Abstract
Background
Dexamethasone is a potent analgesic and antiemetic. However, the benefit of dexamethasone after TKA is unclear, as is the efficacy in a current multimodal regime.
Questions/purposes
We determined (1) whether the addition of dexamethasone to a protocol including ramosetron further reduces postoperative emesis compared with ramosetron alone; (2) whether it reduces postoperative pain; and (3) whether it increases the risk for wound complications in a current multimodal regime after TKA.
Methods
We randomized 269 patients undergoing TKAs to receive dexamethasone (10 mg) 1 hour before surgery and ramosetron immediately after surgery (Dexa-Ra group, n = 135), or ramosetron alone (Ra group, n = 134). We recorded the incidence of postoperative nausea and vomiting (PONV), severity of nausea, incidence of antiemetic requirement, complete response, pain level, and opioid consumption. Patients were assessed 0 to 6, 6 to 24, 24 to 48, and 48 to 72 hours postoperatively. In addition, patients were evaluated for wound complications and periprosthetic joint infections at a minimum of 1 year after surgery.
Results
The Dexa-Ra group had a lower incidence of PONV during the entire 72-hour evaluation period and experienced less severe nausea for the first 6 hours after TKA, although not between 6 to 72 hours. Overall use of a rescue antiemetic was less frequent, and complete response was more frequent in the Dexa-Ra group. Patients in the Dexa-Ra group experienced lower pain and consumed less opioids during the 6- to 24-hour period and during the overall study period. No differences were found in wound complications between the groups, and each group had one case of periprosthetic joint infection.
Conclusions
Patients who received prophylactic dexamethasone in addition to ramosetron had reduced postoperative emesis and pain without increased risks for wound complications, compared with patients who received ramosetron alone in patients managed using a multimodal regimen after TKA.
Level of Evidence
Level I, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
A TKA is one of the most efficacious surgical treatments for advanced knee arthritis [6, 7, 14]. During the past decade, the number of TKAs performed has increased substantially, and future demand is projected to rise rapidly [26, 31, 35]. However, as a TKA involves extensive bone resection and soft tissue manipulation, patients can experience severe pain during the early postoperative period [13, 42, 47–49]. Furthermore, many patients experience postoperative nausea and vomiting (PONV) after TKA, owing to not only anesthesia and surgery but also the various agents used for pain control [8, 10, 20, 27]. Inadequate management of postoperative pain and PONV can lead to several systemic complications and delays recovery [13, 16, 34, 48]. Moreover, pain and PONV are strongly associated with patient dissatisfaction [11, 32, 39, 41, 43]. Although management of pain and PONV after TKA is of paramount importance, no gold standard protocols for reducing pain and PONV have been developed, and their management remains a challenging issue for patients and healthcare providers.
Corticosteroids have potent antiinflammatory and antiemetic effects and have been widely used in various perioperative settings such as abdominal, cardiac, ear-nose-throat, gynecologic, and plastic surgery for reducing postoperative pain and PONV [9, 19, 21, 22, 46]. Previous studies have found that perioperative use of single, low-dose corticosteroids significantly decreased inflammatory markers after TKA [23, 24, 49]. However, despite the potential benefits and reported efficacy in numerous surgical procedures, only a few small studies involving less than 25 patients per group evaluated the efficacy and safety of corticosteroids after TKA [15, 24, 38, 40]. Moreover, heterogeneity among studies regarding type, dosage, and administration protocol of corticosteroids and concomitant pain control regime make it difficult to judge the practical value of corticosteroid use after TKA. In addition, concerns regarding increased risk for infection have hampered the widespread use of corticosteroids in multimodal regimens for pain and PONV after TKA. Therefore, although the results of corticosteroid use after TKA seem promising, additional evidence based on prospective studies with sufficient power are required to determine its benefit.
In a previous study, we found a significant decrease in the incidence and severity of PONV during the 6- to 24-hour period in patients treated using a multimodal pain and PONV protocol that included ramosetron, a 5-hydroxytryptamine type 3 (5-HT3) receptor antagonist, and prophylaxis [27]. However, although the pain and emesis relief provided by this regimen were superior to that of traditional measures, a substantial proportion of patients experienced PONV during the first 6 hours. Furthermore, although it is documented that prophylaxis with concurrent dexamethasone and 5-HT3 antagonist provides a superior antiemetic effect compared with a 5-HT3 antagonist alone [21, 22], to our knowledge, no previous study has examined the antiemetic efficacy of concurrent dexamethasone and ramosetron after TKA.
Thus, we sought to determine (1) whether the addition of prophylactic single, low-dose dexamethasone to a protocol including ramosetron further reduced PONV compared with ramosetron alone; (2) whether preemptive use of dexamethasone provided additional analgesic effect; and (3) whether dexamethasone increased the risk for wound complications in patients managed using a multimodal regimen that included ramosetron prophylaxis after TKA.
