior cingulate cortex, conflict monitoring, and levels of processing. NeuroImage. 2001;14:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Zysset S., Schroeter M.L., Neumann J., von Cramon D.Y. Stroop interference, hemodynamic response and aging: an event-related fMRI study. Neurobiol. Aging. 2007;28:937–946. doi: 10.1016/j.neurobiolaging.2006.05.008. [DOI] [PubMed] [Google Scholar]