Abstract
Background
The pathogenesis of tendinopathy is complex and incompletely understood. Although significant advances have been made in terms of understanding the pathological changes in both the extracellular matrix and the cells involved, relatively little is known about the role of neuronal regulation in tendinopathy. The frequent mismatch between tendon pathology and pain may be explained, in part, by differences in the peripheral neuronal phenotype of patients.
Questions/purposes
The primary purpose of this review was to determine whether evidence exists of changes in the peripheral neuronal phenotype in painful human tendinopathy and, if so, to identify the associated histological and molecular changes. The secondary purpose was to determine if any changes in the peripheral neuronal phenotype reported correlate with pain symptoms.
Methods
We conducted a systematic review of the scientific literature using the PRISMA and Cochrane guidelines. The Medline and Embase databases were searched using specific search criteria. Only studies analyzing the peripheral tissue of patients with the clinical diagnosis of tendinopathy were included. Inclusion was agreed on by two independent researchers on review of abstracts or full text.
Results
Overall in the 27 included studies, there was clear evidence of changes in the peripheral neuronal phenotype in painful human tendinopathy. The excitatory glutaminergic system was significantly upregulated in seven studies, there was a significant increase in sensory neuropeptide expression in four studies, and there were significant changes in the molecular morphology of tenocytes, blood vessels, and nerves. In rotator cuff tendinopathy, substance P has been shown to correlate with pain and the neural density in the subacromial bursa has been shown to correlate with rest pain.
Conclusions
The peripheral neuronal phenotype is an important factor in the pathogenesis of painful human tendinopathy. Further research in this area specifically correlating tissue changes to clinical scores has great potential in further developing our understanding of the disease process.
Introduction
Painful tendinopathy is a frequently encountered clinical problem, the three most common sites affected being the Achilles, patellar, and rotator cuff tendons [62]. Rotator cuff tendinopathy (RCT) is the most common of all with an annual incidence of over 1% that greatly increases with age [20, 60]. Other tendons affected include those around the elbow (medial and lateral epicondylitis) and around the wrist. There is a significant disease burden attached to painful tendinopathy not only in terms of healthcare costs, but also in terms of time off work and impact on patients’ quality of life [44]. Therefore, the effective treatment of painful tendinopathy has a huge potential to save money and improve quality of life [38]. In general, the etiology of these tendinopathies remains unclear, although the commonly affected tendons all experience high levels of mechanical stress [48] and overuse is a frequently implicated risk factor [26].
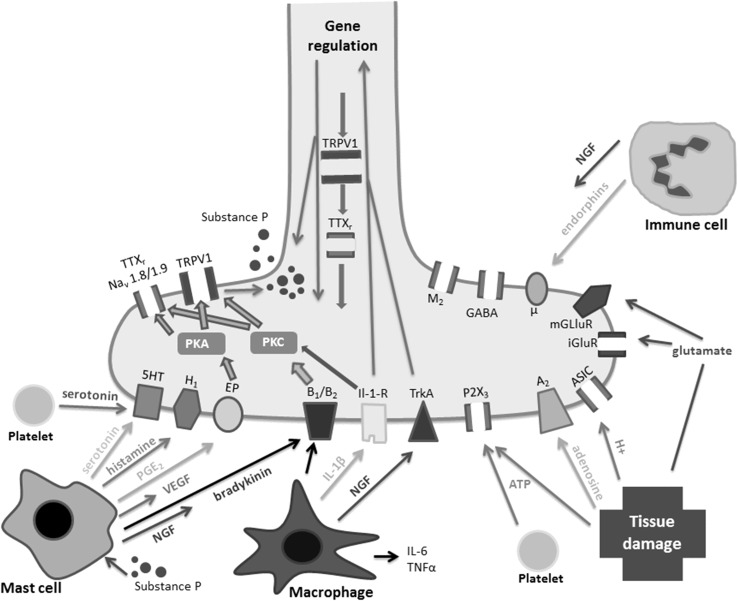
The molecular pathophysiology of tendinopathy has become clearer in recent years [28, 51]. Tendinopathy has characteristic histopathological, clinical, and radiological findings [62]. Clinical symptoms including pain are often poorly matched to the histopathological and radiological findings, meaning that a high proportion of patients with a tendon that is both histopathologically and radiologically abnormal have no pain or other symptoms [22, 27, 66]. The reasons for this mismatch between pathology and perceived pain are poorly understood [27]; however, recent research has identified the peripheral and central pain processing pathways as good candidates for an explanation [32, 35]. It appears that the presence of pain in tendinopathy not only requires mechanical changes in the tendon, but also alterations in the way the local cells and the peripheral nerves react to this change, thus resulting in the nociceptive pathways to higher centers being activated [27]. Some of the mechanisms that drive increased peripheral nerve sensitivity (peripheral sensitization) are depicted (Fig. 1).
Fig. 1.
A schematic diagram depicting mechanisms of peripheral sensitization and inflammation at the peripheral nerve terminal. ASIC = acid sensing ion channel; iGluR = inotropic glutamate receptor; IL-1β = interleukin-1β; IL-6 = interleukin-6; mGluR = metabotropic glutamate receptor; NGF = nerve growth factor; PGE2 = prostaglandin E2; PKA = protein kinase A; PKC = protein kinase C; TNF-α = tumor necrosis factor α; TrkA = tyrosine kinase receptor A; TTXr = tetrodotoxin-resistant sodium channel; μ = mu-opioid receptor; M2 = muscarinic receptor; 5-HT3 = serotonin; H1 = histamine receptor; B1/B2 = bradykinin receptors; A2 = adenosine receptor; GABAR = γ-aminobutyric acid receptor; EP = prostaglandin receptor; VEGF = vascular endothelial growth factor. Reproduced with permission from Dean BJ, Gwilym SE, Carr AJ. Why does my shoulder hurt? A review of the neuroanatomical and biochemical basis of shoulder pain. Br J Sports Med. 2013 Feb 21 [Epub ahead of print] [27].
The peripheral nervous system plays an important role in tissue homeostasis and tendon healing [2]. The neuronal response to tendon injury involves nerve in-growth during the initial inflammatory phase; the subsequent proliferative and remodeling phases are regulated by sensory nerves as well as the glutaminergic and autonomic systems. Sensory neuropeptide expression has been shown to be associated with both failed healing and pain in an animal model of tendon injury [45]; they have also been causally linked with tendinopathy-like changes in animal models [8, 10]. The application of neuropeptides such as substance P has shown promise in improving tendon healing in an animal model of tendon rupture [19], but this has not been taken further into in vivo human studies. Neuropeptides have pro-proliferative, angiogenic, and stem cell-stimulating properties in vitro [11], therefore implicating them further in potentially aiding tendon healing.
