Abstract
OBJECTIVES:
The combination of computed tomography with hepatic arteriography and arterial portography (CTHA/CTAP) can detect additional hepatocellular carcinoma (HCC) nodules undetected by conventional dynamic CT.
METHODS:
In this single-center, randomized, open-label, controlled trial, we randomly assigned 280 patients who were diagnosed as having HCC by conventional dynamic CT, and eligible for radiofrequency ablation (RFA), to undergo CTHA/CTAP before treatment, or to the control group. Newly detected HCC nodules by CTHA/CTAP were intended to be ablated completely. The primary end point was recurrence-free survival and the key secondary end point was overall survival. The analysis was conducted on an intention-to-treat basis. Those with nonablated nodules were treated as for recurrence.
RESULTS:
A total of 75 nodules were newly diagnosed as HCC by CTHA/CTAP in 45 patients. Three patients (one in the CTHA/CTAP group and two in the control group) who refused treatment were excluded from all analyses. The cumulative recurrence-free survival rates at 1, 2, and 3 years were 60.1, 29.0, and 18.9% in the CTHA/CTAP group and 52.2, 29.7, and 23.1% in the control group, respectively (P=0.66 by log-rank test; hazard ratio, 0.94 for CTHA/CTAP vs. control; 95% confidence interval (CI), 0.73–1.22). The cumulative overall survival rates at 3 and 5 years were 79.7 and 56.4% in the CTHA/CTAP group and 86.8 and 60.1% in the control group, respectively (P=0.50; hazard ratio, 1.15, 95% CI, 0.77–1.71).
CONCLUSIONS:
CTHA/CTAP may detect recurrent lesions earlier. However, CTHA/CTAP before RFA did not improve cumulative recurrence-free survival or overall survival.
INTRODUCTION
Hepatocellular carcinoma (HCC) ranks as the fifth most common cancer worldwide (1). In Japan, ∼35,000 patients die from HCC every year (2), and the main cause of HCC is hepatitis C virus infection. In chronic hepatitis patients, screening of HCC is usually performed by ultrasonography, and the diagnosis is confirmed by contrast-enhanced dynamic computed tomography (CT). Hyperattenuation in the arterial phase and hypoattenuation in the equilibrium phase are considered to be definitive signs of HCC ((3,4,5,6,7)). Hyperattenuation in the arterial phase is more emphasized when contrast material is injected from the hepatic artery through a catheter, because dilution of contrast material in the systemic circulation is avoided, thus keeping a high concentration of contrast material in the liver. This technique is called CT during hepatic arteriography (CTHA) ((6,8,9,10)). Similarly, hypoattenuation in the equilibrium phase is accentuated after injection of contrast material into the superior mesenteric artery, which is referred to as CT during arterial portography (CTAP) ((11,12,13,14)). The combination of CTHA and CTAP gives higher sensitivity and specificity for HCC detection than conventional dynamic enhanced CT (8).
If new HCC nodules are detected with CTHA/CTAP, in addition to those detected with dynamic CT, the treatment of choice may be changed ((15,16)). For example, surgical resection and liver transplantation are usually contraindicated for multinodular HCC; that is, exceeding three nodules. Percutaneous tumor ablation methods, such as ethanol injection and microwave coagulation, have played an important role as nonsurgical treatments that can achieve high local cure rates without affecting background liver function ((17,18,19,20)). Radiofrequency ablation (RFA) is currently considered to be the most effective first-line percutaneous ablation protocol because of its greater efficacy in terms of local cure as compared with ethanol injection ((21,22,23,24)). However, even after complete ablation, patients frequently encounter intrahepatic tumor recurrence at a rate of 50% in 2 years, the majority of which occurs at locations distant from the primary ablated site (25). Considering the tumor doubling time, many nodules diagnosed as recurrent within 2 years were probably present at the time of first ablation. If nodules that are undetectable by conventional dynamic CT could be detected and ablated, the recurrence rate would be decreased.
Although CTHA/CTAP is one of the most sensitive techniques available for detection of small HCC, its disadvantages include invasiveness, high cost, and a high false-positive rate (26). The indication for CTHA/CTAP can be justified only when the expected benefits exceed the risk and cost of the procedure. We conducted a single-center, randomized, open-label, controlled trial to assess the utility of CTHA/CTAP before RFA in patients with early-stage HCC by comparing recurrence-free and overall survival.
METHODS
Patients
The study population consisted of patients with early-stage HCC with an indication for RFA. Those who met the following criteria were enrolled between September 2004 and February 2009: (i) diagnosis of typical HCC on dynamic CT performed within 2 weeks, i.e., hyperattenuation during the arterial phase and hypoattenuation during the equilibrium phase ((5,6)); (ii) tumor size ≤3.0 cm and no more than three tumor nodules; (iii) Child–Pugh class A liver function; and (iv) age >20 years. Exclusion criteria were: allergy to contrast media; portal or hepatic vein tumor thrombosis; extrahepatic metastasis; diffuse and infiltrative tumors; renal failure (serum creatinine >2.0 mg/dl, or serum urea nitrogen >30 mg/dl); impaired coagulation (e.g., platelet count <50 × 103/μl, or prothrombin activity <50%); pregnancy; or past history of choledochojejunostomy. We included those with previous treatments as well as treatment-naive cases provided that there was no local recurrence at enrollment. These inclusion criteria and the study design did not change till the study completely ended. The study design conformed to the Declaration of Helsinki Principles and was approved by the ethics committee of our institution. The study was registered at the University Hospital Medical Information Network (UMIN) Clinical Trial Registry (UMIN-CTR000000070). Written informed consent was obtained from each patient. This study complied with the CONSORT guidelines for reporting of clinical trials (27).
Study design
Before receiving RFA, patients were randomly assigned to undergo CTHA/CTAP or not in equal numbers. Patient registration and randomization were performed by computer-generated allocation at a web-based data center (Internet Data and Information Center for Medical Research) administered by UMIN. At the time of randomization, patients were stratified either as treatment naive, for whom RFA was planned as an initial treatment for HCC, or recurrent, for whom RFA was planned for recurrent HCC. The randomization was based on the Efron's biased-coin design (28). In principal, the assignment was not blinded to the investigators and the participants. The interval between random assignment and implementation of treatment for HCC was <4 weeks. CTHA/CTAP was performed on the assigned patients on the second day of admission, and RFA was performed 2 or 3 days later, given that the total number of HCC nodules remained <4. When ≥4 HCC nodules were detected on CTHA/CTAP, patients first received transarterial chemoembolization (TACE) immediately after CTHA/CTAP, followed later by RFA to achieve complete ablation of the tumor nodules.
