Abstract
Backgrounds
Helicobacter pylori cagA can be classified into mainly two types (East-Asian-type and Western-type cagA) according to the repeat regions located in the 3′ region. Recent studies showed that the Western-type cagA in strains from Okinawa, Japan formed a different cluster (J-Western-type cagA subtype). We also reported that J-Western-type cagA possess a 12-bp insertion located in the 5′ region of cagA sequence.
Methods
The prevalence of 12-bp insertion in cagA in Okinawa and the United States (U.S.) was examined by DNA sequencing. We then designed the primer pair which can detect the 12-bp insertion only by polymerase chain reaction (PCR). The prevalence of strains with 12-bp insertion was examined in 336 strains isolated from Okinawa by PCR.
Results
In case of Western-type cagA/vacA s1m2 strains, the prevalence of 12-bp insertion was significantly higher in strains isolated from Okinawa than that from the U.S. (P = 0.002). Phylogenetic tree showed that strains with 12-bp insertion formed two individual clusters within J-Western-type cagA subtype; one is from Okinawa and another is from the U.S. Our designed primer set showed high sensitivity (100%) and specificity (90.8%) in Okinawa. The 12-bp insertion was found in 23.7%, 14.3%, 4.2%, and 4.0% of strains with duodenal ulcer (DU), gastritis, gastric cancer (GC), and gastric ulcer (GU), respectively (P < 0.001 for DU vs. GU) in Okinawa.
Conclusions
Although the mechanisms are unknown, the presence of 12-bp insertion was associated with the presence of DU and might have a suppressive action on GU and GC.
Keywords: Helicobacter pylori, 12-bp insertion, Okinawa, cagA
Introduction
Helicobacter pylori infection is now accepted as the major cause of chronic gastritis. Several epidemiological studies have shown that H. pylori infection is also linked to severe gastritis associated diseases, including peptic ulcer and gastric cancer (GC) 1. In 1994, the International Agency for Research on Cancer categorized H. pylori as a group I carcinogen 2. Although GC is one of the most common cancers, only a minority of individuals with H. pylori infection ever develop it. The prevalence of GC is approximately 3% in H. pylori-positive patients 3. In addition to environmental factors (eg, diet) and host factors, virulence factors of H. pylori, such as cagA and vacA, have been demonstrated to be predictors of gastric atrophy, intestinal metaplasia, and severe clinical outcomes 4. The most studied virulence factor of H. pylori is cagA, which is located at the end of an approximately 40-kb cluster of genes called cag pathogenicity island (PAI). cag PAI encodes a type-IV secretion system and transfers CagA protein into host cells 5. CagA protein is believed to have oncogenic potential 6, 7, and cagA-positive strains are reported to be associated with severe clinical outcomes 4. cagA can be classified into mainly two types (East-Asian-type cagA and Western-type cagA) according to the sequence located in the 3′ region of cagA8-10.
Okinawa consists of small islands (2,276 km2) in southwestern Japan. Though the prevalence of H. pylori in Okinawa is not significantly different from other parts of Japan 11,12, the incidence of GC (6.3 deaths/100,000 population) in Okinawa is the lowest in Japan (mean mortality rate of Japan; 11.8 deaths/100 000 population in 2009) (Center for Cancer Control and Information Services, National Cancer Center, Japan, [http://www.ncc.go.jp/]). Okinawa was under the rule of the United States (U.S.) after World War II (WWII) until 1972, and there are still many U.S. populations (the number of U.S. residents in Okinawa, military personnel, civilian employees, and their families, are estimated as 48,490 in 2009) (http://www.pref.okinawa.jp/annai/index.html). The different environmental factors and diets in Okinawa compared with mainland Japan are thought to be one reason for the lower incidence of GC 13. Furthermore, we recently reported that different incidence of GC between Okinawa and mainland Japan might be explained by the high prevalence of Western-type cagA strains in Okinawa compared with other areas in Japan 14. Intriguingly, recent studies using cagA full-sequenced data showed that the Western-type cagA detected in strains from Okinawa formed a different cluster compared to the original Western-type cagA and it was named the J-Western-type cagA subtype 15. We also examined the cagA sequence data deposited in the GenBank and found that J-Western-type cagA strains possess a 12-bp insertion located in the 5′ region of cagA sequence compared to the original Western-type cagA strains 16. However, the significance of 12-bp insertion, especially for clinical outcomes remains unclear. In this study, we designed the primer pair to be able to detect the 12-bp insertion by polymerase chain reaction (PCR) without DNA sequencing. In addition, we examined the prevalence of 12-bp insertion in Okinawa and the association between 12-bp insertion and clinical outcomes.