In this context, the primary purpose of this systematic review of the literature was to determine whether there was evidence of changes in the peripheral neuronal phenotype in painful human tendinopathy. The term “peripheral neuronal phenotype” is used in this context to refer to specific characteristics of the peripheral nervous system including the nerves, the neuronal mediators, and the receptors for these mediators in peripheral tissue. We also aimed to describe the relevant histological and molecular changes to the peripheral neuronal phenotype as part of this primary purpose. A secondary purpose was to determine whether any changes in peripheral neuronal phenotype were correlated to pain symptoms.
Materials and Methods
This systematic review used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and the Cochrane handbook as guidelines in the development of the study protocol and the report of the current study [21, 43]. The inclusion criteria and methods of analysis were specified in advance and documented in a protocol.
Information Sources and Search Strategy
Studies were identified by searching the Medline (1951 to present) and Embase (1980 to present) electronic databases. The search was undertaken in October 2012. The following search terms were used: tendinopathy AND pain, nerve, neuronal, innervation, somatic, autonomic, sympathetic, parasympathetic, nerve growth factor, NGF, TrkA, p75, neuropeptide*, NPY, adrenaline, noradrenaline, adrenoceptors, VIP, acetycholine, substance P, NK1, CGRP, calcitonin, RAMP, IB4, galanin, receptor*, nocicept*, mechanorecept*, proprioceptor*, Ruffini, Meissner, golgi, opio*, neurohistolog*, somatostatin, encephalin, endorphin, neurokinin, NMDA, AMPA, mGluR, glutam*, metabotropic, ionotropic, neurotrophins, Nav1.8, sodium channels, histology, histological.
Additional studies were located by searching reference lists of short-listed articles. The citations identified from the searches were combined and duplicates excluded. All citations for papers clearly referring to a topic other than painful human tendinopathy were excluded as were others whose title clearly showed that the paper was not relevant to the current study. Papers had to relate to peripheral neuronal phenotype, which included innervation, peripheral neuronal mediators, and their receptors, including gene expression. Studies had to be of human participants who had been diagnosed with painful tendinopathy based on a clinician’s history and clinical examination findings with or without radiological assessment.
Case reports and review articles were excluded. In vitro studies and animal studies were excluded. Studies relating to patients without pain were excluded as were studies relating to changes after surgical intervention. Studies that did not analyze changes in the peripheral tissue were excluded. Studies that did not use control groups were also excluded. Studies that could not be obtained in English were excluded.
The search, selection of studies, and data analysis were performed independently by two individuals (BD, SF). Agreement on inclusion was achieved after review of the full-text articles and a joint decision by both individuals based on the inclusion/exclusion criteria. The data were then extracted using a spreadsheet designed by two authors (BD, SF); this included data relating to study heterogeneity and methodological quality. The data extracted included patient demographics, symptom duration, investigations, control group type, tissue analysis method, and statistical methods. Methodological quality was assessed using a 10-point scale based on the scale used by Hegedus et al. [36] (Table 1).
Table 1.
Methodological quality assessment document (the number of yes answers was counted for each study to give a total score out of 10)
| Number | Criteria | Yes | No | Unclear |
|---|---|---|---|---|
| 1 | Inclusion/exclusion criteria clearly described | |||
| 2 | Study population clearly described | |||
| 3 | Study population representative of those in clinical practice | |||
| 4 | Control group clearly described | |||
| 5 | Sampling method clearly described | |||
| 6 | Live study subjects | |||
| 7 | Live control subjects | |||
| 8 | Quantitative method or semiquantitative method using minimum of two independent observers | |||
| 9 | Reliability and/or validity of methods described | |||
| 10 | Study limitations addressed |
Study Selection
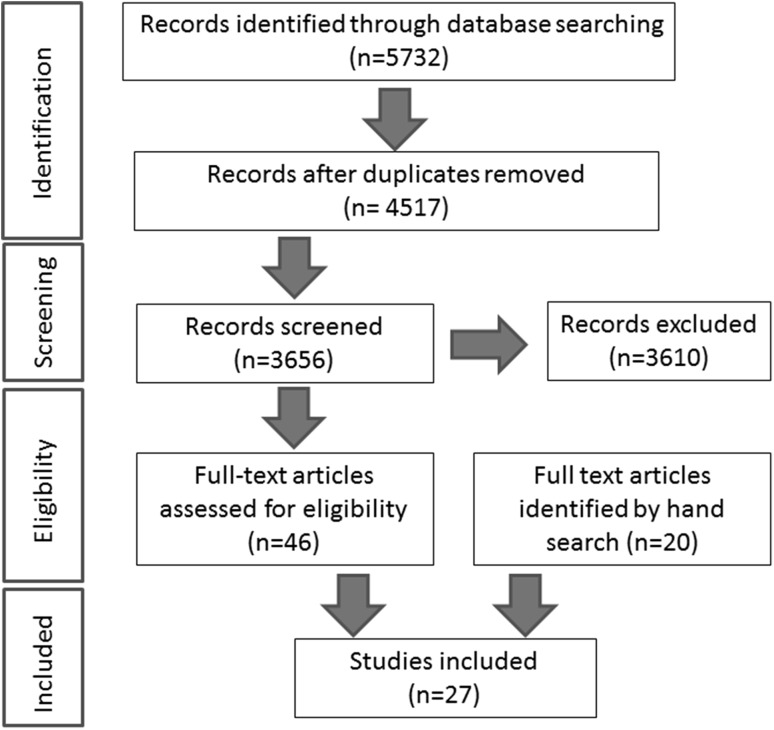
The search strategy revealed a total of 5732 results (Fig. 2). After removal of 1215 duplicate entries, 4517 unique papers remained. Screening of the titles and abstracts revealed 46 papers eligible for inclusion and the hand search of references revealed another 20 papers potentially eligible for inclusion. Further assessment of eligibility, based on full-text papers, led to the exclusion of 39 of these 66 papers. The reasons for the exclusion of these 39 papers were as follows: no control group (three), reviews (17), animal studies (13), or non-neuronal tissue changes (six). This left 27 papers meeting our criteria for inclusion, which are summarized in Appendix 1 [3–7, 9, 12–17, 23–25, 29, 34, 42, 53–56, 58, 59, 61, 64, 68].
Fig. 2.
A flow diagram demonstrating the systematic review protocol.
