Abstract
Background
Rotational kinematics has become an important consideration after ACL reconstruction because of its possible influence on knee degeneration. However, it remains unknown whether ACL reconstruction can restore both rotational kinematics and normal joint contact patterns, especially during functional activities.
Questions/purposes
We asked whether knee kinematics (tibial anterior translation and axial rotation) and joint contact mechanics (tibiofemoral sliding distance) would be restored by double-bundle (DB) or single-bundle (SB) reconstruction.
Methods
We retrospectively studied 17 patients who underwent ACL reconstruction by the SB (n = 7) or DB (n = 10) procedure. We used dynamic stereo x-ray to capture biplane radiographic images of the knee during downhill treadmill running. Tibial anterior translation, axial rotation, and joint sliding distance in the medial and lateral compartments were compared between reconstructed and contralateral knees in both SB and DB groups.
Results
We observed reduced anterior tibial translation and increased knee rotation in the reconstructed knees compared to the contralateral knees in both SB and DB groups. The mean joint sliding distance on the medial compartment was larger in the reconstructed knees than in the contralateral knees for both the SB group (9.5 ± 3.9 mm versus 7.5 ± 4.3 mm) and the DB group (11.1 ± 1.3 mm versus 7.9 ± 3.8 mm).
Conclusions
Neither ACL reconstruction procedure restored normal knee kinematics or medial joint sliding.
Clinical Relevance
Further study is necessary to understand the clinical significance of abnormal joint contact, identify the responsible mechanisms, and optimize reconstruction procedures for restoring normal joint mechanics after ACL injury.
Introduction
Abnormal knee rotation occurs after ACL injury and is not corrected by conventional single-bundle (SB) ACL reconstruction [1, 8, 13, 15, 29, 30, 32, 33]. Abnormal knee kinematics (translations, rotations) has been implicated in the initiation and progression of knee osteoarthritis secondary to altered normal joint contact [5].
Double-bundle (DB) ACL reconstruction was introduced in an effort to better restore native ACL anatomy and improve knee kinematics [7, 39]. Recent reports suggest DB reconstruction may restore normal rotational kinematics during functional activity [18, 26]. Restoration of normal joint contact mechanics may be a primary determinant for long-term joint health, especially when meniscus and/or cartilage are not damaged at the time of injury. This could be especially important for highly active patients, for whom the combination of abnormal contact mechanics and larger cumulative joint loading could increase risk for cartilage degeneration. However, it remains unclear whether restoration of rotational kinematics is sufficient to reestablish normal joint contact mechanics.
Most previous studies exploring joint contact mechanics after ACL injury and reconstruction have focused on the tibial surface [6, 27, 28, 37], though joint contact mechanics is defined between two articulating surfaces. Joint sliding distance, which compares femoral and tibial joint contact paths, was introduced as a kinematic surrogate for joint shear stress [21]. By accounting for both motion and joint surface geometry, joint sliding distance is more closely related to cartilage contact mechanics than simple rotational/translational knee kinematics.
We therefore determined whether anatomic ACL reconstruction (using either the SB or DB technique) was sufficient to restore normal knee kinematics (tibial anterior translation and axial rotation) and joint contact mechanics (tibiofemoral sliding distance).
Patients and Methods
We retrospectively recruited 17 patients who previously underwent anatomic ACL reconstruction. During the period from July 2007 to December 2009, more than 300 patients who had primary ACL reconstruction were screened. Those who passed screening criteria (Table 1) and agreed to participate were included in the study. In eight of 17 patients, the choice of SB versus DB reconstruction was based on the size of the native ACL footprint (measured intraoperatively), using a previously described decision algorithm [36]. For the remaining patients, the choice of surgical procedure was at the discretion of the surgeon and patient. Consequently, seven patients had SB ACL reconstruction (SB group), while 10 patients had DB reconstruction (DB group). There were differences in sex, age, weight, height, and duration after surgery between groups (Table 2). An a priori power analysis to determine sample size was not performed for this study. However, this sample size was justified based on previous studies using similar measurement techniques that have identified differences between ACL-reconstructed and contralateral (uninjured) limbs in as few as six patients after nonanatomic, SB reconstruction [13, 32, 33]. This study was approved by our institution’s institutional review board, and informed consent was acquired from all study participants.
Table 1.
Inclusion and exclusion criteria
| Type of criteria | Criteria |
|---|---|
| Inclusion | 4 months to 2 years postsurgery |
| Finished rehabilitation and will be able to perform daily activities without symptoms, including at least light running | |
| Rest of the knee ligaments will be intact; however, subjects may have sustained meniscal damage with or without repair or partial meniscectomy affecting not more than 1/3 of the meniscus. | |
| At least 18 years of age | |
| BMI between 20 and 35 | |
| Free of obvious joint effusion and capable of performing the laboratory tasks without difficulty, limping, or substantial pain | |
| Exclusion | Any prior surgical procedure(s) that grossly disrupt(s) anatomy or function of either lower extremity, with the exception of the over-the-top group, which will allow previous ACL reconstruction |
| Other known injury or disease that would interfere with lower-extremity function | |
| Knee pain determined to substantially affect gait | |
| Pregnancy; women of childbearing potential will be screened for pregnancy before each test and any woman testing positively for pregnancy at any time during the study will be excluded from further study participation for the duration of her pregnancy | |
| Receiving radiation therapy |
Table 2.
