Abstract
We compared protein expression by Western blot analysis in four areas of postmortem brain from patients with schizophrenia and control subjects for several proteins that are often used as controls for Western blot studies: β-tubulin, actin, glyceraldehyde-3-phosphate dehydrogenase, and valosin-containing protein. We did not detect any differences in expression between subjects with schizophrenia and a comparison group. These results suggest that all four proteins are suitable loading controls for postmortem studies of schizophrenia.
Keywords: VCP, GAPDH, Anterior cingulate cortex, Dorsolateral prefrontal cortex, Hippocampus, Primary visual cortex
Introduction
Western blotting is a common technique used to measure protein levels. To ensure even loading, blotting, and processing between samples, an internal control is utilized. These loading controls are usually so-called housekeeping proteins measured in the same lane of the gel for each sample, and are assumed to have constant between-sample expression relative to the amount of sample loaded. Commonly used loading controls include the cytoskeletal structural molecules β-tubulin and actin, and the glycolytic enzyme glyceralde-hyde-3-phosphate dehydrogenase (GAPDH).
Valosin-containing protein (VCP) is a 97 kDa protein used as a loading control in one postmortem schizophrenia study (Stan et al. 2006). VCP is an ATPase involved in the ubiquitin-proteasome degradation pathway, membrane fusion, transcription activation, cell cycle control, apoptosis, and molecular chaperoning (Wang et al. 2004). Although it is not predicted to be affected in this disease, it has not been determined whether VCP is abnormally regulated in schizophrenia. In this study, we examined expression of VCP, β-tubulin, actin, and GAPDH in patients with schizophrenia and control subjects.
Materials and methods
Subjects
Subjects from the Mount Sinai Medical Center Department of Psychiatry Schizophrenia Brain Bank were studied (Table 1), including 39 individuals diagnosed with schizophrenia and 41 comparison subjects. Subjects were diagnosed with schizophrenia if the presence of schizophrenic symptoms were documented before age 40, the medical records contained evidence of psychotic symptoms and at least 10 years of psychiatric hospitalization with diagnosis of schizophrenia, and a DSM-III-R diagnosis of schizophrenia was agreed upon by two experienced clinicians. Diagnostic groups were matched for age, postmortem interval, and tissue pH. Upon neuropathological examination, no evidence of Alzheimer or other neurodegenerative disease was found. The brain banking procedures were approved by the Mount Sinai School of Medicine Institutional Review Board.
Table 1.
Subject Characteristics
| Comparison group | Schizophrenia | |
|---|---|---|
| n | 41 | 39 |
| Age | 78.4 ± 13.6 | 74.3 ± 11.7 |
| Sex | 23 F/18 M | 12 F/27 M |
| PMI (hours) | 8.1 ± 6.3 | 13.7 ± 7.8 |
| Tissue pH | 6.44 ± 0.25 | 6.41 ± 0.29 |
| Antipsychotic Medication | NA | 23 typical, 9 atypical, 3 both, 1 unmedicated, 3 unknown |
F female, M male, PMI postmortem interval
Tissue preparation
Brains were obtained after autopsy and one hemisphere was cut coronally into ~0.8–1 cm3 slabs and flash frozen. Tissue was dissected from anterior cingulate cortex (ACC) (n = 68), dorsolateral prefrontal cortex (DLPFC) (n = 66), hippocampus (n = 49), and primary visual cortex (PVC) (n = 46). Approximately, 1 cm3 of frozen tissue was first pulverized, then homogenized (10% wt/vol) in 5 mM Tris–HCl (pH 7.4) with 320 mM sucrose and one protease inhibitor tablet per 10 mL (Complete mini, Roche Diagnostics, Manheim, Germany) for 30 s with a polytron homogenizer (Fisher Scientific, Pittsburgh, PA) and stored at −80°C in 0.5 mL aliquots. For our loading control, tissue from the frontal cortex was dissected from a 9.8-year-old female pig-tail macaque (Macaca nemestrina) provided by the University of Washington Regional Primate Research Center. The animal was sacrificed as part of a protocol (which did not require brain tissue) unrelated to the present study. Protocols involving this animal were reviewed by the Washington Primate Research Center Research Review Committee and the University of Washington IACUC. Frozen macaque cortex was thawed on ice, cut into pieces of approximately 1 cm3, and homogenized as described above and stored at −80°C in 1.2 mL aliquots. To determine protein concentrations, assay by the Bradford method (Bradford 1976) was performed on these homogenates.