Study Characteristics
Of the 27 studies included, 11 related to the Achilles tendon, seven to the patellar tendon, two to both the Achilles and patellar tendons, five to RCT, and two to medial/lateral humeral epicondylitis. All studies related to patients with painful tendinopathy and all studies stated specific diagnostic criteria. The majority of these studies specified that the tendinopathy was of a chronic duration (25 of 27 studies); the remaining two studies did not specify the duration of symptoms. The mean ages of the overall patient groups were as follows: 28 years (patellar), 40 years (medial/lateral epicondylitis), 46 years (Achilles), and 56 years (RCT).
In 23 of the 27 studies, the tissue samples were obtained as part of the surgical treatment of their tendinopathy, whereas in the remaining four, tissue samples were taken as open biopsies with no surgical treatment being performed. In 23 studies, an open biopsy was performed in isolation, in two studies microdialysis was performed in isolation, whereas in the remaining two studies, both an open biopsy and microdialysis were performed.
The control group consisted of healthy asymptomatic patients in 21 studies, cadaveric controls in three studies, patients undergoing surgery for shoulder instability in two studies, and patients with Achilles tendon rupture in one study. Nine studies used clinical pain scores to measure pain levels in individual patients; of note, only two of these studies correlated tissue findings with the pain scores.
Study Methodology and Assessing the Risk of Bias
All included studies stated clear diagnostic criteria, described their study population and control groups, and clearly described method(s) of tissue analysis. The results of the methodological quality assessment are detailed in Appendix 2. The median score was 9 (range, 7–10). Five studies produced quantitative results (four of which used microdialysis and one radioimmunoassay). Microdialysis is a minimally invasive sampling technique by which extracellular fluid is sampled and analyzed. Fourteen studies produced semiquantitative results (13 of which used immunohistochemistry and one histology), whereas eight were purely descriptive studies. Twelve of these 14 studies clearly described their method of semiquantitative analysis; two involved nerve counts and the other 10 described objective methods using more than one observer or computer analysis. Two studies did not clearly describe the semiquantitative methods and were therefore not included in the quantitative section of the results. Levels of statistical significance were stated in 16 of the 19 studies, which used quantitative or semiquantitative analysis. Of these 16, 12 studies used the 95% significance level with four studies using the 99% level. Studies that used descriptive nonquantitative methods only and/or studies that did not state the statistical significance of their results were excluded from the quantitative section of the results. Studies that did not have control groups for comparison were excluded. This methodological assessment means that the quantitative results have only included studies with minimal levels of bias.
Results
There were clear changes in the peripheral neuronal phenotype in painful human tendinopathy (Tables 2, 3). Studies have consistently shown increased overall neural ingrowth in tendinopathy versus control [6, 42, 59, 64]. Specifically there was also strong quantitative evidence showing a significant upregulation of the glutaminergic system; this relates not only to the overall tissue, but also to the nerves, blood vessels, and tenocytes (Table 3). Glutamate NMDAR1 receptors have been frequently noted in morphologically altered tenocytes in tendon tissue proper and in the peritendinous connective tissue [4, 54]. These NMDAR1 receptors have been colocalized with AChE and are frequently seen in free-nerve fibers. Tenocytes also showed a significant increase in the expression of NPY-1 and CB-1 receptors in tendinopathy. There was also good evidence that the sensory system is upregulated with substance P levels increased in tendinopathy as well as increased numbers of substance P and CGRP (calcitonin gene-related peptide)-positive nerves. There was conflicting evidence relating to the autonomic system with one study showing a significant decrease in tyrosine hydroxylase-positive nerves and another showing no change in tyrosine hydroxylase-positive nerves.
Table 2.
Summary of the descriptive results
| Pathway | Type | Nerve | Tenocyte | Overall tissue |
|---|---|---|---|---|
| General | PGP 9.5 ↑↑ [6, 42] | |||
| Sensory | Sensory | Substance P ↑ [42] | Substance P ↑ [9] NK-1R ↑ [9] |
|
| Opioid | ||||
| Autonomic | Sympathetic | Tyrosine hydroxylase ↑ ↑ [24, 68] α1 adrenoceptors↑ [16] Tyrosine hydroxylase and NPY → [16] |
Tyrosine hydroxylase and NPY → [16] | |
| Parasympathetic | VAChT ↑↑ ChAT ↑↑ M2R ↑↑ [23, 25] AChE → [23] ChAT ↑VAChT ↑ [17] |
ChAT absent [68] | ||
| Excitatory | Glutaminergic | |||
| Other | Neurotrophins | NGF ↑ BDNF↑ p75↑ [13] TrkA → TrkB → [13] |
BDNF ↑ [12] |
PGP = protein gene product; GAP = growth-associated product; NK-1R = neurokinin-1 receptor; NPY = neuropeptide Y; VAChT = vesicular acetylcholine transporter; ChAT = choline acetyltransferase; M2R = M2 muscarinic acetylcholine receptor; AChE = acetycholine esterase; NGF = nerve growth factor; BDNF = brain-derived neurotrophic factor; p75 = low-affinity NGF receptor; TrkA = neurotrophic tyrosine kinase receptor type 1; TrkB = neurotrophic tyrosine kinase receptor type 2.
Table 3.
Summary of the quantitative results
| Pathway | Type | Nerve | Blood vessel | Tenocyte | Overall tissue |
|---|---|---|---|---|---|
| General | ↑neural elements [59] PGP9.5↑ [59, 64] GAP43↑ [64] ↑ neural elements with rest pain [61] |
||||
| Sensory | Sensory | Substance P ↑ [55] CGRP ↑ [59] Tyrosine hydroxylase ↓ [42] Tyrosine Hydroxylase → [55] |
Substance P ↑ [34, 54] Substance P/CGRP → [58] |
||
| Opioid | CB1R ↑ [14] | ||||
| Autonomic | Sympathetic | NPY-1R ↑ [15] | |||
| Parasympathetic | |||||
| Excitatory | Glutaminergic | Glutamate↑ [53] NMDAR1↑ [53] |
Glutamate↑ [53] NMDAR1↑ [53] |
NMDAR1 ↑ [53] Glutamate ↑ [53] VGluT2 ↑ [56] VGluT1 → [56] |
Glutamate↑ ↑↑↑ [3–5, 7] NMDAR1↑↑ → [3, 54] pNMDAR1 ↑ → [54] mGluR5 ↑ [54] mGluR6 → [54] |
CGRP = calcitonin gene-related peptide; CB1R = cannabinoid receptor 1; NPY-1R = neuropeptide Y receptor 1; NMDAR1 = NMDA receptor (type of glutamate receptor); VGluT1/2 = vesicular glutamate transporter 1/2; pNMDAR1 = phospho-NMDA receptor 1.
