Abstract
Background
The durability and risks associated with total hip arthroplasty (THA) for patients with a history of Legg-Calvé-Perthes disease (LCPD) are not well known.
Questions/purpose
We sought to (1) determine the survivorship of THAs performed for LCPD; (2) assess hip scores and complications associated with THA in this patient population; and (3) compare results between patients who had undergone surgery in childhood with patients who had conservative treatment.
Methods
We reviewed 99 primary THAs performed in 95 patients with a history of LCPD with minimum 2-year followup (mean ± SD, 8 ± 5 years). Mean age at THA was 48 ± 15 years.
Results
A total of 10 revisions were performed. Using revision for any reason as the end point, the 8-year survival rate was 90% (95% confidence interval [CI], 76%–96%) for cementless implants compared with 86% (95% CI, 57%–96%) for hybrid implants. The mean Harris hip score improved by 31 ± 16 (n = 76). Complications occurred in 16% of hips. The most common major complication was intraoperative fracture (eight femoral, one acetabular). Three patients developed sciatic nerve palsy after a mean lengthening of 2.2 ± 1 cm compared with a mean of 1.4 ± 1 cm in patients with intact sciatic nerve (p = 0.3).
Conclusions
Cementless THAs for the sequelae of LCPD demonstrate 90% survival from any revision at 8 years followup. THAs for the sequelae of LCPD can be complicated and technically difficult. Intraoperative fractures and nerve injuries are common. Care should be taken to avoid excessive limb lengthening.
Level of Evidence
Level IV, retrospective case series. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Legg-Calvé-Perthes disease (LCPD) is believed to represent idiopathic avascular necrosis of the femoral head, which typically develops in children aged 3 to 10 years [7, 15]. Treatments for this condition are diverse, partly because the long-term outcomes of the disease are poorly understood [7]. Historic treatments involved bracing, prolonged bedrest, and immobilization [16]. Prospective data support the use of femoral or pelvic osteotomies for children older than the age of 6 to 8 years at disease onset [8, 9, 21]. A recent prospective series found a 5% rate of THA at 20 years after nonoperative treatment of LCPD [12]. Nevertheless, there are limited data available in the literature regarding the characteristics and complications of THA in patients with a history of LCPD [4, 18, 19]. To refine treatment techniques, it is important to understand the longevity of hips affected by LCPD and identify any risk factors associated with THA in this population.
We undertook a retrospective review of a large total joint arthroplasty registry at a tertiary referral center. In our study, we sought to (1) determine the survivorship of THAs performed for LCPD; (2) assess hip scores and complications associated with THA in this patient population; and (3) compare results between patients with a history of surgery for LCPD at childhood with patients who had been treated conservatively.
Patients and Methods
We performed a review of an established computerized arthroplasty registry at a single tertiary care center and identified all patients with LCPD treated with THA between 1989 and 2009. Over this period, 161 hips in 153 patients with an associated diagnosis of LCPD underwent THA. During this timeframe, a total of 16,051 THAs were performed at our high-volume arthroplasty center. Those patients who did not have a documented childhood history of treated LCPD were excluded (56 hips in 52 patients). The hips that merely had the appearance of previous LCPD at the time of arthroplasty were excluded. This left a total of 105 hips in 101 patients who underwent THA for a confirmed childhood history of LCPD. Therefore, hips replaced as a result of the sequelae of LCPD represented 0.6% of all primary hip arthroplasties performed at our center during the study period.
At our institution, patients undergoing arthroplasty are followed prospectively at regular intervals (3 months, 6 months, 1 year, 5 years, 10 years, and 20 years) [1]. Six patients did not achieve a minimum of 2 years followup and were excluded. This left 95 patients (99 hips) with a minimum of 2 years followup (range, 2–21 years). All patients either had a pediatric radiographic record of childhood LCPD or reported a history of undergoing operative or nonoperative treatment for LCPD. Time to followup is calculated as time from surgery to most recent visit. Fifteen patients had undergone childhood treatment at our center. Thirty hips (30%) had undergone previous childhood surgery, including pelvic osteotomy (five), femoral osteotomy (12), femoral osteotomy plus soft tissue procedure (four), femoral and pelvic osteotomy (one), and soft tissue procedure (eight).
