Abstract
Overexpression of epidermal growth factor receptors (ErbB) is frequently seen in inflammatory breast cancer (IBC). Treatment with ErbB1/2-targeting agents (lapatinib) mediates tumor apoptosis by downregulating ErbB1/2 phosphorylation and downstream survival signaling. In this study, using carboxy-H2DCFDA, DHE, and MitoSOX Red to examine changes in hydrogen peroxide radicals, cytoplasmic and mitochondrial superoxide, respectively, we observed that GW583340 (a lapatinib-analog) increases reactive oxygen species (ROS) in two models of IBC (SUM149, SUM190) that are sensitive to ErbB1/2 blockade. This significant increase in ROS levels was similar to those generated by classical oxidative agents H2O2 and paraquat. In contrast, minimal to basal levels of ROS were measured in a clonal population of GW583340-resistant IBC cells (rSUM149 and rSUM190). The GW583340-resistant IBC cells displayed increased SOD1, SOD2, and glutathione expression, which correlated with decreased sensitivity to the apoptotic-inducing effects of GW583340, H2O2, and paraquat. The ROS increase and cell death in the GW583340-sensitive cells was reversed by simultaneous treatment with a superoxide dismutase (SOD) mimic. Additionally, overcoming the high levels of antioxidants using redox modulators induced apoptosis in the GW583340-resistant cells. Taken together, these data demonstrate a novel mechanism of lapatinib-analog-induced apoptosis and indicate that resistant cells have increased antioxidant potential, which can be overcome by treatment with SOD modulators.
Keywords: Reactive oxygen species, SUM149, SUM190, IBC, XIAP, Lapatinib, Superoxide, SOD, Antioxidants, Therapeutic resistance, Redox modulators, Caspases
Introduction
Inflammatory breast cancer (IBC) is an aggressive, highly invasive tumor affecting younger women with racial disparity, which has one of the worst clinical outcomes among breast cancers [1, 2]. The majority of IBC tumors are negative for estrogen receptor (ER) and have overexpression or activation of epidermal growth factor receptors 1 and/or 2 (ErbB1, ErbB2) [3]; therefore, ErbB1/2-targeting agents including lapatinib (a dual ErbB1/2 tyrosine kinase inhibitor) are approved for treatment of IBC patients. The primary mechanism of action of lapatinib lies in its ability to downregulate ErbB1 and ErbB2 receptor phosphorylation, which leads to inhibition of downstream survival signaling via the PI3K-AKT and MAPK pathways [4]. However, recent data suggest that lapatinib’s mechanism of action is more multifactorial than was first appreciated. Studies have demonstrated that lapatinib affects survivin stability [5], multidrug resistance proteins [6], efflux and uptake transporters [7], and cell metabolism [8]. Although all of these mechanisms can potentially lead to cell death, and the clinical response (CR) rate of patients receiving lapatinib monotherapy has been remarkable (50% CR rate in ErbB2-overexpressing IBC patients [9]), a significant number of patients do not respond to lapatinib monotherapy, and acquired resistance is frequent [10]. Therefore, studies elucidating mechanisms of lapatinib resistance are clinically significant. Acquired resistance to lapatinib occurs despite the decrease in ErbB1/2 phosphorylation and downstream AKT activation [11, 12]. Additionally, resistance does not seem to be due to receptor mutation or increased downstream signaling as is seen with another ErbB2 targeting agent (Trastuzumab—a humanized ErbB2 monoclonal antibody), but rather has been observed to occur via maintenance or upregulation of key survival/anti-apoptotic proteins such as survivin [12], Mcl-1 [13], XIAP [11], and NFκB [14].
Activation of AMPK, a kinase that responds to cellular stress and leads to ATP depletion [15], has been reported in lapatinib-sensitive AU565 breast cancer cells and cardiomyocytes [8, 16]. A recent study from our lab using two IBC cellular models of acquired resistance to a research grade lapatinib-analog, GW583340, identified that expression of the most potent cellular caspase inhibitor, XIAP, is critical for survival of these resistant cells [11]. Upregulation of XIAP in the acquired lapatinib-resistant IBC cellular models was shown to be due to non-canonical protein translation via its IRES (internal ribosomal entry site) element, indicating activation of a stress response pathway [11, 17].
Oxidative stress, where there is an imbalance between reactive oxygen species (ROS) and the cell’s antioxidant capacity [18], is one of the classical stress response mechanisms that modulate apoptosis and a critical path of therapeutic-agent induced stress in cancer cells [19]. Increase in p-AMPK, which has been observed post-lapatinib treatment [8, 16], can be due to increased oxidative stress [20]. Moreover, previous studies have shown that in cardiomyocytes, blockade of the ErbB2 receptor leads to cell death via ROS [21]. Therefore, the present study aimed to understand the effects of the ErbB1/2 dual kinase inhibitor lapatinib on oxidative stress-induced apoptosis in IBC cells.
The results from this study show that the lapatinib-analog GW583340 induces generation of ROS, including hydrogen peroxide radicals, cytoplasmic and mitochondrial superoxide, in IBC cells responding to GW583340-mediated apoptosis. Sensitivity to GW583340 and generation of ROS was reversed in the presence of a potent antioxidant (SOD mimic, MnTnHex-2-PyP). ROS levels were minimal in GW583340-resistant IBC cells (rSUM149 and rSUM190), corresponding to high levels of SOD1, SOD2, and glutathione content. Moreover, cells with acquired resistance to GW583340 had low levels of ROS and resistance to classical ROS mediators, potentially due to high basal levels of antioxidants. Overcoming the high levels of antioxidants in the resistant cells using SOD modulators was able to induce apoptosis in the presence of ROS generators. These data reveal a novel mechanism of lapatinib-analog-induced apoptosis and acquired resistance, whereby cells gain antioxidant capacity, making them cross-resistant to other oxidative stress inducers.
Materials and methods
Cell culture
SUM149 and SUM190 cells were obtained from Asterand, Inc. (Detroit, MI). GW583340-resistant rSUM149 and rSUM190 were generated as described previously [11], and all cells were cultured as described previously [11, 22].
ROS measurements
Cells were cultured in six-well plates (Corning Incorporated, Corning, NY) in regular growth media until reaching 70–80% confluence. Then, cells were treated with hydrogen peroxide (H2O2), paraquat, and GW583340 alone or GW583340 in combination with an SOD mimic (MnTnHex-2-PyP) for 1 (H2O2) or 24 h (GW583340 and paraquat). After incubation with agents, cells were harvested and incubated for 30 min with dyes to detect ROS [10 μM dihydroethidium (DHE), 10 μM carboxy-H2-DCFDA, and 10 μM MitoSOX Red Molecular Probes, Carlsbad, CA]. Cells were then washed twice with 1% BSA/PBS and analyzed for fluorescence by flow cytometry. At least 25,000 events were collected on a FACScalibur flow cytometer (Beckton Dickinson, Rockville, MD) and analyzed using Cellquest (Beckton Dickinson). High fluorescence was calculated by setting a gate on the control cells where the peak reached a minimum, and all experimental samples were compared to this control gate.