Patients and Methods
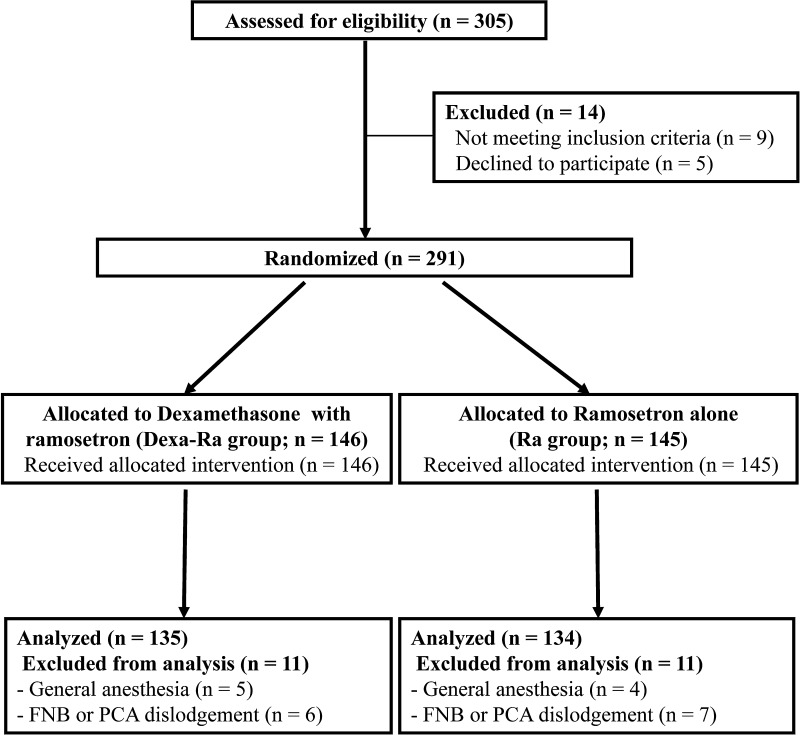
We randomized 269 patients undergoing TKAs between February and December 2011 to receive either intravenous dexamethasone (dexamethasone; Yuhan, Seoul, Korea) once 1 hour before surgery with intravenous ramosetron (Nasea, Astellas, Tokyo, Japan) once immediately after surgery (Dexa-Ra group; as the experimental group, n = 135) or intravenous ramosetron alone once immediately after surgery (Ra group; the control group, n = 134). Eligible patients included those 18 years or older who were scheduled for unilateral TKA for primary osteoarthritis. Exclusion criteria included a history of intolerance or allergy to any drug used in the current study; severe impairment of bowel motility; administration of another antiemetic drug or systemic steroid 24 hours before surgery; history of cardiovascular or respiratory disease, or alcohol or opioid dependence; and impairment of renal or hepatic function. Furthermore, patients were excluded if regional anesthesia was contraindicated, spinal anesthesia failed, or the femoral nerve block and/or intravenous patient-controlled analgesia (PCA) was discontinued before the planned schedule. Patients who declined to participate in the trial or who were unable to provide informed consent were excluded. A computer-generated randomization table permuted into blocks of four and six was used to randomly assign patients to either the Dexa-Ra group or Ra group. One of the authors (JHL) who was not involved in patient recruitment for this trial telephoned a surgeon for allocation assignments 1 week before surgery. The patients and an independent investigator who prospectively collected the clinical information were unaware of group assignments until the final data analyses were completed. A total of 146 patients initially were allocated to the Dexa-Ra group and 145 were assigned to the Ra group. We excluded 11 patients from each group according to the defined exclusion criteria. Thus 269 patients (Dexa-Ra, 135; Ra, 134) were included in the final analysis (Fig. 1). We found no differences in demographic characteristics or number of PONV risk factors between the groups (Table 1). The study protocol was approved by our institutional review board and registered at ClinicalTrials.gov (NCT01612702).
Fig. 1.
A flow diagram shows the study design. FNB = femoral nerve block; PCA = patient-controlled analgesia.
Table 1.
Demographic data and risk factors for PONV
| Parameter | Dexa-Ra (n = 135) |
Ra (n = 134) |
Significance (p value) |
|---|---|---|---|
| Demographic data* | |||
| Age (years) | 72.0 (6.7) | 72.0 (6.2) | 0.978 |
| Sex (female) | 117 (87%) | 119 (89%) | 0.364 |
| Height (cm) | 152.8 (7.4) | 152.6 (7.1) | 0.865 |
| Weight (kg) | 61.5 (9.7) | 60.9 (9.3) | 0.606 |
| BMI (kg/m2) | 26.3 (3.6) | 26.1 (3.2) | 0.559 |
| Risk factors for PONV | |||
| Duration of surgery (minutes) | 106.8 (14.0) | 107.0 (14.1) | 0.917 |
| Risk factors identified† | 0.819 | ||
| With one factor | 8 (4) | 5 (6) | |
| With two factors | 12 (9) | 12 (9) | |
| With three factors | 112 (86) | 115 (83) | |
| With four factors | 3 (2) | 2 (2) | |
| Calculated mean risk‡ | 56.3 (11.2) | 57.0 (9.7) | 0.575 |
PONV = postoperative nausea and vomiting; Dexa-Ra = dexamethasone and ramosetron; Ra = ramosetron; * data presented as means (SDs) with the exception for gender, which is presented as number of female patients (percentage); †data presented as number of patients with risk factors with proportions in parentheses, risk factors were described by Apfel et al. [3]; ‡data presented as calculated mean risk for PONV, determined using the simplified risk scoring system of Apfel et al., with SDs in parentheses [3].
We performed an a priori power analysis based on the results of a previous study showing a PONV incidence of 42% in 60 patients who received ramosetron prophylaxis alone during a 48-hour study period [27]. We calculated that 238 patients (119 in each group) were required to detect a 50% reduction in the incidence of PONV at an alpha level of 0.05 and a power of 90% using a two-sided test. To allow for exclusions and dropouts, we enrolled 291 patients in the current trial.
All surgeries were performed by one of two surgeons (TKK and CBC) using the standard medial parapatellar arthrotomy with a tourniquet. A posteriorly stabilized prosthesis (Genesis II; Smith & Nephew, Memphis, TN, USA) was implanted in all patients. The patella was resurfaced, and cement fixation was used for all components in all cases.
All patients received the same anesthetic and multimodal regimen to manage pain and PONV, with the exception that only the Dexa-Ra group received 10 mg intravenous dexamethasone 1 hour before surgery. One hour before surgery, multimodal oral analgesic drugs (10 mg sustained-release oxycodone, 200 mg celecoxib, 75 mg pregabalin, and 650 mg acetaminophen) were administered for preemptive analgesia on a call basis. All patients received 1.0 g cefazolin as antimicrobial prophylaxis, were premedicated using midazolam (0.03 mg/kg) before induction, and received continuous femoral nerve block (0.2% ropivacaine solution at 5 mL/hour). Spinal anesthesia using 0.5% bupivacaine was administered by one of two anesthesiologists. Anesthesia was maintained with propofol (target blood concentration, 0.8–1.5 μg/mL) using a target-controlled device (Orchestra; Fresenius Kabi, Bad Homburg, Germany), and O2 was delivered at 5 L/minute (FiO2 0.4) through a partial rebreathing mask bag. After all prostheses had been fixed with cement, all patients received periarticular injections of a multimodal drug cocktail comprising 300 mg ropivacaine, 10 mg morphine sulfate, 30 mg ketorolac, 300 μg of 1:1000 epinephrine, and 750 mg cefuroxime [29]. At the end of surgery, 0.3 mg ramosetron was administered intravenously to all patients. Postoperatively, all patients received intravenous PCA, which was programmed to deliver 1 mL of a 100-mL solution containing 2000 μg fentanyl for patients 70 years or younger, or 1500 μg for patients older than 70 years when patients depressed a button. There was a 10-minute lockout period without basal flow. When patients resumed oral intake, 200 mg celecoxib, 75 mg pregabalin, and 650 mg acetaminophen were administered every 12 hours. An intramuscular injection of ketoprofen (100 mg) was used as an acute analgesic when a patient reported severe pain greater than level 6 on a 0 to 10 VAS. The continuous femoral nerve block and intravenous PCA typically were discontinued on the third and fourth postoperative days, respectively, or sooner if the intravenous PCA pump had been emptied. Continuous femoral nerve block and intravenous PCA were maintained throughout the entire study in all patients. An intravenous injection of metoclopramide (10 mg) was used as a first-line antiemetic rescue treatment when patients experienced two or more episodes of PONV or had severe nausea (> 4 on a 0–10 VAS). If severe nausea persisted after administration of two consecutive boluses of metoclopramide in a 30-minute interval, 4 mg of ondansetron was administered intravenously as the second-line treatment.