Western blot analysis
For gel electrophoresis, tissue samples were adjusted to a concentration of 0.8 μg/μl with sterile water, reducing buffer (Invitrogen), and denaturing buffer (Invitrogen). Samples were then incubated at 70°C for 10 min.
The Novex Mini Cell NuPAGE system (Invitrogen) with 4–12% Bis–Tris gradient polyacrylamide gels (Invitrogen) was used. A 8 μg of denatured protein homogenate was run in each lane. Samples were loaded in duplicate in adjacent lanes, and a molecular weight standard was run on each gel. A lane containing 8 μg of homogenized macaque cortex was also loaded onto each gel to control for interblot variability. Gels were suspended in a bath of NuPAGE MES SDS running buffer (Invitrogen) with 500 μl NuPAGE antioxidant (Invitrogen) during electrophoresis.
Following electrophoresis, proteins were transferred onto Immobilon-FL PVDF membranes (Millipore) using a semi-dry transfer apparatus (Bio-Rad). After electroblot transfer of the protein, membranes were washed twice and incubated with Odyssey Blocking Buffer (Li-Cor Biosciences) for 1 h at room temperature with rocking to block non-specific antibody binding. Membranes were exposed to the primary polyclonal antibody diluted 1:10,000 for actin (Chemicon MAB150R), VCP (Abcam ab11433), and β-tubulin (Upstate 05-661), and 1:20,000 for GAPDH (Sigma G9545) in blocking buffer with 0.1% tween overnight at 4°C with rocking. Next, the membranes were washed three times for 10 min in tris-buffered saline with 0.1% tween (TBST), then rocked for 1 h with anti-mouse IR-Dye 680 or 800 CW secondary antibody (Li-Cor Biosciences) diluted 1:10,000 in blocking buffer with 0.1% tween. Membranes were washed three times for 10 min in TBST then washed five times in high purity water and allowed to dry for 3–5 min before scanning (infrared imaging system; Li-Cor Biosciences). We pre-tested the β-tubulin, actin, GAPDH, and VCP Western blot assays using varying concentrations of protein from a human cortical tissue homogenate sample. These experiments demonstrated that each assay was linear with protein concentrations found in this study (VCP: R = 0.99, P < 0.01; β-tubulin: R = 0.99, P < 0.01; actin: R = 0.95, P = 0.01; GAPDH: R = 0.98, P < 0.01).
Data analysis
Membranes were scanned using a Li-Cor Odyssey scanner, and the intensity value for each protein band was measured using the Odyssey 2.1 software package. Specific protein values were corrected for lane background and divided by intensity values for the macaque cortex homogenate lane used as an internal loading control. Because all of the proteins studied are typically used as loading controls, we did not divide these values by another within-lane control value. Finally, adjusted intensity values from duplicate lanes were averaged for each subject.
Statistical analysis
All statistical analyses were performed using Statistica (StatSoft, Tulsa, Oklahoma). Outliers more than six standard deviations from the mean were excluded. Correlation analysis was performed to analyze associations between protein expression and age, postmortem interval, and pH. We analyzed protein expression using analysis of variance (ANOVA) or with analysis of covariance (ANCOVA) when significant correlations were detected.