Viability studies
Viability was assessed using the trypan blue exclusion assay as previously described [11]. Briefly, cells were treated with 0–20 μM 2-methoxyestradiol (2-ME; Sigma, Saint Louis, MO) or 0–100 μM sodium diethyldithiocarbamate trihydrate (DETC; Sigma) for 24 h alone and in combination with paraquat, and viability was assessed using trypan blue exclusion. In some studies, viability and cell injury was assessed using the mitochondrial membrane potential marker tetramethylrhodamine, ethyl ester, perchlorate (TMRE, Molecular Probes). Cells were treated with the indicated concentrations of GW583340, paraquat, and SOD mimic for 24 h. Then cells were harvested and incubated for 30 min with 500 nM TMRE. Cells were washed twice with 1% BSA/PBS and analyzed for fluorescence by flow cytometry.
Apoptosis assay
Cells were seeded in 96-well plates (Corning Incorporated), and after 20 h incubation with drugs, nucleosome enrichment was determined by the Cell Death Detection ELISAPLUS (Roche Applied Science; Mannheim, Germany) as per the manufacturer’s instructions. Nucleosome enrichment was calculated by: (mU sample − Blank)/(mU untreated − Blank) × 100.
Western immunoblot
Western immunoblot analysis was carried out as described previously [11]. Primary antibodies were: AMPK, p-AMPK (Cell Signaling, Danvers, MA), SOD1, SOD2 (BD Bioscience, San Jose, CA), and actin (Santa Cruz, Santa Cruz, CA). Stripping of membranes for detection of total protein was done as described previously [23]. Densitometric analysis was performed using the NIH ImageJ software (http://rsb.info.nih.gov/ij/).
Glutathione assay
Reduced glutathione levels were assessed using the GSHGloTM Glutathione Assay (Promega, Madison, WI) as per the manufacturer’s instructions.
Statistical analysis
The statistical analyses were performed using Graphpad InStat Student’s two-tailed t-test and Anova (Turkey–Kramer multiple comparison test). Differences were considered significant at P < 0.05.
Results
Treatment with an ErbB1/2 kinase inhibitor induces ROS production in IBC cells
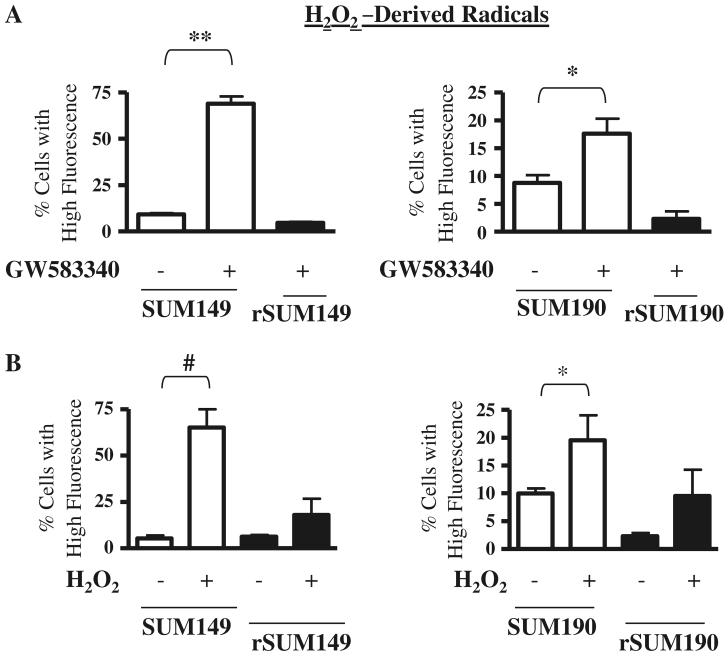
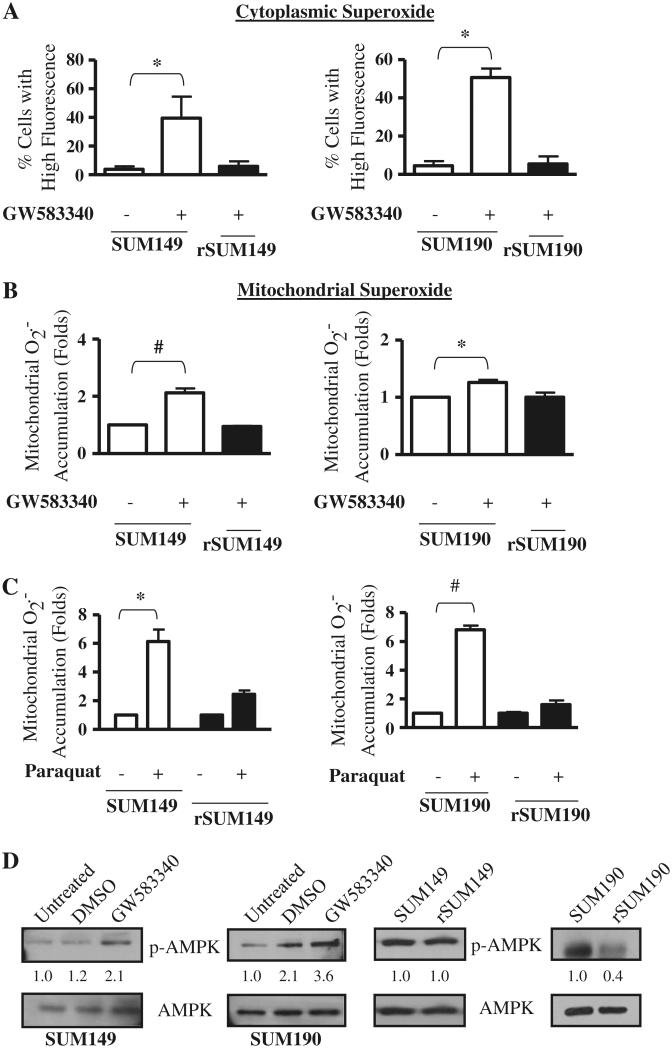
We hypothesized that the cell death effect of ErbB1/2 kinase inhibition corresponds with ROS accumulation. In order to evaluate this, the ErbB1/2 dual kinase small molecule inhibitor GW583340 (research grade lapatinib) was tested for oxidative stress response in two sets of isogenic IBC models of GW583340-sensitivity and acquired resistance. These are: ErbB1 activated, triple negative, basal-like cell pairs (SUM149, rSUM149); and ErbB2 overexpressing, ER–ve, PR–ve cell pairs (SUM190, rSUM190). The GW583340-resistant clonal population of rSUM149 and rSUM190 were generated in the laboratory as previously reported [11] and maintained in 7.5 and 2.5 μM GW583340, respectively. The cells were treated with GW583340 for 24 h at the indicated concentrations, which induce apoptosis in the sensitive cells [11]. Results demonstrate that GW583340 treatment caused a significant increase in ROS accumulation in both SUM149 and SUM190 cells: Fig. 1a: H2O2-derived radicals; Fig. 2a: cytoplasmic superoxide anions (O2−); and Fig. 2b: mitochondrial O2−, which were measured by flow cytometry using carboxy-H2DCFDA, dihydroethidium (DHE), and MitoSOX Red, respectively. The levels of ROS accumulation were similar to treatment with classical ROS inducing agents—H2O2 (Fig. 1b) [24] and paraquat (Fig. 2c), a well known O2−-generating agent [25].
