Abstract
Background
Extracts of Tripterygium wilfordii Hook F (TWHF), a traditional Chinese herb, have been reported to show efficacy in patients with rheumatoid arthritis (RA). Since RA is not only characterized by inflammation but also by synovial proliferation in the joints, we examined whether triptolide (a constituent of TWHF) could influence the proliferation of rheumatoid synovial fibroblasts (RSF) by induction of apoptosis.
Results
RSF were obtained from RA patients during surgery and were treated with triptolide under various conditions. The viability and proliferation of RSF were measured by the 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate (WST-1) assay and by 5-bromo-2'-deoxyuridine incorporation, respectively. Apoptosis was identified by detection of DNA fragmentation using an enzyme-linked immunosorbent assay and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL). The role of caspases in apoptosis of RSF was analyzed by measuring caspase-3 activity. Activation of the peroxisome proliferator-activated receptor (PPAR) γ was assessed by a luciferase reporter gene assay using RSF transfected with a plasmid containing the peroxisome proliferator response element. Triptolide decreased viability, inhibited proliferation, and induced apoptosis of RSF in a concentration-dependent manner at very low (nM) concentrations. Caspase-3 activity was increased by treatment with triptolide and was suppressed by caspase inhibitors. Although PPARγ activation was induced by 15-deoxy-Δ12,14-prostaglandin J2, triptolide did not induce it under the same experimental conditions. An extract of TWHF also induced DNA fragmentation in RSF.
Conclusion
The mechanism of action remains to be studied; however, triptolide may possibly have a disease-modifying effect in patients with RA.
Background
Extracts of Tripterygium wilfordii Hook F (TWHF) have been reported to show efficacy in patients with a variety of inflammatory and autoimmune diseases, including rheumatoid arthritis (RA) [1-3]. Previous studies have shown that several TWHF extracts can exert immunosuppressive and anti-inflammatory effects in vivo. A chloroform extract of TWHF suppresses type II collagen-induced arthritis in mice [4], while carrageenan-induced inflammation in rats is suppressed by the ethyl acetate extract of TWHF [5].
The mechanism of action of these TWHF extracts has been investigated in vitro. It has been shown that a multi-glycoside chloroform/methanol extract of TWHF (GTW) significantly inhibits proliferation and interleukin (IL)-2 productions by activated T cells [6]. GTW also inhibits the production of IL-1, IL-6, IL-8, tumor necrosis factor (TNF)-α, and prostaglandin (PG) E2 by human peripheral blood monocytes, as well as IgG, IL-2, and IL-4 production by human peripheral blood lymphocytes [7]. Likewise, a chloroform/methanol extract known as T2, significantly inhibits the release of PGE2 and IL-2 from human peripheral blood mononuclear cells [8]. We have previously reported that the anti-rheumatic effect of GTW or TWHF might be partly mediated through inhibition of PGE2 production by human synovial cells due to down-regulation of IL-1β-induced cyclooxygenase (COX)-2 mRNA expressions, possibly via the inhibition of nuclear factor (NF)-κB activity [9]. TWHF also inhibits T cell proliferation via the induction of apoptosis [6].
Triptolide is an active compound that was identified in extracts of TWHF [10,11], and its actions have been reported to be as follows: inhibition of IL-2 production by mouse T cell hybridomas [12], human peripheral blood lymphocytes, and Jurkat cells through nuclear inhibition of transcriptional activation of NF-κB [13]; inhibition of vascular endothelial growth factor expression [14]; suppression of NF-κB in T lymphocytes [15]; inhibition of IL-8 expression in human bronchial epithelial cells [13]; reduction of PGE2 production in human monocytes and RA synovial fibroblasts (RSF) [16]; and inhibition of pro-matrix metalloproteinase-1 and -3 mRNA expression [17]. Taken together, these results suggest that triptolide may be an active compound from TWHF extracts with immunosuppressive and anti-inflammatory effects. However, it has not been clarified whether triptolide exerts a disease-modifying effect on the pathophysiology of RA. Since induction of apoptosis in synovial cells may be a possible therapeutic strategy for RA, we examined whether triptolide could induce the apoptosis of RSF.
Results
Effect of triptolide on RSF viability
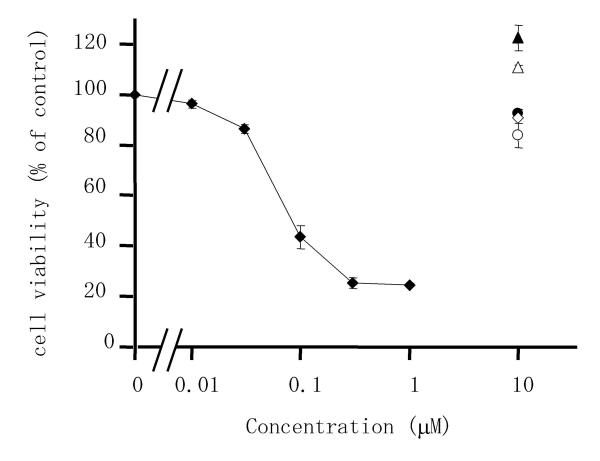
To determine whether triptolide had an influence on the viability of RSF, we first evaluated the effects of various anti-rheumatic drugs on cell viability using the 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate (WST-1) assay. Triptolide caused a marked decrease of cell viability in a concentration-dependent manner (Figure 1), while bucillamine, D-penicillamine, methotrexate, sulphasalazine, and sodium aurothiomalate did not decrease viability at a concentration of 10 μM. The mean (± S.D.) value of 50% inhibitory concentration (IC50) of 6 independent experiments was 74.3 ± 9.5 nM.
Figure 1.
Effects of triptolide and anti-rheumatic drugs on the viability of rheumatoid synovial fibroblasts (RSF). Cells were incubated with triptolide (closed diamond), bucillamine (closed triangle), methotrexate (closed circle), D-penicillamine (open triangle), sodium aurothiomalate (open diamond), or sulphasalazine (open circle) for 24 h. Then viability was measured by the WST-1 assay and calculated as a percentage of the control value (untreated cells). Data are the mean ± S.D. for triplicate cultures and representative results from 6 independent experiments are shown.
