History and Physical Examination
An 80-year-old woman presented to her primary care physician with chronic knee pain of more than 2 years’ duration. Her medical history was significant only for trauma to her knee, approximately 2 years prior, at which time she presented to the emergency room and was diagnosed with a knee sprain and arthritis, and was told she had no evidence of fracture observed on her radiographs. The patient returned to her primary care physician because of an increase in her baseline knee pain level which was not well controlled with her usual dose of pain medication. Her pain was mainly vague, although it localized to the anterior aspect of her knee more than elsewhere. The pain improved with rest and became worse with activity and walking. She reported no history of fever, fatigue, or malaise. On physical examination, her vital signs were stable and she was in no distress. She had tenderness to palpation over the distal femur and pain with axial load of the left femur. There was no limitation in ROM, although ROM was painful. Her motor examination was 5/5 and sensation was intact throughout all dermatomes. No soft tissue mass was present around the knee, and there was no evidence of lymphadenopathy in the inguinal or popliteal region.
Laboratory examination showed a normal white blood cell count of 8.2 × 103/μL, but an elevated sedimentation rate of 19 mm/hour. All blood cultures were negative.
Imaging studies included AP and lateral radiographs of the knee (Fig. 1), MRI (Fig. 2), and radionuclide Tc-99 m methylene diphosphonate bone scan (Fig. 3).
Fig. 1A–B.
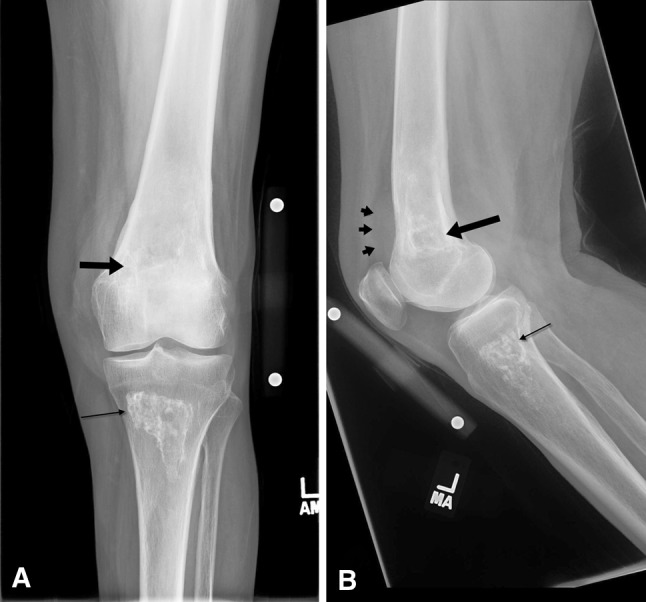
(A) AP and (B) lateral radiographs of the patient’s left knee show an ill-defined lucent lesion with a serpentine sclerosing border (large black arrow) in the distal femur. An adjacent soft tissue density (black arrowheads) is causing anterior displacement of the muscular shadow. The well-defined sclerotic region with a sclerosing border seen in the proximal tibia represents bone infarction (thin black arrow).
Fig. 2A–F.