Fig. 1.
Effect of GW583340 and H2O2 on H2O2-derived radical accumulation in IBC cells a SUM149 (left panel) and SUM190 (right panel) cells were treated with 7.5 and 2.5 μM GW583340, respectively, for 24 h, and H2O2-derived radical accumulation was compared to rSUM149 and rSUM190 cells growing in GW583340. b SUM149 and rSUM149 cells were treated with 500 μM H2O2 (left panel) and SUM190 and rSUM190 cells were treated with 100 μM H2O2 (right panel), and H2O2-derived radical accumulation was assessed. Bars represent mean ± SEM of the percentage of cells with high carboxy-H2DCFDA fluorescence (n = 3; *P < 0.05, **P < 0.01, #P < 0.005)
Fig. 2.
Effect of GW583340 and paraquat on superoxide accumulation in IBC cells a SUM149 (left panel) and SUM190 (right panel) cells were treated with 7.5 and 2.5 μM GW583340, respectively, for 24 h, and cytoplasmic superoxide accumulation was compared to rSUM149 and rSUM190 cells growing in GW583340. Bars represent mean ± SEM of the percentage of cells with high DHE fluorescence (n = 3; *P < 0.05). b SUM149 (left panel) and SUM190 (right panel) cells were treated with 7.5 and 2.5 μM GW583340, respectively, for 24 h, and mitochondrial superoxide accumulation was compared to rSUM149 and rSUM190 cells growing in GW583340. Bars represent mean ± SEM MitoSOX Red fluorescence normalized to DMSO (n = 3; *P < 0.05, #P < 0.005). c SUM149 and rSUM149 cells were treated with 5 mM paraquat (left panel) and SUM190 and rSUM190 cells were treated with 0.5 mM paraquat (right panel), and superoxide accumulation was assessed. Bars represent mean ± SEM MitoSOX Red fluorescence normalized to DMSO (n = 3; *P < 0.05, #P < 0.005). d p-AMPK expression in SUM149 and SUM190 cells treated for 1 h with 7.5 and 2.5 μM GW583340, respectively (left panel). p-AMPK expression was assessed in rSUM149 and rSUM190 cells growing in GW583340 (right panel) and compared to their parental counterparts. Blots were stripped and reprobed for total AMPK. Numbers represent densitometric analysis of p-AMPK normalized to total AMPK levels
In contrast, the isogenic GW583340-acquired resistance models (rSUM149, rSUM190) of each of these parental IBC lines [11] showed only a slight increase or basal levels of ROS accumulation in the presence of similar GW583340 concentrations, indicating that GW583340 sensitivity correlates with ROS generation. Additionally, treatment of an ErbB2 low breast cancer cell line (SUM44), which is not sensitive to the death-inducing effects of GW583340, did not increase ROS in this cell line (data not shown), demonstrating the specificity of this phenomenon.
Further, GW583340 treatment in the parental cells caused p-AMPK activation (Fig. 2d), a marker of oxidative stress [20], similar to a previous report in lapatinib-sensitive AU565 breast cancer cells [8, 16]. In contrast, there was no increase in p-AMPK expression in the rSUM149 and rSUM190 cells that are maintained in GW583340, indicating the partial loss of a stress response to GW583340 in the IBC cells with acquired resistance. Preliminary experiments in rSUM149 cells wherein the drug GW583340 was removed for a period of 2 months and the cells were re-exposed to 7.5 μM GW583340 (the concentration in which rSUM149 cells are routinely maintained in culture), revealed an increase in ROS generation and p-AMPK comparable to parental SUM149 cells (data not shown), suggesting a resistance reversal mechanism that needs to be studied further. Taken together, these data demonstrate that GW583340 increases oxidative stress by increasing accumulation of ROS in sensitive IBC cells.
ErbB1/2 kinase inhibitor-mediated cell death regulated by ROS
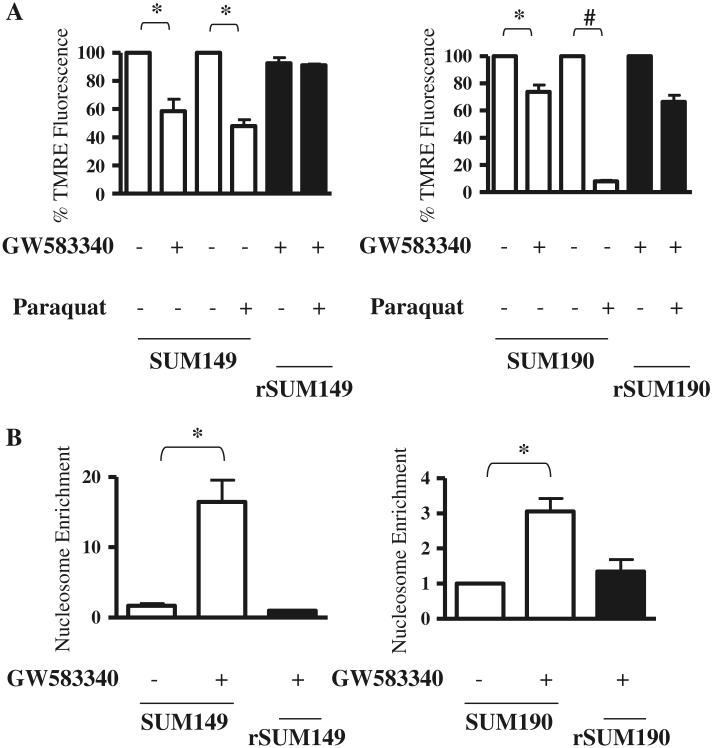
Since GW583340 treatment causes apoptosis in the parental SUM149 and SUM190 cells [11] and data in Figs. 1 and 2 show decreased ROS levels in the resistant counterparts, cell death/apoptosis was assessed in these cells by measuring mitochondrial membrane potential using TMRE, a dye that is sequestered by active mitochondria, and cytoplasmic nucleosomes. ROS generation in the cytoplasm and mitochondria of sensitive IBC cells treated with GW583340 or paraquat correlated with a significant loss of mitochondrial membrane potential (Fig. 3a). A corresponding increase in cytosolic nucleosome enrichment was observed in the sensitive cells with high ROS levels (Fig. 3b), which was similar to cytosolic nucleosome enrichment mediated by the positive control staurosporine (shown previously in [11]). In contrast, the clonal isogenic population of GW583340-resistant cells (rSUM149, rSUM190), which showed only a minimal increase in the levels of ROS, displayed mostly intact mitochondrial integrity, and basal or near basal levels of cytosolic nucleosomes.