Demographic data of the SB and DB groups
| Variable | SB group | DB group | Difference |
|---|---|---|---|
| Number of patients | 7 | 10 | |
| Age (years)* | 28 ± 10 | 36 ± 10 | 8 (95% CI, 3–18) |
| Sex (male:female) (number of patients) | 2:5 | 5:5 | p = 0.62† |
| Height (cm)* | 169 ± 10 | 171 ± 13 | 3 (95% CI, 9–15) |
| Weight (kg)* | 64 ± 11 | 73 ± 19 | 11 (95% CI, 6–27) |
| Time after surgery (months)* | 14 ± 4 | 13 ± 6 | 0 (95% CI, 5–6) |
* Values are expressed as average ± SD; †tested by Fisher’s exact test; SB = single bundle; DB = double bundle.
ACL reconstruction was performed by several different surgeons within the same institution, using a three-portal independent drilling technique [12, 17]. DB reconstruction was performed according to previous reports [9, 14, 36, 37]. The femoral tunnel in the SB group and the posterolateral (PL) tunnel in the DB group were drilled through the anteromedial (AM) portal. The femoral AM tunnel was drilled through the PL tibial tunnel in eight patients in the DB group, while transportal drilling was used to drill the femoral AM tunnel in the other two patients [37]. The native insertion sites of the AM and PL bundle footprints were identified and marked by a thermal device (Arthrocare, Austin, TX, USA) on both the femur and the tibia, and the tunnels were created at the center of each AM and PL bundle footprint for the DB procedure or between the AM and PL bundle footprint centers for the SB procedure. Femoral/tibial tunnel diameter was 7.5 to 9 mm in the SB group and 6 to 7 mm for the PL bundle and 7 to 8 mm for the AM bundle in the DB group. A single tunnel was placed in the middle of the native ACL footprint for SB procedures, while two tunnels for the AM and PL bundles were located in the middle of each bundle for DB procedures (Fig. 1). Allograft was used in all patients in the DB group and four patients in the SB group, while autografts were utilized in three patients in the SB group (two hamstrings, one patellar tendon). The EndoButton® CL (Smith & Nephew Inc, Memphis, TN, USA) was used for fixation of the femoral side and a bioabsorbable screw (Smith & Nephew) was used for the tibial side. A standardized postoperative rehabilitation program was prescribed for both groups [22]. All the subjects in this study were closely monitored to ensure they successfully completed their routine rehabilitation program and could perform daily activities without symptoms, including at least light running.
Fig. 1A–B.
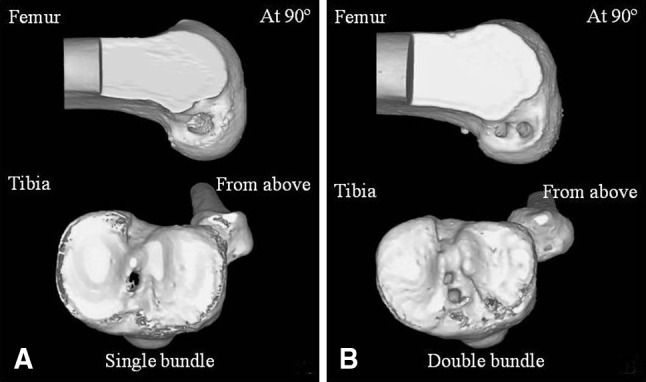
Typical CT images of ACL-reconstructed knees using the (A) SB and (B) DB techniques are shown.
In vivo knee kinematics data were obtained during downhill running using a dynamic stereo x-ray (DSX) system and model-based tracking technique, as previously reported [2, 3, 13, 21, 32, 33] and briefly described below. The patients performed moderate-speed running (2.5 m/second) on an instrumented dual-belt treadmill (Bertec Corp, Columbus, OH, USA) with a 10° downward slope (Fig. 2). We selected downhill running for the testing activity because it generates greater shear stress on the knee [25] and has been used previously to identify abnormal knee kinematics after conventional SB reconstruction [32, 33]. We collected three trials for each limb of each patient. For each trial, pairs of radiographic images were acquired simultaneously by the DSX system during the stance phase of running (0.6-second acquisitions). The DSX system consisted of two custom-built gantries configured with beam paths intersecting at 60° in a plane parallel to the floor (Fig. 2). Each gantry contained an x-ray source driven by a 100-kW pulsed generator (CPX 3100CV; EMD Technologies, Inc, Saint-Eustache, Quebec, Canada), a 40-cm image intensifier (Thales, Neuilly-sur-Seine, France), and a high-speed 4-megapixel digital video camera (Phantom® v10; Vision Research, Wayne, NJ, USA). Images were acquired at 150 Hz with a 90-kVp/150-mA protocol, using 1-millisecond pulsed exposures to reduce exposure and minimize motion blur. High-resolution (0.625-mm slices, 0.5-mm in-plane resolution) CT scans were acquired from 10 cm above to 10 cm below the joint line, along with single slices through the femoral head and ankle to establish long bone axes (LightSpeed® 16; GE Healthcare, Waukesha, WI, USA). Three-dimensional (3-D) bone models were generated using medical imaging software (Mimics®; Materialise Inc, Leuven, Belgium) to segment bone from soft tissue. Position and orientation of the tibia and femur were determined for each frame using a model-based tracking technique to align the radiographic image pairs to digitally reconstructed radiographs generated by projection through the 3-D bone model as described previously [2].