Radiographic procedures
For the diagnosis of HCC at study entry, intravenous contrast-enhanced dynamic CT was performed on an outpatient basis using an X-ray CT device with 4, 8, or 16 detector rows (Aquilion 4/16; Toshiba, Tokyo, Japan; LightSpeed Qx/I, LightSpeed Ultra; GE Healthcare, Milwaukee, WI). Images were obtained during the early arterial, late arterial, and equilibrium phases at 28, 40, and 120 s after starting the intravenous bolus injection of iopamidol (Iopamiron; Nihon Schering, Osaka, Japan) or iohexol (Omnipaque; Daiichi Sankyo, Tokyo, Japan) at a rate of 2.3–3.3 ml/s with a power injector. The total dose of iodine was 0.7 g/kg body weight, with an upper limit of 37 g iodine. The injection time for the contrast material was 30 s. Images were reconstructed with a section thickness of 2.5 mm and a reconstruction interval of 1.5 mm, and were reviewed by experienced radiologists.
CTHA/CTAP was performed on an inpatient basis. First, a 4-Fr modified Shepherd-hook catheter and a 4-Fr hepatic-curve catheter were placed in the celiac artery and superior mesenteric artery, respectively, through bilateral femoral arteries, according to Seldinger's method. Digital subtraction angiography was performed from the celiac artery to evaluate hepatic artery anatomy. A microcatheter was inserted through the 4-Fr catheter and placed in the proper or common hepatic artery for hepatic arteriography.
The CTAP catheter was placed in the superior mesenteric artery in all cases. In the case of a replaced or accessory right hepatic artery, the catheter was inserted well beyond the origin of the hepatic artery to prevent contrast medium overflow into the hepatic artery. Less than 30 ml of contrast agent, which was diluted to 100 mg I/ml, was used before the CTHA/CTAP study. First, CTAP was performed using 90 ml nonionic contrast medium diluted to 100 mg I/ml, and then CT scanning was performed 30 s after the start of the injection at a rate of 3.0 ml/s. Multidetector-row CT images were obtained during a single breath hold in a longitudinal direction with collimation of 1 mm, table speed of 30 mm/s, 120 kVp, and 300 mAs. CTHA was performed at least 5 min after CTAP, using the same parameters. CT scanning was performed at 10 and 45 s after the start of contrast medium injection into the microcatheter at a rate of 2.0–2.5 ml/s. A total of 30–50 ml contrast agent diluted to 100 mg I/ml was used. When the liver was perfused by two or more hepatic arteries such as a replaced right hepatic artery, accessory right hepatic artery, or left hepatic artery downstream of the left gastric artery, CTHA was performed from each of the respective arteries. A diagnosis of typical HCC on CTHA/CTAP was defined as a round hypervascular nodule on CTHA with a defect on CTAP, accompanied by corona enhancement during the second phase of CTHA or hypoattenuation during the equilibrium phase of prior dynamic CT ((10,29)).
TACE was additionally performed when ≥4 HCC nodules were detected on CTHA/CTAP, as evaluated at the time by the operating radiologist. The procedure used 3.0 ml contrast medium, 30 mg doxorubicin (Adriacin; Kyowahakko Kirin, Tokyo, Japan), and 3.0 ml iodized oil (Lipiodol Ultra-Fluid; Guerbet Japan, Tokyo, Japan). The amounts of contrast medium and iodized oil in this suspension were arbitrarily adjusted according to tumor size. This agent was injected into each feeder of the HCC, followed by infusion of 2-mm-diameter gelatin sponge particles (Gelpart; Nihonkayaku, Tokyo, Japan).
CTHA/CTAP images were scrutinized by two experienced radiologists, who made the final diagnosis. The radiologists were not blinded to information regarding the preceding conventional dynamic CT. Preceding intravenous contrast-enhanced dynamic CT was retrospectively reviewed for nodules newly diagnosed by CTHA/CTAP to determine whether the nodules could have been detected on dynamic CT.
Radiofrequency ablation
RFA was performed on an inpatient basis. The precise procedure of RFA is described elsewhere (30). All RFA procedures were performed percutaneously under ultrasonographic guidance. We used a 17-gauge cooled-tip electrode (Cool-Tip; RF Ablation System, Covidien, Boulder, Colombia, CO) for RFA. Radiofrequency energy was delivered for 6–12 min for each application. For large tumors, the electrode was repeatedly inserted into different sites, such that the entire tumor could be enveloped by assumed necrotic volumes. A CT scan with a 5-mm section thickness was performed 1–3 days after RFA to evaluate technical effectiveness. Complete ablation was defined as hypoattenuation of the entire tumor. We intended to ablate not only the tumor but also some of the liver parenchyma surrounding it. When we suspected that some portion of tumor remained nonablated, RFA was repeated. We did not predefine the procedure number in a treatment: treatment was generally continued until CT imaging demonstrated necrosis of the entire tumor.
Follow-up
The follow-up regimen after RFA consisted of blood tests and monitoring of tumor markers in an outpatient setting. Ultrasonography and dynamic CT were performed every 4 months. Tumor recurrence was defined as a newly developed lesion on a dynamic CT that showed hyperattenuation in the arterial phase with washout in the late phase. Recurrent site was categorized as intrahepatic recurrence distant from ablated nodules, local tumor progression defined as the appearance of viable cancer tissue touching the ablated nodules, and extrahepatic metastasis (31). The follow-up was censored in February 2011 when 2 years had passed after the enrollment of patient 280. No interim analysis was specified in the protocol.
End points
The primary end point was recurrence-free survival, where both recurrence and death were treated as an event. We intended to ablate all detected nodules in both groups. When additional nodules were detected by CTHA/CTAP, the newly detected nodules were also ablated. When >3 nodules were diagnosed as HCC by CTHA/CTAP, we performed TACE and subsequently intended to ablate all of the nodules. When nonablated viable tumors were detected by CT for treatment evaluation, those cases were treated as an event 120 days after randomization. Even when newly detected nodules showed dense Lipiodol deposits after TACE, the nodules were considered as viable if the nodules were nonablated.