Effect of triptolide on RSF proliferation
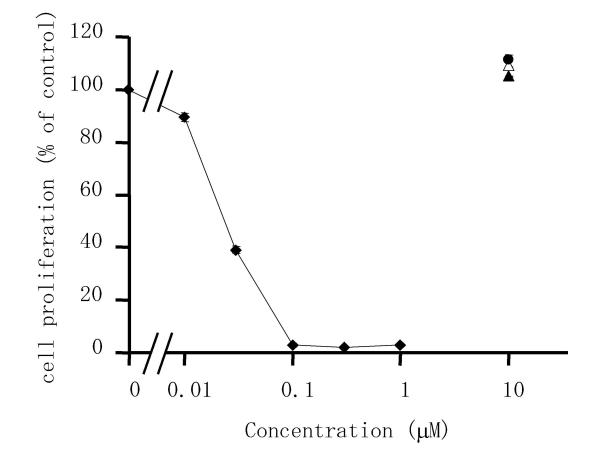
Since triptolide reduced the viability of RSF, we also examined its effect on cell proliferation (DNA synthesis) by measuring the incorporation of 5-bromo-2'-deoxyuridine (BrdU) (Figure 2) using an enzyme-linked immunosorbent assay (ELISA). Triptolide caused marked suppression of cell proliferation in a concentration-dependent manner and it was almost completely inhibited at 100 nM. In contrast, the other drugs that were tested, such as bucillamine, D-penicillamine, and methotrexate, did not suppress the proliferation of RSF. The mean (± S.D.) value of IC50 of 7 independent experiments was 20.4 ± 2.4 nM.
Figure 2.
Effects of triptolide and anti-rheumatic drugs on the proliferation of RSF. Cells were incubated with triptolide (closed diamond), bucillamine (closed triangle), methotrexate (closed circle), or D-penicillamine (open triangle) for 24 h. Then cell proliferation was estimated from the incorporation of BrdU and calculated as a percentage of the control value (untreated cells). Data are the mean ± S.D. for triplicate cultures and representative results from 7 independent experiments are shown.
Induction of apoptosis in RSF by triptolide
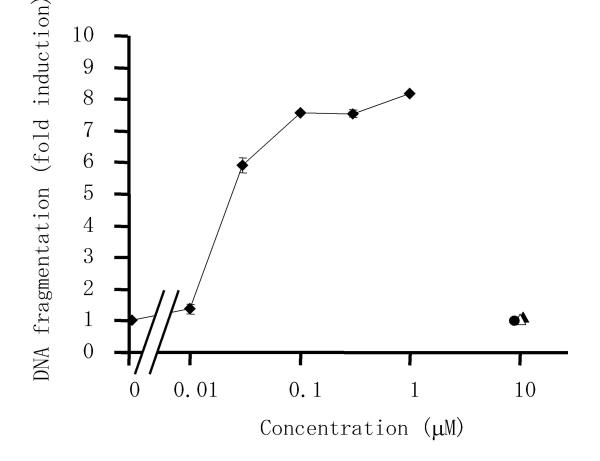
To determine whether the decrease of viability and suppression of proliferation when RSF were treated with triptolide was related to apoptosis, we examined the effect of triptolide on DNA fragmentation (a hallmark of apoptosis). An ELISA that specifically detected cytoplasmic histone-associated DNA fragments, mononucleosomes and oligonucleosomes, was used to quantitatively analyze DNA fragments. As shown in Figure 3, triptolide induced DNA fragmentation in cultured RSF, while bucillamine, D-penicillamine, and methotrexate did not affect cellular DNA. The mean (± S.D.) value of triptolide concentration at a half of maximum fold induction of 5 independent experiments was 35.9 ± 16.3 nM.
Figure 3.
Effects of triptolide and anti-rheumatic drugs on DNA fragmentation of RSF. Cells were incubated with triptolide (closed diamond), bucillamine (closed triangle), methotrexate (closed circle), or D-penicillamine (open triangle) for 24 h, after which fragmented DNA in the cytoplasm was measured by enzyme immunoassay and the fold induction of DNA fragmentation was calculated relative to the control value (untreated cells). Data are the mean ± S.D. for triplicate cultures and representative results from 5 independent experiments are shown.
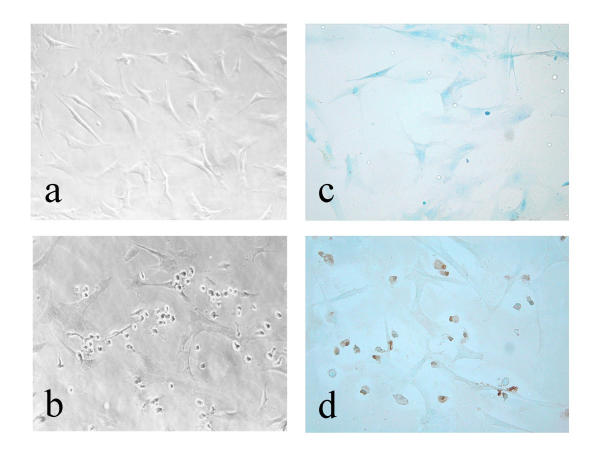
Cell morphology was observed with a light microscope after treatment of RSF with triptolide (Figure 4). When synovial cells were incubated with triptolide (100 nM) for 24 h, distinctive morphological changes occurred, such as cellular rounding, shrinkage, and membrane blebbing, and the cells became separated from neighbouring cells (panel b). The terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL) assay was used to confirm the apparent pro-apoptotic effect of triptolide. As shown in panel d, TUNEL-positive cells were observed after incubation with 100 nM triptolide for 24 h, accounting for 66 % of the cultured RSF. These results suggested that triptolide caused a decrease of RSF proliferation by the induction of apoptosis.
Figure 4.