A clinical investigator (YGK) who was blind to the group assignments reviewed the diagnosis and medical histories, and prospectively collected demographic data. Risk factors for PONV were assessed using predesigned datasheets according to the recommendations of the consensus guidelines for management of PONV [4, 18]. Risk factors and calculated mean risks for PONV were evaluated using the simplified risk scoring system devised by Apfel et al. [3]. This scoring system includes four risk factors: female gender, history of PONV or motion sickness, nonsmoking status, and the use of postoperative opioids. The predicted incidences of PONV given the presence of one, two, three, or four of these risk factors are 21%, 39%, 61%, and 78%, respectively [2, 3].
The primary outcome variable was incidence of PONV and the secondary outcome variables were severity of nausea, rescue antiemetic requirement and complete response, pain level, amount of opioid consumption, and incidence of wound complications. A blinded investigator (YGK) recorded all episodes of nausea and vomiting, severity of nausea, rescue antiemetic requirement, and complete response during four postoperative periods (0–6 hours, 6–24 hours, 24–48 hours, and 48–72 hours) to evaluate the antiemetic efficacy. Nausea was defined as a subjective unpleasant sensation associated with awareness of the urge to vomit, and vomiting as the forceful expulsion of gastric contents from the mouth [51]. Before surgery, a blinded investigator (YGK) instructed all patients to mark their level of perceived symptom intensity on the VAS on the predesigned data sheet. The incidence of nausea and vomiting was determined for each of the four periods and during the entire study by calculating the proportion of patients who experienced PONV. The severity of nausea was assessed by patients using a 0 to 10 VAS, where 0 corresponded to no nausea and 10 corresponded to the worst imaginable nausea during each of the four study periods. Complete response to an administered rescue antiemetic was defined as no additional episodes of PONV, with no need for another rescue antiemetic [33].
Pain level and amount of opioid consumption (determined using the intravenous PCA pump) were recorded to evaluate the analgesic effect. Pain levels during the four periods were estimated using a VAS that ranged from 0 (no pain) to 10 (worst imaginable pain) during each of the four study periods. Opioid (fentanyl) consumption was recorded for each of the four periods and summed to obtain consumption during the entire72-hour study period.
Wound complications including periprosthetic joint infection and inadequate wound healing (including delayed wound healing or wound dehiscence) were evaluated by one of two surgeons (TKK and CBC) at 2 weeks, 6 weeks, 3 months, 6 months, and 1 year after surgery at followups. Periprosthetic joint infection was diagnosed using the criteria outlined by the Musculoskeletal Infection Society [44].
We compared the primary and secondary outcomes between the Dexa-Ra and Ra groups. Chi-square or Fisher’s exact tests were used to determine the statistical significance of differences in the categorical variables, namely sex, presence of PONV risk factors, incidence of PONV, requirements for rescue antiemetics, proportion of complete responses, and incidence of wound complications. Continuous variables were analyzed with Student’s t-test (age, height, weight, BMI, duration of surgery, and calculated mean risk) or the Wilcoxon signed-rank test (VAS pain scores and opioid consumption). The variables subjected to multiple between-group comparisons included the incidence of PONV, severity of nausea, requirement for rescue antiemetics, proportion of complete responses, VAS pain scores, and amount of opioid consumption; these variables were analyzed using repeated-measures ANOVA, followed by the Bonferroni corrected post hoc test. Statistical analyses were conducted using the Statistical Package for the Social Sciences for Windows version 17 (SPSS Inc, Chicago, IL, USA).
Results
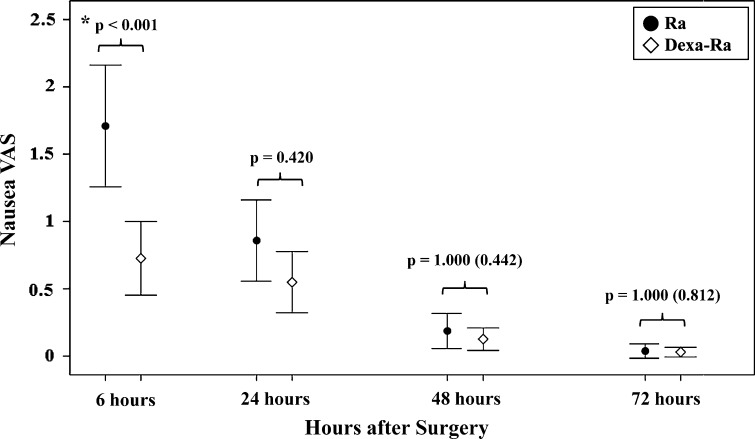
Prophylactic use of concurrent dexamethasone and ramosetron reduced the overall incidence of PONV, rescue antiemetic requirement, and improved the overall complete response during the entire 72-hour evaluation period and reduced the severity of nausea during the first 6-hours. During the whole evaluation period, the incidences of postoperative nausea (24% versus 40%; p = 0.004) and vomiting (7% versus 21%; p = 0.001) (Table 2) were lower in the Dexa-Ra group, and fewer patients in the Dexa-Ra group required rescue antiemetics (17% versus 35%; p = 0.001). More patients in the Dexa-Ra group had a complete response compared with the Ra group (76% versus 60%; p = 0.006) (Table 3). In addition, the severity of nausea was lower in the Dexa-Ra than in the Ra group during the first 6 hours (1.6 versus 2.6; p < 0.001) (Fig. 2). No between-group differences in incidence of PONV or nausea severity were found during the 6- to 72-hour period.
Table 2.