Secondary end points were the number of additional nodules detected by CTHA/CTAP, the proportion of patients with complete ablation, overall survival, and safety of CTHA/CTAP and RFA. Complications were defined according to the guidelines of the Society of Interventional Radiology (32). According to the guidelines, major complications were defined as those that required therapy or prolonged hospitalization, or left permanent adverse sequelae, or death.
Statistical analysis
This study was designed to detect a 15% increase in 2-year recurrence-free survival in the CTHA/CTAP group from an anticipated 35% in the control group. To detect this difference with a power of 80% and type I error of 5% (two-sided test), we needed 280 patients (140 for each arm). Differences between groups for each characteristic were tested for significance with Fisher's exact test for categorical variables and t-test for continuous variables. All data necessary for analysis was corrected in the main computer server system of University of Tokyo, Department of Gastroenterology.
Recurrence-free survival and overall survival were calculated using the Kaplan–Meier method and were compared by the log-rank test. Cox proportional hazard regression was used to calculate hazard ratios with 95% confidence interval (CI) between the groups in univariate and multivariate settings. The primary end point was evaluated in subgroups according to the following characteristics: age, sex, body mass index, treatment naivety, hepatitis B surface antigen (HBsAg) positivity, hepatitis C virus antibody positivity, tumor size, tumor number, platelet count, tumor marker positivity for α-fetoprotein (AFP), lens culinaris agglutinin-reactive fraction of AFP, and des-γ-carboxy prothrombin. An adjusted hazard ratio comparing the groups was calculated using multivariate Cox regression with factors that showed significance in univariate analysis. Data at entry were used for the analyses. A post hoc analysis comparing the recurrence-free survival of those with and without newly diagnosed HCC in the CTHA/CTAP group was performed.
All analyses were performed on an intention-to-treat basis. Differences with a two-sided P value of <0.05 were considered statistically significant. Data processing and analysis were performed with S-PLUS ver. 7 (TIBCO Software, Palo Alto, CA). Finally, all authors had access to the study data and had reviewed and approved the final manuscript.
RESULTS
Patient enrollment
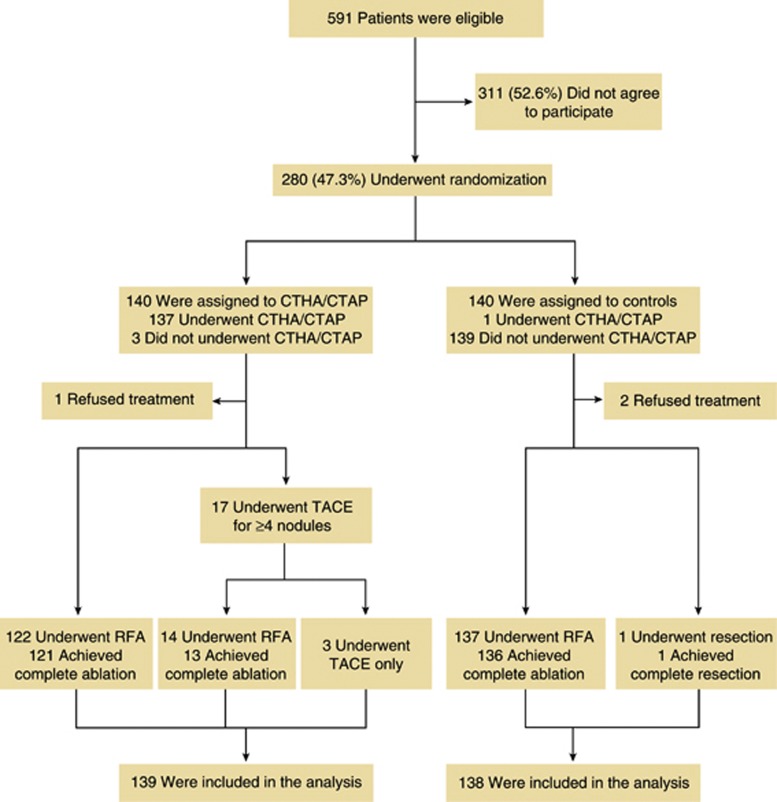
According to the study protocol, the registration started from September 2004 for 5 years and the follow-up was censored in February 2011 when 2 years had passed after the enrollment of patient 280. During the study period, 280 of 591 (47.4%) eligible patients agreed to participate in the trial, and 140 of these were randomly assigned to undergo CTHA/CTAP before RFA. Three patients declined to undergo CTHA/CTAP after assignment. A total of 140 patients were randomly assigned to the control group. One patient assigned to the control group received CTHA/CTAP because of strong preference (Figure 1).
Figure 1.
Patient enrollment and outcomes. CTAP, computed tomography during arterial portography; CTHA, computed tomography during hepatic arteriography; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
Treatment
In 45 (32.4%) patients, 75 nodules with a median diameter of 8 mm (range, 2–20) were additionally diagnosed by experienced radiologists as definite HCC on CTHA/CTAP. The detailed characteristics of newly diagnosed nodules have been reported previously (33). In 17 patients, the number of HCC nodules exceeded 3 after CTHA/CTAP, and TACE was performed subsequently. We intended to ablate all nodules by RFA including additionally detected nodules. In 122 patients, there were ≤3 HCC nodules, and complete ablation was obtained in 121 patients (99.2%). Among 17 patients treated with TACE, 14 (82.4%) subsequently underwent RFA and complete ablation was obtained in 13 (92.9%) patients. The remaining 3 patients (17.6%) did not undergo RFA because of tumor nodule multiplicity in 2 patients and simultaneously diagnosed malignant B-cell lymphoma in the third patient. Among 140 patients who were assigned to the control group, 137 (97.9%) were treated with RFA, and complete ablation was obtained in 136 (99.3%) patients. One patient withdrew consent and underwent hepatic resection. Two patients refused to receive any treatment and were lost to follow-up. Finally, 139 (99.3%) patients in the CTHA/CTAP group and 138 (98.6%) patients in the control group were included in the analysis.