Morphological changes of RSF (a, b) and detection of apoptosis by the TUNEL assay (c, d). Cells were incubated for 24 h without (a, c) or with (b, d) triptolide at 100 nM. Brown cells are apoptotic cells that show TUNEL staining, while blue cells are normal cells stained with methylgreen. Original magnification × 200.
Effect of triptolide on caspase activity in RSF
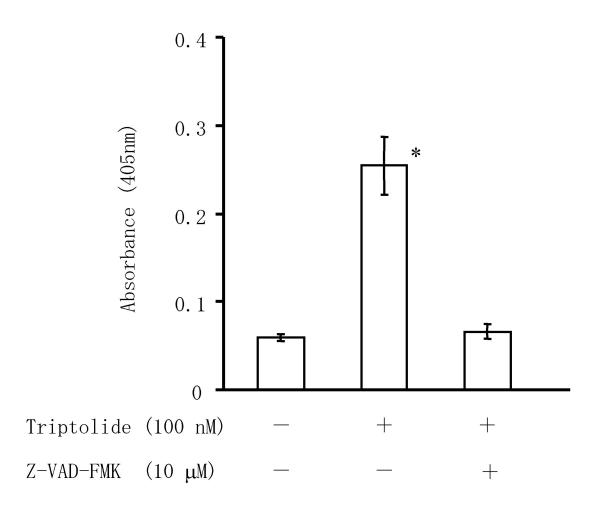
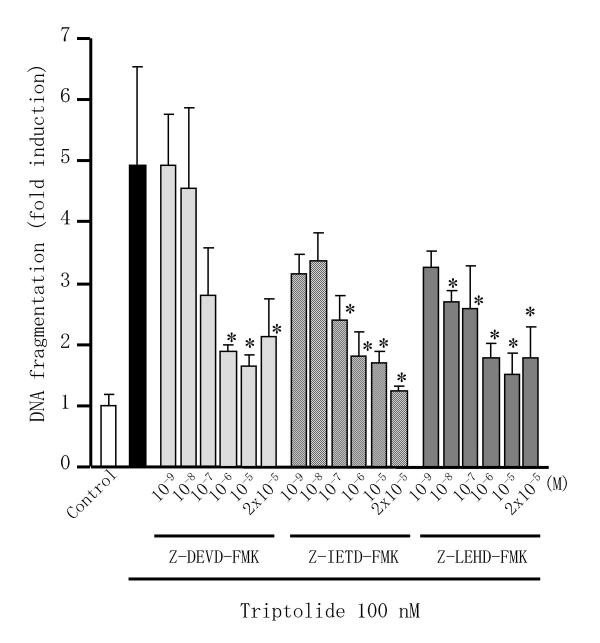
Caspases are responsible for many of the biochemical and morphological changes that occur during apoptosis, so we investigated whether triptolide induced the activation of caspase-3 in RSF. As shown in Figure 5, incubation of RSF with 100 nM triptolide for 24 h induced the activation of caspase-3, while activation was completely blocked by incubation with the pan-caspase inhibitor Z-VAD-FMK. We then examined effects of caspase inhibitors on triptolide induced-apoptosis in RSF. Triptolide-induced DNA fragmentation in RSF was suppressed by Z-DEVD-FMK, Z-IETD-FMK, and Z-LEHD-FMK, inhibitors of caspases-3, -8 and -9, respectively, in concentration dependent manner (Figure 6).
Figure 5.
Effect of triptolide on caspase activity in RSF. RSF were cultured without agents for 24 h, or were incubated with 100 nM triptolide or 100 nM triptolide plus 10 μM Z-VAD-FMK, and then caspase activity was measured by the CaspACE™ assay system (Promega) as the absorbance at 405 nm. Data are the mean ± S.D. for 3 independent experiments. *, p < 0.01 versus untreated control cells and versus cells treated with triptolide plus Z-VAD-FMK. Significance was evaluated by Student's t-test with a Bonferroni's correction.
Figure 6.
Effects of triptolide and caspase inhibitors on DNA fragmentation in RSF. Cells were incubated with triptolide with/without caspase inhibitors for 24 h, after which fragmented DNA in the cytoplasm was measured by enzyme immunoassay and the fold induction of DNA fragmentation was calculated relative to the control value (untreated cells). Data are the mean ± S.D. for triplicate cultures and representative results from 3 independent experiments are shown. *, p < 0.05 versus cells treated with triptolide alone. Significance was evaluated by Student's t-test with a Bonferroni's correction in each caspase inhibitor study.
Effect of triptolide on PPARγ activation in RSF
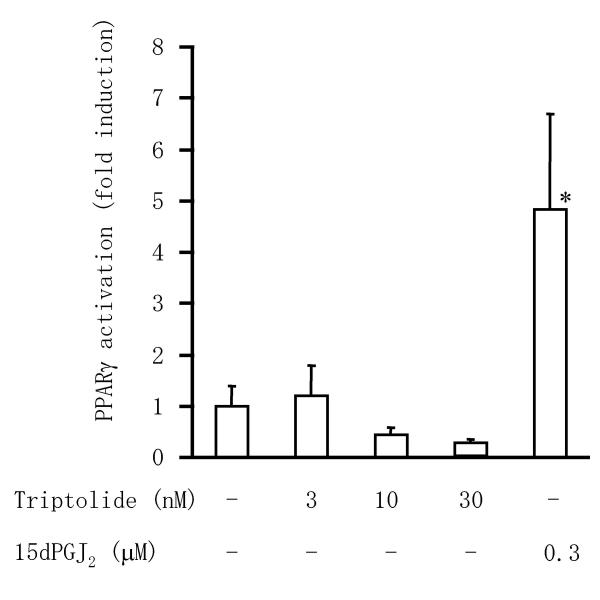
To explore whether proliferator-activated receptor (PPAR)γ activation was involved in the apoptotic effect of triptolide, we performed a luciferase reporter gene assay by co-transfection of RSF with a peroxisome proliferator response element (PPRE)-driven luciferase reporter plasmid and a PPARγ expression plasmid (Figure 7). Incubation with 15-deoxy-Δ12,14-prostaglandin J2 (15dPGJ2), a PPARγ ligand, significantly induced PPRE-driven luciferase activity in this system. In contrast, triptolide had little influence on PPARγ activation despite causing the apoptosis of RSF.
