Abstract
Rationale
Atrial fibrillation (AF) is the most common cardiac arrhythmia, however the mechanism(s) causing AF remain poorly understood and therapy is suboptimal. The ryanodine receptor (RyR2) is the major calcium (Ca2+) release channel on the sarcoplasmic reticulum (SR) required for excitation-contraction coupling in cardiac muscle.
Objective
In the present study we sought to determine whether intracellular diastolic SR Ca2+ leak via RyR2 plays a role in triggering AF and whether inhibiting this leak can prevent AF.
Methods and Results
We generated three knock-in mice with mutations introduced into RyR2 that result in leaky channels and cause exercise induced polymorphic ventricular tachycardia in humans [catecholaminergic polymorphic ventricular tachycardia (CPVT)]. We examined AF susceptibility in these three CPVT mouse models harboring RyR2 mutations to explore the role of diastolic SR Ca2+ leak in AF. AF was stimulated with an intra-esophageal burst pacing protocol in the three CPVT mouse models (RyR2-R2474S+/−, 70%; RyR2-N2386I+/−, 60%; RyR2-L433P+/−, 35.71%), but not in wild type (WT) mice (P<0.05). Consistent with these in vivo results, there was a significant diastolic SR Ca2+ leak in atrial myocytes isolated from the CPVT mouse models. Calstabin2 (FKBP12.6) is an RyR2 subunit that stabilizes the closed state of RyR2 and prevents a Ca2+ leak through the channel. Atrial RyR2 from RyR2-R2474S+/− mice were oxidized and the RyR2 macromolecular complex was depleted of calstabin2. The Rycal drug S107 stabilizes the closed state of RyR2 by inhibiting the oxidation/phosphorylation induced dissociation of calstabin2 from the channel. S107 reduced the diastolic SR Ca2+ leak in atrial myocytes and decreased burst pacing-induced AF in vivo. S107 did not reduce the increased prevalence of burst pacing-induced AF in calstabin2-deficient mice, confirming that calstabin2 is required for the mechanism of action of the drug.
Conclusions
The present study demonstrates that RyR2-mediated diastolic SR Ca2+ leak in atrial myocytes is associated with AF in CPVT mice. Moreover, the Rycal S107 inhibited diastolic SR Ca2+ leak through RyR2 and pacing-induced AF associated with CPVT mutations.
Keywords: CPVT, atrial fibrillation, SR Ca2+ leak, Ca2+ sparks, ryanodine receptor
INTRODUCTION
Atrial fibrillation (AF) is the most common arrhythmia and is especially prevalent in the elderly 1. AF accounts for more than one-third of all arrhythmia-related hospitalizations 2. Although AF itself is not typically lethal, complications related to AF including thromboembolism, hemodynamic compromise and arrhythmogenesis make it one of the leading causes of cardiovascular morbidity and mortality. Current clinical management of AF is focused on rate control and chronic anticoagulation with concomitant potential bleeding risk and impaired cardiac function, especially in patients with congestive heart failure (CHF).
The mechanism of AF is not well understood despite more than 100 years of study. Most hypotheses regarding the induction of AF are based on observational studies in patients with chronic AF or studies of chronic AF animal models. Based on these studies, structural remodeling including atrial enlargement and fibrosis are proposed to play important roles in both triggering and maintaining AF 3. However, it has proven difficult to distinguish whether these factors are the cause or the consequence of AF. Structural changes may directly or indirectly induce atrial electrical abnormalities leading to atrial ectopic events and AF. More recently, the role of Ca2+ in AF has been explored as a possible contributing factor to the well-known reentry mechanism and in ‘triggered activity’ models 4.
Our laboratory has reported that SR Ca2+ leak via PKA hyperphosphroylated and/or oxidized RyR2 channels contributes to heart failure (HF) progression 5 and triggers ventricular arrhythmias 6–8. Furthermore, we showed that CPVT-linked RyR2 mutations cause a diastolic SR Ca2+ leak, delayed after depolarizations (DADs) and lethal ventricular arrhythmias in mice 6. Recent reports demonstrate that patients with CPVT mutations have AF 9–13. Mouse models harboring CPVT mutations recapitulate the ventricular human phenotype manifesting exercise-induced polymorphic VT and sudden death and studies with knock-in mice have helped to establish the role of diastolic SR Ca2+ leak through mutant RyR2 in ventricular arrhythmias 6, 14–16. The normal cardiac structure and function of CPVT mouse models makes them ideal tools to study the role of diastolic SR Ca2+ leak via RyR2 in triggering AF in the absence of structural cardiac defects.
In the present study, AF was inducible by burst atrial pacing in mice harboring CPVT mutations in vivo but not in WT littermates. Moreover, we examined the difference in diastolic SR Ca2+ leak between atrial and ventricular myocytes isolated from both WT and CPVT mice. Consistent with in vivo study results, there was an increase in diastolic SR Ca2+ leak in atrial myocytes isolated from RyR2-R2474S+/−, RyR2-R2386I+/−, and RyR2-L433P+/− mice compared to atrial myocytes from WT mice. Increased diastolic SR Ca2+ leak, associated with depletion of calstabin2 (FKBP12.6) from the RyR2 channel complex, was observed in atrial myocytes from RyR2-R2474S+/− mice, but not in WT mice. The small molecule Rycal S107, which stabilizes RyR2-calstabin2 interactions, significantly decreased the diastolic SR Ca2+ leak in RyR2-R2474S+/− mice at the cellular level and prevented burst pacing-induced AF in vivo. These data suggest a role for diastolic SR Ca2+ leak in initiating AF. Furthermore, inhibiting diastolic SR Ca2+ leak with a Rycal could be a potential therapeutic approach for preventing AF.
METHODS
Detailed methods are provided in the Online Supplement including generation of RyR2- knock-in mice, murine atrial myocytes isolation, intracellular Ca2+ measurements, measurement of total SR Ca2+ leak, intra-esophageal burst pacing, intra-cardiac burst pacing ECG recording, immunoprecipitation and immunoblot analyses.
S107 and Metoprolol treatment
S107 and metoprolol were diluted in drinking water at concentrations of 0.25 mg/ml and 0.1 mg/ml respectively. The drinking water was changed every week and the water consumption was recorded. There were no differences in water consumption between vehicle, S107 or metoprolol treated groups.
Statistical analysis
Data are reported as mean ± s.e.m unless otherwise indicated. In vivo AF stimulation studies were analyzed with chi-square. P < 0.05 was accepted as statistically significant. All experiments with animals were approved by Columbia University’s Institutional Animal Care and Use Committee.
RESULTS
Atrial fibrillation (AF) in CPVT mouse models
Diastolic SR Ca2+ leak via mutant RyR2 triggers lethal ventricular arrhythmias during stress in CPVT patients 6–8, 14. However, whether these mutant RyR2 in atria lead to atrial arrhythmias is not clear, although there are several clinical case reports demonstrating atrial premature complexes which may trigger atrial tachycardia, atrial flutter or AF in CPVT patients 9–13, 17. As we have shown previously, RyR2-R2474S+/− mice developed typical bidirectional ventricular tachycardia (VT) and polymorphic VT during stress, which mimics the human CPVT phenotype 6. To further study the molecular mechanism of CPVT, we generated two new mice harboring human CPVT mutations: RyR2-N2386I+/− and RyR2-L433P+/−. Using stress protocols we confirmed the phenotype of these two new CPVT mouse models (supplemental figure I).