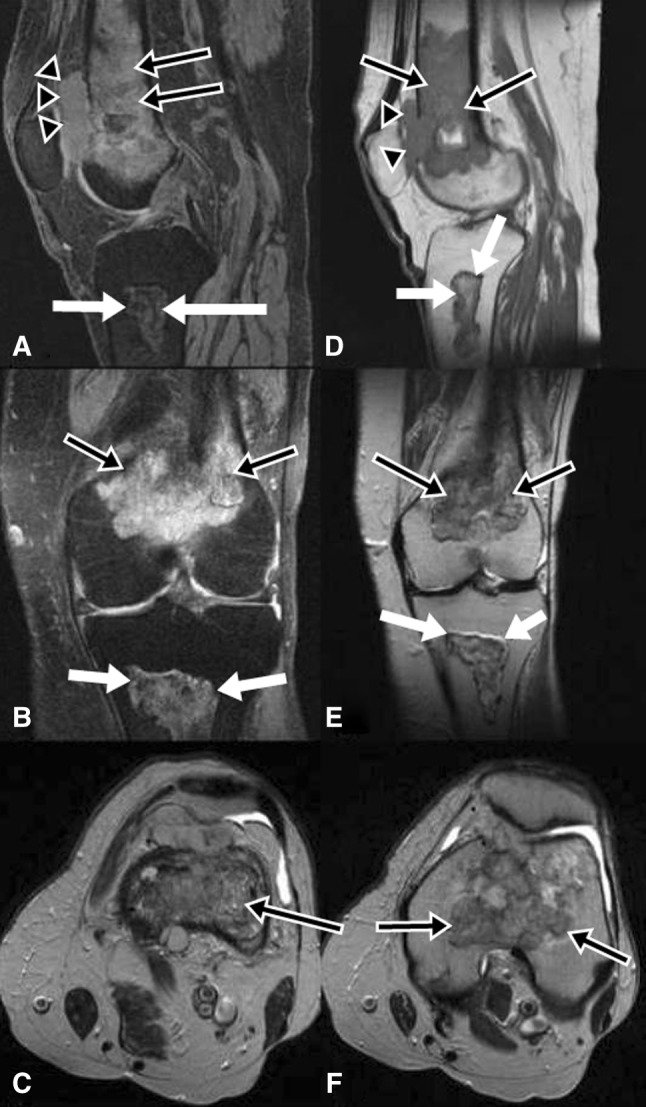
(A) Sagittal T2-weighted with fat saturation, (B) coronal T2-weighted with fat saturation, (C) axial T2-weighted, (D) sagittal T1-weighted, (E) coronal T2-weighted, and (F) axial T2-weighted MR images are shown. An area of ill-defined increased signal intensity is seen on the T2-weighted images and decreased signal on the T1-weighted images (black arrows with white margin) in the distal femur. There is an adjacent soft tissue density (arrowheads) that can be seen in Illustrations A and D. There is endosteal scalloping, periosteal reaction, and cortical destruction associated with the soft tissue mass with cortical break-through. An area of well-defined bone infarct is seen in the proximal tibia (white arrows).
Fig. 3A–B.
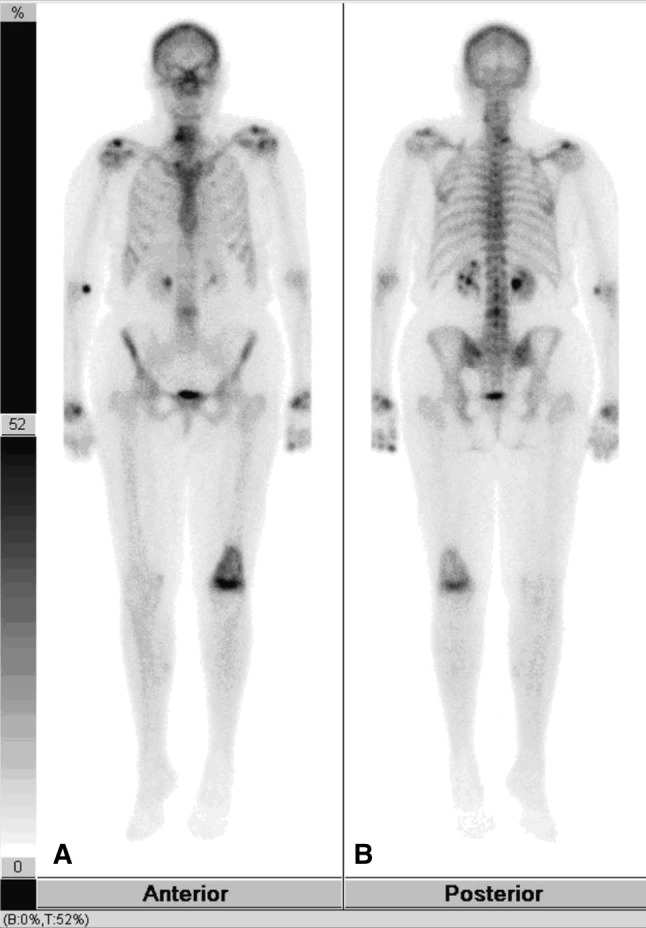
A bone scan was performed with technetium 99-labeled methylene disphosphonate. (A) The anterior projection shows increased uptake in the left proximal femur. There is no uptake in the left proximal tibia. (B) The posterior projection also shows intense uptake in the distal left femur.