Incidence of PONV*
| Parameter | Dexa-Ra (n = 135) |
Ra (n = 134) |
Significance† (p value) |
|---|---|---|---|
| Nausea | 33 (24) | 54 (40) | 0.004 |
| 0–6 hours | 29 (22) | 49 (37) | 0.028 |
| 6–24 hours | 26 (19) | 33 (25) | 1.000 (0.305) |
| 24–48 hours | 9 (7) | 9 (7) | 1.000 |
| 48–72 hours | 3 (2) | 2 (1) | 1.000 |
| Vomiting | 9 (7) | 28 (21) | 0.001 |
| 0–6 hours | 4 (3) | 28 (21) | < 0.001 |
| 6–24 hours | 5 (4) | 7 (5) | 1.000 (0.571) |
| 24–48 hours | 1 (1) | 1 (1) | 1.000 |
| 48–72 hours | 0 (0) | 0 (0) | – |
PONV = postoperative nausea and vomiting; * data presented as number (percentage) of patients who experienced nausea or vomiting; Dexa-Ra = dexamethasone and ramosetron; RA = ramosetron; †p values in parentheses are the uncorrected values before Bonferroni analysis. The incidence of vomiting during 48 to 72 hours was not statistically analyzed.
Table 3.
Requirement for rescue antiemetics and frequency of complete response*
| Parameter | Dexa-Ra (n = 135) |
Ra (n = 134) |
Significance† (p value) |
|---|---|---|---|
| Rescue antiemetics requirement | 23 (17) | 47 (35) | 0.001 |
| 0–6 hours | 20 (15) | 42 (31) | 0.004 |
| 6–24 hours | 16 (12) | 24 (18) | 0.696 |
| 24–48 hours | 3 (2) | 1 (1) | 1.000 (0.622) |
| 48–72 hours | 1 (1) | 1 (1) | 1.000 |
| Complete response‡ | 102 (76) | 80 (60) | 0.006 |
| 0–6 hours | 106 (79) | 85 (64) | 0.028 |
| 6–24 hours | 111 (83) | 101 (76) | 0.728 |
| 24–48 hours | 125 (93) | 124 (93) | 1.000 |
| 48–72 hours | 132 (98) | 132 (99) | 1.000 |
* Data presented as number of patients (percentage); Dexa-Ra = dexamethasone and ramosetron; Ra = ramosetron; †p values in parentheses are the uncorrected values before Bonferroni analysis; ‡complete response was defined as no additional postoperative nausea and vomiting or no requirement for rescue antiemetics.
Fig. 2.
A graph shows nausea severity according to VAS scores during the 72 hours after surgery. Patients in the Dexa-Ra group experienced less severe nausea than those in the Ra group during the first 6 hours after surgery. Error bars represent 95% CIs. *p < 0.05; p values in parentheses are uncorrected values before the Bonferroni analysis.
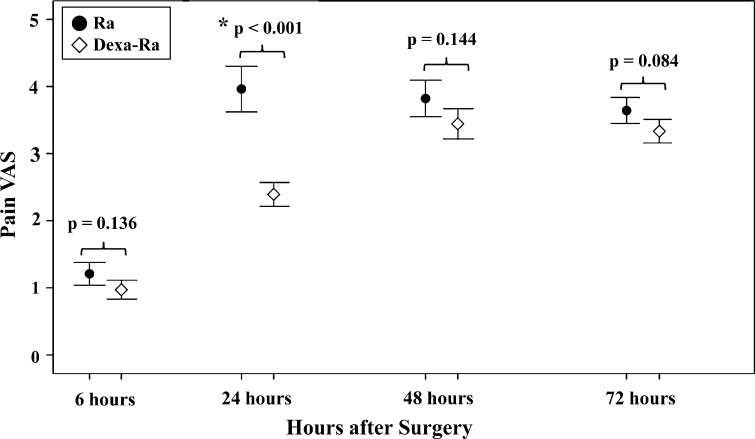
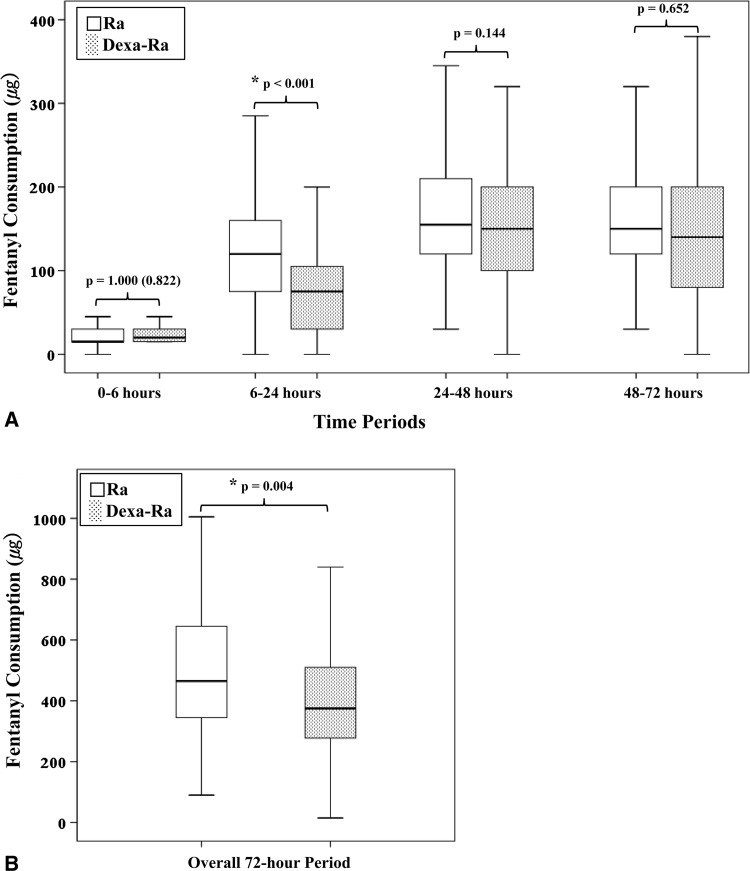
Preemptive use of dexamethasone reduced postoperative pain and opioid consumption during the 6- to 24-hour period and reduced overall opioid consumption during the entire 72-hour period. The mean VAS pain score (2.4 versus 4.0; p < 0.001) (Fig. 3) and opioid consumption during the 6- to 24-hour period (73.5 versus 128.3 μg; p < 0.001) (Fig. 4A), and overall opioid consumption (406.2 versus 500.1 μg; p = 0.004) (Fig. 4B) were lower in the Dexa-Ra group compared with the Ra group. However, no between-group differences were observed during the whole study period (p > 0.1 for all comparisons), except during the 6- to 24-hour postoperative period (pain VAS and opioid consumption during the 6- to 24-hour period in the Dexa-Ra group versus Ra group were 2.4 versus 4.0 [p < 0.001] and 73.5 versus 128.3 μg [p < 0.001], respectively).
