Abstract
Although globalization can contribute to increased blood pressure by spreading unhealthy behaviors, it also provides powerful means to tackle hypertension. The dissemination of information about and advice on cardiovascular prevention and facilitated contact with health services are valuable resources. To investigate the effects of urbanization, geographical area, and air temperature on hypertension burden and kidney damage, a survey was performed in 2008 with a door-to-door approach among urban and rural adult dwellers of three geographic areas (capital, inland, coast) of Yemen. Subjects (n=10 242) received two visits several days apart to confirm the diagnosis of hypertension. Proteinuria (dipstick test ⩾+1) was used as a marker of kidney damage. Prevalence rates were weighted to represent the Yemen population aged 15–69 years in 2008. Rates of hypertension and proteinuria progressively increased from the capital (6.4% 95% confidence level (CI) 5.8–7.0 and 5.1% 4.4–5.9, respectively), to inland areas (7.9% 7.0–8.7 and 6.1% 5.1–7.1), to the coastal area (10.1% 8.9–11.4 and 8.9% 7.3–10.4). When compared with urban dwellers, rural dwellers had similar hypertension prevalence (adjusted odds ratios (ORs) 1.03; 95% CI 0.91–1.17) but higher proteinuria rates (adjusted ORs 1.55; 1.31–1.85). Overall, home temperature was associated with a lower hypertension rate (adjusted OR 0.98; 0.96–0.99). This large population study reveals that the highest burden of hypertension and kidney damage is detectable in remote areas of the country.
Keywords: developing countries, epidemiology, epidemiological transition, HYDY study, proteinuria, urbanization
Introduction
Globalization can contribute to increased prevalence of high blood pressure (BP) by spreading unhealthy lifestyle and dietary habits to developing countries.1, 2, 3 However, globalization may also offer favorable opportunities to countries with fewer resources by improving the dissemination of knowledge and achieving a high level education as well as increasing contact with health services.4, 5, 6 The effects of globalization are generally more evident in urban areas. However, the effect of geographical area might also be relevant in developing countries. In Yemen, a country characterized by a highly traditional lifestyle, the capital, Sana'a, is located at an altitude of 2300 m (7500 ft) in the highlands, one of the highest capital cities in the world. The absence of railways and the poor road transportation system make communications with the coastal plains difficult. Thus, the rural area surrounding Sana'a might differ from rural areas of remote regions of the country. Information regarding prevalence, awareness, treatment and control rates of hypertension in Yemen is limited to the capital area.7, 8, 9 The possibility of extending those data at a national level might lead to biased results because sociocultural and environmental factors and traditional or religious habits may influence the final development of biological risk factors. Therefore, further epidemiological studies are needed. Finally, although a negative correlation between BP reading values and environmental temperature was consistently reported,10, 11, 12 the potential effect of temperature on hypertension burden estimation in epidemiological surveys performed with a door to door approach has not been investigated.
The country was thus stratified into three different geographic areas, and the prevalence of hypertension was estimated on the basis of two visits as recommended by current clinical guidelines.13, 14
Methods
Study sites and study population
A multi-stage stratified sampling method was used. In the first stage, the country was stratified into three regions: the capital area, the inland area and the coastal area. The governorate of Sana'a (capital area), the governorate of Taizz (inland), and the governorates of Al Hudaydah and Hadramaut (coast) were selected to be representative of the geographic, economic and climatic characteristics of the three regions. In the second stage, rural and city regions were identified from each study area. In the third stage, districts were arbitrarily identified within each urban and rural region, and boundaries were defined using local maps or in consultation with the local health workers. The total number of districts within each study area (20 in the capital area, 12 in the inland area and 8 in the coastal area) was proportional to the estimated population size of the area. In the final stage, because of the lack of a national population register,15 a cluster of 250 adult participants, equally stratified by gender and age (15–24, 25–34, 35–44, 45–54 and 55–69 years) to a total of 10 strata, was made for each district. All male and female subjects aged 15–69 years who were permanent residents in the study areas, with the exception of pregnant women, were eligible. All study personnel successfully completed the specific 1-week training program and pilot testing in Sana'a (December 2007).16 The study was approved by the ethical committee of the University of Science and Technology, Sana'a, Yemen. Informed consent was obtained from each participant before data collection.
Data collection
The burden of hypertension on the population was assessed by taking BP measurements in triplicate on two home visits, separated by a few days as previously reported.17 During the first visit (visit 1), three measurements of BP and pulse rate were taken 2-min apart on the dominant arm after a rest of at least 15 min, with subjects in a seated position.13, 18 Readings were obtained with a clinically validated semiautomatic sphygmomanometer (HEM 705 IT; Omron Matsusaka, Mie, Japan). The averages of the last two readings for systolic and diastolic BP were defined as SBP1 and DBP1, respectively. Anthropometric measurements (weight, height, and waist and hip circumference) were taken.17 Then, finger prick blood samples were obtained from fasting (>8 h) subjects aged ⩾15 years to measure fasting glucose (FG1) (Accutrend system, Roche Diagnostics, Mannheim, Germany), cholesterol and triglyceride blood values (MultiCare-in, Biochemical System International, Arezzo, Italy) using dry chemistry.19 A urine sample was collected and a dipstick test (Auction sticks, A Menarini Diagnostics, Florence, Italy)17 was immediately performed. The results were determined by careful visual comparison of the test strip with a color chart provided on the bottle label. All subjects were then visited again within the next 10 days by the same survey team using the same measurement devices and procedures for a second session of BP (SBP2 and DBP2) and fasting glucose (FG2) measurements. Air temperature was measured at the homes of the participants during the two visits using digital thermometers (Checktemp, Hanna Instruments, Padova, Italy) (accuracy: ±0.3 °C; range −20 to 90 °C).