Based on the history, physical examination, laboratory studies, and imaging studies, what is the differential diagnosis?
Image Interpretation
The AP and lateral radiographs of her left knee showed an area of ill-defined lucency with a serpentine sclerotic border (Fig. 1) in the distal femur. A soft tissue density was seen adjacent to this area of lucency and was causing anterior displacement of the muscular shadow. Another well-defined sclerotic region with a serpentine sclerotic border was seen in the proximal tibia. This was believed to be a bone infarction.
T2-weighted MR images showed an area of ill-defined increased signal and the T1-weighted images showed decreased signal with a serpentine border (Fig. 2) in the distal femur. There was endosteal scalloping, periosteal reaction, and cortical destruction associated with a soft tissue mass with cortical break-through. Heterogenous enhancement with central necrosis was observed. An area of well-defined bone infarct was seen in the proximal tibia. A technetium 99-labeled methylene diphosphonate bone scan showed intense uptake in the distal left femur (Fig. 3).
Differential Diagnosis
Bone infarction
Primary sarcomas: osteosarcoma and fibrosarcoma
Osteomyelitis
Malignant fibrous histiocytoma (MFH; pleomorphic undifferentiated sarcoma)
Metastasis
Primary lymphoma
A CT-guided core needle biopsy of the lesion was performed and the histologic features of the lesion were examined (Fig. 4).
Fig. 4A–B.
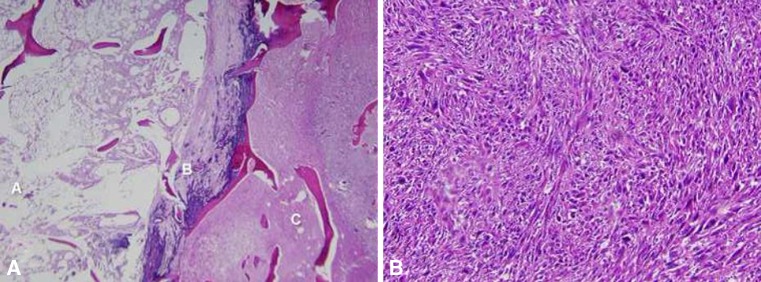
(A) In section A, a central bone infarct can be seen consisting of fat necrosis with dystrophic calcifications and bone necrosis on the left. In section B, a necrotic sarcoma is evident and in section C, a viable sarcoma is present (Stain, hematoxylin and eosin; original magnification, × 10). (B) A storiform arrangement of the sarcoma with pleomorphic malignant cells was seen (Stain, hematoxylin and eosin; original magnification, × 200).
Based on the medical history, physical examination, laboratory studies, imaging studies, and histologic picture, what is the diagnosis and how should the patient be treated?
Histology Interpretation
The gross pathology examination revealed a 4-cm nodular mass protruding from the anteromedial aspect of the femur (Figs. 4 and 5). The femur contained a 4.5 × 2.5 × 2.5-cm destructive lesion abutting the cortex located at the border of metadiaphyseal junction (Figs. 4 and 5). Histopathologic examination showed three different areas: a central bone infarct consisting of fat necrosis with dystrophic calcifications and bone necrosis, necrotic sarcoma, and viable sarcoma comprising approximately 40% of the lesion (Fig. 4). Immunohistochemical stains for cytokeratins, desmin, muscle specific actin, and S100 protein were negative. No osteoid production was seen.
Fig. 5A–C.