Fig. 3.
Pain level according to VAS scores during the 72 hours after surgery is shown. Patients in the Dexa-Ra group experienced less severe pain than those in the Ra group during the 6- to 24-hour period. Error bars represent 95% CIs. *p < 0.05; p values in parentheses are uncorrected values before the Bonferroni analysis.
Fig. 4A–B.
Fentanyl consumption via intravenous PCA, with 95% CIs, during (A) each period and during the (B) whole study period are shown. Patients in the Dexa-Ra group consumed less opioid during the 6- to 24-hour period and less overall opioid during the whole 72-hour period. Values with a significant difference (p < 0.05) are marked with an asterisk. P values in parentheses are the uncorrected values before Bonferroni analysis.
The use of dexamethasone did not increase the risk for wound complications or infections. Each group had one patient who had a periprosthetic joint infection develop (Dexa-Ra group, 0.7% versus Ra group, 0.7%; p > 0.1). There was no difference in the incidence of wound complications between the two groups. The Dexa-Ra group had two patients with inadequate wound healing and the Ra group had three (Dexa-Ra group, 1.5% versus Ra group, 2.2%; p > 0.1) during the first year after TKA. The two patients who had periprosthetic joint infections were treated with débridement, polyethylene component exchange, and antibiotics; at 1 year, the infections had not recurred. All patients in both groups who had inadequate wound healing were treated successfully with conservative management. A power analysis for comparison of wound complication incidence showed that this study (3% in Ra group) had 80% power to detect the difference at a 10% increase of wound complication incidence at an alpha level of 0.05 using a two-sided test. There was no differential loss to followup between groups; eight patients from the Dexa-Ra group (5.9%) and six from the Ra group (4.8%) were unable to return for followup at 1 year (p > 0.1). All patients who did not return for followup at 1 year were contacted by telephone to assess for the presence of periprosthetic joint infection.
Discussion
Given well-documented analgesic and antiemetic effects, corticosteroids have been widely used in various perioperative settings. However, whether incorporation of single, low-dose dexamethasone in a current multimodal regimen to manage pain and PONV after TKA provides additional clinical benefit remains unclear because there is limited evidence from prospective studies with sufficient power to support its utility. We therefore determined (1) whether the addition of prophylactic single, low-dose dexamethasone further reduced PONV; (2) whether preemptive use of dexamethasone provided additional analgesia; and (3) whether dexamethasone increased the risk for wound complications in patients managed using a multimodal regimen that included ramosetron prophylaxis after TKA.
Our study has several limitations. First, our study participants all were Korean and most were women (88%). Thus, our findings may not be widely generalizable because female gender is a well-established risk factor for PONV [3, 17, 18], and pain may be manifested differently in various ethnic populations [12]. Second, our postoperative analgesic and antiemetic regimen included extensive multimodal pain control drugs and modalities such as preemptive analgesic medication, femoral nerve block, periarticular injection and intravenous PCA and ramosetron, a recently developed potent, long-acting 5-HT3 antagonist. It is important to take this into account when considering our results in the context of other multimodal regimens after TKA. We felt we needed to use a multimodal regimen for ethical reasons related to patient comfort after surgery. We suspect, but cannot prove, that the synergistic benefits we observed likely would be similar with other similarly designed multimodal protocols that, like ours, address inflammatory responses as a main goal of the treatment approach. Third, 14 of 269 patients (5.2%) could not complete the 1-year followup visit (eight patients from the Dexa-Ra group [5.9%] and six patients from Ra group [4.5%]). However, all of them completed the 6-month followup visit and all were contacted by telephone to assess the presence of a periprosthetic joint infection at 1 year after TKA. Fourth, despite the numerous potential side effects associated with corticosteroids [22, 45, 46], we assessed only the incidence of postoperative wound complications because they are the most serious complication associated with TKA. Although previous studies using higher doses of corticosteroids reported no increase in other potential side effects [22, 46], additional study is necessary to investigate potential side effects of corticosteroid use after TKA. Finally, this study had limited power to detect a subtle difference in postoperative wound complications because the sample size of the study was determined based on the primary efficacy outcome, that is, the incidence of PONV. A power analysis for comparison of the incidence of wound complications showed this study would have only 33% power to detect the difference at a 5% wound complication incidence at an alpha level of 0.05 using a two-sided test. More large prospective series are needed to ascertain whether corticosteroid use is associated with increased risks for wound complications in patients undergoing TKA.
Our study suggests that the antiemetic efficacy provided by concurrent dexamethasone and ramosetron prophylaxis is superior to ramosetron alone. Our results showed that prophylaxis with dexamethasone and ramosetron was more effective than ramosetron alone in decreasing the incidence and severity of PONV, the rescue antiemetic requirement, and in obtaining a higher complete response during the first 6 hours and during the entire 72-hour study period. Our findings concur with those of previous studies showing the superior antiemetic effect of concurrent dexamethasone and 5-HT3 antagonist to the 5-HT3 antagonist alone [21, 22], but no previous study has investigated the antiemetic efficacy of concomitant dexamethasone and ramosetron after TKA. Two previous studies reported that ramosetron monoprophylaxis after TKA provided a limited antiemetic effect during the 2- to 48-hour or 6- to 48-hour period compared with ondansetron or no prophylaxis [20, 27]. Our findings, together with those of previous studies, suggest that prophylaxis with concomitant dexamethasone and ramosetron has a potent antiemetic effect on early PONV which is not provided by ramosetron alone. Meanwhile, our findings also indicate that ramosetron alone reduced PONV effectively during the 6- to 72-hour period after TKA in patients who are at high risk for PONV (Table 1). Therefore, although concurrent dexamethasone and ramosetron prophylaxis did not show superior antiemetic efficacy during the 6- to 72-hour period compared with ramosetron alone, our findings suggest concurrent dexamethasone and ramosetron prophylaxis is effective in reducing PONV during the 72-hour period after TKA in patients who are at high risk for PONV.