Diagnostic criteria and definition of variables
Outcome variables included hypertension, awareness, treatment and control. Arterial hypertension was defined as (1) SBP1 and SBP2⩾140 mm Hg and/or DBP1 and DBP2⩾90 mm Hg and/or (2) self-reported use of antihypertensive drugs at the time of the interview.13 Average SBP and DBP values measured at the two visits were categorized according to the guidelines of the European Society of Hypertension/European Society of Cardiology (ESH/ESC).13 Awareness of hypertension was defined as self-report of any prior diagnosis of hypertension by a health-care professional among the population defined as having hypertension. Hypertension treatment was defined as a self-reported use of antihypertensive drugs within the 2 weeks preceding the interview. A prevalence estimate of treatment was relative to the number of hypertensive patients. Hypertension control was defined as SBP1 and SBP2<140 mm Hg and DBP1 and DBP2<90 mm Hg. The proportion of hypertension control was relative to the number of hypertensive patients treated with drugs.20
Covariates included gender, age in decades, living area (rural/urban), geographical area (capital, inland, coast), air temperature measured at home (average of the two visits) and education level. The model was also adjusted for potential confounders including comorbidities (diabetes mellitus (DM), overweight or obesity, high cholesterol and high triglycerides) and behavioral factors (sedentary lifestyle, smoking, fruit and vegetable consumption). DM was defined as (1) FG1 and FG2 ⩾7.0 mmol l−1 and/or (2) self-reported use of hypoglycemic medications at the time of the interview; impaired FG as FG⩾5.6 mmol l−1 and <7.0 mmol l−1 in the absence of hypoglycemic medications; and normal FG as FG1 and FG2 <5.6 mmol l.21 The results of dipstick urinalysis were recorded as follows: (1) urinary proteins: negative (0), trace (±) or proteinuria (⩾1+). The reliability of the measurements performed by visual inspection of strips was previously assessed.22
Overweight and obesity were defined as a body mass index of 25.0–29.9 kg m–2 and ⩾30 kg m–2, respectively. Gender-specific tertiles for body mass index were calculated using data from adult subjects without hypertension, normal FG, cholesterol <5.0 mmol l−1 and triglycerides <1.7 mmol l−1. The resulting cutoffs were 20.0 and 23.2 kg m–2 for men and 20.5 and 24.3 kg m–2 for women. Abdominal obesity was defined as waist circumference >102 cm in men or >88 cm in women. Subjects were classified as having high cholesterol (>5.0 mmol l−1) or high triglycerides (>1.7 mmol l−1).13 A smoker was defined as one who smoked any form of tobacco on a daily (daily smoker) or a non-daily (occasional smoker) basis. Those who had smoked but had quit were designated as former smokers, and those who had never smoked at all were designated as non-smokers. Fruit and vegetable consumption was classified as (1) ⩽1 day per week; (2) 2–4 days per week; (3) ⩾5 days per week. Participants were categorized as sedentary if they walked or cycled for <10 min daily, if their job did not involve intense physical activity, and if they did not regularly participate in sports or recreational physical activities.
Statistical methods
Description and validation of the database can be found elsewhere.16 Data are expressed as means±s.d. with 95% confidence level (CI) for continuous variables and as rates with 95% CI for categorical variables. A sample size of at least 1117 subjects was required to achieve a 1% precision at an estimated prevalence of DM of 3% with 95% CI. The estimated required sample size for two-sample comparison of diabetes prevalence of 2.25% vs. 3.75% with the assumption of 0.05 alpha (two-sided), 80% power, was 2161 subjects for each group.23
Directly age-standardized prevalence rates for the age ranges 15–69 years and 35–69 years in the overall population were calculated using the WHO World Standard Population.24 Where unspecified, all calculations presented were weighted to represent the total Yemen populations aged between 15 and 69 years. Weights were calculated on the basis of data from the 2008 estimated Yemen population census.15 Using gender-specific population figures for Yemen, directly age-standardized prevalence rates were calculated for men and women, urban or rural locations, and the three geographic areas.15, 23 The rate of hypertension awareness and treatment was calculated on the sub-population of hypertensive patients, and hypertension control was considered among the subgroup of treated subjects. As no national data were available for these two sub-populations for the purpose of weighting, we did not weigh the data at this stage of analysis.
Associations between variables and the prevalence of hypertension were preliminarily explored with univariate logistic regression analysis, and the diagnosis of hypertension was entered as a dependent dichotomous variable. Associations were then investigated with multivariate logistic regression analysis using three different models (independent variables included as a block with forced entry):
adjusted for age, gender, urban or rural location, geographical area (capital, inland, coast) and level of education (illiterate, can read and write, primary school, secondary school, high school, college or postgraduate);
adjusted for age, gender, urban or rural location, geographical area (capital, inland, coast), level of education (illiterate, primary or secondary school, high school or more), and average air temperature at the two survey visits;
adjusted for age, gender, urban or rural location, geographical area (capital, inland, coast), level of education (illiterate, can read and write, primary school, secondary school, high school, college or postgraduate), average air temperature at the two survey visits, self-reported sedentary lifestyle (vs. active), smoking habits (never, past, occasional, daily), vegetable and fruit consumption (categories), obesity (body mass index tertiles), blood glucose categories, high cholesterol and high triglycerides.