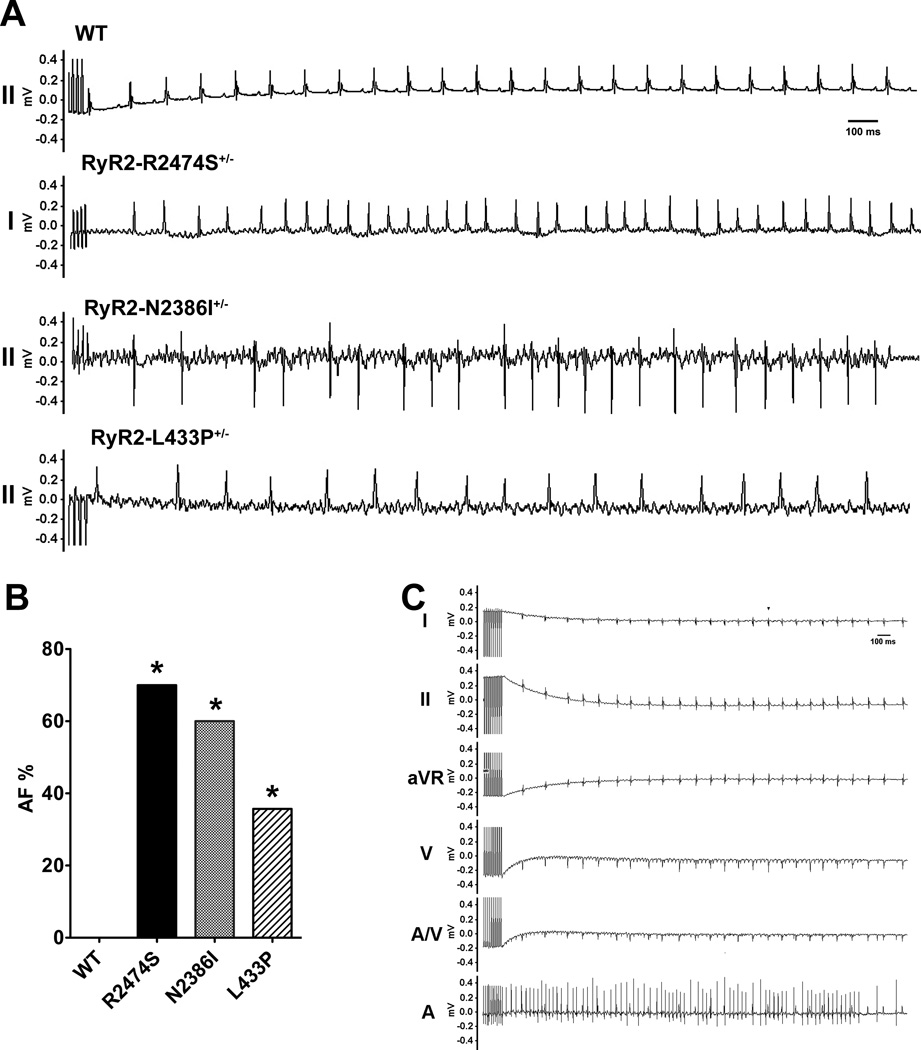
An intra-esophageal pacing method was developed to verify the role of diastolic SR Ca2+ leak via RyR2 in AF using three CPVT knock-in mouse models. In these experiments, the left atrium was paced via an intra-esophageal pacing catheter using a previously reported atrial burst pacing protocol 18. Compared to WT mice which exhibited no AF under these conditions, mice harboring CPVT mutations could be stimulated into AF by atrial burst pacing (RyR2-R2474S+/−; 7/10, RyR2-N2386I+/−; 9/15, RyR2-L433P+/−; 5/14 (Figures 1 A and B). The duration of AF and heart rate during atrial burst pacing-induced AF were similar among the groups (data not shown). To further verify the AF induced by intra-esophageal burst pacing, an octapolar catheter was introduced into the right ventricle via the jugular vein to record intra-cardiac ECGs during intra-esophageal pacing in three RyR2-N2386I+/− mice. AF events recognized on surface ECGs in these mice were confirmed by the presence of typical irregular atrial waves recorded from the intra-cardiac atrial leads (Figure 1C).
Figure 1. Intra-esophageal burst pacing induces AF in three murine CPVT models.
A) Representative surface ECG traces from WT, RyR2-R2474S+/−, RyR2-N2386I+/−, and RyR2-L433P+/− mice during intra-esophageal burst pacing. B) Prevalence of AF in WT (n=33), RyR2-R2474S+/− (n=10), RyR2-N2386I+/− (n=15), and RyR2-L433P+/− (n=14) mice during intra-esophageal burst pacing. *, P<0.05 vs. WT. C) Representative AF in surface and intra-cardiac ECG trace from a RyR2-N2386I+/− mouse after intra-esophageal burst pacing stimulation (I, II, and aVR, surface leads; V, ventricular leads; A/V, atrioventricular node leads; A, atrial leads).
Sarcoplasmic reticulum Ca2+ leak in atrial myocytes from CPVT mice
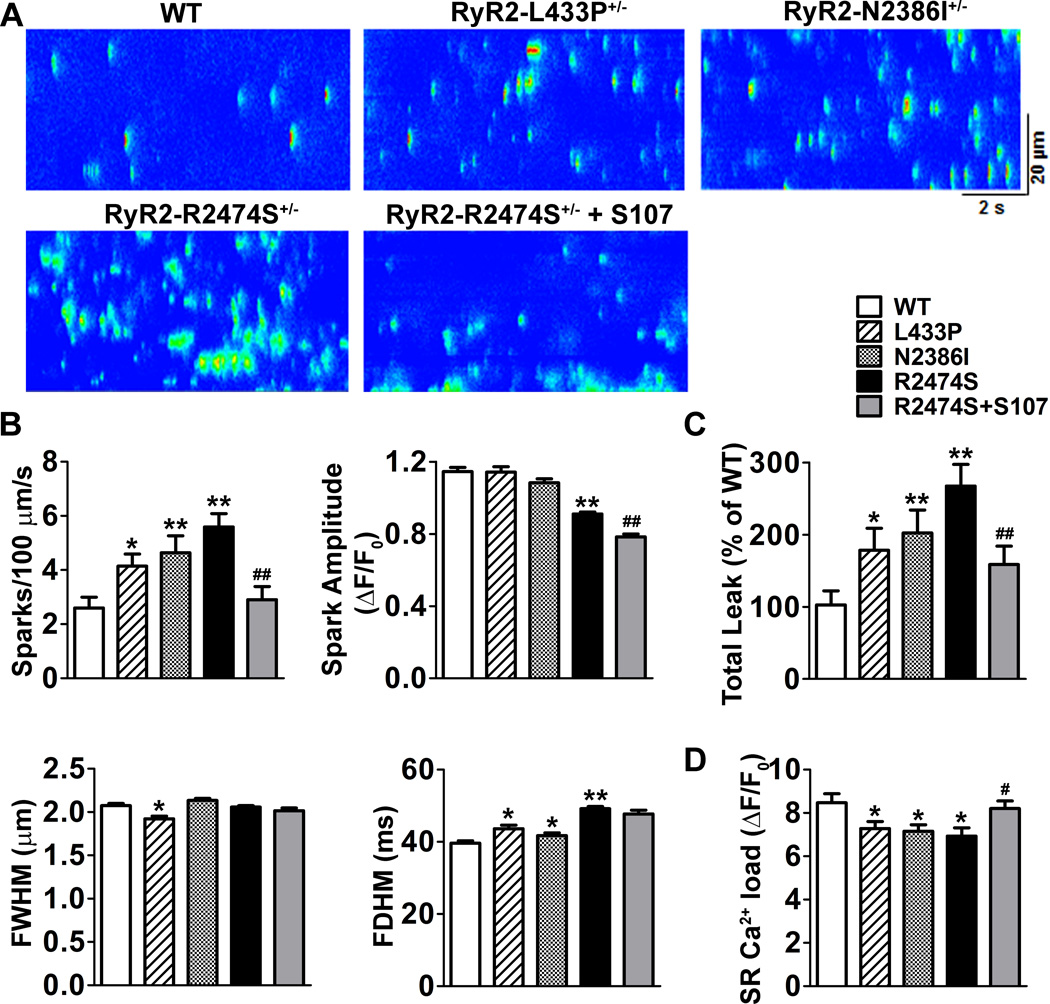
To further explore the mechanism of increased prevalence of atrial burst pacing-induced AF in CPVT mice, we characterized the SR Ca2+ release in atrial myocytes isolated from CPVT and WT mice. Ca2+ spark frequencies were significantly increased in atrial myocytes from RyR2-R2474S+/− (5.59±0.49 /100 µm/s), RyR2-N2386I+/− (4.63±0.63 /100 µm/s) and RyR2-L433P+/− (4.14±0.45 /100 µm/s) compared to WT myocytes (2.60±0.39 /100 µm/s, P<0.05, Figure 2B). As the morphologies of Ca2+ sparks showed profound variations between these groups (Figure 2A), we developed a new parameter (methods described in supplemental data) to quantitatively assess the total SR Ca2+ leak between different groups. The results of total SR Ca2+ leak were similar to but more profound than that measured by Ca2+ spark frequencies. In agreement with the results of diastolic SR Ca2+ leak, the SR Ca2+ content in atrial myocytes isolated from RyR2-R2474S+/−, RyR2-N2386I+/−, and RyR2-L433P+/− mice were decreased compared to WT atrial myocytes (ΔF/F0: 6.93±0.38, 7.16±0.30, and 7.28±0.32 vs 8.47±0.42 in WT, P<0.05, Figure 2C).