Our findings support the hypothesis that the pain relief and opioid-sparing effect are enhanced by the addition of preemptive dexamethasone to the multimodal analgesic regimen. Patients who received dexamethasone experienced lower pain and consumed less opioid during the 6- to 24-hour postoperative period compared with patients who received ramosetron alone. These findings agree with those of two recent studies that showed that 125 mg of methylprednisolone reduced resting pain for 2 to 48 hours and reduced opioid consumption for the first 24 hours in 24 patients who had unilateral TKA [38], and that 300 mg hydrocortisone reduced postoperative pain and epidural medication for the first 24 hours in 17 patients who had bilateral TKAs concurrently treated with a multimodal analgesic regimen [24] (Table 4). Our results and those of previous studies [24, 38] suggest that incorporation of corticosteroids in a multimodal analgesic regimen provides synergistic analgesia and opioid-sparing effects. We noted that pain occurring between 6 and 24 hours after surgery may be breakthrough pain from local anesthetics included in the periarticular injection. A temporary increase in pain during 12 to 24 hours after surgery has been observed with multimodal regimens that involve periarticular injection, regardless of the surgery type or concomitant analgesic regimen [27–30, 37].We believe the addition of dexamethasone to the current multimodal analgesic regimen after TKA may have potent analgesic effects in combating breakthrough pain caused by components of the multimodal regimen.
Table 4.
Summary of randomized controlled trials of corticosteroid use after TKA
| Study | Patients and study design | Equivalency to dexamethasone | Anesthesia and pain control protocol | Clinical benefits and safety |
|---|---|---|---|---|
| Fujii et al. [15] |
80 unilateral TKAs 3 groups of 20 dexamethasone (4 mg, 8 mg, 16 mg) versus 20 placebo |
4, 8, 16 mg | General anesthesia Continuous epidural infusion |
Study period: 0–24 hours; PONV incidence: lower in 8 mg & 16 mg dexamethasone (control vs 8 mg vs 16 mg = 65% vs 30% vs 25%); no wound complication |
| Lunn et al. [38] |
48 unilateral TKAs 24 methyprednisolone (125 mg) versus 24 placebo |
25 mg | Preemptive gabapentin, acetaminophen, celecoxib; spinal anesthesia; periarticular injection; rescue sulfentanyl and oral oxycodone; regular celecoxib, acetaminophen, gabapentin |
Study period: 0–4, 4–6, 6–24, & 24–48 hours; PONV Incidence: lower with methylprednisolone 24–48 hours (0% vs 21%); nausea severity: lower with methylprednisolone 0–24 hours; rescue: lower with methylprednisolone 0–48 hours; pain: lower with methylprednisolone 2–48 hours; opioid use: lower with methylprednisolone 0–24 hours; no wound complications |
| Jules-Elysee et al. [23] | 34 simultaneous bilateral TKAs 17 hydrocortisone (300 mg) versus 17 placebo |
11.3 mg | Combined spinal-epidural anesthesia; Continuous femoral nerve block; epidural PCA; rescue Percocet (oxycodone + acetaminophen) |
Study period: 0–12, 12–24 hours; PONV incidence: no difference (18% vs 18%); pain: lower with hydrocortisone 0–24 hours; opioid use: lower with hydrocortisone 0–24 hours; no wound complications |
| Current study | 269 unilateral TKAs 135 dexamethasone (10 mg) with ramosetron (0.3 mg) versus 134 ramosetron (0.3 mg) |
10 mg | Preemptive oxycodone, celecoxib, gabapentin, acetaminophen; spinal anesthesia; continuous femoral nerve block; periarticular injection; intravenous PCA; regular celecoxib, gabapentin, acetaminophen |
Study period: 0–6, 6–24, 24–48, 48–72 hours; PONV incidence: lower in dexamethasone 0–6 hours & overall 72 hours (22% vs 37% & 24% vs 40%, respectively); nausea severity: lower with dexamethasone 0–6 hours; rescue: lower with dexamethasone 0–6 hours & overall 72 hours; pain: lower with dexamethasone 6–24 hours; opioid use: lower with dexamethasone 6–24 hours & overall 72 hours; no differences in wound complications |
PONV = postoperative nausea and vomiting; vs = versus; PCA = patient-controlled analgesia; Percocet (Endo Pharmaceuticals, Malvern, PA, US).
Our results indicate that prophylactic use of dexamethasone does not increase the risks for wound complications after TKA. The incidence of postoperative wound complications within 1 year was similar between the Dexa-Ra and Ra groups. Our findings agree with those of previous small series [15, 36, 38, 40] and reviews of numerous perioperative settings [19, 21, 22, 46] that found no association between corticosteroid use and increased risk for postoperative wound infection. Furthermore, studies using higher corticosteroid doses than that used in our study showed no increase in postoperative wound infection after major orthopaedic surgeries [1, 5, 19, 25, 46]. The rate of periprosthetic joint infections after primary TKA at our institute was 0.6% (8/1323 patients) between 2006 and 2009 [50], and we have maintained the same operating room environment, surgical scrub, skin preparation, and antibiotic prophylactic protocol since 2003. Although our study had limited power to detect clinical significance, the incidence of periprosthetic joint infections in our study participants was comparable to those at our institute. Nevertheless, the evidence supporting the safety of corticosteroids regarding wound infection risk after TKA is inconclusive, and more research is needed to clarify the potential association between postoperative wound infection and corticosteroid use.
Concomitant use of dexamethasone further reduces postoperative pain and PONV after TKA without increased risks for wound complications in patients managed using a multimodal pain and PONV regimen. We propose incorporating concomitant use of single, low-dose dexamethasone in a current multimodal regime after TKA because it is a simple, effective, and inexpensive intervention that is not associated with any apparent increased risk of infection or wound complications, although this last issue should be confirmed in larger multicenter trials or meta-analyses of randomized trials.
Acknowledgments
We thank Jung-Hee Ryu MD of the Department of Anesthesiology and Pain Medicine for anesthesia and Yeon Gwi Kang MS of Seoul National University Bundang Hospital for data collection.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution has approved the human protocol for this
investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
This work was performed at the Joint Reconstruction Center, Seoul National University Bundang Hospital, Seongnam-si, Korea.