Associations were investigated among all participants (for hypertension diagnosis and the self-report of having BP measured at least one time during the previous year), among the subgroup of subjects with hypertension diagnosis (for hypertension awareness and pharmacologic treatment), and among the subgroup of 528 hypertensive subjects treated with antihypertensive drugs (for BP control).
Results are expressed as odds ratio (OR) with 95% confidence limits (95% CI). The test of the hypothesis was performed at a two-sided significance level of 0.05. The Statistical Package for the Social Sciences software (SPSS, Chicago, IL, USA), version 19.0, was used for statistical analyses.
Results
Characteristics of cohort participants
The main characteristics of the 10 242 study participants (5063 men and 5179 women) are reported in Table 1. Age-specific and age-weighted SBP and DBP values in HYDY (hypertension and diabetes in Yemen) study participants are reported in Supplementary Table 1S. The average number of years spent in school was 9.4 (95% CI 9.1–9.6) for men and 6.1 (5.8–6.4) for women, and 35.5% of the women and 10.3% of the men were illiterate. Approximately 5.4% and 2.2% of men had obesity or abdominal obesity, respectively. The obesity or abdominal obesity prevalence in women was 10.5% and 19.5%, respectively. The average air temperatures recorded at participants' homes during the two sessions of BP measurements were 22.0 °C (95% CI 21.9–22.1 °C; n=4977) in the capital area, 25.4 °C (25.3–25.5 °C; n=3039) inland and 28.3 °C (28.1–28.4 °C; n=2147) in the coastal area.
Table 1. Age-weighted characteristics of HYDY participants by gender.
|
Men |
Women |
|||
|---|---|---|---|---|
| Characteristics | n | % (95% CI) | n | % (95% CI) |
| Subjects | 5063 | 50.81 (50.79–50.83) | 5179 | 49.19 (49.17–49.21) |
| Age category (years) | ||||
| 15–24 | 1016 | 21.49 (21.46–21.51) | 1058 | 21.34 (21.32–21.37) |
| 25–34 | 1020 | 15.56 (15.54–15.58) | 1047 | 14.11 (14.09–14.13) |
| 35–44 | 1008 | 7.32 (7.30–7.33) | 1021 | 7.83 (7.81–7.85) |
| 45–54 | 990 | 5.53 (5.51–5.54) | 1057 | 6.32 (6.31–6.34) |
| 55–69 | 1029 | 4.20 (4.19–4.21) | 996 | 4.49 (4.48–4.51) |
| Education level | ||||
| Illiterate | 986 | 10.3 (9.5–11.0) | 2666 | 35.5 (34.0–37.0) |
| Can read and write | 830 | 9.2 (8.5–9.9) | 375 | 6.7 (6.0–7.5) |
| Primary school | 685 | 16.8 (15.3–18.2) | 661 | 17.1 (15.7–18.5) |
| Secondary school | 832 | 23.8 (22.0–25.5) | 542 | 15.8 (14.4–17.2) |
| High school | 774 | 19.8 (18.2–21.3) | 504 | 14.6 (13.2–15.9) |
| College or post | 950 | 20.2 (18.8–21.6) | 428 | 10.3 (9.3–11.4) |
| Smoking status | ||||
| Never | 3048 | 68.6 (67.2–69.9) | 4371 | 88.4 (87.6–89.3) |
| Past | 344 | 4.5 (4.0–5.1) | 192 | 2.4 (2.1–2.8) |
| Current occasional | 243 | 4.1 (3.5–4.7) | 158 | 2.5 (2.0–2.9) |
| Current daily | 1428 | 22.8 (21.6–24.0) | 458 | 6.6 (6.0–7.3) |
| Sedentary lifestyle | 4079 | 83.2 (80.3–86.2) | 3573 | 72.1 (69.4–74.8) |
| Fruit consumption | ||||
| ⩽1 day per week | 2357 | 47.6 (45.4–49.9) | 2470 | 47.1 (45.0–49.3) |
| 2–4 days per week | 1947 | 38.0 (36.0–40.0) | 1962 | 38.4 (36.5–40.4) |
| ⩾5 days per week | 744 | 14.4 (13.2–15.6) | 740 | 14.4 (13.2–15.6) |
| Vegetable consumption | ||||
| ⩽1 day per week | 1101 | 22.0 (20.5–23.5) | 1040 | 19.3 (17.9–20.6) |
| 2–4 days per week | 1362 | 27.6 (25.9–29.3) | 1437 | 28.1 (26.4–29.8) |
| ⩾5 days per week | 2597 | 50.4 (48.1–52.7) | 2692 | 52.6 (50.4–54.9) |
| Comorbidities | ||||
| Obesity | 349 | 5.4 (4.7–6.1) | 695 | 10.5 (9.7–11.4) |
| Abdominal obesity | 171 | 2.2 (1.8–2.6) | 1372 | 19.5 (18.4–20.7) |
| High cholesterol | 593 | 8.5 (7.7–9.4) | 803 | 13.0 (11.9–14.0) |
| High triglycerides | 2007 | 35.3 (33.5–37.2) | 1887 | 31.8 (30.2–33.4) |
| Impaired fasting glucose | 647 | 11.2 (10.2–12.3) | 728 | 12.0 (11.0–13.0 |
| Diabetes mellitus | 257 | 2.9 (2.5–3.3) | 281 | 3.4 (3.0–3.9) |
| Self-reported myocardial infarction | 23 | 0.25 (0.13–0.36) | 16 | 0.20 (0.09–0.32) |
| Self-reported stroke | 16 | 0.15 (0.08–0.23) | 13 | 0.15 (0.07–0.23) |
Abbreviation: CI, confidence level.