Patient characteristics
There was no statistically significant difference in patient characteristics between the groups ( Table 1). Median age at enrollment was 70 years, and approximately two-thirds of patients were male. Approximately 55% of patients were treatment-naive cases and the remaining patients had a history of previous treatment. Among those previously treated patients, the median interval between the initial treatment and the study enrollment was 42 (interquartile range, 22–65) months in the CTHA/CTAP group and 30 (20–61) months in the control group. There was no statistically significant difference between the two groups (P=0.72). The total number of HCC nodules detected in original contrast-enhanced dynamic CT was 197 (101 patients were uninodular and the rest were multinodular) in the CTHA/CTAP group and 196 (98 patients were uninodular and the rest were multinodular) in the control group.
Table 1. Baseline characteristics of the patientsa.
| Characteristics | CTHA/CTAP (N=139) | Control (N=138) | P value |
|---|---|---|---|
| Age, years | 70 (63–74) | 70 (64–75) | 0.43 |
| Male, n (%) | 93 (67) | 86 (62) | 0.42 |
| Alcohol >80 g/day, n (%) | 23 (17) | 20 (15) | 0.82 |
| BMI (kg/m2) | 23.1 (21.4–25.1) | 23.4 (21.2–25.3) | 0.48 |
| Viral markers | |||
| HCVAb positive, n (%) | 104 (75) | 99 (72) | 0.59 |
| HBsAg positive, n (%) | 21 (15) | 20 (14) | 1 |
| Serum albumin (g/dl) | 3.8 (3.6–4.1) | 3.9 (3.6–4.1) | 0.20 |
| Total bilirubin (mg/dl) | 0.8 (0.6–1.0) | 0.8 (0.6–1.0) | 0.31 |
| AST (IU/l) | 56 (34–69) | 57 (33–70) | 0.84 |
| ALT (IU/l) | 54 (29–63) | 57 (27–73) | 0.61 |
| Platelet count (× 103/μl) | 128 (89–163) | 130 (91–159) | 0.88 |
| Prothrombin activity (%) | 80 (72–90) | 81 (74–87) | 0.39 |
| Treatment-naive case, n (%) | 77 (55) | 74 (54) | 0.81 |
| Previously treated case, n (%) | 62 (45) | 64 (46) | |
| Resection, n (%)b | 15 (24) | 16 (25) | 0.27 |
| RFA, n (%)b | 46 (74) | 45 (70) | |
| Ethanol injection, n (%)b | 10 (16) | 3 (4.6) | |
| TACE, n (%)b | 11 (18) | 7 (11) | |
| Tumor size (cm) | 1.6 (1.2–2.0) | 1.7 (1.2–2.0) | 0.91 |
| Single nodule, n (%) | 101 (73) | 98 (71) | 0.76 |
| AFP >100 ng/ml, n (%) | 23 (17) | 24 (17) | 0.85 |
| DCP >100 mAU/ml, n (%) | 16 (12) | 22 (16) | 0.28 |
| AFP-L3 >15%, n (%) | 16 (12) | 15 (11) | 0.86 |