Figure 2. SR Ca2+ leak in atrial myocytes isolated from CVPT mice.
A) Representative raw line scan of WT, RyR2-L433P+/−, RyR2-N2386I+/−, RyR2-R2474S+/−, and S107 treated RyR2-R2474S+/− atrial myocytes. B) Ca2+ leak parameters for different groups, n=20~25 cells in each group. C), SR Ca2+ contents, n=7~14 cells in each group. * P<0.05 and ** P<0.01 compared to WT; # P<0.05 and ##, P<0.01 compared to RyR2-R2474S+/−.
S107 prevents SR Ca2+ leak in atrial myocytes from RyR2-R2474S+/− mice
Calstabin2 binding to the RyR2 channel complex stabilizes the closed state of the channel thereby preventing pathological diastolic SR Ca2+ leak 7, 8, 19. Calstabin2 knock-out mice exhibit a higher prevalence of AF induced by endocardial burst pacing and diastolic SR Ca2+ leak in isolated atrial myocytes 20. To test whether calstabin2 plays a role in diastolic SR Ca2+ leak in atrial myocytes of RyR2-R2474S+/− mice we treated isolated atrial myocytes with the Rycal S107, a 1,4-benzothiazepine, which is known to inhibit diastolic SR Ca2+ leak via RyR2 by inhibiting dissociation of calstabin2 from RyR2 21. Pre-incubation with S107 (10 µM) for 2 hrs significantly reduced the total diastolic SR Ca2+ leak in RyR2-R2474S+/− group by 46% (Figure 2B). Consistent with the reduction in diastolic SR Ca2+ leak, SR Ca2+ content was returned to normal by S107 (ΔF/F0: 8.47±0.42 in WT, 6.93±0.38 in RyR2-R2474S+/−, P<0.05, and 8.21±0.35 in S107 treated RyR2-R2474S+/−, P=NS, Figure 2C).
Decreased calstabin2 binding to RyR2 in atria but not ventricles isolated from RyR2-R2474S+/− mice
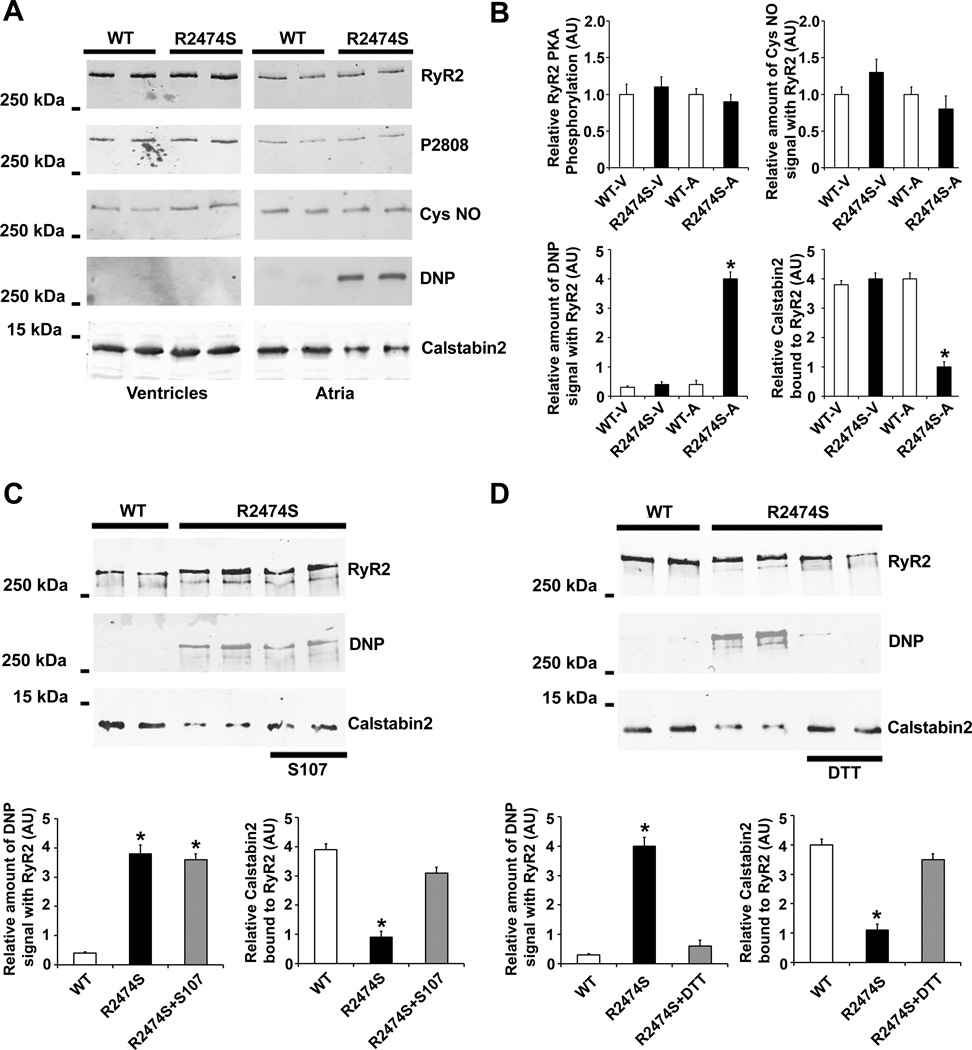
As we previously reported, PKA phosphorylation of RyR2 and castabin2 binding to RyR2 were unchanged in ventriclular cardiomyocytes from RyR2-R2474S+/− mice compared to WT littermates 6. Stress-induced PKA phosphorylation of the channel depletes calstabin2 from RyR2 causing diastolic SR Ca2+ leak and ventricular arrhythmias 6. In the present study atrial burst pacing did not cause PKA phosphorylation of RyR2 (Figure 3A and 3B); instead, it caused CaMKII phosphorylation of RyR2 as previously reported 22, 23 (supplemental figure XII). However, compared to WT mice, the calstabin2 level was significantly decreased in atrial RyR2 immunoprecipitated from RyR2-R2474S+/− mice. This was not the case in ventricular immunoprecipitates as we previously reported 6.
Figure 3. Atrial RyR2 channel complex remodeling in RyR2-R2474S+/− mice.
A) Representitive immunoprecipitation of RyR2 from ventricular and atrial tissues from WT and RyR2-R2474S+/− mice showing oxidation (DNP) and calstabin2 dissociation from RyR2 channel complex only in atria of RyR2-R2474S+/− mice. B) Pooled data from 4 separate immunoblots. V, ventricles; A, atria; *, P<0.05 vs. WT. C) Representitive immunoprecipitation of RyR2 from atrial tissues from WT and RyR2-R2474S+/− treated vehicle or S107. Bottom, pooled data from 3 separate immunoblots, *, P<0.05 vs. WT. D) Representitive immunoprecipitation of RyR2 from atrial tissues from WT, RyR2-R2474S+/− and RyR2-R2474S+/− following in vitro treatment with DTT. Bottom, pooled data from 3 separate immunoblots, *, P<0.05 vs. WT.