Fig. 3.
Effect of GW583340 and paraquat on mitochondrial membrane potential and apoptosis of IBC cells. Parental cells treated with GW583340 (SUM149: 7.5 μM GW583340; SUM190: 2.5 μM GW583340) were compared to rSUM149 and rSUM190 cells growing in the same concentration of GW583340. Mitochondrial membrane potential (a) and apoptosis (b) were assessed by TMRE staining and nucleosome enrichment, respectively (n = 2, *P < 0.05, #P < 0.005)
Antioxidant expression is higher in IBC cells resistant to the ErbB1/2 kinase inhibitor
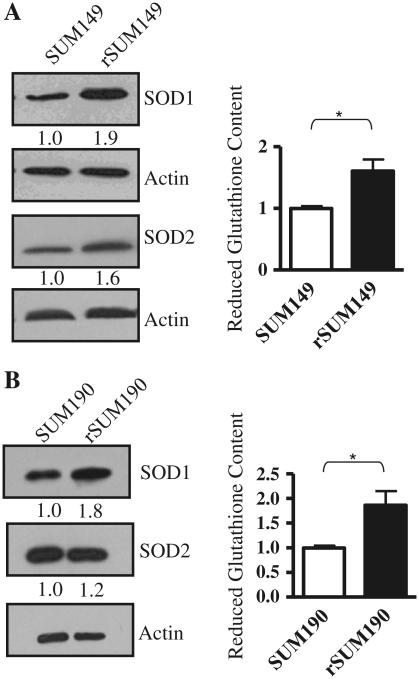
Data in Figs. 1 and 2 indicate that cytoplasmic and mitochondrial ROS are not induced by GW583340 treatment in the acquired resistant IBC cells (rSUM149 and rSUM190). We thereby characterized the levels of key cellular antioxidants (SOD1/Cu, ZnSOD, SOD2/MnSOD, and reduced glutathione) in the rSUM149 and rSUM190 cells. SODs catalyze dismutation of superoxide into hydrogen peroxide and oxygen; SOD1 is located in the cytoplasm, nucleus and mitochondrial intermembrane space, whereas SOD2 is located in the mitochondrial matrix [26]. Additionally, reduced glutathione is the major cellular antioxidant that is critical for cellular detoxification of H2O2 [27]. Data in Fig. 4a demonstrate that compared to parental SUM149 cells, rSUM149 cells maintained in GW583340 have increased basal levels of SOD1 and SOD2 protein expression as measured by immunoblot analysis. Further, reduced glutathione content, as measured by the GSH-GloTM detection kit, was significantly (P < 0.05) increased in rSUM149 cells compared to parental SUM149 cells. Similarly, rSUM190 cells displayed increased SOD1, SOD2, and reduced glutathione expression compared to SUM190 cells (Fig. 4b). These data show that cells with acquired GW583340 resistance have increased antioxidant expression.
Fig. 4.
Antioxidant expression in parental and GW583340-resistant IBC cells a Left panel immunoblot analysis of SOD1 and SOD2 in rSUM149 vs. SUM149 cells. Actin was used as a loading control. Numbers represent densitometric analysis of SOD1 or SOD2 normalized to actin. Right panel reduced glutathione content in SUM149 and rSUM149 cells. Bars represent mean ± SEM luciferase RLU normalized to SUM149 (n = 2; *P < 0.05). b Left panel immunoblot analysis of SOD1 and SOD2 in rSUM190 vs. SUM190 cells. Actin was used as a loading control. Numbers represent densitometric analysis of SOD1 or SOD2 normalized to actin. Right panel reduced glutathione content in SUM190 and rSUM190 cells. Bars represent mean ± SEM luciferase RLU normalized to SUM190 (n = 2; *P < 0.05)
Redox modulators reverse resistance to the ErbB1/2 kinase inhibitor
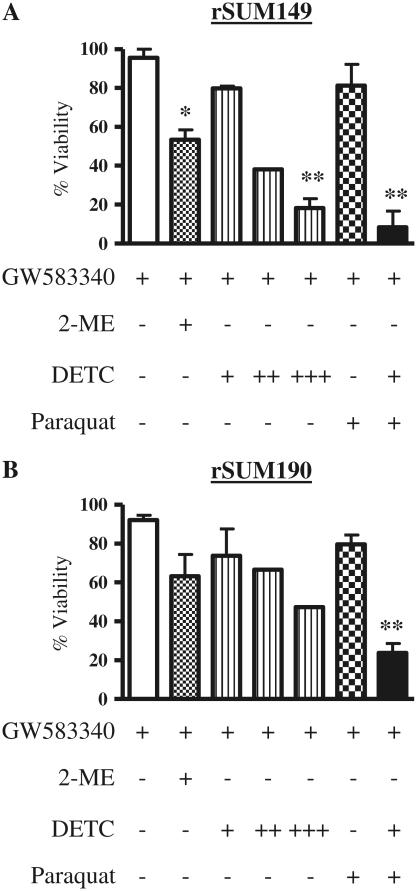
GW583340-resistant cells have high expression of antioxidants (Fig. 4), corresponding with no significant increase in ROS accumulation after treatment with ROS-generating agents, H2O2, and paraquat (Figs. 1b, 2c). Therefore, we evaluated the effect of modulation of the redox pathway on the viability and apoptosis of rSUM149 and rSUM190 cells. For this, two different agents were used: 2-methoxyestradiol (2-ME) and sodium diethyldithiocarbamate trihydrate (DETC). 2-ME has been shown to increase superoxide accumulation [28] and is in clinical trials for a variety of cancers [29], and DETC is a copper chelator with a similar mechanism of action as tetrathiomolybdate, an agent currently in clinical trials for breast cancer [29]. Data in Fig. 5a and b show the effect of 2-ME and DETC on cell viability of rSUM149 and rSUM190 cells, respectively, as measured by trypan blue exclusion. The rSUM149 and rSUM190 cells are maintained in GW583340 with no inhibitory effect on viability; however, addition of 2-ME (1 μM) and 100 μM DETC (10–100 μM dose range tested) showed a decrease in viability, which was significant in the rSUM149 cells. In addition, when paraquat was added along with a suboptimal concentration of DETC (10 μM), a significant decrease in viability was observed (black bar) compared to paraquat alone (checked bar) in the rSUM149 and rSUM190 cells maintained in GW583340. These data indicate that redox modulators can reverse the resistance of rSUM149 and rSUM190 cells to ROS-mediated apoptosis.