Methods
Patients and H. pylori
H. pylori strains were obtained from the gastric mucosa of H. pylori-infected patients who underwent endoscopy at University of the Ryukyus (Okinawa, Japan) between February 1993 and March 2005 and Michael E. DeBakey Veterans Affairs Medical Center (Houston, TX) between January 2001 and June 2006. Samples from Okinawa are same as our previous study 14. Presentations included gastritis, duodenal ulcer (DU), gastric ulcer (GU), and GC. Gastric biopsy specimens were taken from the antrum. DU, GU, and GC were identified by endoscopy, and gastric cancer was further confirmed by histopathology 17. Gastritis was defined as H. pylori gastritis in the absence of peptic ulcer or gastric malignancy. Patients with a history of partial gastric resection were excluded. Written informed consent was obtained from each participant, and the protocol was approved by the Ethics Committee of University of the Ryukyus (Japan) and Michael E. DeBakey Veterans Affairs Medical Center (U.S.).
Isolation and genotyping of H. pylori
Gastric biopsy specimens were taken from the antrum (pyloric gland area) and the corpus (fundic gland area). Antral biopsy specimens were used for the isolation of H. pylori using standard culture methods as previously described 18. H. pylori DNA was extracted from confluent plate cultures using a commercially available kit (QIAGEN Inc., Santa Clarita, CA). The status of cagA based on the 3′ region (East-Asian-type or Western-type) and vacA (genotypes of s and m region; s1 or s2 and m1 or m2) was determined as described in our previous study 14. To detect the 12-bp insertion, the new forward primer was designed on the basis of the sequence data we obtained. Forward primer was located the sequences including 12-bp insertion and was CAGYMF (+) (5′-AAT GGA GAG CCTACT GGA GAG CC-3′). The cagA M1(-) primer was used as reverse primer 19. The PCR conditions were initial denaturation for 5 min at 95°C, 35 amplification steps (95°C for 30 s, 52°C for 30 s, and 72°C for 30 s), and a final extension cycle of 7 min at 72°C, using Blend Taq® DNA polymerase (TOYOBO, Japan). The expected lengths of PCR products amplified with the primers CAGYMF and cagA M1(-) was approximately 400 bp. The amplified fragment was detected by a 1.5% agarose gel electrophoresis using an ultraviolet transilluminator.
Nucleotide sequencing
The presence of 12-bp insertion was examined by sequencing using primer set cagA L2 (+) and cagA M1(-) as described previously 19. PCR products (approximately 1,000 bp) were purified with QIAquick Purification Kit (QIAGEN) according to the manufacturer's instructions and DNA direct sequencing was performed using AB 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions.
Phylogenetic analysis
For construction of phylogenetic tree based on the sequence of cagA including 12-bp insertion, cagA sequences of 14 strains with typical cagA genotypes based on the 3′ region (6 Western-type cagA strains [26695, NCTC11637, NCTC11638, F79, F80, and OK111], 2 J-Western-type cagA [J99 and OK180], 3 East-Asian-type cagA strains [F16, F37 and OK101], and 3 Amerindal-type cagA strains [Shi257, Shi329 and Shi417]) were obtained from Genbank that were deposited by other group. Among the strains, OK101, OK111 and OK180 were isolated from Okinawa. These sequences were compared with our data obtained from strains in Okinawa. Neighbor joining tree was constructed by MEGA 5.0 with 3,000 bootstrappings and using Kimura-2 parameters 20, 21.