Medical records and radiographs were reviewed for all patients. The radiographs were reviewed before arthroplasty and at latest postoperative followup. Latest radiographs were reviewed by an orthopaedist specializing in adult reconstruction (TMM) for osteolysis around cemented and cementless stems according to Engh et al. and Gruen et al. criteria [2, 5], osteolysis around cemented and cementless cups according to Hodgkinson et al. and Udomkiat et al. criteria [10, 20], and polyethylene wear [13] on AP pelvic radiographs. The leg lengthening assessment was performed by measuring the leg length discrepancy pre- and postoperatively. The leg length discrepancy is measured from the line intersecting the ischial tuberosities to the midpoint of the lesser trochanter on both sides. The total of pre- and postoperative discrepancies represents the leg lengthening. Radiographic measures were multiplied by 0.85 to correct for magnification error. Preoperative, postoperative, and change in leg length discrepancy were recorded.
Our study cohort is comprised of 95 patients (99 hips) who had THA for the sequelae of LCPD with minimum 2-year followup (range, 2–21 years). There were 76 men (79 hips) and 19 women (20 hips). The mean patient age at arthroplasty was 48 ± 15 years with a mean followup of 8 ± 5 years. The mean body mass index (BMI) was 29 ± 6 kg/m2. Thirty hips had a history of childhood surgery. Patients with a history of previous childhood hip surgery were significantly younger at the time of arthroplasty compared with patients who were treated nonoperatively as a child (p = 0.0006). There were no other significant demographic differences between the childhood operative and nonoperative cohorts (Table 1).
Table 1.
Patient demographics based on the childhood management for LCPD
| Demographic | History of surgically treated patients | History of conservatively treated patients | p value |
|---|---|---|---|
| Number of hips (number of patients) | 30 (29) | 69 (66) | |
| Male | 25 | 54 | 0.56 |
| Female | 5 | 15 | |
| Age* at THA (years) | 40 ± 14 | 51 ± 14 | 0.0006 |
| Operative time* (minutes) | 168 ± 65 | 172 ± 63 | 0.75 |
| Blood loss* (mL) | 700 ± 350 | 780 ± 734 | 0.47 |
| Height* (m) | 1.74 ± 0.12 | 1.72 ± 0.09 | 0.28 |
| Weight* (kg) | 91 ± 26 | 85 ± 21 | 0.39 |
| Body mass index* (kg/m2) | 30 ± 7 | 29 ± 6 | 0.67 |
| Reported preoperative Harris hip score (HHS) | 21 | 58 | |
| Preoperative HHS* | 55 ± 8 | 57 ± 10 | 0.47 |
| Time* from THA (years) | 6 ± 4 | 8 ± 5 |
* Value expressed as mean ± SD; LCPD = Legg-Calvé-Perthes disease.
The mean operative time at THA was 171 ± 63 minutes. The mean blood loss was 757 ± 643 mL. Three patients had retained implants requiring removal at the time of arthroplasty. Three patients underwent a transtrochanteric approach. The remaining hips were exposed with either an anterolateral approach (40 hips) or posterior approach (56 hips). The implant design, fixation technique, and bearing surface type were also reviewed (Table 2). The bearing surface was metal on polyethylene in 79 hips, metal on metal in seven hips, ceramic on ceramic in seven hips, and ceramic on polyethylene in six hips. The constructs were cementless in 76 hips, hybrid (cemented stem and cementless cup) in 21 hips, and all cemented in the remaining two hips. The mean clinical followup for cementless and hybrid constructs were 7 ± 5 and 8 ± 4 years, respectively.
Table 2.
Component details
| Cup components |
| DePuy, Warsaw, IN, USA |
| Pinnacle (23), ASR (1), Duraloc Option (1) |
| Howmedica, Mahwah, NJ, USA |
| Trident Hemispherical (6) |
| Implex, Allendale, NJ, USA |
| Hedrocel (11), Elliptical (2) |
| Osteonics, Mahwah, NJ, USA |
| Omnifit PSL (12), Omnifit Dual Geometry (4), Secur Fit Cluster (2), Micro Dual Geometry (1), Omnifit (1), Omnifit Spherical (1), PTH Concentric (1), Trident Hemispherical (1) |
| Richards (Smith & Nephew), Andover, MA, USA |
| Reflection (8) |
| Stryker, Mahwah, NJ, USA |
| Tritanium Hemispherical (1) |
| Zimmer, Warsaw, IN, USA |
| Trilogy (19), HGP II (4) |
| Stem components |
| DePuy |
| Summit (10), Prodigy (6), Solution (3), AML (2) |
| Howmedica |
| Secur-Fit (2), Exeter V40 (1) |
| Implex |
| F-220 Proxilock (8), F-130 Smooth (2), Cobrex (1) |
| J&J, Warsaw, IN, USA |
| S-ROM (8), Summit (2), PFC (1) |
| Osteonics |
| Secur-Fit HA (6), Omnifit HA-M (4), Omniflex Microstructured (3), Secur-Fit HA Plus (3), Omnifit EON Plus (3), Omnifit Plus (2), Omnifit C (2), Omnifit Collared (2), Omnifit EON (2), Omnifit HA (2), HA Omnifit (2), Hemi-Hip (1), Hydroxylapatite Omniflex (1), ODC FX (1), Omnifit (1), Omniflex (1), Omniflex HA (1), Secur-Fit (1), Secur-Fit Plus (1) |
| Zimmer |
| Mayo/Morrey (4), Mayo Conservative (3), Harris Precoat Plus (2), Centralign Option (2), Centralign Precoat (2), HA Proxilock (1) |
Perioperative complications (fractures, sciatic nerve injury, and thromboembolic event), reoperations without component exchange (irrigation and débridement and reduction for hip instability), and revision surgery with component exchange were also reviewed. Harris hip score was calculated preoperatively and at the time of latest clinical followup [6].