Fig. 5.
Effect of redox modulators on viability of rSUM149 and rSUM190 IBC cells. rSUM149 (a) and rSUM190 (b) cells growing in GW583340 (white bar) were treated with 1 μM 2-ME (dotted bar), 10–100 μM DETC (striped bars: +, 10 μM; ++, 50 μM; +++, 100 μM), paraquat (checked bar), and paraquat and 10 μM DETC in combination (black bars), and viability was assessed using trypan blue exclusion. Bars represent mean ± SEM% viable cells normalized to control cells (n = 2; *P < 0.05, **P < 0.01)
SOD mimic/antioxidant reverses ROS-mediated apoptosis
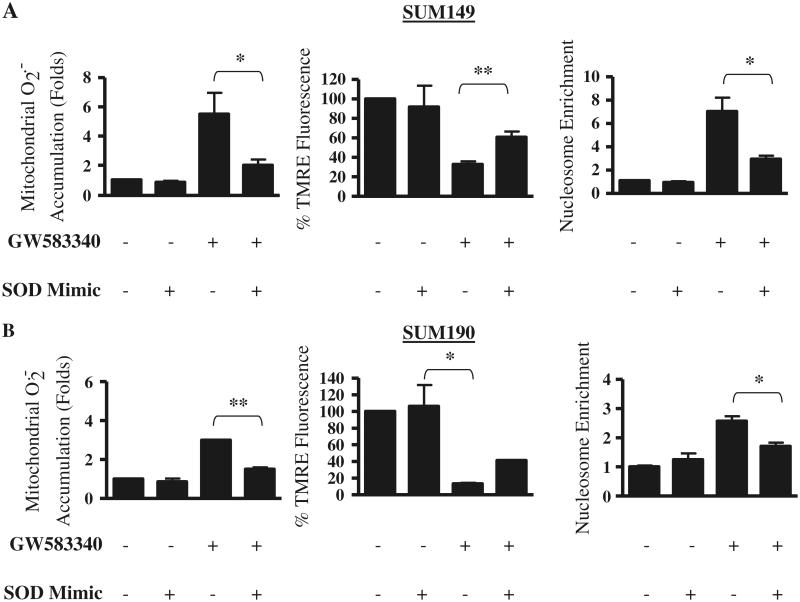
In order to directly correlate ROS generation with sensitivity to GW583340 in the parental IBC cells, we tested the effect of a SOD mimic (MnTnHex-2-PyP) on GW583340-mediated apoptosis. The SOD mimic (MnTnHex-2-PyP) has been previously shown to be a potent antioxidant both in vitro and in vivo [30-32] and can reverse the effects of the oxidizing agent paraquat in SUM149 cells (data not shown). Data in Fig. 6a (SUM149) and b (SUM190) show that in these two GW583340-sensitive parental cells, treatment for 24 h with 30 μM SOD mimic alone had no effect on basal ROS levels in the mitochondria, cell viability, or apoptosis compared to GW583340 alone (Fig. 6a, b, left panel) as observed by TMRE staining and apoptosis quantitated by nucleosome enrichment. In contrast, combination treatment of 20 μM GW583340 and 30 μM SOD mimic for 24 h significantly reversed the generation of ROS levels compared to GW583340 alone. Further, the combination (GW583340 + SOD mimic) displayed 50–70% viability compared to 15–30% viability in cell treated with GW583340 alone. Taken together, these data indicate the specificity of GW583340-mediated ROS-induced apoptosis in sensitive cells and demonstrate that this observation can be reversed using a SOD mimic.
Fig. 6.
Effect of GW583340 and SOD mimic alone and in combination on ROS production, mitochondrial membrane potential, and apoptosis in parental IBC cells. SUM149 (a) and SUM190 (b) cells were treated with 30 μM SOD mimic and 20 μM GW583340 alone or in combination for 24 h, and mitochondrial superoxide generation (left panels), mitochondrial membrane potential (middle panels), and apoptosis (right panels) were assessed using MitoSOX Red, TMRE, and cytoplasmic nucleosome enrichment, respectively. Bars represent mean ± SEM normalized to untreated or DMSO (n = 2; *P < 0.05, **P < 0.005)
Discussion
We report herein a novel mechanism of apoptosis induced by ErbB1/2-targeting via ROS production in two cellular models of IBC. Treatment of IBC cells that are sensitive to the apoptotic-inducing effects of an ErbB1/2 dual kinase small molecule inhibitor, GW583340, caused a marked increase in ROS, which included H2O2-derived radicals, cytosolic and mitochondrial superoxide. Further, this increase in ROS and apoptosis in the presence of GW583340 was reversible in the presence of a potent antioxidant/SOD mimic (MnTnHex-2-PyP). In contrast, isogenic cells with acquired resistance to GW583340 (rSUM149 and rSUM190) showed only minimal ROS accumulation, which corresponded with high antioxidant protein expression. Modulation of SOD activity using clinically relevant copper chelators/SOD inhibitors sensitized the resistant cells to ROS-induced cell death.