Figure 7.
Effect of triptolide on activation of PPARγ in RSF. Cells were co-transfected with a PPRE-driven luciferase reporter plasmid, a PPARγ expression plasmid, and an internal control plasmid. Then the transfected cells were treated with known PPARγ ligand or triptolide and luciferase activity was determined as described in Materials and Methods. Data are the mean ± S.D. for triplicate cultures and representative results from 3 independent experiments are shown. *, p < 0.05 versus other groups. Significance was evaluated by Student's t-test with a Bonferroni's correction.
Induction of apoptosis in RSF by GTW
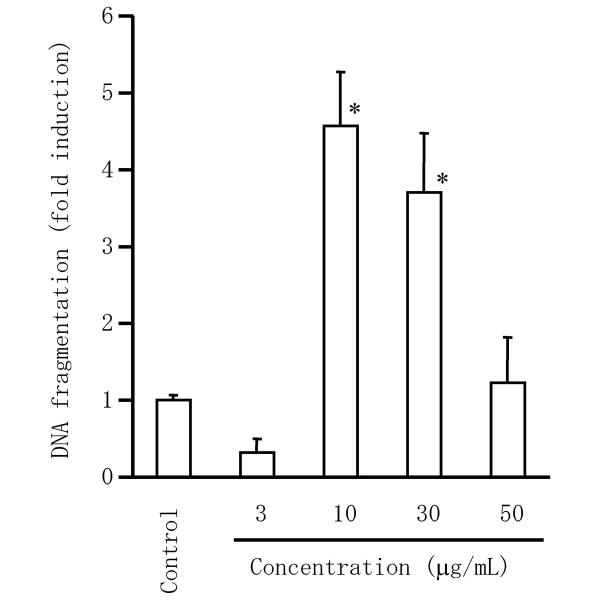
Since triptolide was regarded as a major active compound of GTW, an extract of TWHF, we examined whether GTW induced apoptosis in RSF. As show in Figure 8, GTW induced DNA fragmentation in RSF at 10 to 30 μg/mL. With GTW at 50 μg/mL, the cells had undergone secondary (type 2) necrosis, which usually occurs after apoptosis during in vitro experiments.
Figure 8.
Effect of GTW on DNA fragmentation in RSF. Cells were incubated with GTW for 24 hr, after which fragmented DNA in the cytoplasm was measured by enzyme immunoassay and the fold induction of DNA fragmentation was calculated relative to the control value (untreated cells). Data are the mean ± S.D. for triplicate cultures and representative results from 3 independent experiments are shown. *, p < 0.01 versus untreated control cells. Significance was evaluated by Student's t-test with a Bonferroni's correction.
Discussion
The present study showed that triptolide, which has been identified as a major active compound in TWHF extract, induced the apoptosis of RSF. Triptolide as well as TWHF extracts have been shown to inhibit cell growth by induction of apoptosis in several kinds of lymphocytes and cancer cell lines, as reviewed by Chen et al. [18]. However, this is the first evidence that triptolide induces apoptosis of RSF.
Extracts of TWHF (T2 and GTW) and triptolide have very similar actions, such as suppressing the production of NF-κB by T lymphocytes [15], IL-8 by human bronchial epithelial cells [13], and PGE2 by human monocytes [16] and RSF [9,16]. Triptolide has been reported to display both immunosuppressive and anti-inflammatory effects [13,16,19,20]. These findings suggest that it is a major active compound of TWHF extracts.
RA is characterized by extensive inflammation and proliferation of the synovium in various joints and synovial hyperplasia leads to joint destruction. Proliferation of synovial cells contributes to hyperplasia of the synovium and to the formation of inflammatory pannus tissue, which exhibits tumor-like proliferation and invades the articular cartilage and surrounding tissues [21]. Nishioka et al. [22] have suggested that the stimulation of proliferation by TNF-α and induction of apoptosis by Fas ligand play an important role in regulating the growth of rheumatoid synovial tissue. Hyperplasia of invasive synovial cells has been suggested to arise from an imbalance between cell proliferation and apoptotic death [23]. Therefore, the identification of agents that induce apoptosis of RSF may be a key step toward the successful treatment of RA [24]. Our finding that triptolide induces the apoptosis of RSF may help to explain the anti-rheumatic effect of TWHF extract in clinical studies [1]. Although we found that triptolide could induce the apoptosis of RSF, standard disease-modifying anti-rheumatic drugs like methotrexate and sulphasalazine did not induce apoptosis. Low-dose methotrexate and sulphasalazine have been reported to inhibit radiographic progression of joint changes in patients with RA [25,26]. We found that triptolide and GTW could induce the apoptosis of RSF, suggesting that triptolide might have a stronger effect than conventional disease-modifying anti-rheumatic drugs. Tao et al. [3] studied the efficacy and safety of TWHF extract in patients with RA. They found that TWHF extract improved the symptoms of RA according to American College of Rheumatology criteria. In addition, it decreased C-reactive protein, rheumatoid factor, and the erythrocyte sedimentation rate, changes that were not seen during treatment with non-steroidal anti-inflammatory drugs.