There was relatively little direct evidence in the literature relating changes in peripheral neuronal phenotype to variations in pain symptomatology among patients; only two of the 27 studies we evaluated provided information relating to this question. One study demonstrated that substance P levels in the subacromial bursa appeared to correlate with pain levels as measured with a visual analog score. Another study demonstrated that an increased neural density in the subacromial bursa correlated with the presence of shoulder pain at rest. Although many of the other studies did not directly investigate the relationship between peripheral neuronal phenotype and pain, all remaining studies (25 of 25) compared a group with painful tendinopathy with a group of pain-free control subjects (admittedly one study’s control group had asymptomatic Achilles tendinopathy that went on to rupture).
Discussion
Our knowledge of the pathogenesis of tendinopathy has advanced much in recent years, but the role of neuronal regulation in this process is still relatively poorly understood. Neuronal regulation is of key importance in tendon homeostasis and healing [2]. We sought to determine whether the peripheral neuronal phenotype is altered in tendinopathy; results from this systematic review suggest this to be the case. Specifically, we found strong evidence of an upregulation of the glutaminergic system in painful human tendinopathy, and we found weaker, but still suggestive, evidence that changes in the peripheral neuronal phenotype were related to variations in pain symptomatology among patients.
A significant limitation to all the research carried out on painful tendinopathy is the adequacy of the control tissue used. The gold standard control tissue would be taken from asymptomatic patients with tendinopathic tendons and this could then be compared with the tendinopathic tissue of patients with pain. Ethically this has not been possible, and the next best option is control tissue taken from healthy asymptomatic patients as was done in 21 of the 27 studies included in the review. In 11 of the 20 studies that related to the Achilles and patellar tendons, radiological assessment was used to confirm that no tendon abnormality was present in the healthy control subjects; however, in the remaining nine studies, no radiological assessment of the control subjects was undertaken, which means that some of these tendons may in fact have been tendinopathic in nature. The consequent problem is that any observed differences in the tissue between disease and control groups may or may not be related to painful tendinopathic changes; they may be related to changes that also occur in asymptomatic tendinopathic patients.
Another limitation to the literature is that the disease subgroups studied have largely been patients undergoing surgery for the failed conservative management of their tendinopathy (23 of 27 studies); this means that the findings cannot necessarily be applied to other disease subgroups. Also, the degree to which the results of the studies were descriptive or quantitative was highly variable. As a result, only studies that used semiquantitative or quantitative methods and that stated levels of statistical significance of p ≤ 0.05 were included in the quantitative section of the results (Table 3). Other less robust methods of result gathering were included in the descriptive results (Table 2). The variable blinding of the observers undertaking the semiquantitative tissue grading does increase the risk of study bias toward positive findings (nine of 14 studies did not state whether observers were blinded). The nature of the study results and their heterogeneity precluded a meta-analysis; the overall descriptive results (Tables 2, 3) are the reasonable limit of any synthesis in our opinion.
The cause of tendinopathy has been the subject of much heated debate over the years [40] and many different theories have been postulated [30, 47, 57, 65]. Certainly there are problems in generalizing from results relating to different tendons. The rotator cuff tendons tend to be affected increasingly in old age, whereas the patellar tendon is more commonly affected in younger age groups. Different tendons exist in their own unique anatomical environments and are subject to different mechanical forces. This implies that there are some significant differences in the etiology of pathology in different tendons. There are, however, numerous similarities between the tendinopathies of different tendons. The histological changes are well characterized and similar across the different tendinopathies [46], whereas many of the molecular changes are also shared in common [28, 51]. Therefore, we would argue that it is valid to summarize the changes in peripheral neuronal phenotype across the different tendinopathies.
There is little doubt that the peripheral neuronal phenotype plays a key role in painful human tendinopathy; in answer to our first key question, we conclude that there is clear evidence of changes in the peripheral neuronal phenotype in painful human tendinopathy. However, the precise role that neuronal regulation plays is not yet clear. Whether changes in neuronal regulation are crucial in driving the development of symptomatic tendinopathy or whether they are more reactive secondary changes is still open to debate. The importance of nerve in growth and neuronal regulation in animal tendon healing has been well demonstrated [2]; interestingly the ingrowth of Substance P and CGRP-positive fibers was associated with increased nociception in the proliferative phase of healing, whereas the occurrence of sympathetic markers in the remodelling phase of healing was associated with decreased nociception. Work on an animal model of osteoarthritis has clearly shown the presence of ectopic neural ingrowth and this has been linked with pain generation [33]. Nerve ingrowth has also been demonstrated in degenerative spinal disease and postulated as a mechanism of pain generation [31]. Increased neural ingrowth in RCT is a consistent finding and may represent attempted tendon healing [59, 64]. The evidence of increased neural ingrowth in Achilles and patellar tendinopathy is weaker and less consistent; some studies have demonstrated increased ingrowth [6, 42] but several studies have not shown any significant differences. The reasons for these inconsistent findings are unclear; one explanation is that nerve ingrowth may diminish as a tendinopathy becomes more chronic, whereas another reasonable explanation is that the properties of the nerves and their consequent sensitivity are more important than simple nerve density.
The consistent finding of increased glutamate levels and an overall upregulation of the excitatory glutaminergic system is of particular interest. Glutamate levels have been shown to be increased in several painful musculoskeletal disorders [39, 52]. Glutamate is a key metabolite and key neurotransmitter involved in the transmission of pain [18, 49]. Not only does glutamate have a vital role in the regulation of peripheral pain fiber sensitivity (peripheral sensitization) through its numerous receptors [18], but it also plays a key role in the sensitivity of the spinal cord and brain to pain transmission from the periphery (“central sensitization”) [63]. There is also some evidence for upregulation of the sensory system (Substance P/CGRP) but this is not as clear a finding as that of the glutaminergic system. An upregulation of the sensory system has been implicated in pain generation in both animal models and human disease [1, 31, 33, 34]. The neuropeptides Substance P and CGRP are both vasodilators and both appear to have important roles in tissue healing [19, 41]. The sensory innervation of tissue appears vital in its ability to proliferate and remodel as part of the normal healing process [37]. The histological and molecular changes in the peripheral neuronal phenotype shown in our study appear consistent with the theory that the failing healing of tendon in painful tendinopathy results in features consistent with a prolonged proliferative phase of healing. The precise reason why this apparent state of failed healing results in pain may relate to the complex dynamic of the peripheral neuronal tissue changes and the effect this has on the sensitivity of the peripheral nerve fibers.