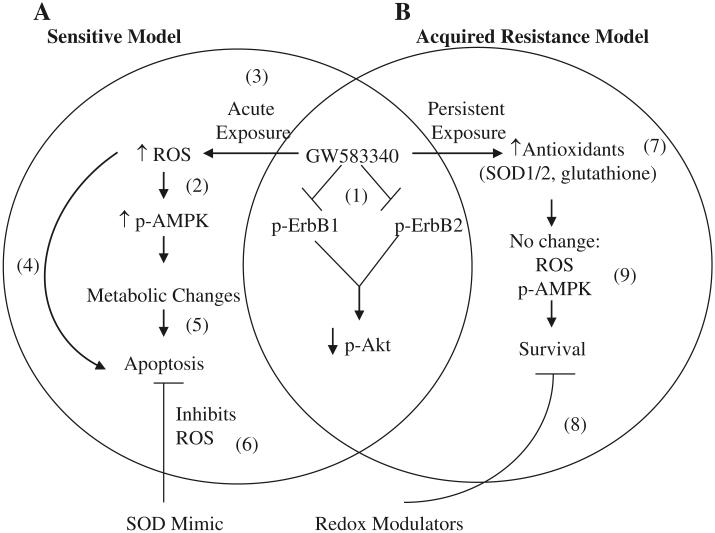
Lapatinib is a fairly selective dual ErbB1/ErbB2 tyrosine kinase inhibitor [33], which binds to the ATP-binding pocket on the receptors to disallow phosphorylation and downstream signaling (Fig. 7(1)) [4]. However, over the past several years, different labs have determined that lapatinib has additional mechanisms of action, including mediating a stress-response [8, 11, 16]. Spector et al. and Shell et al. identified that activation of AMPK is one of these stress response mechanisms [8, 16]. AMPK is activated due to changes in the AMP:ATP ratio, and oxidative stress is one of the stressors that causes a shift in this ratio (Fig. 7(2)) [15]. In the IBC cell lines studied here, we observed an increase in p-AMPK 1 h post-GW583340 treatment (Figs. 1, 2a). Interestingly, both SUM149 and SUM190 cells have mutated p53 [34], and therefore changes in AMPK may not change the metabolic status of these cells [35] like AU565 cells, which express wildtype p53 [36]. Additionally, there was no increase in p-AMPK expression in GW583340-resistant cells compared to their parental counterparts. Interestingly, p-AMPK was significantly downregulated in rSUM190 cells compared to the parental SUM190 cells. This may be due to a decrease in LKB1 (the kinase known to regulate AMPK activation), which is a known tumor suppressor whose loss can lead to highly invasive cancer [37]. Genotyping studies are currently ongoing in our lab to assess changes in gene expression of important metabolic parameters in the parental and isogenic GW583340-acquired resistant IBC cell models. Nevertheless, these data indicate that acute exposure (Fig. 7(3)) may be a mechanism of lapatinib-induced apoptosis in sensitive cellular models, whereby a significant increase in ROS leads to phosphorylation and activation of AMPK, both of which can lead to apoptosis (Fig. 7(4, 5)) [19, 38].
Fig. 7.
Schematic representation of mechanism of action of GW583340 in sensitive (a) and acquired resistance (b) IBC models. (1) GW583340 binds to and inhibits phosphorylation of ErbB1 and ErbB2 and subsequently decreases p-AKT expression in both sensitive and acquired resistance models [4, 11, 12]. However, in sensitive cells, GW583340 treatment causes an acute stress by increasing ROS (3), which in turn can increase AMPK activation (2) [20]. Both a substantial increase in ROS [42] and activation of AMPK [8, 16] and changes in metabolism [8] can independently lead to apoptosis of sensitive cells (4, 5). Treatment of sensitive cells in the presence of a SOD mimic can reverse this ROS-induced apoptosis (6). In contrast, cells with acquired GW583340 resistance have higher antioxidant expression (7) corresponding with no change in ROS accumulation or p-AMPK activation (9) in the presence of GW583340 and cell survival. Modulation of the redox pathway using 2-ME or DETC can sensitize these cells to ROS-generating agents(8)
To provide sufficient evidence that oxidative stress is indeed in question, an SOD mimic, Mn(III) meso-tetrakis(N-n-hexylpyridinium-2yl)porphyrin was employed [30, 39]. Relative to other SOD mimics reported thus far, this compound is identified as a potent compound that mimics the ability to catalyze O2− dismutation [30] and has enhanced efficacy both in in vitro and in vivo models due to its enhanced lipophilicity. It is efficient in suppressing oxidative stress in vivo at low concentrations (0.05 mg/kg) with single or multiple injections [39]. Its appropriate lipophilicity along with cationic charge allow its accumulation in mitochondria and the nucleus, where it acts presumably in a MnSOD fashion [40, 41]. The data herein demonstrate that GW583340 increases superoxide, and treatment of cells with GW583340 in combination with the SOD mimic can significantly reverse increase in mitochondrial superoxide, correlating with a decrease in cell death and apoptosis (Fig. 7(6)). The combination was not fully able to return ROS levels to basal levels, which may be due to either cytosolic superoxide or H2O2-derived radicals that are not decreased by treatment with the SOD mimic. Interestingly, treatment of cells with the combination of GW583340 and a different SOD mimic (MnTE-2-PyP5+) [30, 39, 41] that preferentially accumulates in the cytosol did not have as significant an effect on ROS levels or viability (data not shown). This suggests that the accumulation of superoxide in the mitochondria due to GW583340 treatment is the insult that pushes the cell over the threshold [42] to undergo apoptosis, which is similar to the study by Gordon et al. that showed that ErbB2 blockade in cardiomyocytes leads to cell death specifically via the mitochondria [21]. ROS in the mitochondria could leak into the cytoplasm, which would increase damage to the cell [43]. Moreover, because lapatinib has been shown to inhibit efflux transporters [6, 7], the ROS accumulation in the cell cannot be efficiently detoxified, which potentially increases cell death. Therefore, it is interesting to speculate that treatment of cells with GW583340 in combination with both mitochondrial and cytosolic SOD mimics would have an even greater affect on GW583340 efficacy.
This report also demonstrated that IBC cells with acquired resistance have increased antioxidant expression and are resistant to other ROS-generating agents, including H2O2 and paraquat. Although there is still debate in the field as to whether increased antioxidants are harmful or beneficial to tumor cells and therapeutic sensitivity [44], our data demonstrate that in terms of acquired resistance to GW583340, increased antioxidant expression allows for the continued survival and evasion of apoptosis of these cells (Fig. 7(7)). Increased SOD1/2 expression can detoxify cells from mitochondrial and cytoplasmic superoxide [26], and increased reduced glutathione is particularly important for detoxification of H2O2 [45]. When both are overexpressed, such as in the case of both GW583340-resistant IBC cell lines, this can lead to increased survival [46], which potentially explains the insensitivity of the GW583340-resistant cells to H2O2 and paraquat.
Importantly, previous studies have shown that increased antioxidant expression can lead to resistance to therapeutic agents whose mechanism of action is in part due to increase in ROS [19], including cisplatin, doxorubicin, arsenic trioxide, and others [47]. Therefore, we can speculate that our GW583340-resistant IBC cellular models are potentially cross-resistant to these ROS generators, although further studies are needed to validate this hypothesis. Because increase in antioxidant expression can decrease the efficacy of some therapeutic agents, a number of inhibitors of the antioxidant system are currently being tested in Phase II trials for a variety of solid tumors [29]. By modulating redox pathways in this study, we were able to decrease viability and increase apoptosis of cells with acquired resistance to GW583340. Both 2-ME, which can increase O2− levels [28], and DETC, a copper chelator similar to tetrathiomolybdate [48] that eliminates the activity of Cu,ZnSOD1 [49], were able to induce cell death in the GW583340-resistant rSUM149 and rSUM190 cells (Fig. 7(8)). Moreover, suboptimal concentrations of DETC were also able to sensitize the cells to paraquat, demonstrating that modulation of the redox pathway in GW583340 cells is a potential therapeutic strategy for sensitization of these cells to ROS-generating agents.
Taken together, the data in this study demonstrate a novel mechanism of lapatinib-analog-induced apoptosis and suggest a novel therapeutic strategy using modulation of the redox pathway for IBC patients with acquired resistance to ErbB1/2 kinase inhibitors.