AFP, α-fetoprotein; AFP-L3, lens culinaris agglutinin-reactive fraction of AFP; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CTHA/CTAP, computed tomography during hepatic arteriography and arterial portography; DCP, des-γ-carboxy prothrombin; HBsAg, hepatitis B surface antigen; HCVAb, hepatitis C virus antibody; RFA, radiofrequency ablation, TACE, transarterial chemoembolization.
Data are expressed as median (25th–75th percentiles) or number (percent).
Including overlap.
Recurrence
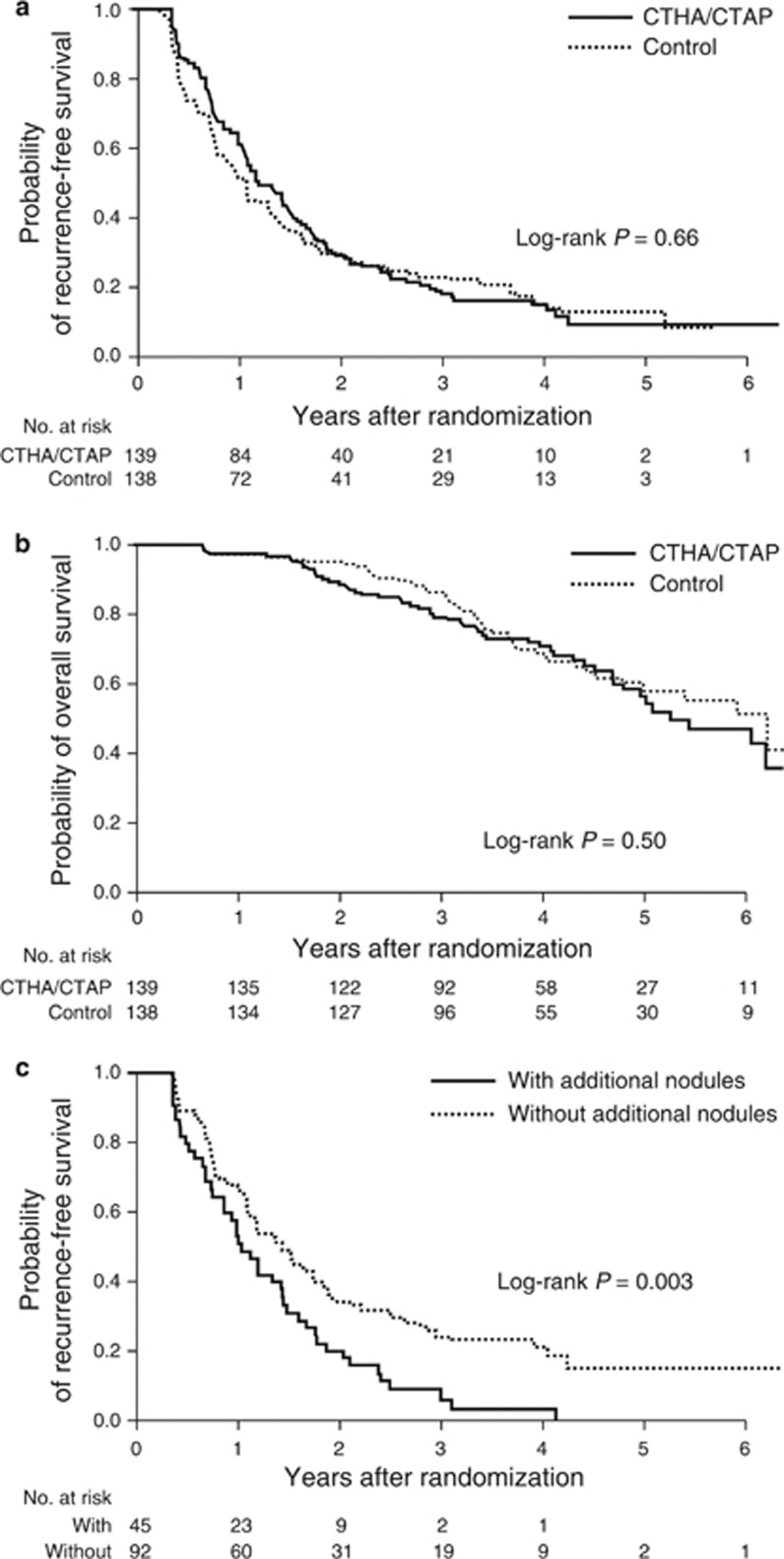
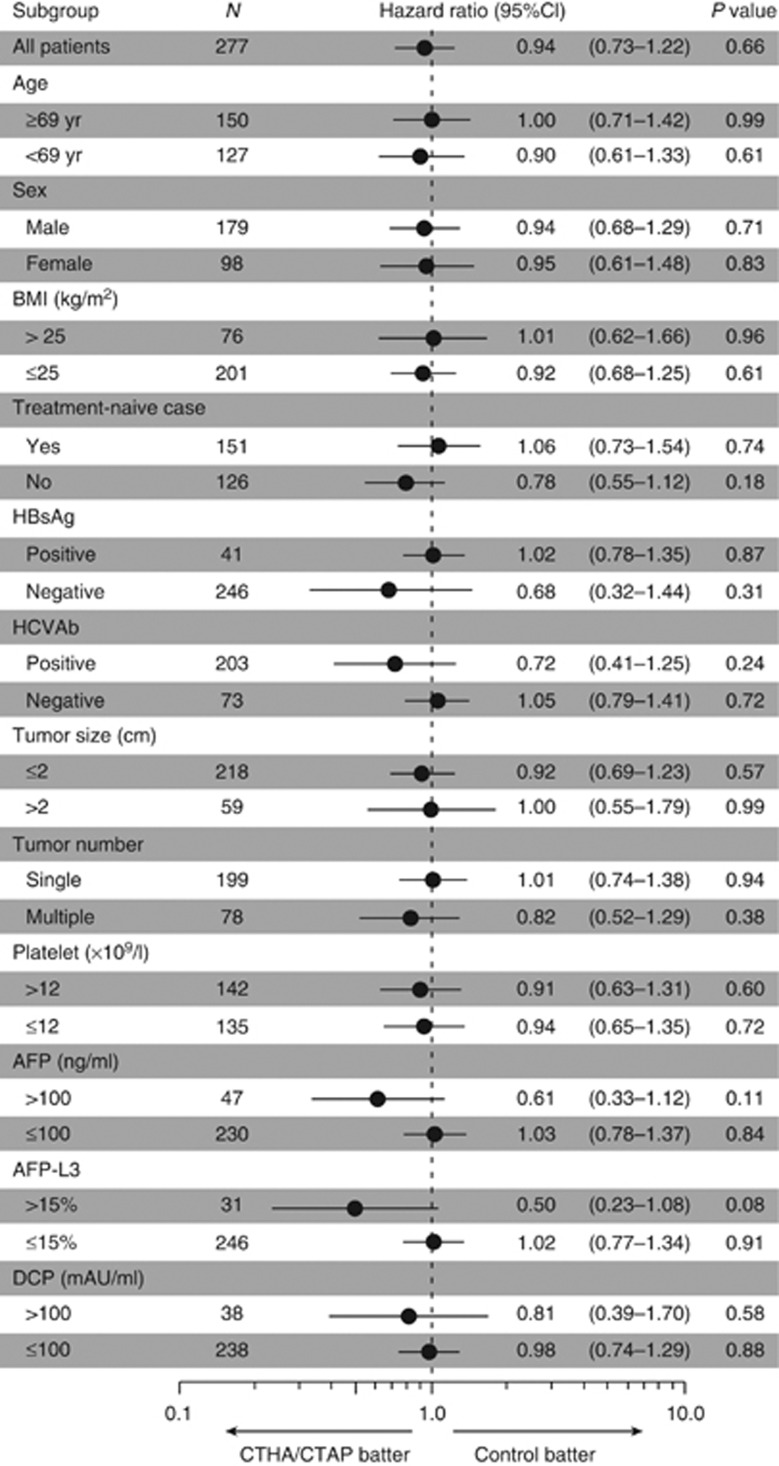
By the end of the follow-up, tumor recurrence was identified in 109 patients (78.4%) in the CTHA/CTAP group and 112 patients (81.2%) in the control group. The distribution of recurrent site was intrahepatic distant recurrence (N=98), local tumor progression (N=7), both (N=1), and extrahepatic metastasis (N=3) in the CTHA/CTAP group and intrahepatic distant recurrence (N=103), local tumor progression (N=4), both (N=2), and extrahepatic metastasis (N=3) in the control group. Five patients (3.6%) in the CTHA/CTAP group and 1 patient (0.7%) in the control group in whom complete ablation could not be obtained by RFA were treated as recurrence on 120 days after randomization when the first follow-up CT would have been scheduled. In each group, four patients died without recurrence. The cumulative recurrence-free survival rates at 1, 2, and 3 years were 60.1, 29.0, and 18.9% in the CTHA/CTAP group and 52.2, 29.7, and 23.1% in the control group, respectively (Figure 2a). The difference between the two groups was not statistically significant (P=0.66 by log-rank test; hazard ratio, 0.94 for CTHA/CTAP vs. control; 95% CI, 0.73–1.22). The CTHA/CTAP group showed better recurrence-free survival with marginal statistical significance in the subgroups with higher AFP or AFP-L3 values (Figure 3).
Figure 2.
Kaplan–Meier estimate of the recurrence-free survival and overall survival. (a) The cumulative recurrence-free survival rates at 1, 2, and 3 years were 60.1, 29.0, and 18.9% in the CTHA/CTAP group and 52.2, 29.7, and 23.1% in the control group, respectively. (b) The cumulative overall survival rates at 3 and 5 years were 79.7 and 56.4% in CTHA/CTAP group and 86.8 and 60.1% in the control group, respectively. (c) Patients with an additional nodule detected by CTHA/CTAP showed significantly poorer recurrence-free survival than those without an additional nodule. CTAP, computed tomography during arterial portography; CTHA, computed tomography during hepatic arteriography.