Finally, in answer to our second question, there is a small amount of evidence that changes in the peripheral neuronal phenotype may be related to variations in pain symptomatology between patients. This is consistent with other areas of research in which changes in the peripheral neuronal phenotype have been associated with pain symptoms [50, 67]. However, we conclude that there is not enough good evidence available to answer our second key question adequately. Ultimately further research is needed to better define the role of neuronal regulation in the disease process and to better relate any changes in the peripheral neuronal phenotype to clinical pain scores.
Future studies that combine tissue analysis with clinical pain scores to study the natural history of tendinopathy and the response to different treatment modalities have the potential to help answer these important questions, including perhaps research that may lead to the identification of novel neuromodulatory drug targets. Another intriguing idea for the future is the measurement of markers of peripheral neuronal phenotype in the clinical setting with the potential of this being used as prognostic aid to guide individual patient treatment or even a surrogate outcome measure in trials.
Acknowledgments
We thank the reviewers and editorial staff who have kindly helped in the production of this final manuscript.
Appendix 1. Study details
| Number | Study | Year | Journal | Pathology | Primary tissue method |
|---|---|---|---|---|---|
| 1 | Alfredson et al. [3] | 2001 | Knee Surg Sports Traumatol Arthrosc | Achilles tendinopathy | Microdialysis via open incision |
| 2 | Alfredson et al. [4] | 2001 | J Orthop Res | Patellar tendinopathy | Microdialysis via open incision |
| 3 | Alfredson et al. [5] | 2000 | Acta Orthop Scand | Humeral epicondylitis | Microdialysis via open incision |
| 4 | Alfredson et al. [6] | 2003 | Knee Surg Sports Traumatol Arthrosc | Achilles tendinopathy | Open biopsy |
| 5 | Alfredson et al. [7] | 1999 | Knee Surg Sports Traumatol Arthrosc | Achilles tendinopathy | Microdialysis via open incision |
| 6 | Andersson et al. [9] | 2008 | Regul Pept | Achilles tendinopathy | Open biopsy |
| 7 | Bagge et al. [13] | 2009 | Histol Histopathol | Achilles tendinopathy | Open biopsy |
| 8 | Bagge et al. [12] | 2012 | Histol Histopathol | Achilles tendinopathy | Open biopsy |
| 9 | Bjorklund et al. [14] | 2011 | Plos One | Achilles tendinopathy | Open biopsy |
| 10 | Bjur et al. [15] | 2009 | Br J Sports Med | Achilles tendinopathy | Open biopsy |
| 11 | Bjur et al. [17] | 2008 | Cell Tissue Res | Achilles tendinopathy | Open biopsy |
| 12 | Bjur et al. [16] | 2008 | Histol Histopathol | Achilles tendinopathy | Open biopsy |
| 13 | Danielson et al. [23] | 2006 | Microsc Res Tech | Patellar tendinopathy | Open biopsy |
| 14 | Danielson et al. [24] | 2007 | Microsc Res Tech | Patellar tendinopathy | Open biopsy |
| 15 | Danielson et al. [25] | 2007 | Life Sci | Patellar tendinopathy | Open biopsy |
| 16 | Forsgren et al. [29] | 2005 | Regul Pept | Achilles and patellar tendinopathy | Open biopsy |
| 17 | Gotoh et al. [34] | 1998 | J Orthop Res | Rotator cuff disease | Open biopsy |
| 18 | Lian et al. [42] | 2006 | Am J Sports Med | Patellar tendinopathy | Open biopsy |
| 19 | Schizas et al. [53] | 2010 | Scand J Med Sci Sports | Patellar tendinopathy | Open biopsy |
| 20 | Schizas et al. [54] | 2012 | J Orthop Res | Patellar tendinopathy | Open biopsy |
| 21 | Schubert et al. [55] | 2005 | Ann Rheum Dis | Achilles tendinopathy | Open biopsy |
| 22 | Scott et al. [56] | 2008 | J Orthop Res | Achilles and patellar tendinopathy | Open biopsy |
| 23 | Singaraju et al. [58] | 2008 | J Shoulder Elbow Surg | Long head of biceps tendon | Open biopsy |
| 24 | Xu et al. [64] | 2011 | Sports Med Arthrosc | Rotator cuff disease | Open biopsy |
| 25 | Zeisig et al. [68] | 2009 | Br J Sports Med | Humeral epicondylitis | Open biopsy |
| 26 | Tomita et al. [61] | 1997 | J Orthop Surg | Rotator cuff disease | Open biopsy |
| 27 | Tamai et al. [59] | 2000 | Clin Orthop Relat Res | Rotator cuff disease | Open biopsy |
Appendix 2. Methodological quality scores for each study (q denotes question number and total denoting total score)
| Author | Year | Journal | q1 | q2 | q3 | q4 | q5 | q6 | q7 | q8 | q9 | q10 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alfredson et al. [3] | 2001 | Knee Surg Sports Traumatol Arthrosc | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Alfredson et al. [4] | 2001 | J Orthop Res | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Alfredson et al. [5] | 2000 | Acta Orthop Scand | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Alfredson et al. [6] | 2003 | Knee Surg Sports Traumatol Arthrosc | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 8 |
| Alfredson et al. [7] | 1999 | Knee Surg Sports Traumatol Arthrosc | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 9 |
| Andersson et al. [9] | 2008 | Regul Pept | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Bagge et al. [13] | 2009 | Histol Histopathol | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 8 |
| Bagge et al. [12] | 2012 | Histol Histopathol | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 8 |
| Bjorklund et al. [14] | 2011 | Plos One | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 9 |
| Bjur et al. [15] | 2009 | Br J Sports Med | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 9 |
| Bjur et al. [17] | 2008 | Cell Tissue Res | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 7 |
| Bjur et al. [16] | 2008 | Histol Histopathol | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 7 |
| Danielson et al. [23] | 2006 | Microsc Res Tech | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 8 |
| Danielson et al. [24] | 2007 | Microsc Res Tech | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 7 |
| Danielson et al. [25] | 2007 | Life Sci | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 8 |
| Forsgren et al. [29] | 2005 | Regul Pept | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 8 |
| Gotoh et al. [34] | 1998 | J Orthop Res | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Lian et al. [42] | 2006 | Am J Sports Med | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Schizas et al. [53] | 2010 | Scand J Med Sci Sports | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 9 |