Statistical analysis
The associations between the presence of 12-bp insertion and clinical outcome were analyzed with the chi-square test and Fisher's exact probability test. A multivariate logistic regression model was used to calculate the odds ratios (OR) of the clinical outcomes to adjust by age, sex, cagA type (Western-type or East-Asian-type), vacA m status (m1 or m2) and the presence of 12-bp insertion. All determinants with P values of < 0.10 were entered together in the full model of logistic regression, and the model was reduced by excluding variables with P values of > 0.10. OR and 95% confidence interval (CI) were used to estimate the risk. A P value less than 0.05 was accepted as statistically significant. The SPSS statistical software package version 19.0 (SPSS, Inc., Chicago, IL) was used for all statistical analyses.
Results
The prevalence of 12-bp insertion in Okinawa and the U.S
We first examined the prevalence of 12-bp insertion in cagA in Okinawa and the U.S. We previously found that 12-bp insertion especially exist in J-Western-type cagA strains 16. In our previous study, we did not distinguish J-Western-type cagA strains from typical Western-type cagA strains, and both types were classified as Western-type strains 14 . Therefore, we randomly selected 41 Western-type cagA strains isolated from Okinawa from the previous study 14 based on the 3′ region of cagA (including both J-Western-type and typical Western-type cagA). We also randomly selected 29 Western-type cagA strains isolated from the U.S. from our previous study 22 As control, we selected 28 East-Asian-type cagA strains based on the 3′ region of cagA (24 from Okinawa and 4 from the U.S.; these were isolated from Vietnamese who live in the U.S.) 14, 22. Total of 98 samples (65 from Okinawa and 33 from the U.S.) were amplified by PCR and sequenced. Among 65 strains from Okinawa, 33 (50.7%) possessed 12-bp insertion (Table 1). On the other hand, the prevalence of 12-bp insertion was 18.1% (6/33) in strains from the U.S. The prevalence in strains from Okinawa was 83.9% (26/31) in Western-type cagA/vacA s1m2 strains, 50% (5/10) in Western-type cagA/vacA s1m1 strains, and 13.3% (2/15) in East-Asian-type cagA/vacA s1m2 strains. None of East-Asian-type cagA/vacA s1m1 strains possessed 12-bp insertion. In the U.S., the 12-bp insertion was only found in Western-type cagA/vacA s1m2 strains. Even in case of Western-type cagA/vacA s1m2 strains, the prevalence of 12-bp insertion was significantly higher in strains from Okinawa than that from the U.S. (83.9% vs. 37.5%, P = 0.002). This 12-bp insertion code the four amino acid PTGE or STGE in strains from Okinawa, whereas PNGE, PNGD and PIRE in strains from the U.S.
Table 1. The prevalence of 12bp insertion by DNA Sequencing.
| Okinawa, Japan | Total | Positive | |
|---|---|---|---|
| East-Asian-type cagA/vacA s1m1 | 9 | 0 | (0.0%) |
| East-Asian-type cagA/vacA s1m2 | 15 | 2 | (13.3%) |
| Western-type cagA/vacA s1m1 | 10 | 5 | (50.0%) |
| Western-type cagA/vacA s1m2 | 31 | 26 | (83.9%) |
|
| |||
| United State | Total | Positive | |
|
| |||
| East-Asian-type cagA/vacA s1m2 | 4 | 0 | (0.0%) |
| Western-type cagA/vacA s1m1 | 12 | 0 | (0.0%) |
| Western-type cagA/vacA s1m2 | 16 | 6 | (37.5%) |