To further explore the cause of this difference between atrial and ventricular RyR2, we examined the PKA phosphorylation, oxidation and nitrosylation of RyR2 in atrial and ventricular tissues from WT and RyR2-R2474S+/− mice. We previous reported that RyR2 are oxidized and nitrosylated in cardiomyocytes from a knock-in mouse harboring RyR2 that mimic constitutively PKA hyperphosphorylated channels, RyR2-S2808D, as well as in human, rat and mouse HF 21. Interestingly, the RyR2 from atrial tissue in RyR2-R2474S+/− mice exhibited increased oxidation but no detectable PKA phosphorylation or nitrosylation. In contrast there was no oxidation, PKA phosphorylation, or nitrosylation of atrial RyR2 from WT mice (Figure 3A and 3B). The cause of atrial RyR2-R2474S+/− channel oxidation is unknown, but provides an explanation for the depletion of calstabin2 from the RyR2 channel complex in atrial tissue of RyR2-R2474S+/− mice.
In order to explore the mechanism underlying S107 treatment in prevention of both burst pacing-induced AF and diastolic R Ca2+ leak in RyR2-R2474S+/− mice, we examined castabin2 binding and oxidation of atrial RyR2 in atrial samples from WT and RyR2-R2474S+/− mice. S107 inhibited depletion of calstabin2 from the RyR2 channel complex without reducing RyR2 oxidation (Figure 3C).
Since oxidation of the RyR2 channel complex was observed in RyR2-R2474S+/− atria, to test whether RyR2 oxidation can cause calstabin2 depletion from the channel complex, we applied the anti-oxidant DTT to atria from RyR2-R2474S+/− mice. Incubation with DTT reversed the oxidation of RyR2 and restored calstabin2 binding to RyR2 to levels comparable to those observed in RyR2 from WT atria (Figure 3D).
Atrial burst pacing-induced AF in PLN-KO and PLN-DM mice
Atrial burst pacing did not induce AF in phospholamban (PLN)-DM mice in which SR Ca2+ content is not increased by adrenergic stimulation due to mutations in the PKA and CaMKII phosphorylation sites in PLN 24 (Table 1). However, in PLN knockout mice, in which SR Ca2+ is maximally loaded, the prevalence of atrial burst pacing-induced AF was 37.5% (3/8) (Table 1). Thus, atrial burst pacing-induced AF in mice can be triggered by leaky RyR2 and/or SR Ca2+ overload and preventing adrenergic or rate related increases in SR Ca2+ content can inhibit AF triggered by leaky RyR2 channels.
Table 1.
Atrial burst pacing-induced AF in WT, PLN KO and PLN-DM mice before and after caffeine treatment
| WT (n=33) | PLN KO (n=8) | PLN-DM (n=8) | |
|---|---|---|---|
| Vehicle (%) | 0 | 37.5* | 0 |
| Caffeine (120 mg/kg) (%) | 45.5 | 62.5 | 50 |
P<0.05 vs. WT
S107 prevents atrial burst pacing-induced AF in RyR2-R2474S+/− and RyR2-N2386I+/− mice
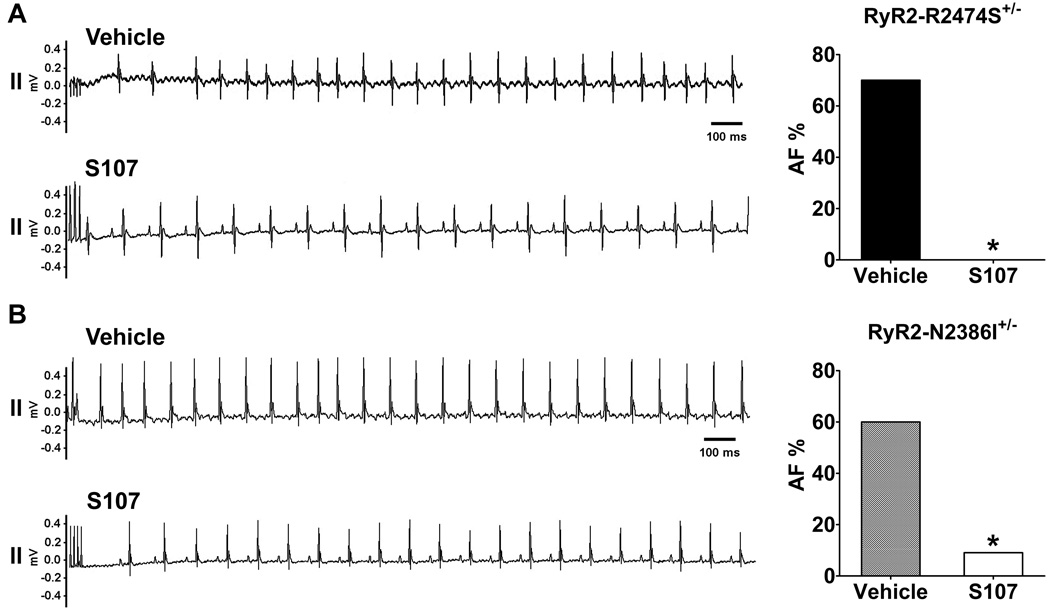
RyR2-R2474S+/− and RyR2-N2386I+/− mice were treated with S107 in the drinking water (20 mg/kg/day) for two weeks. Compared to control groups, S107 treatment significantly decreased the susceptibility to AF in both RyR2-R2474S+/− (from 70% to 0%, Figure 4A) and RyR2-N2386I+/− (from 60% to 9.1%, Figure 4B) mice, indicating that diastolic SR Ca2+ leak plays a major role in atrial burst pacing-induced AF in these CPVT mice.
Figure 4. S107 prevents atrial burst pacing-induced AF in RyR2-R2474S+/− and RyR2-N2386I+/− mice.
A) Left panel: representative ECG traces from RyR2-R2474S+/− mice during intra-esophageal burst pacing in vehicle or S107 treatment groups. Right panel: prevalence of AF in RyR2-R2474S+/− mice treated with vehicle (n=10) or S107 (n=10). B) Left panel: representative ECG traces of RyR2-N2386I+/− mice during intra-esophageal burst pacing in vehicle or S107 treatment groups. Right panel: prevalence of AF in RyR2-N2386I+/− mice treated with vehicle (n=15) or S107 (n=11). *, P<0.05 vs. vehicle treatment group.
Increased SR Ca2+ leak in atrial vs. ventricular myocytes from RyR2-R2474S+/− mice
The difference in calstabin2 binding to RyR2 and increased oxidation in atrial RyR2 but not in ventricular RyR2 in RyR2-R2474S+/− mice could result in differences in diastolic SR Ca2+ leak between atria or ventricular cardiomyocytes. We isolated atrial and ventricular cardiomyocytes from WT and RyR2-R2474S+/− mice and compared diastolic SR Ca2+ leaks. As reported previously 25, ventricular myocytes isolated from RyR2-R2474S+/− mice exhibited higher Ca2+ spark frequencies compared to ventricular myocytes isolated from WT mice. Interestingly, the Ca2+ spark frequencies of atrial myocytes were higher than their ventricular counterparts in both WT and RyR2-R2474S+/− mice (Figure 5A), indicating an intrinsic difference in Ca2+ cycling between atria and ventricles. These findings are consistent with in vivo programmed electrical stimulation results in which the same burst pacing protocol introduced via both atrial (intra-esophageal) and ventricular (endocardial) routes in RyR2-R2474S+/− mice resulted in only atrial but not ventricular burst pacing-induced arrthythmias (Figure 5B). In addition, the Ca2+ spark frequencies in atrial myocytes isolated from WT mice and ventricular myocytes from RyR2-R2474S+/− mice were similar indicating a higher resting diastolic SR Ca2+ leak rate in atrial myocytes vs. ventricular myocytes. These data are consistent with in vivo data showing that without stress (exercise + epinephrine), none of RyR2-R2474S+/− or WT mice can be stimulated into ventricular arrhythmias and AF, respectively.