Figure 3.
Recurrence-free survival of subgroups by Cox proportional hazard regression according to clinical characteristics at study entry. AFP, α-fetoprotein; BMI, body mass index; CI, confidence interval; CT, computed tomography; CTAP, computed tomography during arterial portography; CTHA, computed tomography during hepatic arteriography; DCP, des-γ-carboxy prothrombin; HBsAg, hepatitis B surface antigen; HCVAb, hepatitis C virus antibody; yr, year.
Univariate Cox regression analysis identified older age (P=0.01), hepatitis C virus antibody positivity (P=0.001), lower albumin level (P=0.04), recurrent cases (P<0.001), multinodular HCC (P<0.001), and higher AFP level (P=0.02) as significant predictors for recurrence-free survival ( Table 2). Adjusted hazard ratio of the CTHA/CTAP group vs. the control group by multivariate Cox regression analysis was 0.86 (95% CI, 0.67–1.12; P=0.27, Table 3).
Table 2. Univariate Cox's proportional hazard regression analysis of the risk for recurrence-free survival.
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| CTHA/CTAP vs. control | 0.94 (0.73–1.22) | 0.66 |
| Age (per year) | 1.02 (1.00–1.04) | 0.01 |
| Female vs. male | 1.02 (0.78–1.34) | 0.88 |
| Alcohol >80 g/day | 1.02 (0.88–1.17) | 0.81 |
| HCVAb positive | 1.69 (1.23–2.31) | 0.001 |
| BMI (per 1.0 kg/m2) | 1.02 (0.98–1.06) | 0.35 |
| Albumin (per 1.0 g/dl) | 0.72 (0.52–0.98) | 0.04 |
| Total bilirubin (per 1.0 mg/dl) | 1.02 (0.97–1.07) | 0.51 |
| AST >40 IU/l | 1.14 (0.99–1.31) | 0.07 |
| ALT >40 IU/l | 1.05 (0.92–1.20) | 0.45 |
| Platelet count >10 × 103/μl | 0.89 (0.78–1.01) | 0.08 |
| Recurrent case | 2.33 (1.79–3.02) | <0.001 |
| Tumor size of maximal nodule >2.0 cm | 0.97 (0.85–1.10) | 0.62 |
| Multinodular | 1.38 (1.20–1.59) | <0.001 |
| AFP >100 ng/ml | 1.21 (1.03–1.43) | 0.02 |
| DCP >100 mAU/ml | 0.99 (0.82–1.20) | 0.93 |
| AFP-L3 >15% | 1.20 (0.99–1.46) | 0.07 |
AFP, α-fetoprotein; AFP-L3, lens culinaris agglutinin-reactive fraction of AFP; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CI, confidence interval; CTHA/CTAP, computed tomography during hepatic arteriography and arterial portography; DCP, des-γ-carboxy prothrombin; HCVAb, hepatitis C virus antibody.
Table 3. Multivariate Cox's proportional hazard regression analysis of the risk for recurrence-free survival.
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| CTHA/CTAP vs. control | 0.86 (0.0.67–1.12) | 0.27 |
| Age (per year). | 1.01 (0.99–1.02) | 0.36 |
| HCVAb positive | 1.36 (0.98–1.89) | 0.07 |
| Albumin (per 1.0 g/dl) | 0.75 (0.53–1.07) | 0.11 |
| Recurrent case | 2.21 (1.69–2.89) | <0.001 |
| Multinodular | 1.69 (1.27–2.25) | <0.001 |
| AFP >100 ng/ml | 1.41 (0.996–1.98) | 0.052 |
AFP, α-fetoprotein; CI, confidence interval; CTHA/CTAP, computed tomography during hepatic arteriography and arterial portography; HCVAb, hepatitis C virus antibody.
Overall survival
By the end of the follow-up, 51 patients (36.7%) in the CTHA/CTAP group and 45 patients (32.6%) in the control group died. The cumulative overall survival rates at 3 and 5 years were 79.7 and 56.4% in the CTHA/CTAP group and 86.8 and 60.1% in the control group, respectively (Figure 2b). There was no statistically significant difference between the groups (P=0.50 by log-rank test; hazard ratio, 1.15, 95% CI, 0.77–1.71).
Safety
No procedural complications attributable to CTHA/CTAP or TACE were observed. Major complications related to RFA were observed in 2 patients (1.4%) in the CTHA/CTAP group (2 with neoplastic seeding) and in 3 patients (2.2%) in the control group (1 each with hepatic infarction, hemothorax, and neoplastic seeding). There was no procedure-related death.
Recurrence-free survival between those with and without additional nodules in CTHA/CTAP group
As a post hoc analysis, we compared the recurrence-free survival between those with (N=45) and without (N=92) additional HCC nodules diagnosed by CTHA/CTAP. As compared with those in whom additional HCC nodules were not detected by CTHA/CTAP, those with additional nodules included more HBsAg-negative patients (97.7 vs. 78.3%, P=0.002), previously treated patients (62.2 vs. 23.9%, P=0.006), and patients with multiple HCC nodules on dynamic CT (44.4 vs. 17.4%, P=0.002). Patients with additional nodule by CTHA/CTAP showed significantly poorer recurrence-free survival than those without additional nodules (P=0.003, Figure 2c).
DISCUSSION
An advance in diagnostic technology generally indicates improved sensitivity or specificity, which corresponds to the detection of smaller lesions with a clearer view in imaging modalities. In our previous study, we showed that 75 nodules with a mean diameter of 8.7 mm (range, 2–20 mm) in 45 (33%) of 139 patients who underwent CTHA/CTAP were additionally diagnosed as definite HCC, compared with dynamic CT examination (33). However, no significant difference was observed in terms of recurrence-free survival between those who did and did not undergo CTHA/CTAP before RFA.
One reason for this discrepancy may be that the impact of CTHA/CTAP on recurrence reduction was diluted by a long-term follow-up of >2 years. It is unlikely that CTHA/CTAP could detect small nodules that would be detected ≥2 years later by conventional dynamic CT. In fact, the number of recurrences identified within 1 year after enrollment was lower in the CTHA/CTAP group than the control group (54 vs. 65, data not shown).