| Western-type cagA/vacA s2m2 | 1 | 0 | (0.0%) |
cagA type was determined based on the 3′ region of cagA sequence
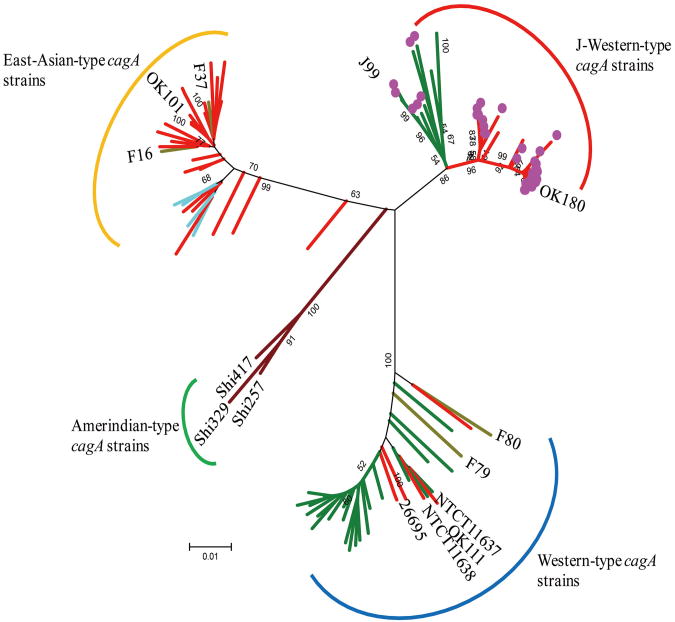
We constructed the phylogenetic tree using cagA sequence (approximately 1,000 bp) including 12-bp insertion (Figure 1). Phylogenetic tree showed that all strains with 12-bp insertion were in the J-Western-type cagA cluster, which was distinct from that of East-Asian-type cagA and typical Western-type cagA from Western countries. Interestingly, strains from Okinawa with 12-bp insertion formed one cluster different from U.S. strains with 12-bp insertion (Figure 1). In case of Okinawa, 2 East-Asian-type cagA strains with 12-bp insertion located in the same cluster with other strains with 12-bp insertion. This suggests that 2 strains have J-Western-type cagA structure in the 5′ region of cagA, whereas East-Asian-type cagA structure in the 3′ region of cagA. Three U.S. strains without 12-bp insertion also belonged to the same cluster with the U.S. strains with 12-bp insertion. Four East-Asian-type cagA strains from Vietnamese who live in the U.S. did not have 12-bp insertion, and were clustered with other East- Asian-type cagA strains.
Fig.1. Phylogenetic tree constructed on the basis of the cagA sequence.
Pylogenetic tree was constructed by using cagA sequence (about 1000 bp) including 12-bp insertion. cagA sequences of 14 strains (J99, 26695, NCTC11637, NCTC11638, F16, F37, F79, F80, OK101, OK111, OK180, Shi257, Shi329, and Shi417) were obtained from Genbank. Neighbor joining tree was constructed in MEGA 5.0 using bootstrapping at 3,000 bootstrap trials and through Kimura-2 parameters. Scale bars indicate the calculated distance. Red branch show the strains from Okinawa. Dark yellow branch show the strains from Fukui. Blue branch show the strains from Western countries. Light blue branch show the strains from Vietnamese who live in the U.S. Brown branch show the strains from Amerindian. Strains with 12-bp insertion were indicated as purple circle. Each cagA type was determined based on the 5′ region of cagA sequence (i.e., 2 East-Asian-type cagA strains based on the 3′ region of cagA sequence with 12-bp insertion located in the same cluster with other strains with 12-bp insertion, suggesting that 2 strains have J-Western-type cagA structure in the 5′ region of cagA, whereas East-Asian-type cagA structure in the 3′ region of cagA).
Construction of primer set to detect the 12-bp insertion
To be able to detect the 12-bp insertion only by PCR, we designed new primer for specific 12-bp insertion on the basis of the sequence data we obtained. In Okinawa, all 34 samples with 12-bp insertion were positive by PCR. On the other hand, 3 out of 33 samples without 12-bp insertion showed the positive band in PCR, which means false-positive because these 3 strains did not have the similar sequence with forward primer. Altogether, sensitivity, specificity and accuracy were 100%, 90.8% and 95.3%, respectively in Okinawa. In the U.S., all samples with 12-bp insertion were positive by PCR. On the other hand, none of samples without 12-bp insertion showed the positive band in PCR. Therefore, sensitivity, specificity and accuracy were all 100% in the U.S.