Risk factor prevalence
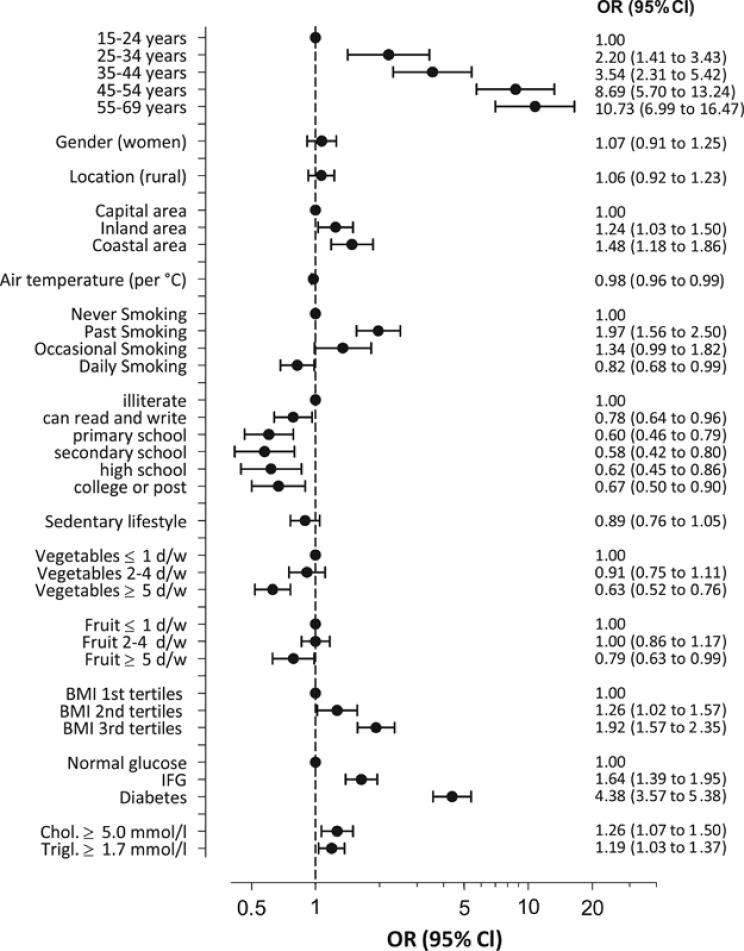
The age-specific rates of hypertension, awareness, treatment and control are reported in Supplementary Table 2S. Overall, 1307 participants fulfilled the criteria for hypertension, with an age-weighted (15–69 years) prevalence of 7.7% (95% CI 7.2–8.1; Table 2). In crude analysis, the odds of being hypertensive in women were 1.2 (95% CI 1.4–1.9) times that of men. This significance of this association was lost after adjusting for education level and other confounders (Figure 1). The capital area had the lowest prevalence of hypertension (Table 2). Differences between geographic areas were independent of urban/rural residency, demographic characteristics, air temperature, health behavior, risk factors and associated clinical conditions at logistic regression analysis (Figure 1, Table 3). Air temperature (although significant) limited only the effect on hypertension prevalence estimation (B coefficient −0.020±0.010; Table 3). When considering only the 1303 subjects with hypertension, self-reported BP measurement, hypertension awareness drug treatment and BP control were also favorably affected by living in the capital area (Table 3). The use of traditional medicine for high BP (self-reported by 74 subjects, 59 with a final diagnosis of hypertension; Supplementary Table 3S) was independent of gender, decades of age, urban/rural setting, and level of education, being more prevalent in the coastal area than in the capital area (adjusted OR 2.5; 1.5–4.3).
Table 2. HYDY participants with hypertension (among all participants), aware of their condition, treated with antihypertensive drugs (among hypertensives) and achieving BP control (among treated hypertensives).