References
- 1.Aminmansour B, Khalili HA, Ahmadi J, Nourian M. Effect of high-dose intravenous dexamethasone on postlumbar discectomy pain. Spine (Phila Pa 1976). 2006;31:2415–2417. [DOI] [PubMed]
- 2.Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, Zernak C, Danner K, Jokela R, Pocock SJ, Trenkler S, Kredel M, Biedler A, Sessler DI, Roewer N. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004;350:2441–2451. doi: 10.1056/NEJMoa032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Apfel CC, Roewer N, Korttila K. How to study postoperative nausea and vomiting. Acta Anaesthesiol Scand. 2002;46:921–928. doi: 10.1034/j.1399-6576.2002.460801.x. [DOI] [PubMed] [Google Scholar]
- 5.Bergeron SG, Kardash KJ, Huk OL, Zukor DJ, Antoniou J. Perioperative dexamethasone does not affect functional outcome in total hip arthroplasty. Clin Orthop Relat Res. 2009;467:1463–1467. doi: 10.1007/s11999-009-0733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callahan CM, Drake BG, Heck DA, Dittus RS. Patient outcomes following tricompartmental total knee replacement: a meta-analysis. JAMA. 1994;271:1349–1357. doi: 10.1001/jama.1994.03510410061034. [DOI] [PubMed] [Google Scholar]
- 7.Chang RW, Pellisier JM, Hazen GB. A cost-effectiveness analysis of total hip arthroplasty for osteoarthritis of the hip. JAMA. 1996;275:858–865. doi: 10.1001/jama.1996.03530350040032. [DOI] [PubMed] [Google Scholar]
- 8.Chen JJ, Frame DG, White TJ. Efficacy of ondansetron and prochlorperazine for the prevention of postoperative nausea and vomiting after total hip replacement or total knee replacement procedures: a randomized, double-blind, comparative trial. Arch Intern Med. 1998;158:2124–2128. doi: 10.1001/archinte.158.19.2124. [DOI] [PubMed] [Google Scholar]
- 9.De Oliveira GS, Jr, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115:575–588. doi: 10.1097/ALN.0b013e31822a24c2. [DOI] [PubMed] [Google Scholar]
- 10.DiIorio TM, Sharkey PF, Hewitt AM, Parvizi J. Antiemesis after total joint arthroplasty: does a single preoperative dose of aprepitant reduce nausea and vomiting? Clin Orthop Relat Res. 2010;468:2405–2409. doi: 10.1007/s11999-010-1357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorr LD, Chao L. The emotional state of the patient after total hip and knee arthroplasty. Clin Orthop Relat Res. 2007;463:7–12. [PubMed] [Google Scholar]
- 12.Edwards CL, Fillingim RB, Keefe F. Race, ethnicity and pain. Pain. 2001;94:133–137. doi: 10.1016/S0304-3959(01)00408-0. [DOI] [PubMed] [Google Scholar]
- 13.Filos KS, Lehmann KA. Current concepts and practice in postoperative pain management: need for a change? Eur Surg Res. 1999;31:97–107. doi: 10.1159/000008627. [DOI] [PubMed] [Google Scholar]
- 14.Fortin PR, Clarke AE, Joseph L, Liang MH, Tanzer M, Ferland D, Phillips C, Partridge AJ, Belisle P, Fossel AH, Mahomed N, Sledge CB, Katz JN. Outcomes of total hip and knee replacement: preoperative functional status predicts outcomes at six months after surgery. Arthritis Rheum. 1999;42:1722–1728. doi: 10.1002/1529-0131(199908)42:8<1722::AID-ANR22>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 15.Fujii Y, Nakayama M. Effects of dexamethasone in preventing postoperative emetic symptoms after total knee replacement surgery: a prospective, randomized, double-blind, vehicle-controlled trial in adult Japanese patients. Clin Ther. 2005;27:740–745. doi: 10.1016/j.clinthera.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Gan TJ. Postoperative nausea and vomiting: can it be eliminated? JAMA. 2002;287:1233–1236. doi: 10.1001/jama.287.10.1233. [DOI] [PubMed] [Google Scholar]
- 17.Gan TJ. Risk factors for postoperative nausea and vomiting. Anesth Analg. 2006;102:1884–1898. doi: 10.1213/01.ANE.0000219597.16143.4D. [DOI] [PubMed] [Google Scholar]
- 18.Gan TJ, Meyer T, Apfel CC, Chung F, Davis PJ, Eubanks S, Kovac A, Philip BK, Sessler DI, Temo J, Tramer MR, Watcha M. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003;97:62–71. doi: 10.1213/01.ANE.0000068580.00245.95. [DOI] [PubMed] [Google Scholar]
- 19.Gilron I. Corticosteroids in postoperative pain management: future research directions for a multifaceted therapy. Acta Anaesthesiol Scand. 2004;48:1221–1222. doi: 10.1111/j.1399-6576.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- 20.Hahm TS, Ko JS, Choi SJ, Gwak MS. Comparison of the prophylactic anti-emetic efficacy of ramosetron and ondansetron in patients at high-risk for postoperative nausea and vomiting after total knee replacement. Anaesthesia. 2010;65:500–504. doi: 10.1111/j.1365-2044.2010.06310.x. [DOI] [PubMed] [Google Scholar]
- 21.Henzi I, Walder B, Tramer MR. Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesth Analg. 2000;90:186–194. doi: 10.1097/00000539-200001000-00038. [DOI] [PubMed] [Google Scholar]
- 22.Holte K, Kehlet H. Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications. J Am Coll Surg. 2002;195:694–712. doi: 10.1016/S1072-7515(02)01491-6. [DOI] [PubMed] [Google Scholar]
- 23.Jules-Elysee KM, Lipnitsky JY, Patel N, Anastasian G, Wilfred SE, Urban MK, Sculco TP. Use of low-dose steroids in decreasing cytokine release during bilateral total knee replacement. Reg Anesth Pain Med. 2011;36:36–40. doi: 10.1097/AAP.0b013e31820306c5. [DOI] [PubMed] [Google Scholar]
- 24.Jules-Elysee KM, Wilfred SE, Memtsoudis SG, Kim DH, Yadeau JT, Urban MK, Lichardi ML, McLawhorn AS, Sculco TP. Steroid modulation of cytokine release and desmosine levels in bilateral total knee replacement: aprospective, double-blind, randomized controlled trial. J Bone Joint Surg Am. 2012;94:2120–2127. doi: 10.2106/JBJS.K.00995. [DOI] [PubMed] [Google Scholar]