Results
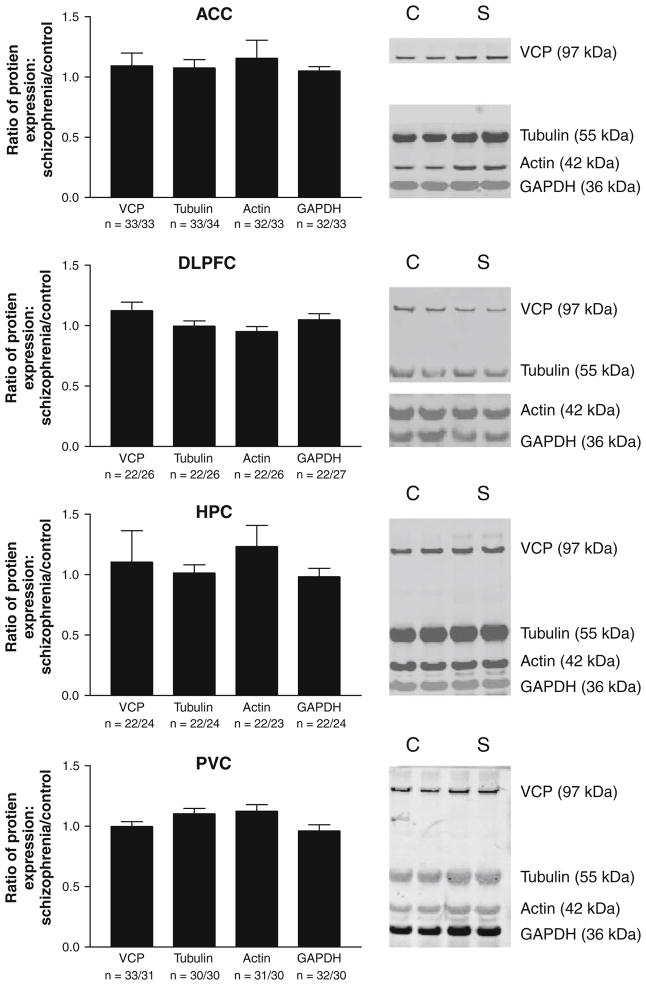
We detected bands for all four proteins at the predicted molecular masses of 97 kDa (VCP), 55 kDa (β-tubulin), 42 kDa (actin), and 36 kDa (GAPDH) (Fig. 1). After exclusion of outliers, the following numbers of subjects remained for analysis (VCP in ACC: 33 control/33 schizophrenia; β-tubulin in ACC: 34 control/33 schizophrenia; actin and GAPDH in ACC: 33 control/32 schizophrenia; VCP, β-tubulin, and actin in hippocampus: 26 control/22 schizophrenia; GAPDH in hippocampus: 27 control/22 schizophrenia; VCP, β-tubulin, and GAPDH in PVC: 24 control/22 schizophrenia; actin in PVC: 23 control/22 schizophrenia; VCP in DLPFC: 31 control/33 schizophrenia; β-tubulin in DLPFC: 30 control/30 schizophrenia; actin in DLPFC: 31 control/30 schizophrenia; GAPDH in DLPFC: 30 control/32 schizophrenia). Regression analysis revealed a correlation between tissue pH and actin expression in PVC (r = 0.33, P < 0.05). No other correlations were detected between age (VCP in ACC: r = 0.10, P = 0.43; VCP in hippocampus: r = 0.03, P = 0.29; VCP in PVC: r = 0.04, P = 0.78; VCP in DLPFC: r = 0.08, P = 0.52; β-tubulin in ACC: r = 0.14, P = 0.24; β-tubulin in hippocampus: r = 0.10, P = 0.51; β-tubulin in PVC: r = 0.13, P = 0.40; β-tubulin in DLPFC: r = 0.09, P = 0.50; actin in ACC: r = 0.07, P = 0.58; actin in hippocampus: r = 0.01, P = 0.95; actin in PVC: r = 0.09, P = 0.54; actin in DLPFC: r = 0.09, P = 0.48; GAPDH in ACC: r = 0.17, P = 0.18; GAPDH in hippocampus: r = 0.03, P = 0.86; GAPDH in PVC: r = 0.07, P = 0.63; GAPDH in DLPFC: r = 0.05, P = 0.69) or pH (VCP in ACC: r = 0.08, P = 0.52; VCP in hippocampus: r = 0.24, P = 0.10; VCP in PVC: r = 0.15, P = 0.32; VCP in DLPFC: r = 0.04, P = 0.77; β-tubulin in ACC: r = 0.06, P = 0.64; β-tubulin in hippocampus: r = 0.11, P = 0.44; β-tubulin in PVC: r = 0.02, P = 0.87; β-tubulin in DLPFC: r = 0.01, P = 0.93; actin in ACC: r = 0.09, P = 0.45; actin in hippocampus: r = 0.27, P = 0.06; actin in DLPFC: r = 0.01, P = 0.94; GAPDH in ACC: r = 0.14, P = 0.27; GAPDH in hippocampus: r = 0.15, P = 0.31; GAPDH in PVC: r = 0.15, P = 0.32; GAPDH in DLPFC: r = 0.20, P = 0.11) for any of the proteins studied. Because sex is a non-continuous variable, we performed ANOVA with sex as the independent variable, and protein expression as the dependent variable to test for effects of sex on protein expression. There was an increase in GAPDH expression in PVC in males compared to females (F = 7.35, P = 0.01), but this effect was not robust enough to withstand a correction for multiple comparisons. No other associations between sex and protein expression were detected (VCP in ACC: F = 0.21, P = 0.65; VCP in hippocampus: F = 0.00, P = 0.99; VCP in PVC: F = 2.37, P = 0.13; VCP in DLPFC: F = 3.03, P = 0.09; β-tubulin in ACC: F = 0.10, P = 0.75; β-tubulin in hippocampus: F = 0.09, P = 0.77; β-tubulin in PVC: F = 2.22, P = 0.14; β-tubulin in DLPFC: F = 0.32, P = 0.57; actin in ACC: F = 0.16, P = 0.67; actin in hippocampus: F = 0.18, P = 0.68; actin in PVC: F = 1038, P = 0.25; actin in DLPFC: F = 0.02, P = 0.90; GAPDH in ACC: F = 0.91, P = 0.34; GAPDH in hippocampus: F = 0.02, P = 0.89; GAPDH in DLPFC: F = 2064, P = 0.11). We did not detect any differences in expression for any of the proteins studied between subjects with schizophrenia and the comparison group in any of the four regions studied (Fig. 1).
Fig. 1.
Housekeeping protein expression in multiple brain regions. Data are expressed as ratio of signal intensity for subjects with schizophrenia divided by signal intensity for comparison subjects. None of these proteins differed between diagnostic groups in any region studied. On the right are representative Western blots for the four proteins studied. ACC anterior cingulate cortex, DLPFC dorsolateral prefrontal cortex, HPC hippocampus, PVC primary visual cortex, C comparison subject, S schizophrenia, VCP valosin-containing protein, GAPDH glyceraldehyde-3-phosphate dehydrogenase, kDa kilodaltons
Discussion
Several studies have shown variations in expression of housekeeping proteins in experimental or disease conditions. GAPDH protein levels in cultured human keratinocytes increased with 2,3,7,8-tetrachlorodibenzo-p-dioxin treatment (McNulty and Toscano 1995), spinal cord injury increased actin expression in rats (Liu and Xu 2006), and actin, β-tubulin and GAPDH were expressed at higher levels in renal tumors compared to normal kidney tissue (Ferguson et al. 2005). Sex differences were found in GAPDH expression in the developing rat brain (Perrot-Sinal et al. 2001). These findings highlight the importance of confirming that the loading controls used for Western blot analyses are not affected by the condition being studied. We did not find differences in the expression of the proteins studied in this cohort in schizophrenia. It is unlikely that the mismatch in sex distribution between groups influenced our results given that we did not find any significant influence of sex on protein expression. Because all of the proteins studied are putative loading controls, we did not normalize to a within-lane comparison protein. However, we normalized to a constant amount of protein loaded on each gel as a control for interblot variability. Our results are consistent with one other study that also found no changes in actin expression in schizophrenia (Eastwood and Harrison 2001).