Pearson’s chi-square test was used to compare binary variables (sex, complications, fracture, and neurological deficit). Two-tailed Student’s t-test was used to compare continuous variables (age, operative time, blood loss, height, weight, BMI, Harris hip score, and leg length discrepancy measurements). Matched pairs analysis was used to compare pre- and postoperative Harris hip scores. A Kaplan-Meier analysis was used to assess the survival at 8 years for THA and for individual components (femoral stem and cup) using revision for any cause as an end point. Log-rank test was used to compare Kaplan-Meier survival curves for stratified factor (history of childhood surgical treatment for LCPD). Significance was set at a p value < 0.05. All statistical analyses were performed using JMP® software (JMP, Version 9.0.1; SAS Institute Inc, Cary, NC, USA).
Institutional review board approval was obtained for all aspects of this study.
Results
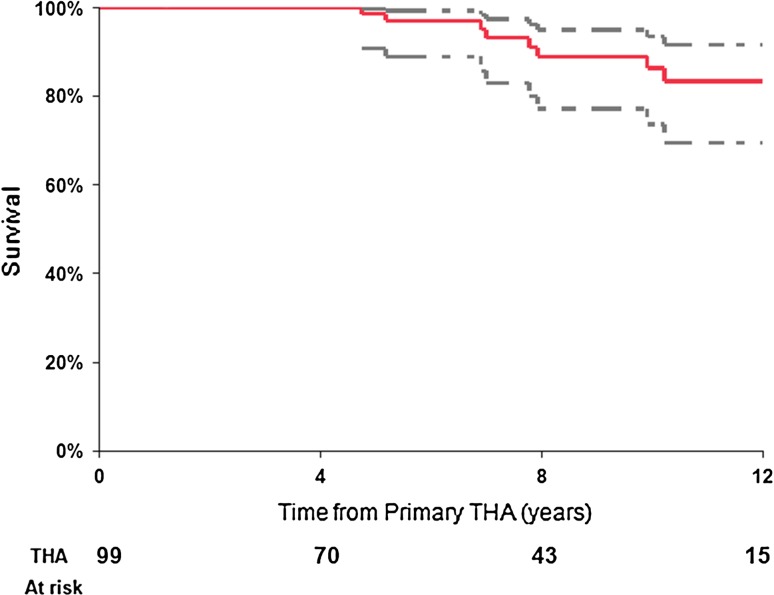
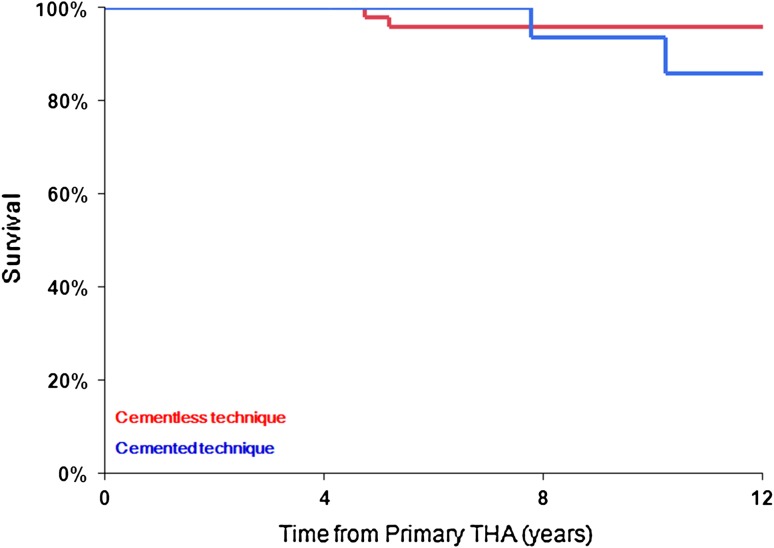
During the followup period, a total of 10 revision surgical procedures were performed (Table 3). Using revision for any reason as an end point, Kaplan-Meier survival estimate at 8 years for the entire cohort (99 THAs) was 89% (95% confidence interval [CI], 77%–95%) (Fig. 1). At 8-year followup, the survival from any revision for cementless implants was 90% (95% CI, 76%–96%); however, hybrid implants showed only 86% survival (95% CI, 57%–96%) (Table 4). Evaluating THA components, the 8-year survival from liner exchange and or acetabular component revision was 94% (95% CI, 83%–98%) and from femoral component revision was 95% (95% CI, 85%–98%). For cementless femoral components, the 8-year survival rate from revision for any reason was 96% (95% CI, 85%–99%) compared with 94% (95% CI, 66%–99%) for cemented femoral components (Fig. 2).