Fig. 2.
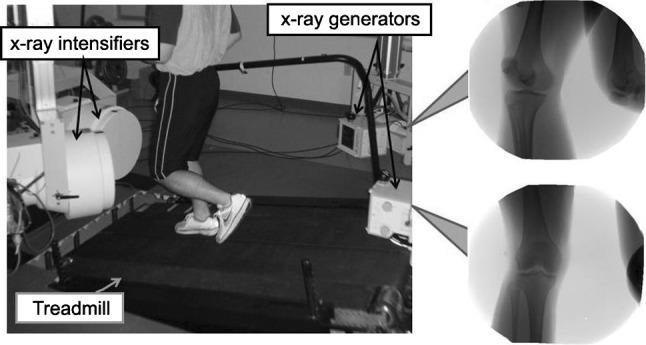
The DSX system is shown. Two-way dynamic x-ray images are captured by high-speed digital video cameras installed on the x-ray intensifiers during downhill running (10° decline).
We defined the anatomic coordinate systems from 3-D bone models as described previously [2, 13, 21, 32, 33]. Tibiofemoral translations were defined as the orthogonal components of a vector from the femoral anatomic origin (midpoint between the centers of the medial and lateral condyles) to the tibial anatomic origin (center of the tibial plateau), expressed in the tibial coordinate system. We calculated knee kinematics using the conventions originally described by Grood and Suntay [16]. This kinematics measurement system has been used extensively for similar studies and is capable of tracking bone motion with precision in the order of 0.2 mm/0.2° [2]. During downhill running, tibial anterior translation and axial rotation were determined [21, 32, 33], and the magnitude of tibial anterior translation and axial rotation from foot strike to 0.1 second after foot strike were calculated for further analysis.
To measure joint contact sliding distance, the joint contact point on the surface of the femur and tibia was estimated using the distance-weighted centroid of the region of closest proximity at each time frame [2, 21]. Tibial joint contact points (xn, yn, zn) were described for the nth frame using a Cartesian coordinate system aligned with the tibial plateau. Femoral contact points (In, Rn, Jn) were described using a cylindrical coordinate system fitted to the distal femoral condyles. Contact path excursions were calculated for the femur (Equation 1) and tibia (Equation 2) in the sagittal plane during early stance (0 to 0.1 second after foot strike) by sequentially summing the sagittal plane distance between the joint contact points at consecutive frames (Fig. 3) as follows:
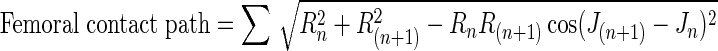 |
1 |
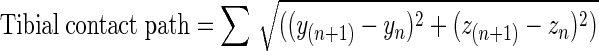 |
2 |
Fig. 3.
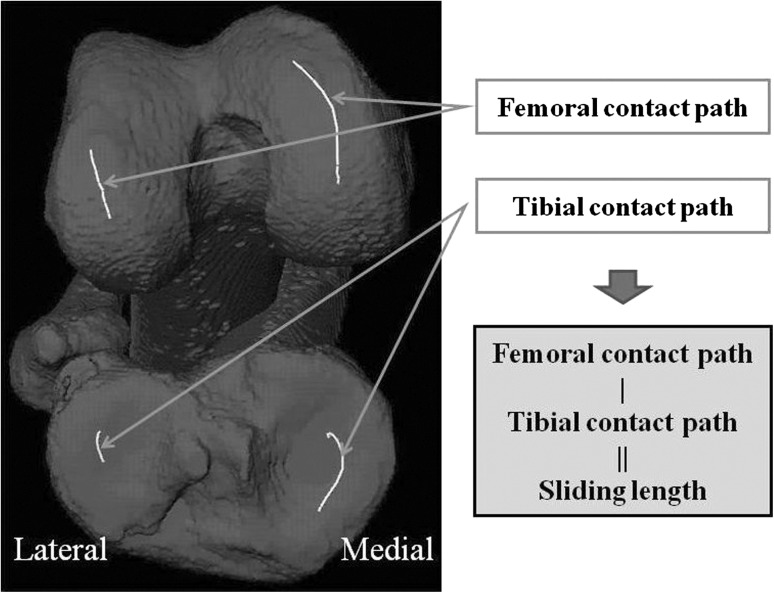
Dynamic joint contact path and sliding distance calculation are shown. Sliding distance is calculated for both compartments.