Another reason could be that fewer patients achieved complete ablation of target nodules in the CTHA/CTAP group than in the control group. The additionally diagnosed HCC nodules were small, and detection of these nodules by ultrasonography was difficult. Recent technologies such as contrast ultrasonography or fusion imaging, which can improve the accuracy of ablation techniques ((34,35,36)), may increase the probability of detection of smaller nodules before RFA.
Precise evaluation of the stage of progression is important for deciding on treatment procedures in HCC management. Seventeen patients in the CTHA/CTAP group were diagnosed with ≥4 nodules by CTHA/CTAP, which is not considered suitable for RFA according to widely used criteria.
In our previous study, we showed that recurrence as opposed to initial occurrence, multinodularity on dynamic CT, and HBsAg negativity were significant predictors for finding additional HCC by CTHA/CTAP (33). In fact, the CTHA/CTAP group showed better outcomes in the subgroups with HBsAg-negative cases, previously treated patients, and multinodular HCC. However, post hoc analysis comparing recurrence-free survival of those with and without additional nodules detected by CTHA/CTAP showed that those with a higher probability of additional nodules were also at a higher risk of recurrence. The advantage of CTHA/CTAP in finding more HCC nodules might be counter balanced by the higher risk of recurrence.
This study has several limitations. First, the additional nodules detected by CTHA/CTAP were not confirmed histologically. Therefore, we cannot exclude the possibility of overdiagnosis. Second, 45% of the patients had a history of previous treatment including resection, RFA, and TACE. Those previous treatments might substantially alter the hemodynamic status in the liver and affect the accuracy of CTHA/CTAP. On the other hand, in the previously treated cases, the radiologists could refer to the past series of dynamic CT during performing CTHA/CTAP, which might improve the accuracy of CTHA/CTAP as compared with treatment-naive cases. Third, 17 patients in the CTHA/CTAP group underwent TACE as a salvage treatment because total number of HCC nodules exceeded 3 after CTHA/CTAP. This might affect the recurrence-free and overall survival in the CTHA/CTAP group.
Our results may be extrapolated to other imaging modalities including gadoxetic acid–enhanced magnetic resonance imaging and second-generation contrast ultrasonography ((37,38)). These newly developed modalities also make possible the detection of small nodules that are invisible by dynamic CT. However, better diagnosis does not necessarily lead to better primary outcome.
In conclusion, CTHA/CTAP before RFA resulted in improved HCC diagnosis and detection of additional nodules in one-third of the study participants. However, it did not improve recurrence-free survival. The indications for CTHA/CTAP should be evaluated carefully.
Study protocol URL: https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&recptno=R000000117&type=summary&language=E.
STUDY HIGHLIGHTS
Guarantor of the article: Ryosuke Tateishi, MD, PhD.
Specific author contributions: Conception and design: R.T., M.A., N.Y., T.G., S.S., H.Y., Y.M., and M.O.; analysis: R.T. and Y.M.; treatment and data collection: T.O., R.T., M.A., S.M., M.S., K.U., T.A., K.E., Y.K., T.G., and S.S.; drafting article: T.O.; critical revision: R.T., M.A., H.Y., K.O., and K.K.
Financial support: This work was supported by Health Sciences Research grants of The Ministry of Health, Labour and Welfare of Japan (Research on Hepatitis). No additional external funding was received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential competing interests: None.
References
- El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- Kiyosawa K, Umemura T, Ichijo T, et al. Hepatocellular carcinoma: recent trends in Japan. Gastroenterology. 2004;127:S17–S26. doi: 10.1053/j.gastro.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Itai Y, Matsui O. Blood flow and liver imaging. Radiology. 1997;202:306–314. doi: 10.1148/radiology.202.2.9015047. [DOI] [PubMed] [Google Scholar]
- Matsui O. Detection and characterization of hepatocellular carcinoma by imaging. Clin Gastroenterol Hepatol. 2005;3:S136–S140. doi: 10.1016/s1542-3565(05)00707-x. [DOI] [PubMed] [Google Scholar]
- Teratani T, Yoshida H, Shiina S, et al. A novel display of reconstruction computed tomography for the detection of small hepatocellular carcinoma. Liver Int. 2004;24:619–624. doi: 10.1111/j.1478-3231.2004.0959.x. [DOI] [PubMed] [Google Scholar]
- Fujishima T, Yoshida H, Obi S, et al. Analysis of factors influencing hepatocellular carcinoma detection: efficient use of computed tomography during arterial portography and during hepatic arteriography. J Gastroenterol. 2005;40:266–273. doi: 10.1007/s00535-004-1533-4. [DOI] [PubMed] [Google Scholar]
- Sangiovanni A, Manini MA, Iavarone M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut. 2010;59:638–644. doi: 10.1136/gut.2009.187286. [DOI] [PubMed] [Google Scholar]
- Murakami T, Oi H, Hori M, et al. Helical CT during arterial portography and hepatic arteriography for detecting hypervascular hepatocellular carcinoma. AJR Am J Roentgenol. 1997;169:131–135. doi: 10.2214/ajr.169.1.9207512. [DOI] [PubMed] [Google Scholar]
- Kanematsu M, Hoshi H, Imaeda T, et al. Detection and characterization of hepatic tumors: value of combined helical CT hepatic arteriography and CT during arterial portography. AJR Am J Roentgenol. 1997;168:1193–1198. doi: 10.2214/ajr.168.5.9129410. [DOI] [PubMed] [Google Scholar]
- Irie T, Takeshita K, Wada Y, et al. CT evaluation of hepatic tumors: comparison of CT with arterial portography, CT with infusion hepatic arteriography, and simultaneous use of both techniques. AJR Am J Roentgenol. 1995;164:1407–1412. doi: 10.2214/ajr.164.6.7754883. [DOI] [PubMed] [Google Scholar]
- Small WC, Mehard WB, Langmo LS, et al. Preoperative determination of the resectability of hepatic tumors: efficacy of CT during arterial portography. AJR Am J Roentgenol. 1993;161:319–322. doi: 10.2214/ajr.161.2.8333369. [DOI] [PubMed] [Google Scholar]