Generally, apoptosis is the result of complex intracellular proteolysis mediated by defined group of intracellular proteases known as caspases. Apoptotic programs are depending on whether the signal originates from inside or outside of the cell [27]. The intrinsic pathway, initiator caspase-9 is activated by the release of mitochondrial components constituted of cytochrome c and ATP. The extrinsic pathway known as death receptor stimulation (Fas, TNF-related apoptosis inducing ligand; TRAIL, TNF receptor-1) requires activation of caspase-8. Then apoptosis was accomplished via activation of caspase-3. In parallel, caspase-8 can cleavage BID into truncated BID (tBID) [28]. The tBID stimulates the release of cytochrome c from mitochondria, leading to activation of caspase-9. Therefore, receptor-mediated death signalling is also connected to the mitochondrial apoptosis signalling pathway. In the present study, activation of caspase-3 was increased when cells were treated with triptolide. This triptolide-induced activation of caspase-3 was suppressed by Z-VAD-FMK, a pan-caspase inhibitor. Therefore, induction of apoptosis by triptolide was dependent on the caspase-3 pathway. Our results showed those inhibitors of not only caspase-3, but also caspases-8 and -9 suppressed triptolide induced-DNA fragmentation in RSF. Yang et al. [12] reported that over expression of Bcl-2, which inhibit mitochondrial intrinsic pathway, suppressed triptolide-induced degradation of poly ADP-ribose polymerase, a caspase substrate, and apoptosis of T lymphocytes. In contrast, our data suggest that triptolide-induced apoptosis in RSF was performed by both intrinsic and extrinsic pathways.
There have been several reports suggesting the mechanism of triptolide-induced apoptosis in cancer cell lines. Jiang et al. [29] found that suppression of p53 expression by an antisense oligonucleotide could prevent triptolide-induced apoptosis, while over-expression of dominant negative p53 abolished the inhibitory effect on NF-κB activation in human gastric cancer cells. In contrast, it was reported that triptolide has a broad spectrum of pro-apoptotic activity against several kinds of cancer cells that express wild-type or mutant forms of p53 [30]. Lee et al. [31] suggested that NF-κB is an important factor for pro-apoptotic action of triptolide on cancer cell lines. Recently, Frese et al. [32] reported that TRAIL-induced apoptosis in lung cancer cells was sensitized by triptolide via activation of ERK2, a member of the mitogen-activated protein kinase family. On the other hand, it was reported [33,34] that 15dPGJ2 and troglitazone, natural and synthetic ligand of PPARγ, respectively, could induce RA synoviocyte apoptosis in vitro. We previously found that some nonsteroidal anti-inflammatory drugs induce the apoptosis of RSF in a COX-2-independent, but PPARγ-dependent, manner [34]. We also reported that celecoxib, a selective COX-2 inhibitor, induce apoptosis and suppress the proliferation of RSF [35] without activation of PPARγ. In the present study, however, triptolide did not induce PPARγ activation. Intracellular mechanism of pro-apoptotic action of triptolide except for PPARγ activation in RSF is to be studied.
In this study, GTW induced apoptosis in RSF in concentration dependent manner. It was reported that triptolide and tripdiolide were purified from aqueous extract of TWHF [11]. Although we did not examine experiments with tripdiolide, one of the major active compounds of GTW might be triptolide. Furthermore, studies should be conducted to clarify the mechanism of the pro-apoptotic action of triptolide in RSF.
Conclusions
We demonstrated that triptolide, an active compound, identified in a traditional Chinese herb, induced apoptosis of RSF. The mechanism of action remains to be studied; however, triptolide may possibly have a disease-modifying effect in patients with RA.
Methods
Chemicals
RPMI-1640 medium, penicillin-streptomycin solution, fetal bovine serum (FBS), and trypsin-EDTA were obtained from Gibco BRL (Gaithersburg, MD, USA). Triptolide was purchased from Biomol Research Laboratories, Inc. (Plymouth Meeting, PA, USA). 15dPGJ2 was obtained from Cayman Chemical (Ann Arbor, MI, USA). Bucillamine, D-penicillamine, and salazosulfapyridine (sulphasalazine) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Sodium aurothiomalate was obtained from Shionogi & Co., Ltd. (Osaka, Japan). Z-DEVD-FMK (a caspase-3 inhibitor), Z-IETD-FMK (a caspase-8 inhibitor) and Z-LEHD-FMK (a caspase-9 inhibitor) were purchased from R&D Systems, Inc. (Minneapolis, MN, USA). GTW powder was kindly provided by Taizhou Pharmaceutical Co. (Jiang Su, China). All other chemicals were purchased from Wako Pure Chemical Industries (Osaka, Japan).
Culture of human RSF
RSF were prepared from synovial tissues, as described previously [36]. The synovial tissues were obtained during total knee replacement from RA patients who fulfilled the revised American Rheumatism Association criteria [37]. Mean (± S.D.) age of 18 patients (14 female) with RA were 64.3 ± 5.8. The protocol for this study was approved by the ethics committee of St. Marianna University and all patients gave written consent to the use of their tissues for this research. Synovial tissues were digested for 2 h with 0.2 % (w/v) bacterial collagenase (Immuno-Biological Laboratories Co., Ltd., Gunma, Japan) and then the synovial cells were suspended in RPMI-1640 medium with 10 % (v/v) FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. The cells were incubated at 37°C in 5 % CO2 for several days, after which nonadherent cells were removed. The fibroblast-like adherent cells were used as RSF within 2 passages. These adherent cells contained no T cells (CD3+) or macrophage/monocytes (CD14+) on two-colour immunofluorescence and flow cytometry. All culture condition of RSF in RPMI-1640 medium containing 1 % (v/v) FBS under an atmosphere of 5 % CO2.
Preparation of drugs
Test drugs except GTW were dissolved in dimethyl sulfoxide (DMSO) as 1000 × stock solutions and then diluted with RPMI-1640 medium containing 1 % (v/v) FBS for cell culture experiments. GTW powder was dissolved in ethanol, filtrated, and then stored at -20°C. Test drug solutions were prepared freshly on the day of use. The final concentration of DMSO or ethanol (just for GTW study) for all treatments (including control cultures) was 0.1 % (v/v).
Cell viability assay
RSF (2 × 104 cells/well) were incubated in 96-well plastic plates with test drugs in the condition described above. After 24 h, cell viability was assessed by measuring mitochondrial NADH-dependent dehydrogenase activity with a Cell Counting Kit (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) using a sulfonated tetrazolium salt, 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt, (WST-1) [38]. Each measurement was done in triplicate and the results are presented as a percentage of the value for untreated control cultures.