Table 3.
Revision surgical procedures
| Procedure | Number |
|---|---|
| Aseptic loose stem component | 5 |
| Liner wear | 3 |
| Aseptic loose cup component | 1 |
| Resection arthroplasty for deep infection | 1 |
| Total | 10 |
Fig. 1.
Kaplan-Meier survival curve of primary THA (99 THAs) performed for the sequelae of LCPD using revision for any reason as an end point. The dashed lines represent 95% CI.
Table 4.
Kaplan-Meier survival estimates for THA using any revision as an end point
| Type of THA | Number of implants | 4 years | 8 years | 12 years | |||
|---|---|---|---|---|---|---|---|
| Survivorship (95% CI) | Implants at risk | Survivorship (95% CI) | Implants at risk | Survivorship (95% CI) | Implants at risk | ||
| Cementless implants | 76 | 100 | 54 | 90 (76–96) | 29 | 90 (76–96) | 10 |
| Hybrid implants | 21 | 100 | 19 | 86 (57–96) | 13 | 68 (38–88) | 4 |
CI = confidence interval.
Fig. 2.
Kaplan-Meier survival curves of femur stem component based on the construction technique and using revision for any reason as an end point (76 cementless and 23 cemented femur stem components).
At latest followup, the Harris hip scores were available pre- and postoperative for 76 hips. They improved by a mean of 31 ± 16 points (p < 0.001), from 56 (range; 25 to 85) to 88 points (range, 50–100) at latest followup. Latest radiographs were also reviewed for patients (Table 5).
Table 5.
Latest radiographic evaluation for survived THA
| Evaluation | Number of THAs |
|---|---|
| Cup component loosening | |
| Zone 1 | 1 |
| Zone 2 | 5 |
| Zone 1, 2 | 1 |
| Zone 1, 3 | 1 |
| Polyethylene wear | 10 |
| Femoral component loosening | 1 (subsided stem) |
| Greater trochanteric osteolysis | 3 |
| Lesser trochanteric osteolysis | 3 |
Complications were common in this series; of the 99 hips, 16 hips (16%) sustained a total of 17 complications (Table 6). Nine THAs were complicated by intraoperative fracture (eight femoral, one acetabular). Five femoral fractures were treated with cables and wires for stabilization, whereas the remaining three fractures were stable. The acetabular fracture underwent cancellous bone grafting. Neurologic complications at the time of THA were frequent. Three patients developed sciatic nerve palsy; one was permanent and two resolved (Fig. 3). The three patients were lengthened by 2, 1.3, and 3.2 cm (mean, 2.2 cm) at the time of THA compared with the mean of 1.4 ± 1 cm in the patients who did not sustain a neurologic injury (p = 0.3) (Fig. 4). Interestingly, for patients with a nerve injury, the arthroplasty side was overlengthened to 1.2 ± 0.4 cm longer than the unaffected side compared with 0.1 ± 1 cm in patients without a nerve injury (p = 0.024). As an additional complication, one patient died from sepsis as a result of an infected primary THA (Table 6).
Table 6.
Complications
| Perioperative complications | Number of hips |
|---|---|
| Fracture | 9 |
| Neurological deficit | 3 |
| Thromboembolic event | 1 |
| Reoperation without components exchange | |
| Irrigation and débridement (subsequent death from sepsis in one patient) | 2 |
| Closed reduction for instability | 1 |
| Adductor tenotomy for contracture | 1 |
| Total | 17 |
Fig. 3A–B.