Femoral contact path calculations were conducted only in the sagittal plane because the mediolateral movement of the contact point was relatively small (1.4 ± 1.1 mm medial, 0.4 ± 1.7 mm lateral) compared to the sagittal-plane movement (8.8 ± 3.5 mm medial, 3.0 ± 4.4 mm lateral) and therefore had minimal influence on the overall shear motion. The joint sliding distance was estimated as described by Hoshino and Tashman [21] to assess shear motions between the femoral and tibial joint surfaces. Typically, the joint contact points on the femur and tibia move backward as the knee flexes after foot strike. For pure rolling, the relative velocity of the tibial and femoral contact points is zero and the contact paths would have equal length. However, surface motion at the knee typically combines both sliding and rolling motions. Therefore, the difference between the lengths of the tibial and femoral contact paths in each medial and lateral compartment is representative of the amount of sliding on each compartment (Fig. 4).
Fig. 4.
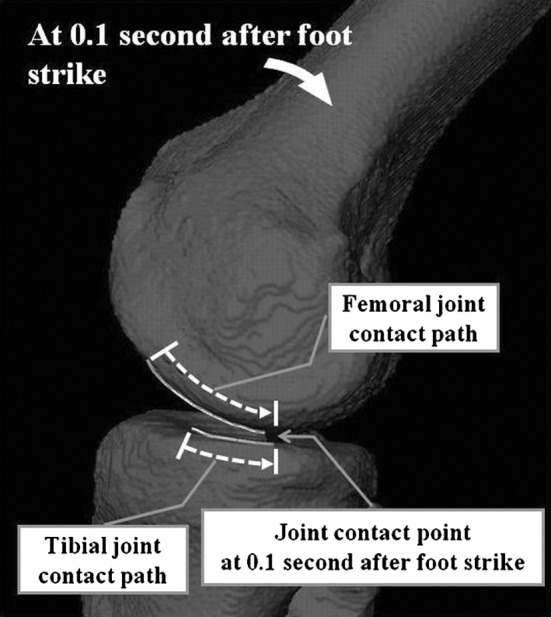
A typical example of the joint contact path on the medial side is shown. After foot strike, the joint contact point moves backward by a combined rolling and sliding movement. Joint contact path is larger on the femoral side.
Separate two-way mixed-model ANOVAs were performed to evaluate differences in tibial anterior translation, axial rotation range, and sliding distance of the medial and lateral compartments between reconstructed and contralateral knees and between SB and DB ACL reconstructions. For compartmental sliding distance, the difference in medial and lateral compartment sliding was determined for each knee. These differences in sliding distance were compared between reconstructed and contralateral knees and between SB and DB ACL reconstructions. In the statistical analyses, main effects were evaluated for side (ACL reconstructed versus contralateral knee), group (SB versus DB), and the interaction between group and side. The a priori alpha level was set at 0.05. We performed all statistical calculations using PASW® Statistics 18 (IBM Corp, Armonk, NY, USA).
Results
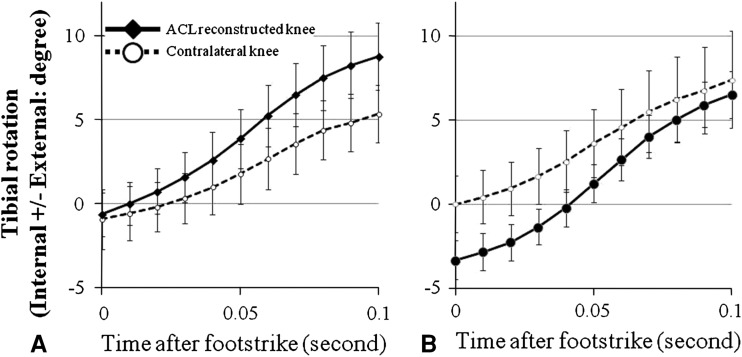
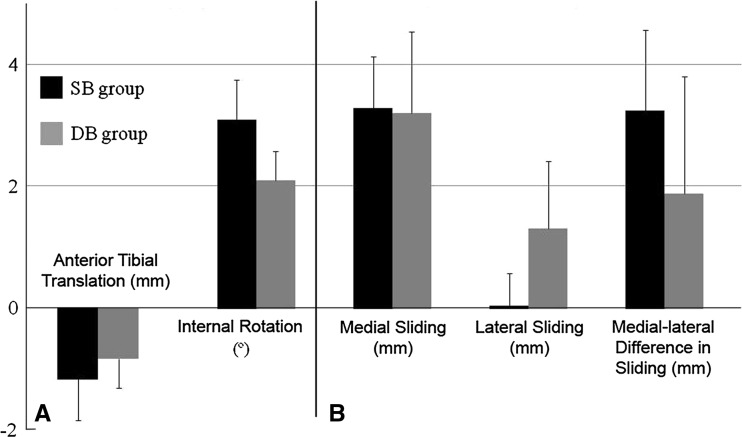
Differences between reconstructed and contralateral knees were observed in tibial anterior translation and tibial rotation for the combined SB and DB groups. There was a decrease (p = 0.028) in tibial anterior translation in the reconstructed knees compared to the contralateral knees. In general, the tibia rotated internally after foot strike (Fig. 5), and tibial internal rotation in the reconstructed knees was larger (p = 0.010) than in the contralateral knees. The sliding distance on the medial compartment was larger (p < 0.001) in the reconstructed knees than in the contralateral knees while the sliding distance on the lateral compartment was similar (p = 0.493) between the reconstructed and the contralateral knees. The difference in the sliding distance between the medial and lateral compartments was also larger (p = 0.029) in the reconstructed knees than in the contralateral, uninjured knees in both the SB and DB groups (Table 3).