(A) A lateral radiograph shows an area of ill-defined lucency (black arrow) in the distal femur with adjacent soft tissue density (black arrowheads). An area of bone infarct is seen in the proximal tibia (white arrow). (B) A sagittal T2-weighted fat saturation MR image shows the area of bone infarct (black arrows) in the distal femur with an adjacent soft tissue density (black arrowheads). The bone infarct is seen in the proximal tibia (white arrow). (C) A sagittal gross section shows the mass in the distal femur arising from a bone infarct (black arrows). The soft tissue mass is seen anteriorly (black arrowheads).
Diagnosis
MFH (pleomorphic undifferentiated sarcoma)
Discussion and Treatment
Numerous items in the differential diagnosis call for consideration and exclusion. The patient, an 80-year-old woman, presented with chronic vague knee pain mainly in the anterior aspect of her knee that did not respond to treatment. She did not have any signs of infection, such as fever, chills, or discharge, therefore osteomyelitis was less likely, but not impossible. Radiographs of her knee showed classic findings of bone infarction with a soft tissue mass adjacent to it. The radiographic findings and the soft tissue mass detected on the radiographs demanded additional evaluation with MRI. The MRI features of this soft tissue mass (high signal on T2-weighted images, low signal on T1-weighted images, and enhancement) and its association with an area of bone infarction suggested the diagnoses of primary bone sarcoma or lymphoma. A biopsy was needed to confirm the diagnosis.
A bone infarction can occur owing to various conditions including corticosteroid therapy, hemoglobinopathy, alcoholism, Gaucher’s disease, dysbaric disorders, trauma, infection, and metabolic disorders [1, 2, 6, 9]. A bone infarction is not associated with a soft tissue mass and therefore it cannot be the only diagnosis in our case.
Osteosarcoma is the most common primary bone malignancy in patients 10 to 20 years old and is an aggressive lesion with bone formation and periosteal reaction. However, secondary osteosarcoma is seen in older patients and therefore osteosarcoma should be in the differential diagnosis. The diagnosis of osteosarcoma is confirmed based on identification of osteoid deposits. The fact that osteoid deposits were not seen under microscopy on the biopsy specimen helps to exclude osteosarcoma, although their absence does not always exclude the diagnosis of osteosarcoma since an osteoid deposit may be scant or focal [12].
Osteomyelitis can cause a sequestrum, which is a segment of dead, sclerotic bone tissue surrounded by granulation tissue. Chronic osteomyelitis frequently is associated with sclerotic margins and endosteal thickening observed on plain radiographs. On MRI, chronic osteomyelitis will show a low signal intensity on T1-weighted images and hyperintensity on T2-weighted images with a rim of enhancement on postcontrast images. A sinus tract may be seen in some patients. Our patient did not have any prior history of infection, which makes osteomyelitis less likely as the diagnosis. Additionally, the patient’s laboratory results (normal WBC, erythrocyte sedimentation rate, and blood cultures) suggested this was not the correct diagnosis.
Fibrosarcoma typically have osteolytic foci and the pattern of bone destruction is a geographic, moth-eaten, or permeative pattern. They occasionally show periosteal reaction and cortical destruction. Excluding fibrosarcoma using only imaging findings is difficult, if not impossible; a biopsy generally is needed, and this was performed. The findings from the biopsy showed the diagnosis to be MFH (now often referred to as pleomorphic undifferentiated sarcoma).
Malignant transformation of bone infarctions to MFH, angiosarcoma, fibrosarcoma, osteosarcoma, and mixed pleomorphic sarcoma is rare, although it has been reported [6, 9, 13]. The earliest report of sarcomas arising in infarcts is from 1960 by Furey et al. [6]. Most lesions arise in long bones with predominance in the femur and most commonly occur in the sixth decade of life [3, 6, 9, 12, 13]. Approximately 2/3 of the patients are male [12]. The pathogenesis of sarcomas arising in bone infarctions is unknown; however theories suggest malignant transformation arises from the cells in the chronic reparative process related to the underlying inflammation [3, 6, 9]. Additionally, given that most infarcts are silent, the rate of malignant transformation cannot be ascertained [3].