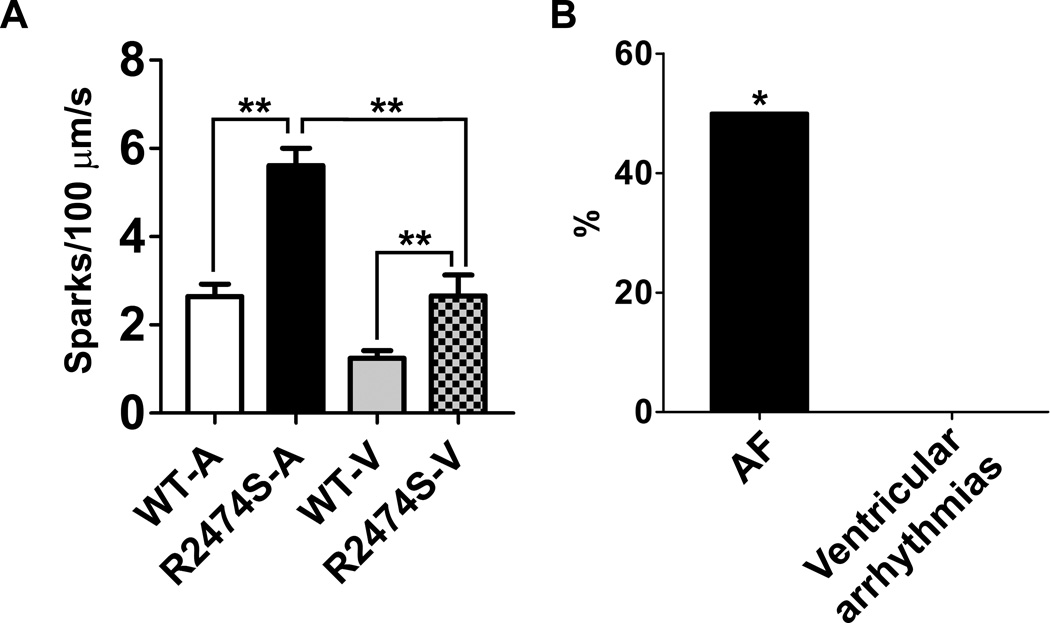
Figure 5. Comparison between atrial and ventricular Ca2+ sparks and arrhythmias in WT and RyR2-R2474S+/− mice.
A) Differences in diastolic SR Ca2+ leak measured as Ca2+ spark frequency in atrial and ventricular cardiomyocytes isolated from WT and RyR2-R2474S+/− mice. For ventricular myocytes, we use the same solutions as those used for atrial cardiomyocytes (see methods) and 1~3 Hz pacing to induce Ca2+ sparks. n=42 atrial and ventricular myocytes from WT mice, n=31 atrial and ventricular myocytes from RyR2-R2474S+/− mice. **, p<0.01. B) Prevalence of burst pacing-induced AF and ventricular arrhythmias in RyR2-R2474S+/− mice. AF was stimulated by a intra-esophageal pacing protocol, ventricular arrhythmias were stiumlated by an intra-cardiac pacing protocol (n=10 in both groups). *, P<0.05 vs. ventricular arrhythmias.
Calstabin2 knockout mice have increased atrial burst pacing-induced AF which was not prevented by S107 treatment
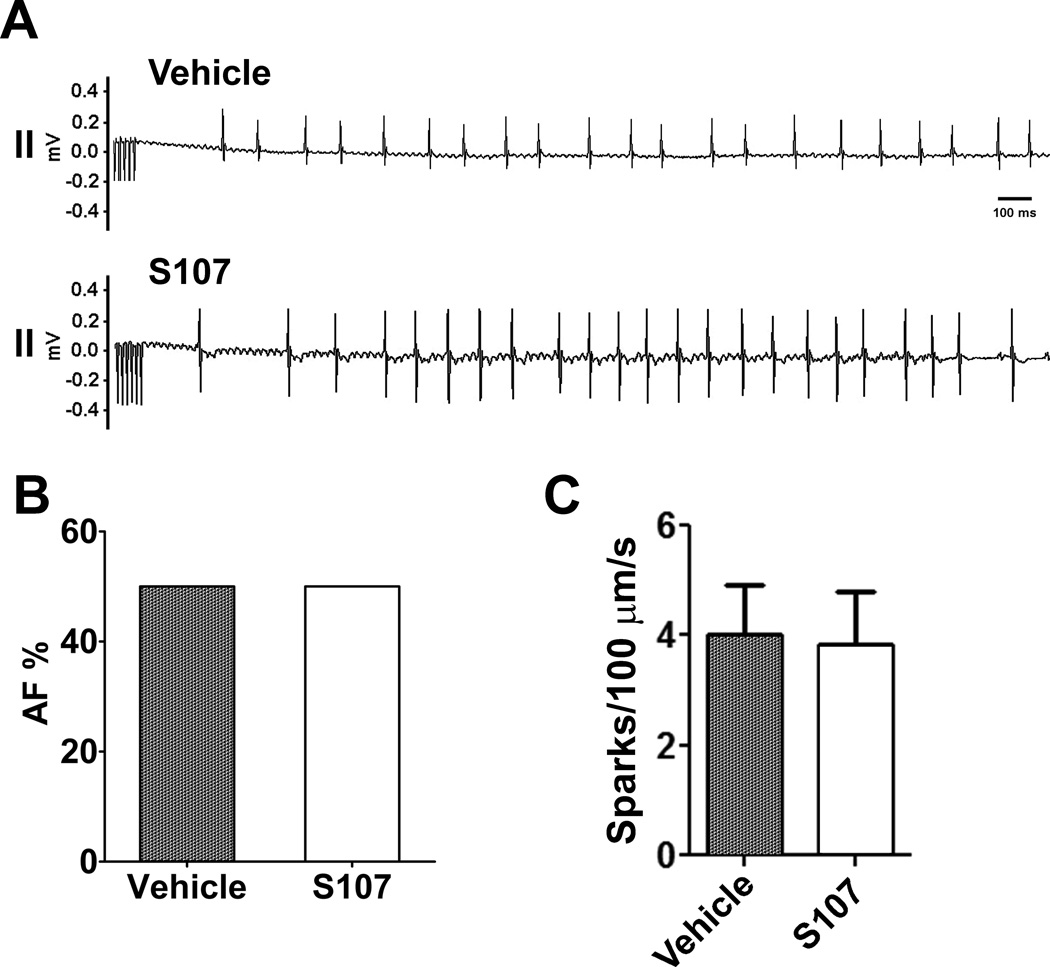
Although our previous work showed that both the 1,4-benzothiazepine JTV519 and the Rycal S107 prevent diastolic SR Ca2+ leak in murine models of heart failure and ventricular arrhythmias by preventing the stress induced depletion of calstabin2 from the RyR2 macromolecular complex 5, 6, 8, 19, 26–29, the mechanism of these drugs continues to be questioned 8. In order to clarify the molecular mechanism of S107 we tested the effect of S107 in calstabin2 knockout mice. Consistent with a previous report 20, compared to WT, calstabin2 knockout mice showed significantly increased prevalence of AF with intra-esophageal burst pacing (Figure 6A and 6B). After 2-weeks of oral S107 (20 mg/kg/day delivered in the drinking water), the incidence of AF by atrial burst pacing was not changed in calstabin2 knockout mice, indicating that the mechanism of action of S107 depends on the presence of calstabin2. Consistent with these in vivo experiments, atrial myocytes isolated from calstabin2 knockout mice showed significantly increased Ca2+ spark frequencies that were not reduced by incubation with S107 (Figure 6C).
Figure 6. S107 has no effect on atrial burst pacing-induced AF in castabin2 KO mice.
A) Representative surface ECG traces from a calstabin2 KO mouse treated with vehicle or S107. B) Prevalence of AF in calstabin2 KO mice in vehicle (n=10) and S107 treatment (n=12) groups during intra-esophageal burst pacing. C) Atrial myocytes isolated from calstabin2 KO mice were incubated with 10 µM S107 for 2 hrs before sampling for Ca2+ spark frequencies (n=13 cells in both groups).