Fig. 5A–B.
Graphs show the tibial axial rotation (0.01-second intervals) during the midstance phase of running (0 to 0.1 second after foot strike) in the (A) SB and (B) DB groups. Values are expressed as average ± standard error.
Table 3.
Results of knee kinematics and sliding distance during the midstance phase of running (0 to 0.1 second after foot strike) in the SB and DB groups
| Variable | SB | DB | p value* | ||
|---|---|---|---|---|---|
| Reconstructed | Contralateral | Reconstructed | Contralateral | ||
| Tibial anterior translation (mm) | 4.7 ± 1.6† | 5.9 ± 1.9 | 4.2 ± 1.6† | 5.1 ± 2.2 | 0.028 |
| Tibial internal rotation (°) | 9.5 ± 3.9† | 7.5 ± 4.3 | 9.4 ± 3.6† | 7.9 ± 3.8 | 0.01 |
| Medial sliding distance (mm) | 9.4 ± 3.5‡ | 6.1 ± 3.3 | 11.1 ± 1.3‡ | 7.9 ± 3.8 | < 0.01 |
| Lateral sliding distance (mm) | 2.5 ± 2.3 | 2.5 ± 4.3 | 4.0 ± 4.4 | 2.7 ± 5.9 | 0.21 |
| Medial-lateral difference in sliding distance (mm) | 6.9 ± 3.4† | 3.7 ± 3.8 | 7.1 ± 4.4† | 5.2 ± 6.0 | 0.029 |
Values are expressed as average ± SD; * determined for side-to-side differences using two-way ANOVA considering both types of ACL reconstruction; †significantly different from contralateral knee (p < 0.05); ‡significantly different from contralateral knee (p < 0.01); SB = single bundle; DB = double bundle.
For surgical and contralateral limbs combined, no effects of the surgical technique, whether SB or DB, were identified for any of the knee kinematics or joint sliding measurements: tibial anterior translation (p = 0.589), axial rotation (p = 0.917), medial sliding distance (p = 0.228), lateral sliding distance (p = 0.677), and sliding distance difference between medial and lateral compartments (p = 0.684) (Table 3).
Side-to-side (reconstructed versus contralateral) differences were similar between SB and DB procedures for tibial anterior translation (p = 0.876), axial rotation (p = 0.642), and sliding distance difference between medial and lateral compartments (p = 0.549) (Fig. 6).
Fig. 6A–B.
Graphs show the side-to-side differences (ACL-reconstructed knees − contralateral knees) in (A) knee kinematics and (B) joint sliding distance during the midstance phase of running (0 to 0.1 second after foot strike) in the SB and DB groups. Values are expressed as average ± standard error. There was no difference between the SB and DB groups.
Discussion
Abnormal rotational knee kinematics occurs after ACL injury and remains even after conventional SB ACL reconstruction [1, 8, 13, 15, 29, 30, 32, 33]. The ability of DB ACL reconstruction to restore normal rotational kinematics during functional activity remains controversial [18, 20, 24, 26]. We therefore determined whether the in vivo knee kinematics (tibial anterior translation and axial rotation) and joint contact mechanics (tibiofemoral sliding distance) during a high-demand, functional activity (downhill running) would be restored to normal by the DB or SB ACL reconstruction. In this study, neither SB nor DB reconstruction restored normal joint kinematics or contact mechanics.