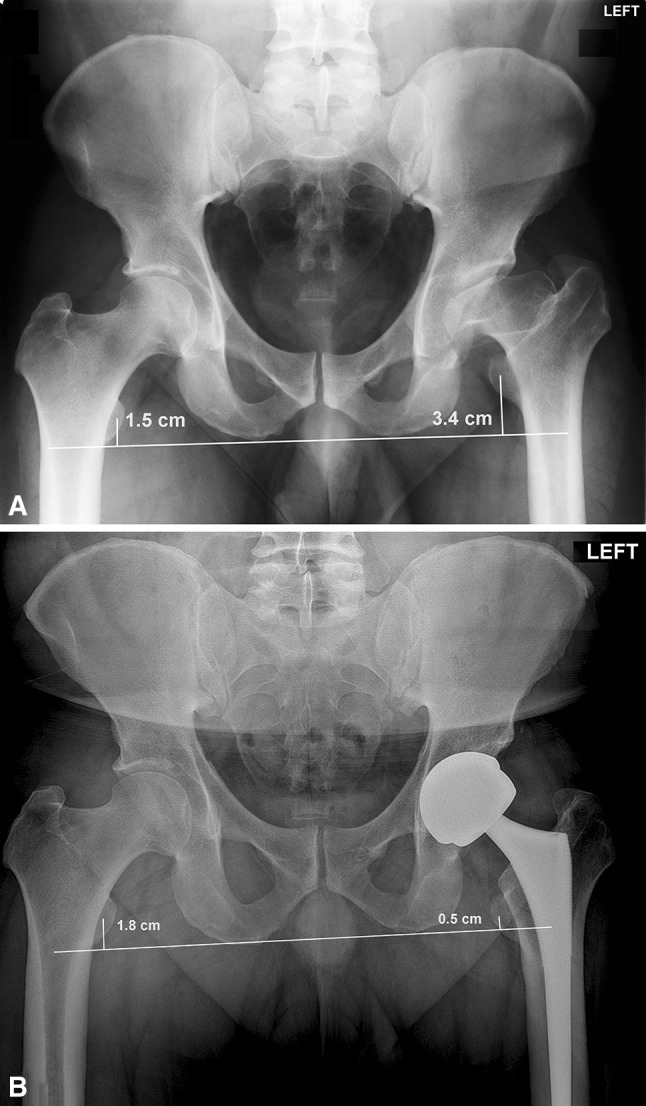
(A) Preoperative AP pelvis film of a 35-year-old man who developed left LCPD at age 5 years treated with bracing and adductor tenotomy. At presentation, the leg length discrepancy is measured at 1.9 cm (3.4–1.5 cm) with the affected leg shorted than the unaffected side. (B) Postoperatively, the leg length discrepancy is 1.3 cm (1.8–0.5) with the affected side now longer than the unaffected side. This represents a total of 3.2 cm of lengthening at the time of arthroplasty. This patient developed a postoperative sciatic nerve injury with foot drop and loss of sensation, which subsequently resolved.
Fig. 4A–B.