|
Hypertension |
Awareness | Treated | Control | ||
|---|---|---|---|---|---|
| Subjects | Prevalencea | subjects | subjects | subjects | |
| Participants | n (%) | % (95% CI) | n (%) | n (%) | n (%) |
| All participants | |||||
| Total | 1307 (12.8) | 7.7 (7.2–8.1) | 582 (44.5) | 528 (40.4) | 127 (24.1) |
| Urban | 631 (12.4) | 7.5 (6.8–8.1) | 322 (51.0) | 288 (45.6) | 72 (25.0) |
| Rural | 676 (13.2) | 7.8 (7.2–8.5) | 260 (38.5) | 240 (35.5) | 55 (22.9) |
| Capital | 573 (11.4) | 6.4 (5.8–7.0) | 297 (51.8) | 273 (47.6) | 78 (28.6) |
| Inland | 422 (13.9) | 7.9 (7.0–8.7) | 168 (39.8) | 154 (36.5) | 33 (21.4) |
| Coast | 312 (14.3) | 10.1 (8.9–11.4) | 117 (37.5) | 101 (32.4) | 16 (15.8) |
| Men | |||||
| Total | 589 (11.6) | 6.7 (6.1–7.3) | 240 (40.7) | 215 (36.5) | 37 (17.2) |
| Urban | 292 (11.6) | 7.0 (6.0–7.9) | 128 (43.8) | 111 (38.0) | 16 (14.4) |
| Rural | 297 (11.7) | 6.5 (5.6–7.3) | 112 (37.7) | 104 (35.0) | 21 (20.2) |
| Capital | 237 (9.5) | 4.7 (4.1–5.4) | 116 (48.9) | 105 (44.3) | 20 (19.0) |
| Inland | 192 (12.6) | 6.9 (5.8–8.1) | 70 (36.5) | 65 (33.9) | 13 (20.0) |
| Coast | 160 (15.2) | 11.0 (9.0–13.0) | 54 (33.8) | 45 (28.1) | 4 (8.9) |
| Women | |||||
| Total | 718 (13.9) | 8.6 (7.9–9.3) | 342 (47.6) | 313 (43.6) | 90 (28.8) |
| Urban | 339 (13.1) | 8.1 (7.1–9.0) | 194 (57.2) | 177 (52.2) | 56 (31.6) |
| Rural | 379 (14.6) | 9.2 (8.2–10.2) | 148 (39.1) | 136 (35.9) | 34 (25.0) |
| Capital | 336 (13.3) | 8.2 (7.2–9.1) | 181 (53.9) | 168 (50.0) | 58 (34.5) |
| Inland | 230 (15.1) | 8.9 (7.6–10.1) | 98 (42.6) | 89 (38.7) | 20 (22.5) |
| Coast | 152 (13.5) | 9.4 (7.7–11.0) | 63 (41.4) | 56 (36.8) | 12 (21.4) |
Abbreviations: BP, blood pressure; CI, confidence level.
Prevalence rates of hypertension (95% CI) are weighted to the Yemen population aged 15–69 years in 2008.
Figure 1.
Association of sociodemographic and environmental factors and comorbidities with hypertension diagnosis (systolic blood pressure (SBP)⩾140 mm Hg and/or diastolic blood pressure (DBP)⩾90 mm Hg at both visits and/or self-reported use of antihypertensive drugs at the time of the interview) on multiple logistic regression analysis (including 9416 study participants). Results are expressed as odds ratios (ORs) with 95% confidence level (CI).
Table 3. Effects of urban/rural residency and geographical area on hypertension burden in Yemen.
| Hypertension | Self-reported BP meas. | Awareness | Treatment | BP control | |
|---|---|---|---|---|---|
| Factors | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Age (per decades) | 1.91 (1.79–2.03) | 1.21 (1.16–1.26) | 1.13 (0.98–1.30) | 1.16 (1.00–1.34) | 0.66 (0.51–0.85) |
| Gender (women) | 1.07 (0.93–1.23) | 1.27 (1.15–1.40) | 1.21 (0.94–1.57) | 1.19 (0.92–1.54) | 1.91 (1.15–3.16) |
| Education (categories) | — | — | — | — | — |
| Illiterate | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Primary or secondary school | 0.73 (0.62–0.86) | 0.88 (0.77–1.00) | 0.90 (0.67–1.22) | 0.84 (0.61–1.14) | 0.94 (0.51–1.74) |
| High school or more | 0.55 (0.45–0.68) | 1.07 (0.94–1.23) | 0.67 (0.45–1.00) | 0.61 (0.40–0.91) | 1.77 (0.86–3.64) |
| Temperature (per °C) | 0.98 (0.96–0.99) | 1.06 (1.04–1.08) | 1.00 (0.97–1.05) | 1.01 (0.97–1.05) | 1.07 (0.99–1.16) |
| Location (rural) | 1.03 (0.91–1.17) | 1.04 (0.95–1.14) | 0.53 (0.42–0.67) | 0.57 (0.45–0.72) | 0.92 (0.60–1.40) |
| Geographical area | — | — | — | — | — |
| Capital | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Inland | 1.36 (1.15–1.60) | 0.68 (0.61–0.77) | 0.59 (0.44–0.80) | 0.60 (0.44–0.81) | 0.54 (0.31–0.93) |
| Coast | 1.53 (1.25–1.84) | 0.83 (0.71–0.96) | 0.52 (0.35–0.77) | 0.49 (0.33–0.73) | 0.23 (0.14–0.52) |
Abbreviations: BP, blood pressure; CI, confidence level; OR, odds ratio.
Logistic regression analyses adjusted for age, gender, education and average air temperature at the two survey visits.
The prevalence of diabetes was 2.9% (2.5–3.3) in men and 3.4% (3.0–3.9) in women (Table 1). Unlike hypertension, diabetes and abdominal obesity were less prevalent in the rural settings than in the urban setting, with no differences between the three geographic areas (Table 4). Overall, 51 of the 538 subjects with diabetes self-reported the use of traditional medicine for DM, and its use was more prevalent in the coastal area than in the capital area (adjusted OR 2.90; 1.52–5.53). Proteinuria (dipstick test ⩾+1) had an estimated prevalence of 6.2% (5.6–6.8) and was more prevalent among rural (7.3% 95% CI 6.4–8.1) than among urban dwellers (5.1% 4.4–5.9) and more prevalent among those living inland (6.1% 5.1–7.1) and on the coast (8.9% 7.3–10.4) than among those the capital area (5.1% 4.4–5.9; Table 4).