The primary aim of this study was to test whether the SB or DB reconstruction procedure would restore the knee kinematics and joint contact mechanics of the affected knees to those of the contralateral normal knees. However, the study was not powered to make direct comparisons between the SB and DB procedures. Also, multiple surgeons performed the surgery. This could have introduced variability within the treatment groups, particularly in regard to tunnel placement. Since development of surgical procedures was ongoing throughout the course of this study, some variation in tunnel placement may have occurred, leading to increased variability of results across patients. Prospective patient recruitment and random assignment of surgical method to reconstruct the ACL with standardized operative techniques are needed to reduce bias. A second limitation of this study was that the study sample size was insufficient to control for potentially confounding variables between the SB and DB groups. Although we attempted to compare SB and DB ACL reconstructions, the main focus of this study was to test the restoration of knee kinematics and joint contact mechanics in two different ACL reconstruction procedures rather than to compare SB and DB procedures. Based on our findings concerning knee kinematics and joint contact kinematics, sufficient power (80%) for a side-to-side comparison of those parameters would have required a sample size of 40 subjects. An even larger number of patients would have been required to directly compare the effects of SB and DB ACL reconstruction and to perform multifactorial analysis concerning other factors, such as sex, age, activity level, and graft material. Such a study can be (and has been) designed based on these results [23]. Third, three of seven patients in the SB group used autograft (two hamstring, one patellar tendon), whereas the other four patients in the SB group and all 10 patients in the DB group utilized allograft. Some would argue allograft may be more likely to be associated with abnormal knee kinematics. Previous reports also used a mixture of different graft materials and surgical procedures to demonstrate abnormal knee kinematics [13, 32, 33]. Allograft might provide some advantage of preserving function of the muscles surrounding the knee, which may have contributed to the lack of side-to-side differences in kinematics in the DB group; however, these individuals still had differences in joint contact mechanics. Also, at the time of this investigation, allograft was often used for DB procedures. It was hard to obtain sufficient graft volume with autograft for DB procedures until we found the quadriceps tendon can constantly provide enough volume of graft material for either SB or DB procedures [35]. Lastly, it remains unknown how much the demonstrated differences in knee rotation and joint sliding distance in this study affect clinical outcome and long-term knee health. Genetic factors, concomitant injuries in meniscus or cartilage, and patient activity level might have greater impact on degenerative change of the knee in the long run. However, it has been reported abnormal knee rotation can lead to cartilage thinning of the joint surfaces [4], and rotational knee kinematics and joint sliding distance balance between the medial and lateral compartments are related during in vivo functional activity [21]. Thus, both abnormal knee rotation and joint sliding distance might impact cartilage degeneration and clinical outcomes. Assessing these effects will require further studies with long-term followup.
Advocates of DB ACL reconstruction propose DB reconstruction could provide better rotational stability than SB reconstruction based on several in vitro studies [7, 31, 39, 40], although other in vitro studies do not support this [19, 20, 24]. Several recent in vivo studies using dynamic, functional activities (ie, jumping and cutting) [13, 18, 26] demonstrated rotational kinematics after DB ACL reconstruction were closer to normal than that after SB ACL reconstruction while other reports of rotational kinematics during walking, descending from a platform, and pivoting [11, 34] have found no differences between SB and DB ACL reconstructions. The disparity of those results could be due largely to the varied loading conditions employed. In this study, we found abnormal knee rotations during downhill running after SB reconstruction, similar to previous reports [32, 33], and after DB reconstruction.
Our findings suggest abnormal knee kinematics (rotations, translations) are associated with altered joint contact kinematics. Analyses that combine bone motion with joint surface geometry are better suited for determining joint contact location and motion and may therefore be more relevant to joint cartilage biology and risk of cartilage degeneration [10]. Andriacchi et al. [5] suggested abnormal knee rotation could lead to initiation and progression of osteoarthritis and subsequently reported abnormal knee rotation was related to cartilage thinning [4]. However, their work was unable to establish specific relationships between altered kinematics and the location of cartilage damage. Previous studies have suggested alteration of the joint contact point and pressure could contribute to cartilage degeneration after ACL injury and reconstruction [6, 27, 28, 37]. These studies, however, have generally investigated only the path of tibial contact and have ignored the complex rolling and sliding components of the articular surface interaction. Given that articular cartilage is more susceptible to damage from shear than from compressive forces [38], the direction and magnitude of stress applied at the area of joint contact may be even more important than its location. The joint sliding distance, calculated from the femoral and tibial joint contact path lengths, reflects shear motion and is therefore related to shear stress. The medial and lateral balance of the joint sliding distance is reportedly related to the knee rotation [21]. Based on the relationship between knee kinematics and joint sliding, abnormal knee rotation is likely associated with abnormal joint sliding distance in either the medial or lateral compartment, as observed in this study. Thus, evaluation of the joint contact dynamics could expose underlying risk factors for cartilage degeneration, even in knees with normally restored knee rotational kinematics.
In conclusion, neither the SB or DB ACL reconstruction procedures restored normal joint kinematics (tibial anterior translation and axial rotation) or joint contact mechanics (tibiofemoral joint sliding distance). Successful restoration of knee translation and rotation are required to obtain full recovery of joint contact sliding. We suspect sliding distance is an important parameter for assessing current ACL reconstructions and for developing future reconstructions.
Acknowledgments
The authors thank Gele Moloney, MD, and Eric Thorhauer, BS, for their contributions to data collection, processing, and analysis.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This study was performed in Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, PA, USA.
Contributor Information
Freddie H. Fu, Email: ffu@msx.upmc.edu.
Scott Tashman, Email: tashman@pitt.edu.