| Schizas et al. [54] | 2012 | J Orthop Res | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Schubert et al. [55] | 2005 | Ann Rheum Dis | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 9 |
| Scott et al. [56] | 2008 | J Orthop Res | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 9 |
| Singaraju et al. [58] | 2008 | J Shoulder Elbow Surg | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 8 |
| Xu et al. [64] | 2011 | Sports Med Arthrosc | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 8 |
| Zeisig et al. [68] | 2009 | Br J Sports Med | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 8 |
| Tomita et al. [61] | 1997 | J Orthop Surg | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 7 |
| Tamai et al. [59] | 2000 | Clin Orthop Relat Res | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 9 |
Footnotes
One or more of the authors are funded by the Musculoskeletal Biomedical Research Unit of the National Institute for Health Research (BD, SF, AC), the Jean Shanks Foundation (BD), and the Orthopaedic Research UK (BD).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
References
- 1.Ackermann PW, Li J, Lundeberg T, Kreicbergs A. Neuronal plasticity in relation to nociception and healing of rat achilles tendon. J Orthop Res. 2003;21:432–441. doi: 10.1016/S0736-0266(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 2.Ackermann PW, Salo PT, Hart DA. Neuronal pathways in tendon healing. Front Biosci. 2009;14:5165–5187. doi: 10.2741/3593. [DOI] [PubMed] [Google Scholar]
- 3.Alfredson H, Forsgren S, Thorsen K, Fahlstrom M, Johansson H, Lorentzon R. Glutamate NMDAR1 receptors localised to nerves in human Achilles tendons. Implications for treatment? Knee Surg Sports Traumatol Arthrosc. 2001;9:123–126. doi: 10.1007/s001670000188. [DOI] [PubMed] [Google Scholar]
- 4.Alfredson H, Forsgren S, Thorsen K, Lorentzon R. In vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in Jumper’s knee. J Orthop Res. 2001;19:881–886. doi: 10.1016/S0736-0266(01)00016-X. [DOI] [PubMed] [Google Scholar]
- 5.Alfredson H, Ljung BO, Thorsen K, Lorentzon R. In vivo investigation of ECRB tendons with microdialysis technique—no signs of inflammation but high amounts of glutamate in tennis elbow. Acta Orthop Scand. 2000;71:475–479. doi: 10.1080/000164700317381162. [DOI] [PubMed] [Google Scholar]
- 6.Alfredson H, Ohberg L, Forsgren S. Is vasculo-neural ingrowth the cause of pain in chronic Achilles tendinosis? An investigation using ultrasonography and colour Doppler, immunohistochemistry, and diagnostic injections. Knee Surg Sports Traumatol Arthrosc. 2003;11:334–338. doi: 10.1007/s00167-003-0391-6. [DOI] [PubMed] [Google Scholar]
- 7.Alfredson H, Thorsen K, Lorentzon R. In situ microdialysis in tendon tissue: high levels of glutamate, but not prostaglandin E2 in chronic Achilles tendon pain. Knee Surg Sports Traumatol Arthrosc. 1999;7:378–381. doi: 10.1007/s001670050184. [DOI] [PubMed] [Google Scholar]
- 8.Andersson G, Backman LJ, Scott A, Lorentzon R, Forsgren S, Danielson P. Substance P accelerates hypercellularity and angiogenesis in tendon tissue and enhances paratendinitis in response to Achilles tendon overuse in a tendinopathy model. Br J Sports Med. 2011;45:1017–1022. doi: 10.1136/bjsm.2010.082750. [DOI] [PubMed] [Google Scholar]
- 9.Andersson G, Danielson P, Alfredson H, Forsgren S. Presence of substance P and the neurokinin-1 receptor in tenocytes of the human Achilles tendon. Regul Pept. 2008;150:81–87. doi: 10.1016/j.regpep.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Backman LJ, Andersson G, Wennstig G, Forsgren S, Danielson P. Endogenous substance P production in the Achilles tendon increases with loading in an in vivo model of tendinopathy-peptidergic elevation preceding tendinosis-like tissue changes. J Musculoskelet Neuronal Interact. 2011;11:133–140. [PubMed] [Google Scholar]
- 11.Backman LJ, Fong G, Andersson G, Scott A, Danielson P. Substance P is a mechanoresponsive, autocrine regulator of human tenocyte proliferation. PloS One. 2011;6:e27209. doi: 10.1371/journal.pone.0027209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagge J, Danielson P, Forsgren S. In situ hybridization studies favouring the occurrence of a local production of BDNF in the human Achilles tendon. Histol Histopathol. 2012;27:1239–1246. doi: 10.14670/HH-27.1239. [DOI] [PubMed] [Google Scholar]
- 13.Bagge J, Lorentzon R, Alfredson H, Forsgren S. Unexpected presence of the neurotrophins NGF and BDNF and the neurotrophin receptor p75 in the tendon cells of the human Achilles tendon. Histol Histopathol. 2009;24:839–848. doi: 10.14670/HH-24.839. [DOI] [PubMed] [Google Scholar]
- 14.Bjorklund E, Forsgren S, Alfredson H, Fowler CJ. Increased expression of cannabinoid CB(1) receptors in Achilles tendinosis. PloS One. 2011;6:e24731. doi: 10.1371/journal.pone.0024731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjur D, Alfredson H, Forsgren S. Presence of the neuropeptide Y1 receptor in tenocytes and blood vessel walls in the human Achilles tendon. Br J Sports Med. 2009;43:1136–1142. doi: 10.1136/bjsm.2008.055780. [DOI] [PubMed] [Google Scholar]
- 16.Bjur D, Danielson P, Alfredson H, Forsgren S. Immunohistochemical and in situ hybridization observations favor a local catecholamine production in the human Achilles tendon. Histol Histopathol. 2008;23:197–208. doi: 10.14670/HH-23.197. [DOI] [PubMed] [Google Scholar]
- 17.Bjur D, Danielson P, Alfredson H, Forsgren S. Presence of a non-neuronal cholinergic system and occurrence of up- and down-regulation in expression of M2 muscarinic acetylcholine receptors: new aspects of importance regarding Achilles tendon tendinosis (tendinopathy) Cell Tissue Res. 2008;331:385–400. doi: 10.1007/s00441-007-0524-1. [DOI] [PubMed] [Google Scholar]
- 18.Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Semin Cell Dev Biol. 2006;17:592–604. doi: 10.1016/j.semcdb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Carlsson O, Schizas N, Li J, Ackermann PW. Substance P injections enhance tissue proliferation and regulate sensory nerve ingrowth in rat tendon repair. Scand J Med Sci Sports. 2011;21:562–569. doi: 10.1111/j.1600-0838.2009.01080.x. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhury S, Gwilym SE, Moser J, Carr AJ. Surgical options for patients with shoulder pain. Nat Rev Rheumatol. 2010;6:217–226. doi: 10.1038/nrrheum.2010.25. [DOI] [PubMed] [Google Scholar]
- 21.Cochrane. Cochrane Handbook for Systematic Reviews of Interventions. Available at: http://www.cochrane.org/training/cochrane-handbook. Accessed March 2013.