Cell proliferation assay
The proliferation of RSF was assessed from the incorporation of 5-bromo-2'-deoxyuridine (BrdU). Cells (1 × 104 cells/well) were incubated in 96-well plastic plates with the test drugs in the condition described above. Then BrdU (10 μM) was added to the medium and the cells were incubated for another 18 h. Subsequently, the cells were fixed and BrdU incorporation was determined with a Cell Proliferation ELISA Kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's instructions. Each measurement was done in triplicate and the results are presented as a percentage of the value for the untreated control culture.
DNA fragmentation assay
DNA fragmentation was detected by ELISA and by TUNEL assay. RSF were plated in 96-well plastic plates (2 × 104 cells/well) and cultured for 24 h. Then the cells were incubated for a further 24 h with test drugs in the condition described above. After incubation, DNA fragmentation was investigated using a Cell Death Detection ELISAPLUS kit (Roche Diagnostics GmbH) [39]. Each measurement was done in triplicate and the results are presented as the fold induction compared with untreated control cultures.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling assay
RSF (2.5 × 104 cells/well) were incubated in 8-well chamber slides (Iwaki, Chiba, Japan) for 24 h with triptolide in the condition described above. Then morphological changes of the cells were observed under a microscope (BX51, Olympus Optical Co., Ltd., Nagano, Japan), after which the cells were fixed with 4 % (v/v) neutral buffered formalin for 10 min at room temperature. Subsequently, apoptotic synovial cells were identified by the TUNEL assay using an Apoptosis in situ Detection Kit (Wako Pure Chemical Industries) according to the manufacturer's instructions. Sections were counterstained with methylgreen (Wako Pure Chemical Industries) before observation.
Caspase activity assay
RSF were incubated in tissue culture flask with or without triptolide and/or Z-VAD-FMK (a pan-caspase inhibitor) in the condition described above. After 24 h, caspase activity in the cells was measured using the CaspACE™ assay system (Promega, Madison, WI, USA) according to the manufacturer's instructions. Cells were washed in ice-cold phosphate-buffered saline (PBS, 9.57 mM, pH 7.35–7.65, Takara Shuzo, Shiga, Japan), and resuspended in cell lysis buffer. After the cells were lysed by freezing and thawing, the lysates were centrifuged and the supernatant was used as the cell extract. The protein content of the extracts was determined by the BCA protein assay (Pierce, Rockford, IL, USA) with bovine serum albumin (BSA) as the standard, and extracts were adjusted to a protein content of 1 mg/ml.
Luciferase reporter assay
A luciferase reporter plasmid, containing 4 copies of the PPRE of the acyl-CoA oxidase gene promoter [40] at the Nhe I restriction site in the firefly luciferase expression vector PGV-P2 (Toyo Ink, Tokyo, Japan), was used to measure PPARγ activation [34]. RSF (6 × 104 cells/well) were seeded into 24-well culture plates in RPMI-1640 medium containing 10 % (v/v) FBS. After culture for 24 h at 37°C under an atmosphere of 5 % CO2, the cells were co-transfected with the reporter plasmid (0.1 μg/well), with a PPARγ expression plasmid containing mouse PPARγ 2 cDNA [41] at the Hind III and Xba I restriction sites in the pRc/CMV expression vector (Invitrogen, Groningen, The Netherlands) (0.1 μg/well), and with an internal control plasmid (pRL-SV40; Promega) (0.01 μg/well) using Effectene Transfection Reagent (12 μg/mL, Qiagen, Hilden, Germany) according to the manufacturer's instructions. After incubation for a further 24 h at 37°C under an atmosphere of 5 % CO2, the transfection mixture was replaced by RPMI-1640 medium containing 1% (v/v) FBS with or without one of the test drugs. After additional incubation for 18 h in the condition described above, luciferase activity was determined using the Dual-Luciferase Reporter Assay System (Promega) and a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA, USA) according to the manufacturer's instructions. Each measurement was done in triplicate and firefly luciferase activity was normalized to Renilla luciferase activity.
Statistical analysis
Data are expressed as the mean ± S.D. Statistical analysis was done using multiple Student's t-tests with a Bonferroni's correction. A probability value of less than 0.05 was taken to indicate a significant difference.
List of abbreviations used
RSF, rheumatoid synovial fibroblasts; PPARγ, peroxisome proliferator-activated receptor γ; COX, cyclooxygenase; PG, prostaglandin; 15dPGJ2, 15-deoxy-Δ12,14-PGJ2; FBS, fetal bovine serum; IL-1β, interleukin-1β; NF-κB, nuclear factor-kappaB; TNF, tumor necrosis factor; TRAIL, TNF-related apoptosis inducing ligand; BrdU, 5-bromo-2'-deoxyuridine; ELISA, enzyme-linked immunosorbent assay; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling; PPRE, peroxisome proliferator response element.
Authors' contributions
NK mainly participated in the experiments and drafted the manuscript. RY participated in the experiments. HK suggested methodology of the experiments. MB and HA provided samples of the patients and discussed the results. SK conceived of the study, and participated in its design and coordination. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We wish to thank Drs. Satoshi Miyamoto, Tadashi Yoshida, and Fumiaki Kojima, as well as Ms. Miyako Kato, Ms. Reiko Oura, Mr. Takeshi Chino, Ms. Eriko Fukazawa, and Ms. Mayumi Hori for excellent technical assistance, and Ms. Sonoko Sakurai for secretarial assistance.
Contributor Information
Natsuko Kusunoki, Email: kusunoki@marianna-u.ac.jp.
Ryuta Yamazaki, Email: ryuta-yamazaki@yakult.co.jp.
Hidero Kitasato, Email: hkita@med.kitasato-u.ac.jp.
Moroe Beppu, Email: m2beppu@marianna-u.ac.jp.
Haruhito Aoki, Email: h2aoki@marianna-u.ac.jp.
Shinichi Kawai, Email: s2kawai@marianna-u.ac.jp.