References
- 1.Abebe ES, Utturkar GM, Taylor DC, Spritzer CE, Kim JP, Moorman CT, 3rd, Garrett WE, DeFrate LE. The effects of femoral graft placement on in vivo knee kinematics after anterior cruciate ligament reconstruction. J Biomech. 2011;44:924–929. doi: 10.1016/j.jbiomech.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderst W, Zauel R, Bishop J, Demps E, Tashman S. Validation of three-dimensional model-based tibio-femoral tracking during running. Med Eng Phys. 2009;31:10–16. doi: 10.1016/j.medengphy.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderst WJ, Tashman S. The association between velocity of the center of closest proximity on subchondral bones and osteoarthritis progression. J Orthop Res. 2009;27:71–77. doi: 10.1002/jor.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006;442:39–44. doi: 10.1097/01.blo.0000197079.26600.09. [DOI] [PubMed] [Google Scholar]
- 5.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32:447–457. doi: 10.1023/B:ABME.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 6.Barrance PJ, Williams GN, Snyder-Mackler L, Buchanan TS. Altered knee kinematics in ACL-deficient non-copers: a comparison using dynamic MRI. J Orthop Res. 2006;24:132–140. doi: 10.1002/jor.20016. [DOI] [PubMed] [Google Scholar]
- 7.Bedi A, Musahl V, O’Loughlin P, Maak T, Citak M, Dixon P, Pearle AD. A comparison of the effect of central anatomical single-bundle anterior cruciate ligament reconstruction and double-bundle anterior cruciate ligament reconstruction on pivot-shift kinematics. Am J Sports Med. 2010;38:1788–1794. doi: 10.1177/0363546510369303. [DOI] [PubMed] [Google Scholar]
- 8.Brandsson S, Karlsson J, Sward L, Kartus J, Eriksson BI, Karrholm J. Kinematics and laxity of the knee joint after anterior cruciate ligament reconstruction: pre- and postoperative radiostereometric studies. Am J Sports Med. 2002;30:361–367. doi: 10.1177/03635465020300031001. [DOI] [PubMed] [Google Scholar]
- 9.Cha PS, Brucker PU, West RV, Zelle BA, Yagi M, Kurosaka M, Fu FH. Arthroscopic double-bundle anterior cruciate ligament reconstruction: an anatomic approach. Arthroscopy. 2005;21:1275. doi: 10.1016/j.arthro.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhari AM, Briant PL, Bevill SL, Koo S, Andriacchi TP. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Med Sci Sports Exerc. 2008;40:215–222. doi: 10.1249/mss.0b013e31815cbb0e. [DOI] [PubMed] [Google Scholar]
- 11.Claes S, Neven E, Callewaert B, Desloovere K, Bellemans J. Tibial rotation in single- and double-bundle ACL reconstruction: a kinematic 3-D in vivo analysis. Knee Surg Sports Traumatol Arthrosc. 2011;19(suppl 1):S115–S121. doi: 10.1007/s00167-011-1568-z. [DOI] [PubMed] [Google Scholar]
- 12.Cohen SB, Fu FH. Three-portal technique for anterior cruciate ligament reconstruction: use of a central medial portal. Arthroscopy. 2007;23:325.e1–5. [DOI] [PubMed]
- 13.Deneweth JM, Bey MJ, McLean SG, Lock TR, Kolowich PA, Tashman S. Tibiofemoral joint kinematics of the anterior cruciate ligament-reconstructed knee during a single-legged hop landing. Am J Sports Med. 2010;38:1820–1828. doi: 10.1177/0363546510365531. [DOI] [PubMed] [Google Scholar]
- 14.Fu FH, Shen W, Starman JS, Okeke N, Irrgang JJ. Primary anatomic double-bundle anterior cruciate ligament reconstruction: a preliminary 2-year prospective study. Am J Sports Med. 2008;36:1263–1274. doi: 10.1177/0363546508314428. [DOI] [PubMed] [Google Scholar]
- 15.Georgoulis AD, Papadonikolakis A, Papageorgiou CD, Mitsou A, Stergiou N. Three-dimensional tibiofemoral kinematics of the anterior cruciate ligament-deficient and reconstructed knee during walking. Am J Sports Med. 2003;31:75–79. doi: 10.1177/03635465030310012401. [DOI] [PubMed] [Google Scholar]
- 16.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105:136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 17.Harner CD, Honkamp NJ, Ranawat A. Anteromedial portal technique for creating the anterior cruciate ligament femoral tunnel. Arthroscopy. 2008;24:113–115. doi: 10.1016/j.arthro.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Hemmerich A, van der Merwe W, Batterham M, Vaughan CL. Double-bundle ACL surgery demonstrates superior rotational kinematics to single-bundle technique during dynamic task. Clin Biomech (Bristol, Avon). 2011;26:998–1004. [DOI] [PubMed]
- 19.Hemmerich A, van der Merwe W, Batterham M, Vaughan CL. Knee rotational laxity in a randomized comparison of single- versus double-bundle anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39:48–56. doi: 10.1177/0363546510379333. [DOI] [PubMed] [Google Scholar]
- 20.Ho JY, Gardiner A, Shah V, Steiner ME. Equal kinematics between central anatomic single-bundle and double-bundle anterior cruciate ligament reconstructions. Arthroscopy. 2009;25:464–472. doi: 10.1016/j.arthro.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Hoshino Y, Tashman S. Internal tibial rotation during in vivo, dynamic activity induces greater sliding of tibio-femoral joint contact on the medial compartment. Knee Surg Sports Traumatol Arthrosc. 2012;20:1268–1275. doi: 10.1007/s00167-011-1731-6. [DOI] [PubMed] [Google Scholar]