A review of pertinent literature from 1978 until 2012 revealed a total of 121 patients presenting with MFH associated with bone infarction (Table 1). Reported cases of osteosarcoma or other variants of sarcoma were excluded. The most common locations were the distal femur (53%) and proximal tibia (38%). Although some patients who present with MFH have the tumor develop in association with other diseases (such as bone infarctions or Paget’s disease), most MFH tumors occur in isolation. The most common radiographic modality used in the cases reported to date has been plain films, and diagnosis generally was done using a core needle biopsy. MRI was used in only two of the reported cases [4, 8]. This may be partly attributable to insufficient familiarity with MRI findings and limited availability of this modality.
Table 1.
Reported cases of malignant fibrous histiocytoma associated with bone necrosis
| Study | Year | Number of patients | Age/sex | Location | Radiologic findings |
|---|---|---|---|---|---|
| Galli et al. [7] | 1978 | 2 | 40/M 33/F | Proximal tibia; proximal humerus | Plain film: medullary infarct associated with soft tissue mass |
| McCarthy et al. [10] | 1979 | 35 | Mean of 34 years | Distal femur; proximal tibia | Plain film: sharply defined areas of increased density with serpiginous margins and peripheral calcifications with cortical erosion, periosteal elevation and soft tissue extension; ill-defined mass |
| Frierson et al. [5] | 1987 | 1 | 42/M | Distal femur | Plain film: sharply demarcated area of increased density with associated soft tissue mass |
| Desai et al. [1] | 1996 | 4 | 39/F 57/M 56/F 46/M | Distal femur (2); proximal tibia (1); proximal humerus (1) | Plain film: symmetric, sharply defined areas of increased density with serpiginous margins and peripheral calcifications surrounded by lytic ill-defined mass with cortical erosion, periosteal elevation, and soft tissue extension. CT: expansile, partly calcified lytic lesion exhibiting destruction of the cortex and soft tissue extension |
| Kenan et al. [8] | 1998 | 1 | 19/M | Midshaft tibia | Plain film: lytic, permeative lesion with cortical thickening, and cortical radiolucencies along the diaphyseometaphyseal section of the bone associated with medullary stenosis. MRI: cortical destructive process extending into the medullary cavity with an extraosseous mass |
| Duong et al. [4] | 2004 | 1 | 69/M | Distal tibia | Plain film: destructive lesion extending from the subchondral end plate to the metaphysis; focal lucency with marginal bone sclerosis. MRI: mass adjacent to the bone infarct extending into soft tissue with edema |
| Domson et al. [2] | 2009 | 12 | 51/F 67/M 59/F 67/M 32/M 56/F 60/M 51/F 48/M 61/F 64/F 61/F | Distal femur (5); proximal femur (1); proximal tibia (6); acetabulum (1) | Plain film: curvilinear radiodensities of the underlying mature bone infarct blending into the permeative destructive areas associated with a soft tissue mass and pathologic fracture |
M = male; F = female.
MFH is an aggressive tumor with a poor prognosis and usually has metastasized at the time of diagnosis, predominately to the lungs [4]. The prognosis is even worse when it is associated with bone infarction [1]. The treatment is a combination of surgery, chemotherapy, and radiation with a 5-year survival rate of 42.9% [4]. The 5-year survival of surgery alone is 18% [4].
MFH arising from bone necrosis has specific radiographic findings. A mature bone infarct appears as a well-defined, sclerotic lesion with a serpentine border with relatively central lucency. Bone infarcts do not result in significant endosteal scalloping. When associated with MFH, curvilinear radiodensities of the underlying mature bone infarct can be seen blending into areas of permeative and lytic destruction attributable to the malignancy [2]. The osteonecrotic lesion frequently will appear larger than usually is seen, and usually is ill defined with cortical erosion, periosteal reaction, a pathologic fracture, and soft tissue extension [4, 5, 7, 8, 10, 11, 13]. Bone destruction may be present. Our patient’s knee radiographs showed large metaphyseal osteonecrotic lesions of the proximal tibia and distal femur with cortical thinning and a questionable ill-defined soft tissue mass anteriorly (Fig. 1). This was associated with periosteal reaction.
On CT scans, MFH arising from a bone infarct typically presents as an expansile partly calcified lytic lesion exhibiting destruction of the cortex and soft tissue extension.