- 25.Kardash KJ, Sarrazin F, Tessler MJ, Velly AM. Single-dose dexamethasone reduces dynamic pain after total hip arthroplasty. Anesth Analg. 2008;106:1253–1257. doi: 10.1213/ANE.0b013e318164f319. [DOI] [PubMed] [Google Scholar]
- 26.Kim S. Changes in surgical loads and economic burden of hip and knee replacements in the US: 1997–2004. Arthritis Rheum. 2008;59:481–488. doi: 10.1002/art.23525. [DOI] [PubMed] [Google Scholar]
- 27.Koh IJ, Chang CB, Jeon YT, Ryu JH, Kim TK. Does ramosetron reduce postoperative emesis and pain after TKA? Clin Orthop Relat Res. 2012;470:1718–1727. doi: 10.1007/s11999-011-2208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koh IJ, Chang CB, Seo ES, Kim SJ, Seong SC, Kim TK. Pain management by periarticular multimodal drug injection after anterior cruciate ligament reconstruction: a randomized, controlled study. Arthroscopy. 2012;28:649–657. doi: 10.1016/j.arthro.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Koh IJ, Kang YG, Chang CB, Do SH, Seong SC, Kim TK. Does periarticular injection have additional pain relieving effects during contemporary multimodal pain control protocols for TKA?: a randomised, controlled study. Knee. 2012;19:253–259. doi: 10.1016/j.knee.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Koh IJ, Kang YG, Chang CB, Kwon SK, Seo ES, Seong SC, Kim TK. Additional pain relieving effect of intraoperative periarticular injections after simultaneous bilateral TKA: a randomized, controlled study. Knee Surg Sports Traumatol Arthrosc. 2010;18:916–922. doi: 10.1007/s00167-010-1051-2. [DOI] [PubMed] [Google Scholar]
- 31.Koh IJ, Kim TK, Chang CB, Cho HJ, In Y. Trends in use of total knee arthroplasty in Korea from 2001 to 2010. Clin Orthop Relat Res. 2013;471:1441–1450. doi: 10.1007/s11999-012-2622-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koivuranta M, Laara E, Snare L, Alahuhta S. A survey of postoperative nausea and vomiting. Anaesthesia. 1997;52:443–449. doi: 10.1111/j.1365-2044.1997.117-az0113.x. [DOI] [PubMed] [Google Scholar]
- 33.Korttila K. The study of postoperative nausea and vomiting. Br J Anaesth. 1992;69(7 suppl 1):20S–23S. doi: 10.1093/bja/69.supplement_1.20S. [DOI] [PubMed] [Google Scholar]
- 34.Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. 2000;59:213–243. doi: 10.2165/00003495-200059020-00005. [DOI] [PubMed] [Google Scholar]
- 35.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 36.Lee Y, Lin YS, Chen YH. The effect of dexamethasone upon patient-controlled analgesia-related nausea and vomiting. Anaesthesia. 2002;57:705–709. doi: 10.1046/j.1365-2044.2002.02572_5.x. [DOI] [PubMed] [Google Scholar]
- 37.Lombardi AV, Jr, Berend KR, Mallory TH, Dodds KL, Adams JB. Soft tissue and intra-articular injection of bupivacaine, epinephrine, and morphine has a beneficial effect after total knee arthroplasty. Clin Orthop Relat Res. 2004;428:125–130. doi: 10.1097/01.blo.0000147701.24029.cc. [DOI] [PubMed] [Google Scholar]
- 38.Lunn TH, Kristensen BB, Andersen LO, Husted H, Otte KS, Gaarn-Larsen L, Kehlet H. Effect of high-dose preoperative methylprednisolone on pain and recovery after total knee arthroplasty: a randomized, placebo-controlled trial. Br J Anaesth. 2011;106:230–238. doi: 10.1093/bja/aeq333. [DOI] [PubMed] [Google Scholar]
- 39.Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg. 1999;89:652–658. doi: 10.1097/00000539-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 40.Miyagawa Y, Ejiri M, Kuzuya T, Osada T, Ishiguro N, Yamada K. Methylprednisolone reduces postoperative nausea in total knee and hip arthroplasty. J Clin Pharm Ther. 2010;35:679–684. doi: 10.1111/j.1365-2710.2009.01141.x. [DOI] [PubMed] [Google Scholar]
- 41.Myles PS, Williams DL, Hendrata M, Anderson H, Weeks AM. Patient satisfaction after anaesthesia and surgery: results of a prospective survey of 10,811 patients. Br J Anaesth. 2000;84:6–10. doi: 10.1093/oxfordjournals.bja.a013383. [DOI] [PubMed] [Google Scholar]
- 42.Park KK, Kim TK, Chang CB, Yoon SW, Park KU. Normative temporal values of CRP and ESR in unilateral and staged bilateral TKA. Clin Orthop Relat Res. 2008;466:179–188. doi: 10.1007/s11999-007-0001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93:1075–1084. doi: 10.2106/JBJS.J.01095. [DOI] [PubMed] [Google Scholar]
- 44.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids: new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 46.Salerno A, Hermann R. Efficacy and safety of steroid use for postoperative pain relief. update and review of the medical literature. J Bone Joint Surg Am. 2006;88:1361–1372. doi: 10.2106/JBJS.D.03018. [DOI] [PubMed] [Google Scholar]
- 47.Shen H, Zhang N, Zhang X, Ji W. C-reactive protein levels after 4 types of arthroplasty. Acta Orthop. 2009;80:330–333. doi: 10.3109/17453670903066596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinatra RS, Torres J, Bustos AM. Pain management after major orthopaedic surgery: current strategies and new concepts. J Am Acad Orthop Surg. 2002;10:117–129. doi: 10.5435/00124635-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Smith C, Erasmus PJ, Myburgh KH. Endocrine and immune effects of dexamethasone in unilateral total knee replacement. J Int Med Res. 2006;34:603–611. doi: 10.1177/147323000603400605. [DOI] [PubMed] [Google Scholar]
- 50.Song KH, Kang YM, Sin HY, Yoon SW, Seo HK, Kwon S, Shin MJ, Chang CB, Kim TK, Kim HB. Outcome of cefazolin prophylaxis for total knee arthroplasty at an institution with high prevalence of methicillin-resistant Staphylococcus aureus infection. Int J Infect Dis. 2011;15:e867–e870. doi: 10.1016/j.ijid.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 51.Watcha MF, White PF. Postoperative nausea and vomiting: its etiology, treatment, and prevention. Anesthesiology. 1992;77:162–184. doi: 10.1097/00000542-199207000-00023. [DOI] [PubMed] [Google Scholar]