Acknowledgments
The authors would like to thank Sharon Peplinski for her technical help with flow cytometry-based experiments and Dr. Neil Spector and Dr. Michael Morse for helpful discussions during the preparation of the manuscript. This work was supported by funding from American Cancer Society RSG-08-290-01-CCE (GRD), Department of Defense Predoctoral award, W81XWH-08-1-0363 (KMA), Viral Oncology Training Grant, 5T32-CA009111-32 (JLA) and SPORE in breast cancer grant (5P50-CA068438) at Duke Comprehensive Cancer Center.
Abbreviations
- AMPK
AMP activated protein kinase
- DHE
Dihydroethidium
- ER
Estrogen receptor
- IBC
Inflammatory breast cancer
- IRES
Internal ribosomal entry site
- NFκB
Nuclear factor kappa B
- PR
Progesterone receptor
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- XIAP
X-linked inhibitor of apoptosis protein
Footnotes
Present Address: K. M. Aird Fox Chase Cancer Center, Philadelphia, PA 19111, USA
References
- 1.Anderson WF, Schairer C, Chen BE, Hance KW, Levine PH. Epidemiology of inflammatory breast cancer (IBC) Breast Dis. 2005;22:9–23. doi: 10.3233/bd-2006-22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodward WA, Cristofanilli M. Inflammatory breast cancer. Semin Radiat Oncol. 2009;19:256–265. doi: 10.1016/j.semradonc.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Van den Eynden GG, Van der Auwera I, Van Laere S, Colpaert CG, van Dam P, Merajver S, Kleer CG, Harris AL, Van Marck EA, Dirix LY, et al. Validation of a tissue microarray to study differential protein expression in inflammatory and non-inflammatory breast cancer. Breast Cancer Res Treat. 2004;85:13–22. doi: 10.1023/B:BREA.0000021028.33926.a8. [DOI] [PubMed] [Google Scholar]
- 4.Xia W, Mullin RJ, Keith BR, Liu LH, Ma H, Rusnak DW, Owens G, Alligood KJ, Spector NL. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 5.Xia W, Bisi J, Strum J, Liu L, Carrick K, Graham KM, Treece AL, Hardwicke MA, Dush M, Liao Q, et al. Regulation of survivin by ErbB2 signaling: therapeutic implications for ErbB2-overexpressing breast cancers. Cancer Res. 2006;66:1640–1647. doi: 10.1158/0008-5472.CAN-05-2000. [DOI] [PubMed] [Google Scholar]
- 6.Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR, Liu DG, Ashby CR, Jr, Huang Y, Robey RW, Liang YJ, et al. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008;68:7905–7914. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polli JW, Humphreys JE, Harmon KA, Castellino S, O’Mara MJ, Olson KL, John-Williams LS, Koch KM, Serabjit-Singh CJ. The role of efflux and uptake transporters in [N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl] amino}methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos. 2008;36:695–701. doi: 10.1124/dmd.107.018374. [DOI] [PubMed] [Google Scholar]
- 8.Spector NL, Yarden Y, Smith B, Lyass L, Trusk P, Pry K, Hill JE, Xia W, Seger R, Bacus SS. Activation of AMP-activated protein kinase by human EGF receptor 2/EGF receptor tyrosine kinase inhibitor protects cardiac cells. Proc Natl Acad Sci USA. 2007;104:10607–10612. doi: 10.1073/pnas.0701286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston S, Trudeau M, Kaufman B, Boussen H, Blackwell K, LoRusso P, Lombardi DP, Ben Ahmed S, Citrin DL, DeSilvio ML, et al. Phase II study of predictive biomarker profiles for response targeting human epidermal growth factor receptor 2 (HER-2) in advanced inflammatory breast cancer with lapatinib monotherapy. J Clin Oncol. 2008;26:1066–1072. doi: 10.1200/JCO.2007.13.9949. [DOI] [PubMed] [Google Scholar]
- 10.Chen FL, Xia W, Spector NL. Acquired resistance to small molecule ErbB2 tyrosine kinase inhibitors. Clin Cancer Res. 2008;14:6730–6734. doi: 10.1158/1078-0432.CCR-08-0581. [DOI] [PubMed] [Google Scholar]
- 11.Aird KM, Ghanayem RB, Peplinski S, Lyerly HK, Devi GR. X-linked inhibitor of apoptosis protein inhibits apoptosis in inflammatory breast cancer cells with acquired resistance to an ErbB1/2 tyrosine kinase inhibitor. Mol Cancer Ther. 2010;9:1432–1442. doi: 10.1158/1535-7163.MCT-10-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia W, Bacus S, Hegde P, Husain I, Strum J, Liu L, Paulazzo G, Lyass L, Trusk P, Hill J, et al. A model of acquired auto-resistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci USA. 2006;103:7795–7800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin AP, Miller A, Emad L, Rahmani M, Walker T, Mitchell C, Hagan MP, Park MA, Yacoub A, Fisher PB, et al. Lapatinib resistance in HCT116 cells is mediated by elevated MCL-1 expression and decreased BAK activation and not by ERBB receptor kinase mutation. Mol Pharmacol. 2008;74:807–822. doi: 10.1124/mol.108.047365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia W, Bacus S, Husain I, Liu L, Zhao S, Liu Z, Moseley MA, 3rd, Thompson JW, Chen FL, Koch KM, et al. Resistance to ErbB2 tyrosine kinase inhibitors in breast cancer is mediated by calcium-dependent activation of RelA. Mol Cancer Ther. 2010;9:292–299. doi: 10.1158/1535-7163.MCT-09-1041. [DOI] [PubMed] [Google Scholar]
- 15.Hardie DG. The AMP-activated protein kinase pathway—new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 16.Shell SA, Lyass L, Trusk PB, Pry KJ, Wappel RL, Bacus SS. Activation of AMPK is necessary for killing cancer cells and sparing cardiac cells. Cell Cycle. 2008;7:1769–1775. doi: 10.4161/cc.7.12.6016. [DOI] [PubMed] [Google Scholar]
- 17.Holcik M, Lefebvre C, Yeh C, Chow T, Korneluk RG. A new internal-ribosome-entry-site motif potentiates XIAP-mediated cytoprotection. Nat Cell Biol. 1999;1:190–192. doi: 10.1038/11109. [DOI] [PubMed] [Google Scholar]
- 18.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 19.Agostinelli E, Seiler N. Non-irradiation-derived reactive oxygen species (ROS) and cancer: therapeutic implications. Amino Acids. 2006;31:341–355. doi: 10.1007/s00726-005-0271-8. [DOI] [PubMed] [Google Scholar]