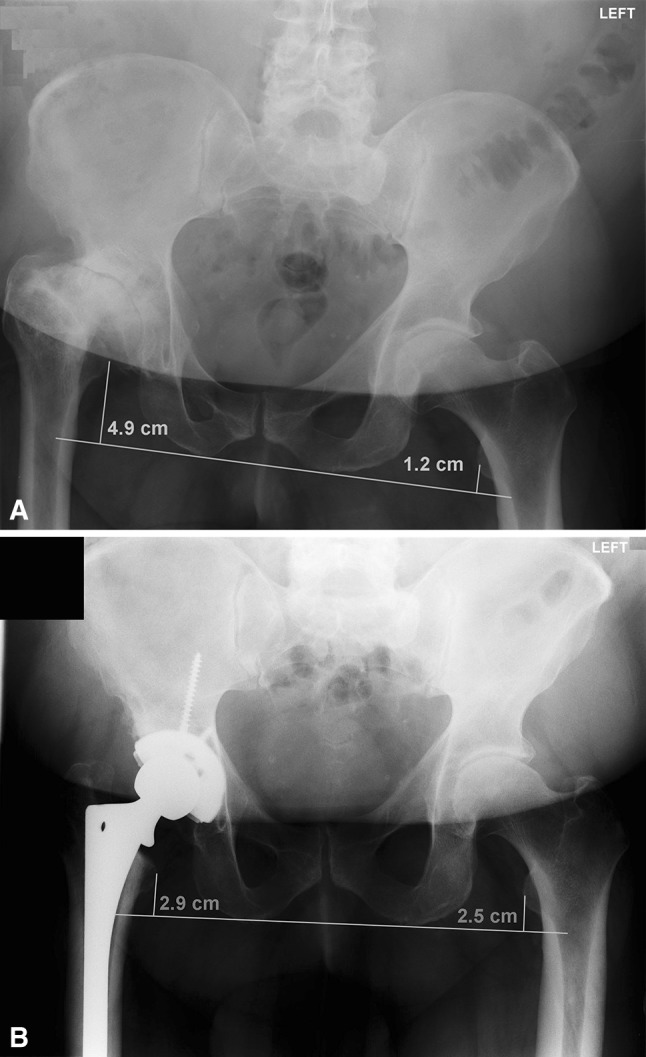
(A) Preoperative AP pelvis film of a 52-year-old man who had right LCPD at age 8 years treated conservatively with a brace. At presentation, the leg length discrepancy is measured at 3.7 cm (4.9–1.2 cm) with the affected leg shorter than the unaffected side. (B) Postoperatively, the leg was lengthened without neurological incident. Now, the leg length discrepancy measured 0.4 cm (2.9–2.5 cm).
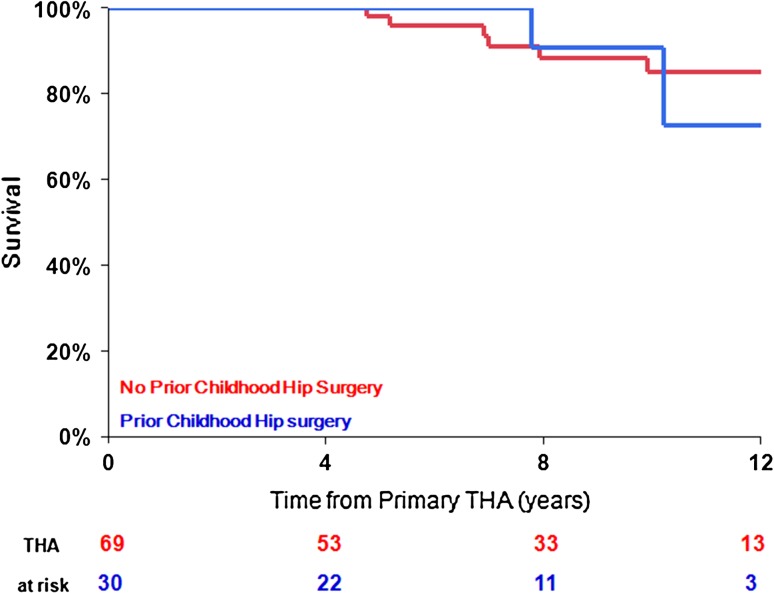
No significant difference in outcomes was detected between patients with LCPD who underwent previous childhood surgery compared with typical patients with LCPD who were treated conservatively (Table 7). However, a higher complication rate and lower implant longevity at 12-year followup were noted in patients with LCPD who underwent previous childhood surgery, although this did not reach statistical significance (Fig. 5).
Table 7.
THA results, complications, and survival from any revision based on the type of childhood management for LCPD
| THA results, general complications, and survival | Surgically treated patients | Conservatively treated patients | p value |
|---|---|---|---|
| Hips | 30 | 69 | |
| General complication rate | 6 (20%) | 10 (14%) | 0.5 |
| Fracture | 2 (7%) | 7 (10%) | 0.6 |
| Neurological deficit | 2 (7%) | 1 (1%) | 0.16 |
| Reported postoperative Harris hip score (HHS) | 29 | 66 | |
| Postoperative HHS | 87 ± 14 | 88 ± 14 | 0.69 |
| Reported HHS change | 20 | 56 | |
| The HHS change | 30 ± 19 | 31 ± 15 | 0.84 |
| THA survival from any revision† at | |||
| 4 years | 100% | 100% | 0.9 |
| THA at risk | 22 | 53 | |
| 8 years | 91% (56–99) | 88% (75–95) | |
| THA at risk | 11 | 33 | |
| 12 years | 73% (32–94) | 85% (70–93) | |
| THA at risk | 3 | 13 | |
* Value expressed as mean ± SD: †values are expressed as the Kaplan-Meier survival estimate, with the 95% confidence interval in parentheses; LCPD = Legg-Calvé-Perthes disease.