Association between the presence of 12-bp insertion and clinical outcomes
The prevalence of strains with 12-bp insertion was examined by PCR using our designed primer set. Total of 336 strains (98 from patients with gastritis, 101 with GU, 114 with DU and 24 with gastric cancer) in Okinawa were included in the final analysis (Table 2). The 12-bp insertion was the most found in 83.8% (31/37) in strains with Western-type cagA/vacA s1m2 genotypes, following 57.1 % (8/14) in those with Western-type cagA/vacA s1m1 genotypes. It was found in 2.3% (5/222) and 10.0% (2/20) of strains with East-Asian-type cagA/vacA s1m1 and vacA East-Asian-type cagA/s1m2, respectively. The prevalence of 12-bp insertion was significantly higher in strains with Western-type cagA/vacA s1m2 than other genotypes (P < 0.001).
Table 2. The prevalence of 12bp insertion by polymerase chain reaction in Okinawa, Japan.
| Total | Positive | ||
|---|---|---|---|
| East-Asian-type cagA/vacA s1m1 | 222 | 5 | (2.3%) |
| East-Asian-type cagA/vacA s1m2 | 20 | 2 | (10.0%) |
| Western-type cagA/vacA s1m1 | 14 | 8 | (57.1%) |
| Western-type cagA/vacA s1m2 | 37 | 31 | (83.8%) |
| Western-type cagA/vacA s2m2 | 0 | 0 | (0.0%) |
| cag A-negative/vacA s2m2 | 43 | 0 | (0.0%) |
cagA type was determined based on the 3′ region of cagA sequence
Next, we examined the association between the presence of 12-bp insertion and clinical outcomes in Okinawa. The prevalence of cagA was significantly higher in strains from GU (89.0%), DU (90.4%) and GC (95.8%) than those from gastritis (77.6%) (P = 0.03, 0.01 and 0.04, respectively) (Table 3). The 12-bp insertion was found in 14.3%, 4.0%, 23.7%, and 4.2% of strains with gastritis, GU, DU, and GC, respectively. The presence of 12-bp insertion was significantly more prevalent in strains from DU than those from GU and GC (23.4 vs. 4.0, 4.2%) (P < 0.001 and P = 0.03, respectively). The presence of 12-bp insertion was significantly more prevalent in strains from gastritis (14.3%) than those from GU (4.0%) (P = 0.01). The presence of 12-bp insertion was tended to be higher in strains from DU than those from gastritis (23.7 vs. 14.3%), although the difference did not reach statistical significance (P = 0.10). In case of cagA-positive subjects, the presence of 12-bp insertion was significantly associated with DU compared with GU after adjustment by age, sex, cagA type, vacA m status in multivariate analysis (odds ratio [OR] = 4.72, 95% confidence interval [CI] = 1.46-15.23). Among 8 Western-type cagA/vacA s1m1strains with 12-bp insertion, 5 strains (62.5%) were isolated from DU. Furthermore, 4 (57.1%) out of 7 East-Asian-type cagA strains with 12-bp insertion were isolated from DU. In addition, the presence of 12-bp insertion was tended to be associated with DU compared with GC after adjustment by age, sex, cagA type, vacA m status in case of cagA-positive subjects in multivariate analysis (OR = 7.76, 95% CI = 0.97-61,66, P = 0.05).
Table 3. The association between the presence of 12-bp insertion and clinical outcomes.
| Gastritis | Gastric ulcer | Duodenal ulcer | Gastric cancer | |||||
|---|---|---|---|---|---|---|---|---|
| % | % | % | % | |||||
| n | 98 | 100 | 114 | 24 | ||||
| mean age | 54.4 | 55.6 | 54.3 | 64.8* | ||||
| male | 47 | (48.0) | 74 | (74.0)* | 69 | (60.5) | 17 | (70.8)* |
| cag A | 76 | (77.6) | 89 | (89.0)* | 103 | (90.4)* | 23 | (95.8)* |
| 12-bp insertion | 14 | (14.3) | 4 | (4.0)* | 27 | (23.7)** | 1 | (4.2)*** |
; p < 0.05 compared with gastritis
; p < 0.05 compared with gastric ulcer
; p < 0.05 compared with duodenal ulcer
Nucleotide sequence
Nucleotide sequence data reported are available under the DDBJ accession numbers AB725775-AB725874.