- 22.Irrgang JJ, Harner CD. Recent advances in ACL rehabilitation: clinical factors that influence the program. J Sport Rehabil. 1997;6:111–124. [Google Scholar]
- 23.Irrgang JJ, Tashman S, Moore C, Fu FH. Challenge accepted: description of an ongoing NIH-funded randomized clinical trial to compare anatomic single-bundle versus anatomic double-bundle ACL reconstruction. Arthroscopy. 2012;28:745–748. doi: 10.1016/j.arthro.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Kanaya A, Ochi M, Deie M, Adachi N, Nishimori M, Nakamae A. Intraoperative evaluation of anteroposterior and rotational stabilities in anterior cruciate ligament reconstruction: lower femoral tunnel placed single-bundle versus double-bundle reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17:907–913. doi: 10.1007/s00167-009-0757-5. [DOI] [PubMed] [Google Scholar]
- 25.Kuster M, Wood GA, Sakurai S, Blatter G. Downhill walking: a stressful task for the anterior cruciate ligament? A biomechanical study with clinical implications. Knee Surg Sports Traumatol Arthrosc. 1994;2:2–7. doi: 10.1007/BF01552646. [DOI] [PubMed] [Google Scholar]
- 26.Lam MH, Fong DT, Yung PS, Ho EP, Fung KY, Chan KM. Knee rotational stability during pivoting movement is restored after anatomic double-bundle anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39:1032–1038. doi: 10.1177/0363546510394430. [DOI] [PubMed] [Google Scholar]
- 27.Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am. 2006;88:1826–1834. doi: 10.2106/JBJS.E.00539. [DOI] [PubMed] [Google Scholar]
- 28.Morimoto Y, Ferretti M, Ekdahl M, Smolinski P, Fu FH. Tibiofemoral joint contact area and pressure after single- and double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2009;25:62–69. doi: 10.1016/j.arthro.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Ristanis S, Giakas G, Papageorgiou CD, Moraiti T, Stergiou N, Georgoulis AD. The effects of anterior cruciate ligament reconstruction on tibial rotation during pivoting after descending stairs. Knee Surg Sports Traumatol Arthrosc. 2003;11:360–365. doi: 10.1007/s00167-003-0428-x. [DOI] [PubMed] [Google Scholar]
- 30.Scanlan SF, Chaudhari AM, Dyrby CO, Andriacchi TP. Differences in tibial rotation during walking in ACL reconstructed and healthy contralateral knees. J Biomech. 2010;43:1817–1822. doi: 10.1016/j.jbiomech.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seon JK, Park SJ, Lee KB, Yoon TR, Seo HY, Song EK. Stability comparison of anterior cruciate ligament between double- and single-bundle reconstructions. Int Orthop. 2009;33:425–429. doi: 10.1007/s00264-008-0530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotation knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:975–983. doi: 10.1177/0363546503261709. [DOI] [PubMed] [Google Scholar]
- 33.Tashman S, Kolowich P, Collon D, Anderson K, Anderst W. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop Relat Res. 2007;454:66–73. doi: 10.1097/BLO.0b013e31802bab3e. [DOI] [PubMed] [Google Scholar]
- 34.Tsarouhas A, Iosifidis M, Kotzamitelos D, Spyropoulos G, Tsatalas T, Giakas G. Three-dimensional kinematic and kinetic analysis of knee rotational stability after single- and double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2010;26:885–893. doi: 10.1016/j.arthro.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 35.van Eck CF, Illingworth KD, Fu FH. Quadriceps tendon: the forgotten graft. Arthroscopy. 2010;26:441?442. [DOI] [PubMed]
- 36.van Eck CF, Lesniak BP, Schreiber VM, Fu FH. Anatomic single- and double-bundle anterior cruciate ligament reconstruction flowchart. Arthroscopy. 2010;26:258–268. doi: 10.1016/j.arthro.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 37.van Eck CF, Schreiber VM, Liu TT, Fu FH. The anatomic approach to primary, revision and augmentation anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2010;18:1154–1163. doi: 10.1007/s00167-010-1191-4. [DOI] [PubMed] [Google Scholar]
- 38.Wilson W, van Burken C, van Donkelaar C, Buma P, van Rietbergen B, Huiskes R. Causes of mechanically induced collagen damage in articular cartilage. J Orthop Res. 2006;24:220–228. doi: 10.1002/jor.20027. [DOI] [PubMed] [Google Scholar]
- 39.Yagi M, Wong EK, Kanamori A, Debski RE, Fu FH, Woo SL. Biomechanical analysis of an anatomic anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30:660–666. doi: 10.1177/03635465020300050501. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto Y, Hsu WH, Woo SL, Van Scyoc AH, Takakura Y, Debski RE. Knee stability and graft function after anterior cruciate ligament reconstruction: a comparison of a lateral and an anatomical femoral tunnel placement. Am J Sports Med. 2004;32:1825–1832. doi: 10.1177/0363546504263947. [DOI] [PubMed] [Google Scholar]