Table 4. Effects of urban/rural residency and geographical area on diabetes, abdominal obesity and proteinuria in Yemen.
| Diabetes | Abdominal obesity | Proteinuria | |
|---|---|---|---|
| Factors | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Age (per decades) | 1.71 (1.56–1.88) | 1.60 (1.51–1.70) | 0.93 (0.86–1.00) |
| Gender (women) | 1.01 (0.83–1.24) | 11.80 (9.87–14.10) | 0.69 (0.57–0.83) |
| Education (categories) | — | — | — |
| Illiterate | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Primary or secondary school | 0.94 (0.75–1.19) | 1.15 (0.97–1.35) | 0.76 (0.60–0.97) |
| High school or more | 0.68 (0.50–0.91) | 0.86 (0.72–1.04) | 0.71 (0.55–0.91) |
| Temperature (per °C) | 1.03 (1.00–1.06) | 0.96 (0.94–0.98) | 0.95 (0.93–0.98) |
| Location (rural) | 0.66 (0.55–0.79) | 0.60 (0.53–0.68) | 1.55 (1.31–1.85) |
| Geographical area | — | — | — |
| Capital | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Inland | 1.00 (0.78–1.27) | 0.85 (0.72–0.99) | 1.52 (1.22–1.88) |
| Coast | 0.88 (0.65–1.20) | 0.88 (0.72–1.08) | 2.36 (1.81–3.09) |
Abbreviations: CI, confidence level; OR, odds ratio.
Logistic regression analyses adjusted for age, gender, education and average air temperature at the two survey visits.
Discussion
According to the present data, hypertension prevalence in Yemeni women is not affected by urbanization, although the hypertension rate increases with increasing remoteness; environmental temperature at the visit session may influence the assessment of hypertension prevalence in population studies.
In Yemen, the prevalence of hypertension is low, probably because the country is behind with regard to the epidemiological transition currently occurring in other countries in the area. The hypertension rates we observed in the capital area are comparable to rates reported in two small surveys investigating self-reported hypertension8 or hypertension diagnosed in the office.9 According to current findings, hypertension prevalence, in Yemen as in Iran,25 is not affected by urban or rural residency. The strong adherence to a traditional lifestyle could have a favorable role in hypertension prevalence, as alcohol is prohibited in countries such as Yemen and Iran.25, 26
One of the most important HYDY findings was regional variation in hypertension prevalence. Hypertension rates are higher in the coastal area than in the capital area. The contribution of well-established risk factors for hypertension, such as age, weight, physical inactivity, diabetes and obesity, was assessed in the HYDY survey. Unlike hypertension, DM and abdominal obesity were more prevalent among urban than among rural residents and did not differ between the geographical areas. The prevalence of diabetes is higher in the middle crescent area than in the Western Regions.2 We can hypothesize that improved nutrition may reveal a genetic predisposition to diabetes. The contributions of salt consumption and urinary sodium excretion, which are difficult to measure in population studies, were not assessed in the present survey.
Regional variations in rates of hypertension were associated with changing rates of awareness, treatment and control. In Yemen, the rate of health services coverage for the population is indeed limited, and long distances are important obstacles to using health facilities. When moving from remote areas of the country to the capital, the self-reporting of having BP measured in the last year, hypertension awareness, drug treatment, and the possibility of achieving BP control markedly increased. The improved access to health-care facilities and increased level of education, two factors that are also associated with urbanization, seem to have positive effects on hypertension burden, especially in women. Hypertension prevalence is higher in women than in men at unadjusted logistic regression. However, when controlling for sociodemographic and environmental factors, women are as likely as men to be hypertensive, aware of their condition and treated. Furthermore, more women than men had their BP measured frequently in the last year and achieved better BP control.
Geographical variations in hypertension burden were also associated with differences in the prevalence of proteinuria, which was higher among rural than urban residents (the highest rates being found in remote areas of the country). The coastal area of Yemen is remote, sparsely populated, hot and humid and is an area with limited resources. Although the reason for the higher prevalence of proteinuria in the coastal than in the capital area requires further investigation, we must consider that poststreptococcal glomerulonephritis continues to have a high incidence in tropical climates where skin infections are common. Serious implications might thus exist for future demand for dialysis in Yemen.
A negative correlation between temperature and BP was reported.10, 11, 12, 27, 28, 29 However, although the possibility of a higher prevalence of hypertension during winter compared with summer was recently reported,29, 30 no population study specifically investigated the possible bias introduced by environmental temperature on hypertension burden assessment in large surveys. The varied Yemen topography corresponds to widespread climate changes, with a temperate climate in the highlands and high temperatures in the coastal region. According to the present data, air temperature was negatively associated with a prevalence of hypertension. Notwithstanding this statistically evident interaction, geographic distribution of hypertension burden follows an opposite direction than average air temperature measured during home visits, as hypertension prevalence is higher in the coast than in the highlands. Air temperature may thus only modulate the contribution of other factors, with a limited although significant final effect.
In conclusion, in Yemen, a country characterized by a highly traditional lifestyle, hypertension burden is favorably affected by urbanization and by living in the capital area. The rates of hypertension prevalence and proteinuria are observed to increase with geographical remoteness. Thus, the capital area cannot be considered representative of the country.