Discussions
cagA, which encodes a highly immunogenic protein (CagA), is the most extensively studied H. pylori virulence factor 23, 24. cagA is a polymorphic gene and classified into mainly two types (East-Asian-type cagA and Western-type cagA) according to the repeat sequence located in the 3′ region of cagA8-10. Some reports showed that individuals infected with East-Asian-type cagA strains have an increased risk of peptic ulcer and/or gastric cancer compared with those infected with non East-Asian-type cagA strains 14, 25, 26.
Recent studies showed that Western-type cagA in Okinawa, Japan was different from that of the typical Western-type cagA found in Western countries according to the full-sequenced cagA, and it was names as J-Western-type cagA15. We recently examined the H. pylori in Okinawa with multi-locus sequence typing (MLST) using seven housekeeping genes 14. Intriguingly, MLST analysis revealed that the majority of Western-type cagA strains in Okinawa formed individual cluster and these did not belonged to cluster of strains isolated from ethnic Europeans, including people from countries colonized by Europeans. These findings supported that the origin of Western-type cagA strains in Okinawa is different from those of Western countries.
Recently, Duncan et al. as well as our group reported that J-Western-type cagA contain unique 12-bp insertion located in the 5′ region of cagA16, 27. In addition, they stated that J-Western-type cagA strains were found not only in Okinawa but also in other parts of the world 27. Finally, they showed that J-Western-type cagA sequences were mainly found in strains possessing vacA m2. In this study, we examined the prevalence of 12-bp insertion in Okinawa and the U.S. As a result, the 12-bp insertion was more prevalent in strains in Okinawa than that from the U.S even among Western-type cagA strains. Intriguingly, the 12-bp insertion was found in 50.0% even in Western-type cagA /vacA s1m1 strains in Okinawa, which was higher than that of Western-type-cagA /vacA s1m2 strains in the U.S (37.5%). These findings suggest that 12-bp insertion was more specific in strains in Okinawa irrespective the status of vacA although it was more common in strains with vacA m2 genotype. In our previous study using MLST, Western-type cagA strains in Okinawa were classified into two groups 14. One is individual group near hpAsia2 (genotype of strains mainly isolated in South Asia), and another was located in hspEAsia (genotype of strains isolated in East Asia). It is not clear whether there is any association between these groups by MLST and 12-bp insertion, which is processing in our subsequent study.
The presence of 12-bp insertion was inversely associated with the presence of GU. The prevalence of 12-bp insertion was the most prevalent in DU subjects, and followed by gastritis, GC and GU subjects. Furthermore, more than half of the strains with 12-bp insertion in Western-type cagA/va cA s1m1 and East-Asian-type cagA genotypes were isolated from DU subjects. This suggests that the presence of 12-bp insertion was associated with the presence of DU and might have a suppressive action on GU and GC. Duodenal ulcer promoting (dupA) gene is also a virulence factor for DU and protective factor for GC 28. In our previous study, in vitro experiments showed that the presence of dupA was associated with increased susceptibility to low pH 28. These findings assume the positive association between the presence of dupA and DU but not GC. It is possible that 12-bp insertion is also related with increased susceptibility to low pH and followed by the development of DU. Further studies will be necessary to investigate the mechanisms how 12-bp insertion of cagA is associated with DU. An additional cagA type, Amerindian cagA, was recently reported from populations of the Peruvian Amazon 29. Amerindian cagA can be divided into 2 types, ie, AM-I and AM-II 30. Interestingly, AM-II cagA has attenuated abilities to stimulate gastric epithelial proliferation and inflammation during infection compared to those of Western-type cagA or East-Asian-type cagA. The Amerindian CagA multimerization segment played an important role in those findings 30. Our phylogenetic tree showed that the strains with 12-bp insertion formed individual cluster different from that of East-Asian-type cagA and typical Western-type cagA from Western countries. Furthermore, Western-type cagA with 12-bp insertion formed two clusters; one is from Okinawa and another is from the U.S. It remained unclear whether there are any differences of the biological activities between typical Western-type cagA and J- Western-type cagA, or two types (Okinawa and the U.S) of J- Western-type cagA. Several studies showed that the importance of N-terminus of CagA on host cell biology 31, 32. However, these did not consider the presence or absence of 12-bp insertion. Low biological activities of cagA including 12-bp insertion may be inversely associated with GU. Further studies are needed to clarify the role of the J-Western-type cagA sequences or 12-bp insertion in vitro study.