Frequently a diphosphonate radionuclide bone scan is performed. The bone scan shows increased uptake. Our patient’s bone scan showed a focus of increased uptake at the distal left femur. Delayed images showed an area of increased uptake in the left distal femur which corresponds to the area of increased uptake on the two prior phases (Fig. 3).
Our routine MRI protocol for imaging tumors includes axial images and an orthogonal plane (either sagittal or coronal planes). An appropriate surface coil should be selected. The most common sequences are a T1-weighted, spin-echo, short T1 inversion recovery (STIR), and/or a T2-weighted turbo spin-echo sequence with fat suppression. The contrast-enhanced axial and sagittal or coronal images should be obtained using T1-weighted spin-echo with fat suppression [8]. The typical findings would be aggressive radiologic features such as endosteal scalloping, periosteal reaction, and cortical destruction or cortical breaking associated with a soft tissue mass which frequently shows cortical break-through. Additionally, typically heterogeneous enhancement associated with central necrosis is observed (Figs. 2 and 5). Because of intraarticular extension of tumor observed on the MR images, the patient underwent an extraarticular wide resection of the left distal femur (Fig. 6). The patient also received
f
our doses of intravenous high-dose methotrexate (8 g/m2) afterward
.
The biopsy tract was kept intact with an ellipse of skin around it. Reconstruction was performed with a distal femoral replacing rotating hinge TKA. At the 6-month followup, the patient’s disease was stable.
Fig. 6.
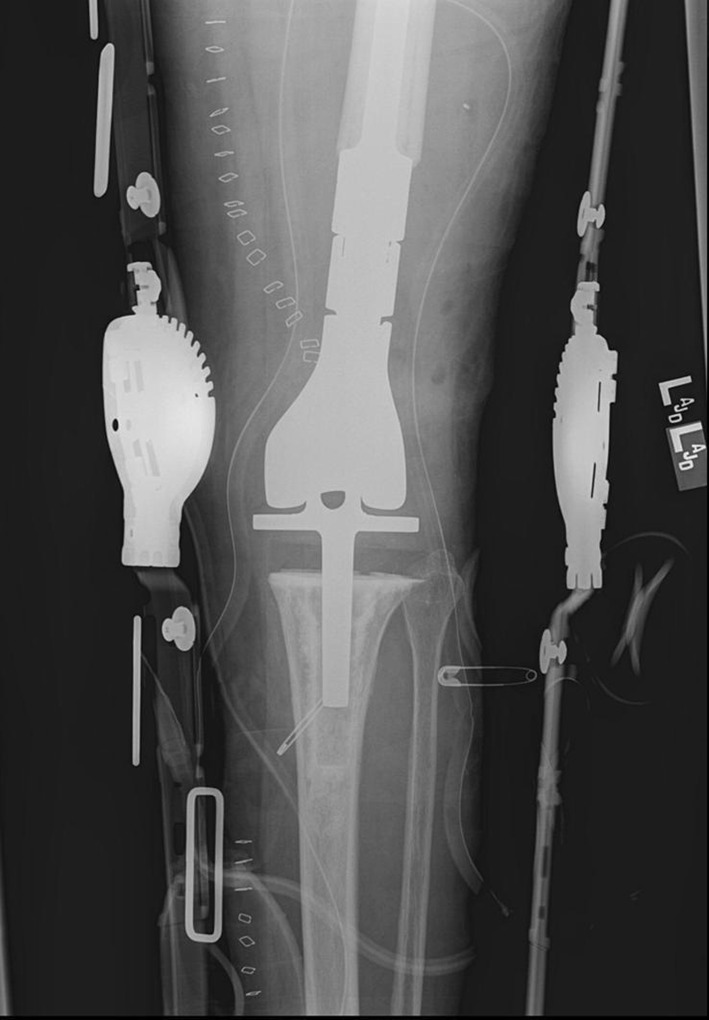
A postoperative plain AP radiograph shows the extraarticular wide resection of the left distal femur with the prosthesis in place.