- 22.Cook JL, Feller JA, Bonar SF, Khan KM. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes’ patellar tendons. J Orthop Res. 2004;22:334–338. doi: 10.1016/j.orthres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Danielson P, Alfredson H, Forsgren S. Immunohistochemical and histochemical findings favoring the occurrence of autocrine/paracrine as well as nerve-related cholinergic effects in chronic painful patellar tendon tendinosis. Microsc Res Tech. 2006;69:808–819. doi: 10.1002/jemt.20351. [DOI] [PubMed] [Google Scholar]
- 24.Danielson P, Alfredson H, Forsgren S. In situ hybridization studies confirming recent findings of the existence of a local nonneuronal catecholamine production in human patellar tendinosis. Microsc Res Tech. 2007;70:908–911. doi: 10.1002/jemt.20495. [DOI] [PubMed] [Google Scholar]
- 25.Danielson P, Andersson G, Alfredson H, Forsgren S. Extensive expression of markers for acetylcholine synthesis and of M2 receptors in tenocytes in therapy-resistant chronic painful patellar tendon tendinosis—a pilot study. Life Sci. 2007;80:2235–2238. doi: 10.1016/j.lfs.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 26.de Jonge S, van den Berg C, de Vos RJ, van der Heide HJ, Weir A, Verhaar JA, Bierma-Zeinstra SM, Tol JL. Incidence of midportion Achilles tendinopathy in the general population. Br J Sports Med. 2011;45:1026–1028. doi: 10.1136/bjsports-2011-090342. [DOI] [PubMed] [Google Scholar]
- 27.Dean BJ, Gwilym SE, Carr AJ. Why does my shoulder hurt? A review of the neuroanatomical and biochemical basis of shoulder pain. Br J Sports Med. 2013 Feb 21 [Epub ahead of print]. [DOI] [PubMed]
- 28.Dean BJF, Franklin SL, Carr AJ. A systematic review of the histological and molecular changes in rotator cuff disease. Bone Joint Res. 2012;1:158–166. doi: 10.1302/2046-3758.17.2000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forsgren S, Danielson P, Alfredson H. Vascular NK-1 receptor occurrence in normal and chronic painful Achilles and patellar tendons: studies on chemically unfixed as well as fixed specimens. Regulat Pept. 2005;126:173–181. doi: 10.1016/j.regpep.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Fredberg U, Stengaard-Pedersen K. Chronic tendinopathy tissue pathology, pain mechanisms, and etiology with a special focus on inflammation. Scand J Med Sci Sports. 2008;18:3–15. doi: 10.1111/j.1600-0838.2007.00746.x. [DOI] [PubMed] [Google Scholar]
- 31.Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O’Brien J, Jayson MI. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178–181. doi: 10.1016/S0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- 32.Gangadharan V, Wang R, Ulzhofer B, Luo C, Bardoni R, Bali KK, Agarwal N, Tegeder I, Hildebrandt U, Nagy GG, Todd AJ, Ghirri A, Haussler A, Sprengel R, Seeburg PH, MacDermott AB, Lewin GR, Kuner R. Peripheral calcium-permeable AMPA receptors regulate chronic inflammatory pain in mice. J Clin Invest. 2011;121:1608–1623. doi: 10.1172/JCI44911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghilardi JR, Freeman KT, Jimenez-Andrade JM, Coughlin KA, Kaczmarska MJ, Castaneda-Corral G, Bloom AP, Kuskowski MA, Mantyh PW. Neuroplasticity of sensory and sympathetic nerve fibers in a mouse model of a painful arthritic joint. Arthritis Rheum. 2012;64:2223–2232. doi: 10.1002/art.34385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gotoh M, Hamada K, Yamakawa H, Inoue A, Fukuda H. Increased substance P in subacromial bursa and shoulder pain in rotator cuff diseases. J Orthop Res. 1998;16:618–621. doi: 10.1002/jor.1100160515. [DOI] [PubMed] [Google Scholar]
- 35.Gwilym SE, Oag HC, Tracey I, Carr AJ. Evidence that central sensitisation is present in patients with shoulder impingement syndrome and influences the outcome after surgery. J Bone Joint Surg Br. 2011;93:498–502. doi: 10.1302/0301-620X.93B4.25054. [DOI] [PubMed] [Google Scholar]
- 36.Hegedus EJ, Cook C, Brennan M, Wyland D, Garrison JC, Driesner D. Vascularity and tendon pathology in the rotator cuff: a review of literature and implications for rehabilitation and surgery. Br J Sports Med. 2010;44:838–847. doi: 10.1136/bjsm.2008.053769. [DOI] [PubMed] [Google Scholar]
- 37.Ivie TJ, Bray RC, Salo PT. Denervation impairs healing of the rabbit medial collateral ligament. J Orthop Res. 2002;20:990–995. doi: 10.1016/S0736-0266(02)00026-8. [DOI] [PubMed] [Google Scholar]
- 38.Kemp KA, Sheps DM, Luciak-Corea C, Styles-Tripp F, Buckingham J, Beaupre LA. Systematic review of rotator cuff tears in workers’ compensation patients. Occup Med (Lond). 2011;61:556–562. doi: 10.1093/occmed/kqr068. [DOI] [PubMed] [Google Scholar]
- 39.Kreiner F, Galbo H. Elevated muscle interstitial levels of pain-inducing substances in symptomatic muscles in patients with polymyalgia rheumatica. Pain. 2011;152:1127–1132. doi: 10.1016/j.pain.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 40.Lewis JS. Rotator cuff tendinopathy. Br J Sports Med. 2009;43:236–241. doi: 10.1136/bjsm.2008.052175. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Kreicbergs A, Bergstrom J, Stark A, Ahmed M. Site-specific CGRP innervation coincides with bone formation during fracture healing and modeling: a study in rat angulated tibia. J Orthop Res. 2007;25:1204–1212. doi: 10.1002/jor.20406. [DOI] [PubMed] [Google Scholar]
- 42.Lian O, Dahl J, Ackermann PW, Frihagen F, Engebretsen L, Bahr R. Pronociceptive and antinociceptive neuromediators in patellar tendinopathy. Am J Sports Med. 2006;34:1801–1808. doi: 10.1177/0363546506289169. [DOI] [PubMed] [Google Scholar]
- 43.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Longo UG, Ronga M, Maffulli N. Achilles tendinopathy. Sports Med Arthrosc Rev. 2009;17:112–126. doi: 10.1097/JSA.0b013e3181a3d625. [DOI] [PubMed] [Google Scholar]