Acknowledgments
We thank the many enthusiastic workers in Yemen who have contributed to the study: Capital area: Hisham Abdulrab Ali Al Qubati, Raed Faisal Nasser, Aziz Hussain Ali Moshoeh, Mohammed Abdulah Ashmeni, Abdulhamed Hulfy Alanashany, Mohammed Ibrahim Ahmed Al Hamali, Naif Radman Hamdan Agial, Sami Abduh Kaid Ahmed Alwessaby, Munera Abduwhab Saleh Yahya, Hyam Abdul Kareem Al Hyfi, Ziad Abdullah Mohammed Al Mass, Liza Nayib Shamiri, Sahar Ahmed Abdullah Moqbel, Abdullah Assan Mohammed Guly, Abdulaziz Saeed Salem Bawazir, Abi Saleh Al Rawi, Kaled Sadeg Ali Al-Shaibari, Abdul Bari Mahfoudh Omar Al Daba, Osama Hadi Abdu Haig, Gamil Al Hamdi Abdo Mohsan. Inland area: Khalil Abdulwahid Ali Al-Kuhlani, Fahd Ahmed Ahmed Ali Al Durafi, Moad Ahmed Abdul Al Karem Al Shorihy, Ashra F Saheed Abdalh, Omar Ahmed Abdul Aziz, Kalil Abdu Mohammed Assufi, Ahmed Saeed Ahmed Al Terin, Mamon Ahdo Alrahmen Alp, Fuad Mohammed Ali Abdu Ghaleb, Mohammed Ali Hussen, Mobark Mohammad Dabwan Quasem, Awad Hamad Mohammad. Coastal area: Yasin Mohammad Al Hay Ibrahim, Abdu Al Aleem Qaid Abdu Al Aleem Safian, Mohammed Ali Mohammed Salman, Abdh Kozim Abdullah Al Nhozy, Mohammed Awad Omer Hydhi, Hussein Omar Al Ahbari, Adel Hai Bin Obedellah, Khalid Hussan Khaleby. We also thank the chiefs and elders of the villages for their help and HE Mario Boffo, Ambassador of Italy to the Republic of Yemen, for assistance. The HYDY project is part of the Executive Program of Scientific and Technological Cooperation between Italy and Yemen for the years 2006–2009. This work was supported by a grant from the Ministero dell'Università e della Ricerca (Direzione Generale per le strategie e lo sviluppo dell'internazionalizzazione della ricerca scientifica e tecnologica), Rome, Italy, and Menarini International Operations Luxembourg SA.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Hypertension Research website (http://www.nature.com/hr)
Supplementary Material
References
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- Global health risks: mortality and burden of disease attributable to selected major risks. WHO, Geneva2009 . http://www.who.int/healthinfo/global_burden_disease/global_health_risks/en/index.html Accessed 23 October 2012.
- Pereira M, Lunet N, Azevedo A, Barros H. Differences in prevalence, awareness, treatment and control of hypertension between developing and developed countries. J Hypertens. 2009;27:963–975. doi: 10.1097/hjh.0b013e3283282f65. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Rizk H, Appel LJ, el Aroussy W, Helmy S, Sharaf Y, Ashour Z, Kandil H, Roccella E, Whelton PK. Hypertension prevalence, awareness, treatment, and control in Egypt. Results from the Egyptian National Hypertension Project (NHP). NHP Investigative Team. Hypertension. 1995;26:886–890. doi: 10.1161/01.hyp.26.6.886. [DOI] [PubMed] [Google Scholar]
- Janghorbani M, Amini M, Gouya MM, Delavari A, Alikhani S, Mahdavi A. Nationwide survey of prevalence and risk factors of prehypertension and hypertension in Iranian adults. J Hypertens. 2008;26:419–426. doi: 10.1097/HJH.0b013e3282f2d34d. [DOI] [PubMed] [Google Scholar]
- Bovet P. Editorial: the cardiovascular disease epidemic: global, regional, local. Trop Med Int Health. 2002;7:717–721. doi: 10.1046/j.1365-3156.2002.00947.x. [DOI] [PubMed] [Google Scholar]
- Motlagh B, O'Donnell M, Yusuf S. Prevalence of cardiovascular risk factors in the Middle East: a systematic review. Eur J Cardiovasc Prev Rehabil. 2009;16:268–280. doi: 10.1097/HJR.0b013e328322ca1b. [DOI] [PubMed] [Google Scholar]
- Gunaid AA. Prevalence of known diabetes and hypertension in the Republic of Yemen. East Mediterr Health J. 2002;8:374–385. [PubMed] [Google Scholar]
- Gunaid AA, Assabri AM. Prevalence of type 2 diabetes and other cardiovascular risk factors in a semirural area in Yemen. East Mediterr Health J. 2008;14:42–56. [PubMed] [Google Scholar]
- Madsen C, Nafstad P. Associations between environmental exposure and blood pressure among participants in the Oslo Health Study (HUBRO) Eur J Epidemiol. 2006;21:485–491. doi: 10.1007/s10654-006-9025-x. [DOI] [PubMed] [Google Scholar]
- Lewington S, Li L, Sherliker P, Guo Y, Millwood I, Bian Z, Whitlock G, Yang L, Collins R, Chen J, Wu X, Wang S, Hu Y, Jiang L, Yang L, Lacey B, Peto R, Chen Z, on behalf of the China Kadoorie Biobank study collaboration Seasonal variation in blood pressure and its relationship with outdoor temperature in 10 diverse regions of China: the China Kadoorie Biobank. J Hypertens. 2012;30:1383–1391. doi: 10.1097/HJH.0b013e32835465b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett AG, Sans S, Salomaa V, Kuulasmaa K, Dobson AJ, WHO MONICA Project The effect of temperature on systolic blood pressure. Blood Press Monit. 2007;12:195–203. doi: 10.1097/MBP.0b013e3280b083f4. [DOI] [PubMed] [Google Scholar]
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Kjeldsen SE, Erdine S, Narkiewicz K, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Cifkova R, Dominiczak A, Fagard R, Heagerty AM, Laurent S, Lindholm LH, Mancia G, Manolis A, Nilsson PM, Redon J, Schmieder RE, Struijker-Boudier HA, Viigimaa M, Filippatos G, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Kiowski W, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O'Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Viigimaa M, Waeber B, Williams B, Zamorano JL, The task force for the management of arterial hypertension of the European Society of Hypertension, The task force for the management of arterial hypertension of the European Society of Cardiology 2007 Guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 1999 World Health Organization International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens. 1999;17:151–183. [PubMed] [Google Scholar]
- U.S. Census Bureau. International Data Base (IDB).Yemen2008 . http://www.census.gov/ipc/www/idb/groups.php Accessed 23 October 2012.