References
- Lipsky PE, Tao XL. A potential new treatment for rheumatoid arthritis: thunder god vine. Semin Arthritis Rheum. 1997;26:713–723. doi: 10.1016/S0049-0172(97)80040-6. [DOI] [PubMed] [Google Scholar]
- Tao X, Cush JJ, Garret M, Lipsky PE. A phase I study of ethyl acetate extract of the Chinese antirheumatic herb Tripterygium wilfordii hook F in rheumatoid arthritis. J Rheumatol. 2001;28:2160–2167. [PubMed] [Google Scholar]
- Tao X, Younger J, Fan FZ, Wang B, Lipsky PE. Benefit of an extract of Tripterygium Wilfordii Hook F in patients with rheumatoid arthritis: a double-blind, placebo-controlled study. Arthritis Rheum. 2002;46:1735–1743. doi: 10.1002/art.10411. [DOI] [PubMed] [Google Scholar]
- Asano K, Matsuishi J, Yu Y, Kasahara T, Hisamitsu T. Suppressive effects of Tripterygium wilfordii Hook f., a traditional Chinese medicine, on collagen arthritis in mice. Immunopharmacology. 1998;39:117–126. doi: 10.1016/S0162-3109(98)00006-X. [DOI] [PubMed] [Google Scholar]
- Tao X, Ma L, Mao Y, Lipsky PE. Suppression of carrageenan-induced inflammation in vivo by an extract of the Chinese herbal remedy Tripterygium wilfordii Hook F. Inflamm Res. 1991;48:139–148. doi: 10.1007/s000110050437. [DOI] [PubMed] [Google Scholar]
- Ho LJ, Chang DM, Chang ML, Kuo SY, Lai JH. Mechanism of immunosuppression of the antirheumatic herb TWHf in human T cells. J Rheumatol. 1999;26:14–24. [PubMed] [Google Scholar]
- Chang DM, Chang WY, Kuo SY, Chang ML. The effects of traditional antirheumatic herbal medicines on immune response cells. J Rheumatol. 1997;24:436–441. [PubMed] [Google Scholar]
- Chou CT, Chang SC. The inhibitory effect of common traditional anti-rheumatic herb formulas on prostaglandin E and interleukin 2 in vitro: a comparative study with Tripterygium wilfordii. J Ethnopharmacol. 1998;62:167–171. doi: 10.1016/S0378-8741(98)00041-5. [DOI] [PubMed] [Google Scholar]
- Maekawa K, Yoshikawa N, Du J, Nishida S, Kitasato H, Okamoto K, Tanaka H, Mizushima Y, Kawai S. The molecular mechanism of inhibition of interleukin-1beta-induced cyclooxygenase-2 expression in human synovial cells by Tripterygium wilfordii Hook F extract. Inflamm Res. 1999;48:575–581. doi: 10.1007/s000110050506. [DOI] [PubMed] [Google Scholar]
- Tao X, Cai JJ, Lipsky PE. The identity of immunosuppressive components of the ethyl acetate extract and chloroform methanol extract (T2) of Tripterygium wilfordii Hook. F. J Pharmacol Exp Ther. 1995;272:1305–1312. [PubMed] [Google Scholar]
- Gu WZ, Chen R, Brandwein S, McAlpine J, Burres N. Isolation, purification, and characterization of immunosuppressive compounds from tripterygium: triptolide and tripdiolide. Int J Immunopharmacol. 1995;17:351–356. doi: 10.1016/0192-0561(95)00022-T. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liu Z, Tolosa E, Yang J, Li L. Triptolide induces apoptotic death of T lymphocyte. Immunopharmacology. 1998;40:139–149. doi: 10.1016/S0162-3109(98)00036-8. [DOI] [PubMed] [Google Scholar]
- Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S, Ng JC, Kao PN. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J Biol Chem. 1999;274:13443–13450. doi: 10.1074/jbc.274.19.13443. [DOI] [PubMed] [Google Scholar]
- Hu KB, Liu ZH, Liu D, Li LS. Inhibition of vascular endothelial growth factor expression and production by triptolide. Planta Med. 2002;68:368–369. doi: 10.1055/s-2002-26737. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu ZH, Chen ZH, Yang JW, Li LS. Triptolide: a potent inhibitor of NF-kappa B in T-lymphocytes. Acta Pharmacol Sin. 2000;21:782–786. [PubMed] [Google Scholar]
- Tao X, Schulze-Koops H, Ma L, Cai J, Mao Y, Lipsky PE. Effects of Tripterygium wilfordii hook F extracts on induction of cyclooxygenase 2 activity and prostaglandin E2 production. Arthritis Rheum. 1998;41:130–138. doi: 10.1002/1529-0131(199801)41:1<130::AID-ART16>3.3.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Lin N, Sato T, Ito A. Triptolide, a novel diterpenoid triepoxide from Tripterygium wilfordii Hook. f., suppresses the production and gene expression of pro-matrix metalloproteinases 1 and 3 and augments those of tissue inhibitors of metalloproteinases 1 and 2 in human synovial fibroblasts. Arthritis Rheum. 2001;44:2193–2200. doi: 10.1002/1529-0131(200109)44:9<2193::AID-ART373>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Chen BJ. Triptolide, a novel immunosuppressive and anti-inflammatory agent purified from a Chinese herb Tripterygium wilfordii Hook F. Leuk Lymphoma. 2001;42:253–265. doi: 10.3109/10428190109064582. [DOI] [PubMed] [Google Scholar]
- Gu WZ, Brandwein SR. Inhibition of type II collagen-induced arthritis in rats by triptolide. Int J Immunopharmacol. 1998;20:389–400. doi: 10.1016/S0192-0561(98)00035-6. [DOI] [PubMed] [Google Scholar]
- Zhao G, Vaszar LT, Qiu D, Shi L, Kao PN. Anti-inflammatory effects of triptolide in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L958–966. doi: 10.1152/ajplung.2000.279.5.L958. [DOI] [PubMed] [Google Scholar]