The role of CaMKII phosphorylation of RyR2 in atrial burst pacing-induced AF in mice with CHF
It has been reported that CaMKII phosphorylation of RyR2 is a major cause of SR Ca2+ leak in atrial myocytes with RyR2 mutations and leads to AF 22, 23, 30. To test the role of CaMKII phosphorylation of RyR2 in triggering AF, we examined AF in a knock-in mouse, RyR2-S2814A, harboring an RyR2 that cannot be CaMKII phosphorylated. In agreement with a previous report by Chelu et al. 22 there was no AF stimulated in RyR2-S2814A mice by our intra-esophageal burst pacing protocol. To further confirm the activation of CaMKII during our intra-esophageal burst pacing procedure, we showed that RyR2 CaMKII phosphorylation at Ser2814 in our WT and CPVT mice was increased22, 23, 31 (supplemental figure XII). However, as we previously reported, RyR2-S2814A knock-in mice were not protected against heart failure after myocardial infarction 32. Moreover, there was no difference in pacing-induced AF between WT and RyR2-S2814A mice (50.0% vs. 63.6%), indicating that in mice with post-MI heart failure, the CaMKII phosphorylation of RyR2 does not play a pivotal role in atrial burst pacing-induced AF (supplemental figure V).
Catecholamines do not play an important role in atrial burst pacing-induced AF in 3 CPVT mouse models
CPVT is characterized by stress-induced polymorphic ventricular arrhythmias. To further explore the role of sympathetic activation in atrial burst pacing-induced AF in CPVT mouse models, we treated CPVT mice with metoprolol, a β-blocker, via drinking water for 4 weeks in a previously reported dose (30 mg/kg/day) 21. Compared to vehicle groups, there was no significant therapeutic effect of metoprolol in the CPVT mice (supplemental figure VI), suggesting that activation of sympathetic system likely does not play an important role in triggering AF in our CPVT mouse models.
DISCUSSION
SR Ca2+ leak triggers AF
Although SR Ca2+ leak has been observed in cardiomyocytes from chronic AF patients33, it is still not clear whether SR Ca2+ leak is the cause of or results from AF. Chronic sustained AF leads to atrial remodeling of both heart structure (increased fibrosis and atrial dilatation) and ion channel function 34. We previously showed that there is RyR2 PKA hyperphosphorylation and calstabin2 dissociation from RyR2 in atrial samples from humans with AF and in an animal model with chronic AF 35. These findings suggested that dynamic molecular changes to RyR2 channels occur during chronic AF and that these changes may relate to the maintenance of AF. In the current study, using knock-in mouse models harboring human CPVT mutations which have diastolic SR Ca2+ leak without structural or functional abnormalities in the heart (supplemental figure IV), we explored the molecular basis of pacing-induced AF in detail. The CPVT mice had a significantly higher prevalence of pacing-induced AF compared to WT littermates. In isolated atrial myocytes from these mice, diastolic SR Ca2+ leak was significantly increased compared to WT atrial myocytes. This increased diastolic SR Ca2+ leak in myocytes isolated from all three CPVT mouse models was associated with decreased SR Ca2+ content compared to WT, indicating that a sustained diastolic SR Ca2+ leak via RyR2 occurs in atrial myocytes.
Calstabin2 dissociation leads to SR Ca2+ leak and burst pacing-induced AF
Both congestive heart failure (CHF) and CPVT are characterized by calstabin2 dissociation from the RyR2 macromolecular complex resulting in “leaky” RyR2 channels. Our previous work showed that RyR2-castabin2 binding stabilizes the RyR2 channel complex and prevents diastolic SR Ca2+ leak in CHF or CPVT 6, 19, 21, 36, 37. The role of castabin2 binding to RyR2 in AF was first reported in myocytes from human chronic AF patients 35 and Sood et al. showed endocardial right atrial burst pacing could induce AF in calstabin2 knockout mice 20. Our intra-esophageal pacing protocol showed a significant increase in AF prevalence in calstabin2 knockout mice and S107 treatment failed to inhibit the burst pacing-induced AF in vivo and diastolic SR Ca2+ leak in vitro in calstabin2 deficient mice. However, in the CPVT models, in contrast to ventricular arrhythmias that are induced by both exercise and epinephrine, AF was induced by fast atrial pacing without any catecholamine treatment. Inhibition of sympathetic activity by the β-blocker metoprolol did not suppress AF in our CPVT mouse models (supplemental figure VI), further indicating that catecholamines may not be important in triggering AF in CPVT. This discrepancy between atrial and ventricular arrhythmias in the CPVT mouse models is likely explained by the depletion of calstabin2 from the RyR2 molecular complex in atrial but not ventricular tissues from resting RyR-R2474S+/− mice (Figure 3). We previously reported that the dissociation of calstabin2 from RyR2 is caused by remodeling of the RyR2 channel complex including PKA phosphorylation, nitrosylation, and oxidation of RyR2 21. We now show that RyR2 from RyR2-R2474S+/− atrial tissue are oxidized at baseline indicating chronic remodeling of the channel in this CPVT mouse. Indeed, clinical reports have suggested a close link between elevated levels of derivatives of reactive oxygen metabolites and persistent AF and AF recurrence after radio frequency catheter ablation in paroxysmal AF patients 38. We previously showed that oxidation and PKA phosphorylation cause calstabin2 depletion from RyR2 21. We now show that calstabin2 dissociation from RyR2 can occur when the channel is oxidized. Using the reducing reagent DTT to inhibit RyR2 oxidation in atrial tissue isolated from RyR2-R2474S+/− mice we further examined the role of RyR2 oxidation in depletion of calstabin2 from the RyR2 channel complex in our CPVT mouse model. It is possible that the CPVT mutations may alter RyR2 conformation rendering it more accessible to oxidation and more sensitive to calstabin2 depletion.
Restoring calstabin2 binding to RyR2 stabilizes the channel and prevents burst pacing-induced AF
As we previously reported 6, 21, 26, 27, S107 stabilizes RyR channels and prevents Ca2+ leak by enhancing RyR-calstabin interactions. Here we show that incubation of atrial myocytes isolated from RyR2-R2474S+/− mice with S107 inhibited diastolic SR Ca2+ leak. S107 treatment of mice also prevented intra-esophageal burst pacing-induced AF in RyR2-R2474S+/−, RyR2-N2386I+/− mice but had no effect in mice lacking calstabin2. The effect of S107 in stabilizing leaky RyR2 channels in the atria indicates that dissociation of calstabin2 from RyR2 channel complex likely plays an important role in AF in these CPVT mouse models. Furthermore, according to the Framingham Study, CHF is the strongest predictor for the development of AF 39. CHF results in RyR2 PKA hyperphosphorylation, oxidation, nitrosylation and calstabin2 dissociation from RyR2 molecular complex. Dissociation of calstabin2 from the RyR2 channel complex could be one of the causes of AF in patients with CHF. Therefore, the RyR2 stabilizing Rycal S107 has potential as a possible therapeutic for the prevention and treatment of AF related to CHF or CPVT.
The role of CaMKII in triggering AF in CPVT
Chelu et al. reported that AF stimulated by intracardiac pacing is associated with CaMKII phosphorylation of RyR2 22. However, the finding that the CaMKII inhibitor KN93 suppressed burst pacing-induced AF does not prove that CaMKII phosphorylation of RyR2 plays a pivotal role in AF since CaMKII phosphorylates other Ca2+ cycling proteins including the L-type Ca2+ channel and PLN which modulates SERCA2a to regulate SR Ca2+ uptake. Consistent with previous reports 22, 23, 31, our experiment using freshly isolated atria from WT and RyR2-R2474S+/− mice with or without atrial burst pacing showed that atrial burst pacing led to RyR2 CaMKII phosphorylation at Ser2814 in both groups (supplemental figure XII), suggesting a role for CaMKII phosphorylation of RyR2 during atrial burst pacing induced AF. To further clarify the role of CaMKII phosphorylation of RyR2 in triggering AF, we used a clinically relevant acute myocardial infarction induced HF model as AF occurs in 15% to 30% of patients with HF40. According to our previously published results, RyR2-S2814A mice showed similar progression of heart failure after myocardial infarction compared to WT littermates 32. In these HF mice, the atrial burst pacing protocol induced similar incidences of AF in both RyR2-S2814A and WT groups, arguing that CaMKII phosphorylation of RyR2 does not play a major role in triggering AF in HF. However, due to the multiple substrates of CaMKII in Ca2+ cycling proteins, the role of pacing-induced activation of CaMKII in modulating Ca2+ cycling requires further study.