- Soyer P, Levesque M, Elias D, et al. Detection of liver metastases from colorectal cancer: comparison of intraoperative US and CT during arterial portography. Radiology. 1992;183:541–544. doi: 10.1148/radiology.183.2.1561365. [DOI] [PubMed] [Google Scholar]
- Soyer P, Levesque M, Elias D, et al. Preoperative assessment of resectability of hepatic metastases from colonic carcinoma: CT portography vs sonography and dynamic CT. AJR Am J Roentgenol. 1992;159:741–744. doi: 10.2214/ajr.159.4.1529835. [DOI] [PubMed] [Google Scholar]
- Matsui O, Kadoya M, Suzuki M, et al. Work in progress: dynamic sequential computed tomography during arterial portography in the detection of hepatic neoplasms. Radiology. 1983;146:721–727. doi: 10.1148/radiology.146.3.6298857. [DOI] [PubMed] [Google Scholar]
- Sherman M, Klein A. AASLD single-topic research conference on hepatocellular carcinoma: conference proceedings. Hepatology. 2004;40:1465–1473. doi: 10.1002/hep.20528. [DOI] [PubMed] [Google Scholar]
- Tateishi R, Shiina S, Ohki T, et al. Treatment strategy for hepatocellular carcinoma: expanding the indications for radiofrequency ablation. J Gastroenterol. 2009;44 (Suppl 19:142–146. doi: 10.1007/s00535-008-2247-9. [DOI] [PubMed] [Google Scholar]
- Ebara M, Ohto M, Sugiura N, et al. Percutaneous ethanol injection for the treatment of small hepatocellular carcinoma. Study of 95 patients. J Gastroenterol Hepatol. 1990;5:616–626. doi: 10.1111/j.1440-1746.1990.tb01115.x. [DOI] [PubMed] [Google Scholar]
- Livraghi T, Giorgio A, Marin G, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197:101–108. doi: 10.1148/radiology.197.1.7568806. [DOI] [PubMed] [Google Scholar]
- Shiina S, Tagawa K, Niwa Y, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma: results in 146 patients. AJR Am J Roentgenol. 1993;160:1023–1028. doi: 10.2214/ajr.160.5.7682378. [DOI] [PubMed] [Google Scholar]
- Seki T, Wakabayashi M, Nakagawa T, et al. Percutaneous microwave coagulation therapy for patients with small hepatocellular carcinoma: comparison with percutaneous ethanol injection therapy. Cancer. 1999;85:1694–1702. [PubMed] [Google Scholar]
- Rossi S, Di Stasi M, Buscarini E, et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996;167:759–768. doi: 10.2214/ajr.167.3.8751696. [DOI] [PubMed] [Google Scholar]
- Allgaier HP, Deibert P, Zuber I, et al. Percutaneous radiofrequency interstitial thermal ablation of small hepatocellular carcinoma. Lancet. 1999;353:1676–1677. doi: 10.1016/S0140-6736(99)00368-2. [DOI] [PubMed] [Google Scholar]
- Livraghi T, Goldberg SN, Lazzaroni S, et al. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655–661. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- Curley SA, Izzo F, Ellis LM, et al. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381–391. doi: 10.1097/00000658-200009000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors Am J Gastroenterol 2012107569–577.quiz 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HJ, Lim JH, Lee SJ, et al. Hepatocellular carcinoma: are combined CT during arterial portography and CT hepatic arteriography in addition to triple-phase helical CT all necessary for preoperative evaluation. Radiology. 2000;215:373–380. doi: 10.1148/radiology.215.2.r00ma30373. [DOI] [PubMed] [Google Scholar]
- Rennie D. CONSORT revised--improving the reporting of randomized trials. JAMA. 2001;285:2006–2007. doi: 10.1001/jama.285.15.2006. [DOI] [PubMed] [Google Scholar]
- Efron B. Forcing a sequential experiment to be balanced. Biometrika. 1971;58:403. [Google Scholar]
- Kitao A, Zen Y, Matsui O, et al. Hepatocarcinogenesis: multistep changes of drainage vessels at CT during arterial portography and hepatic arteriography--radiologic-pathologic correlation. Radiology. 2009;252:605–614. doi: 10.1148/radiol.2522081414. [DOI] [PubMed] [Google Scholar]
- Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201–1209. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20:S377–S390. doi: 10.1016/j.jvir.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:S199–S202. doi: 10.1097/01.rvi.0000094584.83406.3e. [DOI] [PubMed] [Google Scholar]
- Ohki T, Tateishi R, Akahane M, et al. Characteristics of hepatocellular carcinoma nodules newly detected by computed tomography during arteriography and arterial portography: preliminary report of a randomized controlled trial Hepatol Int 2011(e-pub ahead of print). [DOI] [PubMed]
- Minami Y, Kudo M. Review of dynamic contrast-enhanced ultrasound guidance in ablation therapy for hepatocellular carcinoma. World J Gastroenterol. 2011;17:4952–4959. doi: 10.3748/wjg.v17.i45.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata K, Fukuda H, Morimoto M, et al. Use of fusion imaging combining contrast-enhanced ultrasonography with a perflubutane-based contrast agent and contrast-enhanced computed tomography for the evaluation of percutaneous radiofrequency ablation of hypervascular hepatocellular carcinoma. Eur J Radiol. 2012;81:2746–2753. doi: 10.1016/j.ejrad.2011.11.052. [DOI] [PubMed] [Google Scholar]
- Masuzaki R, Shiina S, Tateishi R, et al. Utility of contrast-enhanced ultrasonography with Sonazoid in radiofrequency ablation for hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:759–764. doi: 10.1111/j.1440-1746.2010.06559.x. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Saito K, Yoshioka N, et al. Detection and characterization of focal liver lesions: a Japanese phase III, multicenter comparison between gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced computed tomography predominantly in patients with hepatocellular carcinoma and chronic liver disease. Invest Radiol. 2010;45:133–141. doi: 10.1097/RLI.0b013e3181caea5b. [DOI] [PubMed] [Google Scholar]
- Hatanaka K, Kudo M, Minami Y, et al. Differential diagnosis of hepatic tumors: value of contrast-enhanced harmonic sonography using the newly developed contrast agent, Sonazoid. Intervirology. 2008;51 (Suppl 1:61–69. doi: 10.1159/000122600. [DOI] [PubMed] [Google Scholar]