- Zvaifler NJ, Firestein GS. Pannus and pannocytes. Alternative models of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1994;37:783–789. doi: 10.1002/art.1780370601. [DOI] [PubMed] [Google Scholar]
- Nishioka K, Hasunuma T, Kato T, Sumida T, Kobata T. Apoptosis in rheumatoid arthritis: A novel pathway in the regulation of synovial tissue. Arthritis Rheum. 1998;41:1–9. doi: 10.1002/1529-0131(199801)41:1<1::AID-ART1>3.3.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Eguchi K. Apoptosis in autoimmune diseases. Intern Med. 2001;40:275–284. doi: 10.2169/internalmedicine.40.275. [DOI] [PubMed] [Google Scholar]
- Kawakami A, Nakashima T, Sakai H, Hida A, Urayama S, Yamasaki S, Nakamura H, Ida H, Ichinose Y, Aoyagi T, Furuichi I, Nakashima M, Migita K, Kawabe Y, Eguchi K. Regulation of synovial cell apoptosis by proteasome inhibitor. Arthritis Rheum. 1999;42:2440–2448. doi: 10.1002/1529-0131(199911)42:11<2440::AID-ANR23>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Cohen S, Cannon GW, Schiff M, Weaver A, Fox R, Olsen N, Furst D, Sharp J, Moreland L, Caldwell J, Kaine J, Strand V. Two-year, blinded, randomized, controlled trial of treatment of active rheumatoid arthritis with leflunomide compared with methotrexate. Utilization of Leflunomide in the Treatment of Rheumatoid Arthritis Trial Investigator Group. Arthritis Rheum. 2001;44:1984–1992. doi: 10.1002/1529-0131(200109)44:9<1984::AID-ART346>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Smolen JS, Kalden JR, Scott DL, Rozman B, Kvien TK, Larsen A, Loew-Friedrich I, Oed C, Rosenburg R. Efficacy and safety of leflunomide compared with placebo and sulphasalazine in active rheumatoid arthritis: a double-blind, randomised, multicentre trial. European Leflunomide Study Group. Lancet. 1999;353:259–266. doi: 10.1016/S0140-6736(98)09403-3. [DOI] [PubMed] [Google Scholar]
- Stennicke HR, Salvesen GS. Caspases – controlling intracellular signals by protease zymogen activation. Biochim Biophys Acta. 2000;1477:299–306. doi: 10.1016/S0167-4838(99)00281-2. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase-8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/S0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Jiang XH, Wong BC, Lin MC, Zhu GH, Kung HF, Jiang SH, Yang D, Lam SK. Functional p53 is required for triptolide-induced apoptosis and AP-1 and nuclear factor-kappaB activation in gastric cancer cells. Oncogene. 2001;20:8009–8018. doi: 10.1038/sj.onc.1204981. [DOI] [PubMed] [Google Scholar]
- Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei XF, Yang J, Underhill CB, Zhang L. Triptolide inhibits the growth and metastasis of solid tumors. Mol Cancer Ther. 2003;2:65–72. [PubMed] [Google Scholar]
- Lee KY, Chang W, Qiu D, Kao PN, Rosen GD. PG490 (triptolide) cooperates with tumor necrosis factor-alpha to induce apoptosis in tumor cells. J Biol Chem. 1999;274:13451–13455. doi: 10.1074/jbc.274.19.13451. [DOI] [PubMed] [Google Scholar]
- Frese S, Pirnia F, Miescher D, Krajewski S, Borner MM, Reed JC, Schmid RA. PG490-mediated sensitization of lung cancer cells to Apo2L/TRAIL-induced apoptosis requires activation of ERK2. Oncogene. 2003;22:5427–5435. doi: 10.1038/sj.onc.1206842. [DOI] [PubMed] [Google Scholar]
- Kawahito Y, Kondo M, Tsubouchi Y, Hashiramoto A, Bishop-Bailey D, Inoue K, Kohno M, Yamada R, Hla T, Sano H. 15-deoxy-delta(12,14)-PGJ(2) induces synoviocyte apoptosis and suppresses adjuvant-induced arthritis in rats. J Clin Invest. 2000;106:189–197. doi: 10.1172/JCI9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki R, Kusunoki N, Matsuzaki T, Hashimoto S, Kawai S. Nonsteroidal anti-inflammatory drugs induce apoptosis in association with activation of peroxisome proliferator-activated receptor g in rheumatoid synovial fibroblasts. J Pharmacol Exp Ther. 2002;302:18–25. doi: 10.1124/jpet.302.1.18. [DOI] [PubMed] [Google Scholar]
- Kusunoki N, Yamazaki R, Kawai S. Induction of apoptosis in rheumatoid synovial fibroblasts by celecoxib, but not by other selective cyclooxygenase-2 inhibitors. Arthritis Rheum. 2002;46:3159–3167. doi: 10.1002/art.10692. [DOI] [PubMed] [Google Scholar]
- Kawai S, Nishida S, Kato M, Furumaya Y, Okamoto R, Koshino T, Mizushima Y. Comparison of cyclooxygenase-1 and -2 inhibitory activities of various nonsteroidal anti-inflammatory drugs using human platelets and synovial cells. Eur J Pharmacol. 1998;347:87–94. doi: 10.1016/S0014-2999(98)00078-8. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Shuman S. Structure, mechanism, and evolution of the mRNA capping apparatus. Prog Nucleic Acid Res Mol Biol. 2001;66:1–40. doi: 10.1016/s0079-6603(00)66025-7. [DOI] [PubMed] [Google Scholar]
- Marcus SL, Miyata KS, Zhang B, Subramani S, Rachubinski RA, Capone JP. Diverse peroxisome proliferator-activated receptors bind to the peroxisome proliferator-responsive elements of the rat hydratase/dehydrogenase and fatty acyl-CoA oxidase genes, but differentially induce expression. Proc Natl Acad Sci USA. 1993;90:5723–5727. doi: 10.1073/pnas.90.12.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]