We believe that for patients with radiographic evidence of bone infarction, any evidence of the above-mentioned aggressive radiographic features and soft tissue mass should be investigated to exclude the presence of an associated sarcoma. MRI is a sensitive and accurate method of investigation in revealing the soft tissue mass, and histopathologic evaluation can confirm the diagnosis.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved or waived approval for the reporting of this case and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Desai P, Perino G, Present D, Steiner GC. Sarcoma in association with bone infarcts: report of five cases. Arch Pathol Lab Med. 1996;120:482–489. [PubMed] [Google Scholar]
- 2.Domson GF, Shahlaee A, Reith JD, Bush CH, Gibbs CP. Infarct-associated bone sarcomas. Clin Orthop Relat Res. 2009;467:1820–1825. doi: 10.1007/s11999-009-0744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douya H, Yokoyama R, Beppu Y, Hasegawa T. Malignant fibrous histiocytoma associated with diaphyseal medullary stenosis. Clin Orthop Relat Res. 2002;400:211–216. doi: 10.1097/00003086-200207000-00026. [DOI] [PubMed] [Google Scholar]
- 4.Duong S, Sallis JG, Zee SY. Malignant fibrous histiocytoma arising within a bone infarct in a patient with sickle cell trait. Int J Surg Pathol. 2004;12:67–73. doi: 10.1177/106689690401200113. [DOI] [PubMed] [Google Scholar]
- 5.Frierson HF, Jr, Fechner RE, Stallings RG, Wang GJ. Malignant fibrous histiocytoma in bone infarct: association with sickle cell trait and alcohol abuse. Cancer. 1987;59:496–500. doi: 10.1002/1097-0142(19870201)59:3<496::AID-CNCR2820590324>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Furey JG, Ferrer-Topper-Torells M, Reagan JW. Fibrosarcoma arising at the site of bone infarcts: a report of 2 cases. J Bone Joint Surg Am. 1960;42:802–810. [PubMed] [Google Scholar]
- 7.Galli S, Weintraub HP, Proppe KH. Malignant fibrous histiocytoma and pleomorphic sarcoma in association with medullary bone infarcts. Cancer. 1978;41:607–619. doi: 10.1002/1097-0142(197802)41:2<607::AID-CNCR2820410227>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 8.Kenan S, Abdelwahab IF, Hermann G, Klein MJ. Malignant fibrous histiocytoma associated with a bone infarct in a patient with hereditary bone dysplasia. Skeletal Radiol. 1998;27:463–467. doi: 10.1007/s002560050420. [DOI] [PubMed] [Google Scholar]
- 9.Matsuno T, Kaneda K, Takeda N. Development of angiosarcoma at the site of a bone infarct. Clin Orthop Relat Res. 1996;327:259–263. doi: 10.1097/00003086-199606000-00032. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy EF, Matsuno T, Dorfman HD. Malignant fibrous histiocytoma of bone: a study of 35 cases. Hum Pathol. 1979;10:57–70. doi: 10.1016/S0046-8177(79)80072-6. [DOI] [PubMed] [Google Scholar]
- 11.Michael RH, Dorfman HD. Malignant fibrous histiocytoma associated with bone infarcts: report of a case. Clin Orthop Relat Res. 1976;118:180–183. [PubMed] [Google Scholar]
- 12.Romeo S, Bovée JV, Kroon HM, Tirabosco R, Natali C, Zanatta L, Sciot R, Mertens F, Athanasou N, Alberghini M, Szuhai K, Hogendoorn PC, Dei Tos AP. Malignant fibrous histiocytoma and fibrosarcoma of bone: a re-assessment in the light of currently employed morphological, immunohistochemical and molecular approaches. Virchows Arch. 2012;461:561–570. doi: 10.1007/s00428-012-1306-z. [DOI] [PubMed] [Google Scholar]
- 13.Torres FX, Kyriakos M. Bone infarct-associated osteosarcoma. Cancer. 1992;70:2418–2430. doi: 10.1002/1097-0142(19921115)70:10<2418::AID-CNCR2820701007>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]