The different characteristics of arrhythmias between atria and ventricles
Increased diastolic SR Ca2+ leak in atrial myocytes isolated from RyR2-R2474S+/− mice is consistent with in vivo intra-esophageal and intra-cardiac burst pacing-induced AF and ventricular arrhythmias respectively in RyR2-R2474S+/− mice (Figure 6). A recent report showed that rat atrial myocytes have higher SR mediated Ca2+ uptake and a ~3-fold higher SR Ca2+ load compared to ventricular myocytes 41. Higher SR Ca2+ load and increased SR Ca2+ uptake may explain the increased Ca2+ spark frequencies in both WT and RyR2-R2474S+/− atrial myocytes compared to their ventricular counterparts and may lower the threshold for induction of atrial arrhythmias induced by burst atrial pacing. The baseline Ca2+ spark frequencies of WT atrial myocytes and RyR2-R2474S+/− ventricular myocytes were comparable (Figure 5, first and fourth bar), indicating comparable diastolic SR Ca2+ leak. This leak by itself is not sufficient to induce AF during in vivo burst pacing stimulation in WT mice, or ventricular arrhythmias in RyR2-R2474S+/− mice. Clinically, VT is observed during exercise in patients with the RyR2-R2474S mutation indicating the importance of sympathetic activation of the SR Ca2+ uptake pathway and loading of the SR to increase the amplitude of the leak. The exact reasons for these differences are still not well understood. However, it is well known that unlike ventricular fibrillation which leads to sudden cardiac death, AF is typically not lethal in the absence of a by-pass tract. Therefore there might be less evolutionary pressure to maintain a higher threshold for arrhythmias in the atria.
Mechanism of AF
Although re-entry and multiple wavelets are observed in AF, the molecular events initiating AF remain uncertain. In the current study using CPVT mouse models with known RyR2 mutations and normal cardiac structure and function (supplemental figure IV), we explored the role of diastolic SR Ca2+ leak via mutant RyR2 in the atria. In agreement with previous reports implicating abnormal Ca2+ handling in AF, we did observe Ca2+ waves (supplemental figure IX) and indirect evidence of Ca2+-activated inward current (DAD, supplemental figure VIII) in our studies. Together with the normal cardiac structure and function of the CPVT mouse models, our data indicate that the diastolic SR Ca2+ leak via RyR2 leads to Ca2+ waves and DADs which form multiple wavelets and possible re-entry loops that trigger atrial tachycardia (AT) and AF.
Limitations and disadvantages
The CPVT RyR2 mutations in our study were originally discovered in CPVT patients. However, due to the low prevalence of CPVT in population and extremely low number of patients with each specific RyR2 mutation there are no reports of AF in patients with RyR2-R2474S, RyR2-N2386I, or RyR2-L433P mutations. Due to technical limitations, it is impossible to pace a single cardiomyocyte at physiologic frequencies (for mice) e.g. 9–10Hz. Therefore, the Ca2+ spark measurements may not reflect physiological conditions in intact atria. Also, the burst pacing protocol needed to elicit AF in mice may not represent maintained AF in humans. In addition, abnormal function of pacemaker cells in the CPVT mouse models is potentially a factor in triggering AF.
Supplementary Material
Novelty and Significance.
What Is Known?
Chronic atrial fibrillation (AF) is associated with increased diastolic sarcoplasmic reticulum (SR) Ca2+ leak in atrial cardiac myocytes.
Catecholaminergic polymorphic ventricular tachycardia (CPVT)-linked ryanodine receptor 2 (RyR2) mutations cause diastolic SR Ca2+ leak in ventricular cardiac myocytes.
Recent reports show that CPVT patients have increased prevalence of AF.
What New Information Does This Article Contribute?
Diastolic SR Ca2+ leak likely plays a critical role in initiating AF in murine models of human CPVT-linked RyR2 mutations.
S107 (Rycal) significantly inhibits diastolic SR Ca2+ leak in atrial myocytes and prevents pacing-induced AF in models of human CPVT-linked RyR2 mutations.
Atrial fibrillation (AF) is the most common cardiac arrhythmia. However, the mechanisms underlying AF are not well understood despite more than 100 years of study. This has impaired the development of therapeutics for AF. Intracellular Ca2+ plays a central role in the action potential and contraction of atrial myocytes. While the role of diastolic SR Ca2+ leak in the generation of ventricular arrhythmias has been demonstrated, the impact of diastolic SR Ca2+ leak in the genesis of AF remains to be established. It has been shown that CPVT-linked RyR2 mutations cause diastolic SR Ca2+ leak, delayed after depolarizations and lethal ventricular arrhythmias. Recent reports demonstrate that patients with CPVT-linked RyR2 mutations have a higher prevalence of AF as well. Murine models harboring CPVT-linked RyR2 mutatoins exhibit increased burst pacing-induced AF. Atrial myocytes isolated from these mice showed increased diastolic SR Ca2+ leak. The rycal drug S107 that inhibits loss of the stabilizing subunit calstabin2 from the RyR2 macromolecular complex inhibited diastolic SR Ca2+ SR leak and prevented burst pacing-induced AF in murine models of CPVT-linked RyR2 mutations. These findings indicate that inhibition of diastolic SR Ca2+ leak with a rycal drug could be a potential therapeutic approach for preventing AF.
ACKNOWLEDGEMENTS
We thank Dr. John Vest for help in analyses of in vivo AF stimulation data.
SOURCES OF FUNDING
This work was supported a grant from the NHLBI to ARM (1R01HL102040-01A1). M.J.B. was supported by a Postdoctoral Fellowship (F32-HL107029) from the NIH.
Non-standard Abbreviations
- AF
atrial fibrillation
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- CHF
congestive heart failure
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- DAD
delayed after depolarizations
- DNP
2, 4-dinitrophenyl
- DTT
dithiothreitol
- HF
heart failure
- PKA
protein kinase A
- PLN
phospholamban
- PLN-DM
mutant PLN in which both phosphorylation residues (Ser16 and Thr17) were replaced by Ala
- RyR2
ryanodine receptor type 2
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase
- SR
sarcoplasmic reticulum
- VT
ventricular tachycardia
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
ARM is a consultant for and owns shares in ARMGO Pharma, Inc. a biotech company targeting RyR2 treatment for prevention of CPVT.