Fig. 5.
Kaplan-Meier survival curve of primary THA (99 THAs) performed for the sequelae of LCPD based on a history of surgical management for LCPD in childhood using revision for any reason as an end point.
Discussion
This study evaluates a population of 95 patients treated in childhood for LCPD who later underwent THA. Evaluation of the treatment efforts for LCPD is made more difficult by the long followup that is called for in these relatively young patients to determine the efficacy of treatment [16]. The goal of childhood LCPD treatment is to prevent end-stage arthritis, which today typically is addressed with THA. The present report focuses on a group of patients with LCPD who went on to THA as a result of their pediatric hip diagnosis. We found that although survivorship of these hip reconstructions was generally good, serious complications were frequent.
There are several limitations to the study. Data regarding the childhood treatment course and early radiographs are limited. Thus, we cannot attempt to correlate pediatric treatment of the disease or disease severity with our reported results of THA, because these data were deficient. Although we have survivorship data by implant type (cementless, hybrid, and cemented), this comparison was not a primary study end point, and a number of confounding variables likely affected these results (including selection bias, implant differences, and modifications in technique over time); thus, we would consider these comparisons hypothesis-generating for future research rather than conclusive.
In our series, modern cementless implants had acceptable survivorship of 90% at 8-year followup. The only previous report of THA survivorship in patients with a history of LCPD cites a 96% survivorship at 15 years for primarily ingrowth components [19]. Schmitz et al. reported a 90% 10-year survival rate for patients younger than age 30 years undergoing cemented arthroplasty for a variety of indications [14]. Takenaga et al. report 86% 10- to 15-year survivorship for uncemented implants in patients younger than age 50 years [17]. Thus, our series compares favorably with other reports of THA in a younger cohort of patients.
However, we found a high rate of neurologic injury in patients with a history of LCPD undergoing THA. Nearly 3% of patients sustained a postoperative neurologic deficit with one patient sustaining a permanent deficit. This is much higher than the reported risk of 0.17% of patients undergoing arthroplasty for any cause [3]. Although hip dysplasia has long been known to be a risk factor for neurologic complications, LCPD has not typically been thought of as a risk factor for sciatic nerve palsy [3]. One small case series also reports a high rate of neurologic deficit in patients with LCPD undergoing arthroplasty (6% [two of 32 patients]) [19]. Perhaps the longstanding nature of the shortened limb may put the patient at increased risk of neurologic injury compared with the typical patient with osteoarthritis. For patients with LCPD with severe length leg discrepancy, shortening osteotomies may be considered, similar to the severe dysplasia population [11]. The other study also found a high risk of femoral fracture (3%) [19] compared with 9% in our series. A history of childhood surgery or femoral osteotomy was not associated with intraoperative fracture rate in our analysis.
Finally, this article sought to compare results in patients with LCPD who had surgical treatment as a child versus those patients who had nonoperative treatment. We found no significant differences between these two cohorts, although patients with childhood surgery were typically younger at the time of arthroplasty, likely reflecting the changing trends in treatment of LCPD over the last decades. Conservative management of LCPD was the standard treatment until recent decades.
Patients with LCPD who undergo THA represent a therapeutic challenge. Fractures and motor nerve palsies are more frequent in this population. Future research to alleviate lifetime morbidity in patients with LCPD should be focused on delaying end-stage arthritis in the young adult and on approaches to THA in the younger patient that will increase the durability of the reconstructions.