In conclusion, we found that 12-bp insertion was more prevalent in strains from Okinawa than those from the U.S., especially in Western-type cagA/vacA s1m2 strains. In addition, we designed the primer pair to be able to detect the 12-bp insertion only by PCR without DNA sequencing. The presence of 12-bp insertion was associated with DU and would be used as protective marker for GU and GC.
Acknowledgments
This report is based on work supported in part by grants from the National Institutes of Health (DK62813), grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (22390085 and 22659087), Special Coordination Funds for Promoting Science and Technology from MEXT of Japan, and Research Fund at the Discretion of the President, Oita University. The funders had no role in study design data collection and analysis, decision to publish, or preparation of the manuscript. The authors would like to thank Ms. Kudo, Ms. Matsuda and Ms. Takahashi for their excellent technical assistance.
Footnotes
Potential competing interests: The authors declare that they have no competing interests.
References
- 1.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–86. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 2.Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 3.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 4.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629–41. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backert S, Selbach M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell Microbiol. 2008;10:1573–81. doi: 10.1111/j.1462-5822.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 6.Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004;4:688–94. doi: 10.1038/nrc1433. [DOI] [PubMed] [Google Scholar]
- 7.Hatakeyama M. Helicobacter pylori CagA -- a bacterial intruder conspiring gastric carcinogenesis. Int J Cancer. 2006;119:1217–23. doi: 10.1002/ijc.21831. [DOI] [PubMed] [Google Scholar]
- 8.Yamaoka Y, Kodama T, Kashima K, Graham D, Sepulveda A. Variants of the 3′ region of the cagA gene in Helicobacter pylori isolates from patients with different H. pylori-associated diseases. J Clin Microbiol. 1998;36:2258–63. doi: 10.1128/jcm.36.8.2258-2263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaoka Y, El-Zimaity H, Gutierrez O, et al. Relationship between the cagA 3′ repeat region of Helicobacter pylori, gastric histology, and susceptibility to low pH. Gastroenterology. 1999;117:342–9. doi: 10.1053/gast.1999.0029900342. [DOI] [PubMed] [Google Scholar]
- 10.Yamaoka Y, Osato M, Sepulveda A, et al. Molecular epidemiology of Helicobacter pylori: separation of H. pylori from East Asian and non-Asian countries. Epidemiol Infect. 2000;124:91–6. doi: 10.1017/s0950268899003209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nobuta A, Asaka M, Sugiyama T, et al. Helicobacter pylori infection in two areas in Japan with different risks for gastric cancer. Aliment Pharmacol Ther. 2004;20(1):1–6. doi: 10.1111/j.1365-2036.2004.01976.x. [DOI] [PubMed] [Google Scholar]
- 12.Ito S, Azuma T, Murakita H, et al. Profile of Helicobacter pylori cytotoxin derived from two areas of Japan with different prevalence of atrophic gastritis. Gut. 1996;39:800–6. doi: 10.1136/gut.39.6.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willcox DC, Willcox BJ, Todoriki H, Suzuki M. The Okinawan diet: health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load. J Am Coll Nutr. 2009;28 Suppl:500S–16S. doi: 10.1080/07315724.2009.10718117. [DOI] [PubMed] [Google Scholar]