REFERENCES
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The anticoagulation and risk factors in atrial fibrillation (atria) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Ruskin JN, Singh JP. Atrial fibrillation endpoints: Hospitalization. Heart Rhythm. 2004;1:B31–B34. doi: 10.1016/j.hrthm.2004.04.004. discussion B34-35. [DOI] [PubMed] [Google Scholar]
- 3.Burstein B, Nattel S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 4.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 5.Wehrens XH, Marks AR. Novel therapeutic approaches for heart failure by normalizing calcium cycling. Nat Rev Drug Discov. 2004;3:565–573. doi: 10.1038/nrd1440. [DOI] [PubMed] [Google Scholar]
- 6.Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, Reiken S, Wronska A, Drew LJ, Ward CW, Lederer WJ, Kass RS, Morley G, Marks AR. Leaky ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest. 2008;118:2230–2245. doi: 10.1172/JCI35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D'Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. Fkbp12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 8.Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 9.Kazemian P, Gollob MH, Pantano A, Oudit GY. A novel mutation in the ryr2 gene leading to catecholaminergic polymorphic ventricular tachycardia and paroxysmal atrial fibrillation: Dose-dependent arrhythmia-event suppression by beta-blocker therapy. Can J Cardiol. 2011 doi: 10.1016/j.cjca.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Nof E, Belhassen B, Arad M, Bhuiyan ZA, Antzelevitch C, Rosso R, Fogelman R, Luria D, El-Ani D, Mannens MM, Viskin S, Eldar M, Wilde AA, Glikson M. Postpacing abnormal repolarization in catecholaminergic polymorphic ventricular tachycardia associated with a mutation in the cardiac ryanodine receptor gene. Heart Rhythm. 2011 doi: 10.1016/j.hrthm.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pizzale S, Gollob MH, Gow R, Birnie DH. Sudden death in a young man with catecholaminergic polymorphic ventricular tachycardia and paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19:1319–1321. doi: 10.1111/j.1540-8167.2008.01211.x. [DOI] [PubMed] [Google Scholar]
- 12.Sumitomo N, Nakamura T, Fukuhara J, Nakai T, Watanabe I, Mugishima H, Hiraoka M. Clinical effectiveness of pulmonary vein isolation for arrhythmic events in a patient with catecholaminergic polymorphic ventricular tachycardia. Heart Vessels. 2010;25:448–452. doi: 10.1007/s00380-009-1214-6. [DOI] [PubMed] [Google Scholar]
- 13.Sumitomo N, Sakurada H, Taniguchi K, Matsumura M, Abe O, Miyashita M, Kanamaru H, Karasawa K, Ayusawa M, Fukamizu S, Nagaoka I, Horie M, Harada K, Hiraoka M. Association of atrial arrhythmia and sinus node dysfunction in patients with catecholaminergic polymorphic ventricular tachycardia. Circ J. 2007;71:1606–1609. doi: 10.1253/circj.71.1606. [DOI] [PubMed] [Google Scholar]
- 14.Cerrone M, Colombi B, Santoro M, di Barletta MR, Scelsi M, Villani L, Napolitano C, Priori SG. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ Res. 2005;96:e77–e82. doi: 10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, Duff HJ, Roden DM, Wilde AA, Knollmann BC. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–383. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi S, Yano M, Uchinoumi H, Suetomi T, Susa T, Ono M, Xu X, Tateishi H, Oda T, Okuda S, Doi M, Yamamoto T, Matsuzaki M. Dantrolene, a therapeutic agent for malignant hyperthermia, inhibits catecholaminergic polymorphic ventricular tachycardia in a ryr2(r2474s/+) knock-in mouse model. Circ J. 2010;74:2579–2584. doi: 10.1253/circj.cj-10-0680. [DOI] [PubMed] [Google Scholar]
- 17.Beery TA, Shah MJ, Benson DW. Genetic characterization of familial cpvt after 30 years. Biol Res Nurs. 2009;11:66–72. doi: 10.1177/1099800409333369. [DOI] [PubMed] [Google Scholar]
- 18.Sampson KJ, Terrenoire C, Cervantes DO, Kaba RA, Peters NS, Kass RS. Adrenergic regulation of a key cardiac potassium channel can contribute to atrial fibrillation: Evidence from an i ks transgenic mouse. J Physiol. 2008;586:627–637. doi: 10.1113/jphysiol.2007.141333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehnart SE, Wehrens XH, Laitinen PJ, Reiken SR, Deng SX, Cheng Z, Landry DW, Kontula K, Swan H, Marks AR. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation. 2004;109:3208–3214. doi: 10.1161/01.CIR.0000132472.98675.EC. [DOI] [PubMed] [Google Scholar]
- 20.Sood S, Chelu MG, van Oort RJ, Skapura D, Santonastasi M, Dobrev D, Wehrens XHT. Intracellular calcium leak due to fkbp12.6 deficiency in mice facilitates the inducibility of atrial fibrillation. Heart Rhythm. 2008;5:1047–1054. doi: 10.1016/j.hrthm.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shan J, Betzenhauser MJ, Kushnir A, Reiken S, Meli AC, Wronska A, Dura M, Chen BX, Marks AR. Role of chronic ryanodine receptor phosphorylation in heart failure and beta-adrenergic receptor blockade in mice. J Clin Invest. 2010;120:4375–4387. doi: 10.1172/JCI37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, Schotten U, Anderson ME, Valderrabano M, Dobrev D, Wehrens XH. Calmodulin kinase ii-mediated sarcoplasmic reticulum ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li N, Wang T, Wang W, Cutler MJ, Wang Q, Voigt N, Rosenbaum DS, Dobrev D, Wehrens XH. Inhibition of camkii phosphorylation of ryr2 prevents induction of atrial fibrillation in fkbp12.6 knockout mice. Circ Res. 2012;110:465–470. doi: 10.1161/CIRCRESAHA.111.253229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brittsan AG, Ginsburg KS, Chu G, Yatani A, Wolska BM, Schmidt AG, Asahi M, MacLennan DH, Bers DM, Kranias EG. Chronic sr ca2+-atpase inhibition causes adaptive changes in cellular ca2+ transport. Circ Res. 2003;92:769–776. doi: 10.1161/01.RES.0000066661.49920.59. [DOI] [PubMed] [Google Scholar]
- 25.Uchinoumi H, Yano M, Suetomi T, Ono M, Xu X, Tateishi H, Oda T, Okuda S, Doi M, Kobayashi S, Yamamoto T, Ikeda Y, Ohkusa T, Ikemoto N, Matsuzaki M. Catecholaminergic polymorphic ventricular tachycardia is caused by mutation-linked defective conformational regulation of the ryanodine receptor. Circ Res. 2010;106:1413–1424. doi: 10.1161/CIRCRESAHA.109.209312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, Matecki S, Lacampagne A, Marks AR. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15:325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, Landry DW, Nieman D, Lehnart SE, Samaru M, LaCampagne A, Marks AR. Remodeling of ryanodine receptor complex causes"leaky" channels: A molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci U S A. 2008;105:2198–2202. doi: 10.1073/pnas.0711074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fauconnier J, Thireau J, Reiken S, Cassan C, Richard S, Matecki S, Marks AR, Lacampagne A. Leaky ryr2 trigger ventricular arrhythmias in duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2010;107:1559–1564. doi: 10.1073/pnas.0908540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wehrens XH, Lehnart SE, Reiken S, van der Nagel R, Morales R, Sun J, Cheng Z, Deng SX, de Windt LJ, Landry DW, Marks AR. Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure. Proc Natl Acad Sci U S A. 2005;102:9607–9612. doi: 10.1073/pnas.0500353102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schondube FA, Hasenfuss G, Maier LS. Camkii-dependent diastolic sr ca2+ leak and elevated diastolic ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–1144. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 31.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D. Enhanced sarcoplasmic reticulum ca2+-leak and increased na+-ca2+-exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kushnir A, Shan J, Betzenhauser MJ, Reiken S, Marks AR. Role of camkiidelta phosphorylation of the cardiac ryanodine receptor in the force frequency relationship and heart failure. Proc Natl Acad Sci U S A. 2010;107:10274–10279. doi: 10.1073/pnas.1005843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hove-Madsen L, Llach A, Bayes-Genis A, Roura S, Rodriguez Font E, Aris A, Cinca J. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation. 2004;110:1358–1363. doi: 10.1161/01.CIR.0000141296.59876.87. [DOI] [PubMed] [Google Scholar]
- 34.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 35.Vest JA, Wehrens XH, Reiken SR, Lehnart SE, Dobrev D, Chandra P, Danilo P, Ravens U, Rosen MR, Marks AR. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–2032. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- 36.Huang F, Shan J, Reiken S, Wehrens XH, Marks AR. Analysis of calstabin2 (fkbp12.6)-ryanodine receptor interactions: Rescue of heart failure by calstabin2 in mice. Proc Natl Acad Sci U S A. 2006;103:3456–3461. doi: 10.1073/pnas.0511282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. Pka phosphorylation dissociates fkbp12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 38.Shimano M, Shibata R, Inden Y, Yoshida N, Uchikawa T, Tsuji Y, Murohara T. Reactive oxidative metabolites are associated with atrial conduction disturbance in patients with atrial fibrillation. Heart Rhythm. 2009;6:935–940. doi: 10.1016/j.hrthm.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 39.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The framingham heart study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 40.Stevenson WG, Stevenson LW, Middlekauff HR, Fonarow GC, Hamilton MA, Woo MA, Saxon LA, Natterson PD, Steimle A, Walden JA, Tillisch JH. Improving survival for patients with atrial fibrillation and advanced heart failure. J Am Coll Cardiol. 1996;28:1458–1463. doi: 10.1016/s0735-1097(96)00358-0. [DOI] [PubMed] [Google Scholar]
- 41.Walden AP, Dibb KM, Trafford AW. Differences in intracellular calcium homeostasis between atrial and ventricular myocytes. J Mol Cell Cardiol. 2009;46:463–473. doi: 10.1016/j.yjmcc.2008.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.