- Modesti PA, Massetti L, Bamoshmoosh M, Baldereschi M, Cambi GE, Rapi S. The impact of fine-tuning of optical recognition system on database reliability. Comput Biol Med. 2012;42:778–783. doi: 10.1016/j.compbiomed.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Modesti PA, Rapi S, Bamoshmoosh M, Baldereschi M, Massetti L, Padeletti L, Gensing G, Zhao D, Al-Hidabi D, Al Goshae H. Impact of one or two visits strategy on hypertension burden estimation in HYDY, a population-based cross-sectional study: implications for healthcare resource allocation decision making. BMJ Open. 2012;2:e001062. doi: 10.1136/bmjopen-2012-001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovet P, Gervasoni JP, Ross AG, Mkamba M, Mtasiwa DM, Lengeler C, Burnier M, Paccaud F. Assessing the prevalence of hypertension in populations: are we doing it right. J Hypertens. 2003;21:509–517. doi: 10.1097/00004872-200303000-00016. [DOI] [PubMed] [Google Scholar]
- Rapi S, Bazzini C, Tozzetti C, Sbolci V, Modesti PA. Point-of-care testing of cholesterol and triglycerides for epidemiologic studies: evaluation of the multicare-in system. Transl Res. 2009;153:71–76. doi: 10.1016/j.trsl.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Muntner P, Gu D, Wu X, Duan X, Wenqi G, Whelton PK, He J. Factors associated with hypertension awareness, treatment, and control in a representative sample of the chinese population. Hypertension. 2004;43:578–585. doi: 10.1161/01.HYP.0000116302.08484.14. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31:S55–S60. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- Rapi S, Bartolini L, Puliti D, Cambi GE, Bamoshmoosh M, Baldereschi M, Massetti L, Modesti PA. Conventional dipsticks in the screening of microalbuminuria and urinary tract infections. Killing 2 birds with one stone. Saudi Med J. 2010;31:708–709. [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL.In: Modern Epidemiology3rd edn.Lippincott Williams & Wilkins: Philadelphia; 2008 [Google Scholar]
- Ahmad O, Boschi-Pinto C, Lopez AD, Murray CJL, Lozano R, Inoue M.Age standardization of rates: a new WHO standard.GPE Discussion Paper Series: No.31,World Health Organization,2001 http://www.who.int/healthinfo/paper31.pdf Accessed 23 October 2012.
- Ebrahimi M, Mansournia MA, Haghdoost AA, Abazari A, Alaeddini F, Mirzazadeh A, Yunesian M. Social disparities in prevalence, treatment and control of hypertension in Iran: second National Surveillance of Risk Factors of Noncommunicable Diseases, 2006. J Hypertens. 2010;28:1620–1629. doi: 10.1097/HJH.0b013e32833a38f2. [DOI] [PubMed] [Google Scholar]
- Murakami S, Otsuka K, Kono T, Soyama A, Umeda T, Yamamoto N, Morita H, Yamanaka G, Kitaura Y. Impact of outdoor temperature on prewaking morning surge and nocturnal decline in blood pressure in a Japanese population. Hypertens Res. 2011;34:70–73. doi: 10.1038/hr.2010.176. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Ohshige K, Sawai A, Yamasue K, Tochikubo O. Seasonal influence on blood pressure in elderly normotensive subjects. Hypertens Res. 2008;31:569–574. doi: 10.1291/hypres.31.569. [DOI] [PubMed] [Google Scholar]
- Yano Y, Kario K. The risk of cold temperature: an important aspect of the determination of morning blood pressure surge. Hypertens Res. 2011;34:36–38. doi: 10.1038/hr.2010.203. [DOI] [PubMed] [Google Scholar]
- Sinha P, Taneja DK, Singh NP, Saha R. Seasonal variation in prevalence of hypertension: implications for interpretation. Indian J Public Health. 2010;54:7–10. doi: 10.4103/0019-557X.70537. [DOI] [PubMed] [Google Scholar]
- Modesti PA, Morabito M, Massetti L, Rapi S, Orlandini S, Mancia G, Gensini GF, Parati G. Seasonal blood pressure changes: an independent relationship with temperature and daylight hours. Hypertension. 2013;61:908–914. doi: 10.1161/HYPERTENSIONAHA.111.00315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.