Footnotes
One of the authors (TMM) is a consultant for Zimmer (Warsaw, IN, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Berry DJ, Kessler M, Morrey BF. Maintaining a hip registry for 25 years. Mayo Clinic experience. Clin Orthop Relat Res. 1997;344:61–68. doi: 10.1097/00003086-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br. 1987;69:45–55. doi: 10.1302/0301-620X.69B1.3818732. [DOI] [PubMed] [Google Scholar]
- 3.Farrell CM, Springer BD, Haidukewych GJ, Morrey BF. Motor nerve palsy following primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87:2619–2625. doi: 10.2106/JBJS.C.01564. [DOI] [PubMed] [Google Scholar]
- 4.Gent E, Clarke NM. Joint replacement for sequelae of childhood hip disorders. J Pediatr Orthop. 2004;24:235–240. doi: 10.1097/01241398-200403000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Gruen TA, McNeice GM, Amstutz HC. ‘Modes of failure’ of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed] [Google Scholar]
- 6.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 7.Herring JA. The treatment of Legg-Calve-Perthes disease. A critical review of the literature. J Bone Joint Surg Am. 1994;76:448–458. doi: 10.2106/00004623-199403000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Herring JA. Legg-Calve-Perthes disease at 100: a review of evidence-based treatment. J Pediatr Orthop. 2011;31:S137–140. doi: 10.1097/BPO.0b013e318223b52d. [DOI] [PubMed] [Google Scholar]
- 9.Herring JA, Kim HT, Browne R. Legg-Calve-Perthes disease. Part II: Prospective multicenter study of the effect of treatment on outcome. J Bone Joint Surg Am. 2004;86:2121–2134. [PubMed] [Google Scholar]
- 10.Hodgkinson JP, Shelley P, Wroblewski BM. The correlation between the roentgenographic appearance and operative findings at the bone-cement junction of the socket in Charnley low friction arthroplasties. Clin Orthop Relat Res. 1988;228:105–109. [PubMed] [Google Scholar]
- 11.Krych AJ, Howard JL, Trousdale RT, Cabanela ME, Berry DJ. Total hip arthroplasty with shortening subtrochanteric osteotomy in Crowe type-IV developmental dysplasia. J Bone Joint Surg Am. 2009;91:2213–2221. doi: 10.2106/JBJS.H.01024. [DOI] [PubMed] [Google Scholar]
- 12.Larson AN, Sucato DJ, Herring JA, Adolfsen SE, Kelly DM, Martus JE, Lovejoy JF, Browne R, Delarocha A. A prospective multicenter study of Legg-Calve-Perthes disease: functional and radiographic outcomes of nonoperative treatment at a mean follow-up of twenty years. J Bone Joint Surg Am. 2012;94:584–592. doi: 10.2106/JBJS.J.01073. [DOI] [PubMed] [Google Scholar]
- 13.McCalden RW, Naudie DD, Yuan X, Bourne RB. Radiographic methods for the assessment of polyethylene wear after total hip arthroplasty. J Bone Joint Surg Am. 2005;87:2323–2334. doi: 10.2106/JBJS.E.00223. [DOI] [PubMed] [Google Scholar]
- 14.Schmitz MW, Busch VJ, Gardeniers JW, Hendriks JC, Veth RP, Schreurs BW. Long-term results of cemented total hip arthroplasty in patients younger than 30 years and the outcome of subsequent revisions. BMC Musculoskelet Disord. 2013;14:37. doi: 10.1186/1471-2474-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoenecker PL. Legg-Calve-Perthes disease. Orthop Rev. 1986;15:561–574. [PubMed] [Google Scholar]
- 16.Stulberg SD, Cooperman DR, Wallensten R. The natural history of Legg-Calve-Perthes disease. J Bone Joint Surg Am. 1981;63:1095–1108. [PubMed] [Google Scholar]
- 17.Takenaga RK, Callaghan JJ, Bedard NA, Liu SS, Klaassen AL, Pedersen DR. Cementless total hip arthroplasty in patients fifty years of age or younger: a minimum ten-year follow-up. J Bone Joint Surg Am. 2012;94:2153–2159. doi: 10.2106/JBJS.L.00011. [DOI] [PubMed] [Google Scholar]
- 18.Thillemann TM, Pedersen AB, Johnsen SP, Soballe K. Implant survival after primary total hip arthroplasty due to childhood hip disorders: results from the Danish Hip Arthroplasty Registry. Acta Orthop. 2008;79:769–776. doi: 10.1080/17453670810016830. [DOI] [PubMed] [Google Scholar]
- 19.Traina F, De Fine M, Sudanese A, Calderoni PP, Tassinari E, Toni A. Long-term results of total hip replacement in patients with Legg-Calve-Perthes disease. J Bone Joint Surg Am. 2011;93:e25. doi: 10.2106/JBJS.J.00648. [DOI] [PubMed] [Google Scholar]
- 20.Udomkiat P, Wan Z, Dorr LD. Comparison of preoperative radiographs and intraoperative findings of fixation of hemispheric porous-coated sockets. J Bone Joint Surg Am. 2001;83:1865–1870. doi: 10.2106/00004623-200112000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Wiig O, Terjesen T, Svenningsen S. Prognostic factors and outcome of treatment in Perthes’ disease: a prospective study of 368 patients with five-year follow-up. J Bone Joint Surg Br. 2008;90:1364–1371. doi: 10.1302/0301-620X.90B10.20649. [DOI] [PubMed] [Google Scholar]