- 20.Choi SL, Kim SJ, Lee KT, Kim J, Mu J, Birnbaum MJ, Soo Kim S, Ha J. The regulation of AMP-activated protein kinase by H(2)O(2) Biochem Biophys Res Commun. 2001;287:92–97. doi: 10.1006/bbrc.2001.5544. [DOI] [PubMed] [Google Scholar]
- 21.Gordon LI, Burke MA, Singh AT, Prachand S, Lieberman ED, Sun L, Naik TJ, Prasad SV, Ardehali H. Blockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial and reactive oxygen species-dependent pathways. J Biol Chem. 2009;284:2080–2087. doi: 10.1074/jbc.M804570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aird KM, Ding X, Baras A, Wei J, Morse MA, Clay T, Lyerly HK, Devi GR. Trastuzumab signaling in ErbB2-over-expressing inflammatory breast cancer correlates with X-linked inhibitor of apoptosis protein expression. Mol Cancer Ther. 2008;7:38–47. doi: 10.1158/1535-7163.MCT-07-0370. [DOI] [PubMed] [Google Scholar]
- 23.Amantana A, London CA, Iversen PL, Devi GR. X-linked inhibitor of apoptosis protein inhibition induces apoptosis and enhances chemotherapy sensitivity in human prostate cancer cells. Mol Cancer Ther. 2004;3:699–707. [PubMed] [Google Scholar]
- 24.Eruslanov E, Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol. 2010;594:57–72. doi: 10.1007/978-1-60761-411-1_4. [DOI] [PubMed] [Google Scholar]
- 25.Elorza A, Hyde B, Mikkola HK, Collins S, Shirihai OS. UCP2 modulates cell proliferation through the MAPK/ERK pathway during erythropoiesis and has no effect on heme bio-synthesis. J Biol Chem. 2008;283:30461–30470. doi: 10.1074/jbc.M805400200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miao L, Clair DK. Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med. 2009;47:344–356. doi: 10.1016/j.freeradbiomed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balendiran GK, Dabur R, Fraser D. The role of glutathione in cancer. Cell Biochem Funct. 2004;22:343–352. doi: 10.1002/cbf.1149. [DOI] [PubMed] [Google Scholar]
- 28.Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–395. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- 29.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 30.Batinic-Haberle I. Manganese porphyrins and related compounds as mimics of superoxide dismutase. Methods Enzymol. 2002;349:223–233. doi: 10.1016/s0076-6879(02)49337-8. [DOI] [PubMed] [Google Scholar]
- 31.Fernandes AS, Gaspar J, Cabral MF, Rueff J, Castro M, Batinic-Haberle I, Costa J, Oliveira NG. Protective role of ortho-substituted Mn(III) N-alkylpyridylporphyrins against the oxidative injury induced by tert-butylhydroperoxide. Free Radic Res. 2010;44:430–440. doi: 10.3109/10715760903555844. [DOI] [PubMed] [Google Scholar]
- 32.Saba H, Batinic-Haberle I, Munusamy S, Mitchell T, Lichti C, Megyesi J, MacMillan-Crow LA. Manganese porphyrin reduces renal injury and mitochondrial damage during ischemia/reperfusion. Free Radic Biol Med. 2007;42:1571–1578. doi: 10.1016/j.freeradbiomed.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 34.Chinnaiyan AM, Prasad U, Shankar S, Hamstra DA, Shanaiah M, Chenevert TL, Ross BD, Rehemtulla A. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci USA. 2000;97:1754–1759. doi: 10.1073/pnas.030545097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Contreras CM, Gurumurthy S, Haynie JM, Shirley LJ, Akbay EA, Wingo SN, Schorge JO, Broaddus RR, Wong KK, Bardeesy N, et al. Loss of Lkb1 provokes highly invasive endometrial adenocarcinomas. Cancer Res. 2008;68:759–766. doi: 10.1158/0008-5472.CAN-07-5014. [DOI] [PubMed] [Google Scholar]
- 38.Hardie DG. AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol. 2007;47:185–210. doi: 10.1146/annurev.pharmtox.47.120505.105304. [DOI] [PubMed] [Google Scholar]
- 39.Batinic-Haberle I, Reboucas JS, Spasojevic I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal. 2010;13:877–918. doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Y, Chaiswing L, Oberley TD, Batinic-Haberle I, Clair W, Epstein CJ, Clair D. A mechanism-based antioxidant approach for the reduction of skin carcinogenesis. Cancer Res. 2005;65:1401–1405. doi: 10.1158/0008-5472.CAN-04-3334. [DOI] [PubMed] [Google Scholar]
- 41.Batinic-Haberle I, Spasojevic I, Tse HM, Tovmasyan A, Rajic Z, Clair DK, Vujaskovic Z, Dewhirst MW, Piganelli JD. Design of Mn porphyrins for treating oxidative stress injuries and their redox-based regulation of cellular transcriptional activities. Amino Acids. 2010 doi: 10.1007/s00726-010-0603-6. doi:10.1007/s00726-010-0603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong Q, Beel JA, Lillehei KO. A threshold concept for cancer therapy. Med Hypotheses. 2000;55:29–35. doi: 10.1054/mehy.1999.0982. [DOI] [PubMed] [Google Scholar]
- 43.Roninson IB, Brown JM, Bredesen DE. Beyond apoptosis: cellular outcomes of cancer therapy. Informa Healthcare; New York: 2008. [Google Scholar]
- 44.Ladas EJ, Jacobson JS, Kennedy DD, Teel K, Fleischauer A, Kelly KM. Antioxidants and cancer therapy: a systematic review. J Clin Oncol. 2004;22:517–528. doi: 10.1200/JCO.2004.03.086. [DOI] [PubMed] [Google Scholar]
- 45.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Clarendon Press, Oxford University Press; Oxford, New York: 1985. [Google Scholar]
- 46.Li S, Yan T, Yang JQ, Oberley TD, Oberley LW. The role of cellular glutathione peroxidase redox regulation in the suppression of tumor cell growth by manganese superoxide dismutase. Cancer Res. 2000;60:3927–3939. [PubMed] [Google Scholar]
- 47.Landriscina M, Maddalena F, Laudiero G, Esposito F. Adaptation to oxidative stress, chemoresistance, and cell survival. Antioxid Redox Signal. 2009;11:2701–2716. doi: 10.1089/ars.2009.2692. [DOI] [PubMed] [Google Scholar]
- 48.Khan G, Merajver S. Copper chelation in cancer therapy using tetrathiomolybdate: an evolving paradigm. Expert Opin Investig Drugs. 2009;18:541–548. doi: 10.1517/13543780902845622. [DOI] [PubMed] [Google Scholar]
- 49.Heikkila RE, Cabbat FS, Cohen G. In vivo inhibition of superoxide dismutase in mice by diethyldithiocarbamate. J Biol Chem. 1976;251:2182–2185. [PubMed] [Google Scholar]