- 14.Matsunari O, Shiota S, Suzuki R, et al. Association between Helicobacter pylori virulence factors and gastroduodenal diseases in Okinawa, Japan. J Clin Microbiol. 2012;50:876–83. doi: 10.1128/JCM.05562-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Truong B, Mai V, Tanaka H, et al. Diverse characteristics of the CagA gene of Helicobacter pylori strains collected from patients from southern vietnam with gastric cancer and peptic ulcer. J Clin Microbiol. 2009;47:4021–8. doi: 10.1128/JCM.00504-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiota S, Matsunari O, Yamaoka Y. Relationship between J-Western CagA subtype and the vacA m2 region of Helicobacter pylori. J Clin Microbiol. 2010;48:3033–4. doi: 10.1128/JCM.00983-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rugge M, Correa P, Dixon MF, et al. Gastric dysplasia: the Padova international classification. Am J Surg Pathol. 2000;24:167–76. doi: 10.1097/00000478-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Yamaoka Y, Kodama T, Kita M, Imanishi J, Kashima K, Graham D. Relationship of vacA genotypes of Helicobacter pylori to cagA status, cytotoxin production, and clinical outcome. Helicobacter. 1998;3:241–53. doi: 10.1046/j.1523-5378.1998.08056.x. [DOI] [PubMed] [Google Scholar]
- 19.Satomi S, Yamakawa A, Matsunaga S, et al. Relationship between the diversity of the cagA gene of Helicobacter pylori and gastric cancer in Okinawa, Japan. J Gastroenterol. 2006;41:668–73. doi: 10.1007/s00535-006-1838-6. [DOI] [PubMed] [Google Scholar]
- 20.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–9. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Nei M, Dudley J, Tamura K. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform. 2008;9:299–306. doi: 10.1093/bib/bbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaoka Y, Orito E, Mizokami M, et al. Helicobacter pylori in North and South America before Columbus. FEBS Lett. 2002;517:180–4. doi: 10.1016/s0014-5793(02)02617-0. [DOI] [PubMed] [Google Scholar]
- 23.Covacci A, Censini S, Bugnoli M, et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993;90:5791–5. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tummuru MK, Cover TL, Blaser MJ. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993;61:1799–809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vilaichone RK, Mahachai V, Tumwasorn S, Wu JY, Graham DY, Yamaoka Y. Molecular epidemiology and outcome of Helicobacter pylori infection in Thailand: a cultural cross roads. Helicobacter. 2004;9:453–9. doi: 10.1111/j.1083-4389.2004.00260.x. [DOI] [PubMed] [Google Scholar]
- 26.Jones KR, Joo YM, Jang S, et al. Polymorphism in the CagA EPIYA motif impacts development of gastric cancer. J Clin Microbiol. 2009;47:959–68. doi: 10.1128/JCM.02330-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duncan SS, Valk PL, Shaffer CL, Bordenstein SR, Cover TL. J-Western forms of Helicobacter pylori cagA constitute a distinct phylogenetic group with a widespread geographic distribution. J Bacteriol. 2012;194:1593–604. doi: 10.1128/JB.06340-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu H, Hsu P, Graham D, Yamaoka Y. Duodenal ulcer promoting gene of Helicobacter pylori. Gastroenterology. 2005;128:833–48. doi: 10.1053/j.gastro.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kersulyte D, Kalia A, Gilman RH, et al. Helicobacter pylori from Peruvian amerindians: traces of human migrations in strains from remote Amazon, and genome sequence of an Amerind strain. PLoS One. 2010;5:e15076. doi: 10.1371/journal.pone.0015076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki M, Kiga K, Kersulyte D, et al. Attenuated CagA oncoprotein in Helicobacter pylori from Amerindians in Peruvian Amazon. J Biol Chem. 2011;286:29964–72. doi: 10.1074/jbc.M111.263715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bagnoli F, Buti L, Tompkins L, Covacci A, Amieva MR. Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proc Natl Acad Sci U S A. 2005;102:16339–44. doi: 10.1073/pnas.0502598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelz C, Steininger S, Weiss C, Coscia F, Vogelmann R. A novel inhibitory domain of Helicobacter pylori protein CagA reduces CagA effects on host cell biology. J Biol Chem. 2011;286:8999–9008. doi: 10.1074/jbc.M110.166504. [DOI] [PMC free article] [PubMed] [Google Scholar]