While actin, β-tubulin, and GAPDH have traditionally been used as loading controls, VCP has a higher molecular weight (97 kDa) which makes it useful on blots probed for smaller proteins. In summary, we found no changes in four different brain regions of expression of three housekeeping proteins that are commonly used to normalize protein data, and a fourth protein, VCP, that has not previously been characterized in schizophrenia. Based on these findings, VCP appears to be an acceptable loading control to use for low molecular weight proteins in postmortem protein studies of schizophrenia.
Acknowledgments
Supported by MH53327 (JMW), MH78378 (DEB), MH064673 & MH066392 (VH) and MH074016 (REM).
Contributor Information
Deborah Elaine Bauer, Email: debbauer@uab.edu, Department of Psychiatry and Behavioral Neurobiology, University of Alabama at Birmingham, SC560, 1530 3rd Ave S., Birmingham, AL 35294-0017, USA. Program in Neuroscience, University of Michigan, Ann Arbor, MI, USA.
Vahram Haroutunian, Department of Psychiatry, Mount Sinai School of Medicine, New York, NY, USA.
Robert E. McCullumsmith, Department of Psychiatry and Behavioral Neurobiology, University of Alabama at Birmingham, SC560, 1530 3rd Ave S., Birmingham, AL 35294-0017, USA
James H. Meador-Woodruff, Department of Psychiatry and Behavioral Neurobiology, University of Alabama at Birmingham, SC560, 1530 3rd Ave S., Birmingham, AL 35294-0017, USA. Program in Neuroscience, University of Michigan, Ann Arbor, MI, USA
References
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Synaptic pathology in the anterior cingulate cortex in schizophrenia and mood disorders. A review and a Western blot study of synaptophysin, GAP-43 and the complexins. Brain Res Bull. 2001;55(5):569–578. doi: 10.1016/s0361-9230(01)00530-5. [DOI] [PubMed] [Google Scholar]
- Ferguson RE, Carroll HP, Harris A, Maher ER, Selby PJ, Banks RE. Housekeeping proteins: a preliminary study illustrating some limitations as useful references in protein expression studies. Proteomics. 2005;5(2):566–571. doi: 10.1002/pmic.200400941. [DOI] [PubMed] [Google Scholar]
- Liu NK, Xu XM. Beta-tubulin is a more suitable internal control than beta-actin in western blot analysis of spinal cord tissues after traumatic injury. J Neurotrauma. 2006;23(12):1794–1801. doi: 10.1089/neu.2006.23.1794. [DOI] [PubMed] [Google Scholar]
- McNulty SE, Toscano WA., Jr Transcriptional regulation of glyceraldehyde-3-phosphate dehydrogenase by 2, 3, 7, 8-tetra-chlorodibenzo-p-dioxin. Biochem Biophys Res Commun. 1995;212(1):165–171. doi: 10.1006/bbrc.1995.1951. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Davis AM, McCarthy MM. Developmental sex differences in glutamic acid decarboxylase (GAD(65)) and the housekeeping gene, GAPDH. Brain Res. 2001;922(2):201–208. doi: 10.1016/s0006-8993(01)03167-5. [DOI] [PubMed] [Google Scholar]
- Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, Tamminga CA. Human postmortem tissue: what quality markers matter? Brain Res. 2006;1123(1):1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Song C, Li CC. Molecular perspectives on p97-VCP: progress in understanding its structure and diverse biological functions. J Struct Biol. 2004;146(1–2):44–57. doi: 10.1016/j.jsb.2003.11.014. [DOI] [PubMed] [Google Scholar]