- 45.Lui PP, Chan LS, Fu SC, Chan KM. Expression of sensory neuropeptides in tendon is associated with failed healing and activity-related tendon pain in collagenase-induced tendon injury. Am J Sports Med. 2010;38:757–764. doi: 10.1177/0363546509355402. [DOI] [PubMed] [Google Scholar]
- 46.Maffulli N, Longo UG, Franceschi F, Rabitti C, Denaro V. Movin and Bonar scores assess the same characteristics of tendon histology. Clin Orthop Relat Res. 2008;466:1605–1611. doi: 10.1007/s11999-008-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neer CS., 2nd Impingement lesions. Clin Orthop Relat Res. 1983;173:70–77. [PubMed] [Google Scholar]
- 48.Neviaser A, Andarawis-Puri N, Flatow E. Basic mechanisms of tendon fatigue damage. J Shoulder Elbow Surg. 2012;21:158–163. doi: 10.1016/j.jse.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newsholme P, Procopio J, Lima MM, Pithon-Curi TC, Curi R. Glutamine and glutamate—their central role in cell metabolism and function. Cell Biochem Funct. 2003;21:1–9. doi: 10.1002/cbf.1003. [DOI] [PubMed] [Google Scholar]
- 50.Orita S, Koshi T, Mitsuka T, Miyagi M, Inoue G, Arai G, Ishikawa T, Hanaoka E, Yamashita K, Yamashita M, Eguchi Y, Toyone T, Takahashi K, Ohtori S. Associations between proinflammatory cytokines in the synovial fluid and radiographic grading and pain-related scores in 47 consecutive patients with osteoarthritis of the knee. BMC Musculoskelet Disord. 2011;12:144. doi: 10.1186/1471-2474-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatol ogy (Oxford). 2004;43:131–142. [DOI] [PubMed]
- 52.Rosendal L, Larsson B, Kristiansen J, Peolsson M, Sogaard K, Kjaer M, Sorensen J, Gerdle B. Increase in muscle nociceptive substances and anaerobic metabolism in patients with trapezius myalgia: microdialysis in rest and during exercise. Pain. 2004;112:324–334. doi: 10.1016/j.pain.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 53.Schizas N, Lian O, Frihagen F, Engebretsen L, Bahr R, Ackermann PW. Coexistence of up-regulated NMDA receptor 1 and glutamate on nerves, vessels and transformed tenocytes in tendinopathy. Scand J Med Sci Sports. 2010;20:208–215. doi: 10.1111/j.1600-0838.2009.00913.x. [DOI] [PubMed] [Google Scholar]
- 54.Schizas N, Weiss R, Lian O, Frihagen F, Bahr R, Ackermann PW. Glutamate receptors in tendinopathic patients. J Orthop Res. 2012;30:1447–1452. doi: 10.1002/jor.22094. [DOI] [PubMed] [Google Scholar]
- 55.Schubert TE, Weidler C, Lerch K, Hofstadter F, Straub RH. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann Rheum Dis. 2005;64:1083–1086. doi: 10.1136/ard.2004.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scott A, Alfredson H, Forsgren S. VGIuT2 expression in painful achilles and patellar tendinosis: evidence of local glutamate release by tenocytes. J Orthop Res. 2008;26:685–692. doi: 10.1002/jor.20536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seitz AL, McClure PW, Finucane S, Boardman ND, III, Michener LA. Mechanisms of rotator cuff tendinopathy: intrinsic, extrinsic, or both? Clin Biomech. 2011;26:1–12. doi: 10.1016/j.clinbiomech.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Singaraju VM, Kang RW, Yanke AB, McNickle AG, Lewis PB, Wang VM, Williams JM, Chubinskaya S, Romeo AA, Cole BJ. Biceps tendinitis in chronic rotator cuff tears: a histologic perspective. J Shoulder Elbow Surg. 2008;17:898–904. doi: 10.1016/j.jse.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 59.Tamai M, Okajima S, Fushiki S, Hirasawa Y. Quantitative analysis of neural distribution in human coracoacromial ligaments. Clin Orthop Relat Res. 2000;373:125–134. doi: 10.1097/00003086-200004000-00015. [DOI] [PubMed] [Google Scholar]
- 60.Tashjian RZ. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med. 2012;31:589–604. doi: 10.1016/j.csm.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 61.Tomita Y, Ozaki J, Sakurai G, Kondo T, Nakagaki K, Tamai S. Neurohistology of the subacromial bursa in rotator cuff tear. J Orthop Sci. 1997;2:295–300. doi: 10.1007/BF02488913. [DOI] [Google Scholar]
- 62.Wilson JJ, Best TM. Common overuse tendon problems: a review and recommendations for treatment. Am Fam Physician. 2005;72:811–818. [PubMed] [Google Scholar]
- 63.Woolf CJ, Costigan M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proc Natl Acad Sci U S A. 1999;96:7723–7730. doi: 10.1073/pnas.96.14.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu Y, Bonar F, Murrell GA. Neoinnervation in rotator cuff tendinopathy. Sports Med Arthrosc Rev. 2011;19:354–359. doi: 10.1097/JSA.0b013e318229d7e3. [DOI] [PubMed] [Google Scholar]
- 65.Xu Y, Murrell GA. The basic science of tendinopathy. Clin Orthop Relat Res. 2008;466:1528–1538. doi: 10.1007/s11999-008-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamaguchi DK, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 2006;88:1699–1704. doi: 10.2106/JBJS.E.00835. [DOI] [PubMed] [Google Scholar]
- 67.Yilmaz Z, Renton T, Yiangou Y, Zakrzewska J, Chessell IP, Bountra C, Anand P. Burning mouth syndrome as a trigeminal small fibre neuropathy: increased heat and capsaicin receptor TRPV1 in nerve fibres correlates with pain score. J Clin Neurosci. 2007;14:864–871. doi: 10.1016/j.jocn.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 68.Zeisig E, Ljung BO, Alfredson H, Danielson P. Immunohistochemical evidence of local production of catecholamines in cells of the muscle origins at the lateral and medial humeral epicondyles: of importance for the development of tennis and golfer’s elbow? Br J Sports Med. 2009;43:269–275. doi: 10.1136/bjsm.2008.054619. [DOI] [PubMed] [Google Scholar]