Abstract
Generalized dystonic syndromes may escalate into persistent episodes of generalized dystonia known as status dystonicus that can be life-threatening due to dystonia-induced rhabdomyolysis and/or respiratory compromise. Treatment of these conditions usually entails parenteral infusion of antispasmodic agents and sedatives and occasionally necessitates a medically induced coma for symptom control. The authors report a series of 3 children who presented with medically intractable, life-threatening status dystonicus and were successfully treated with bilateral pallidal deep brain stimulation. Bilateral globus pallidus internus stimulation appears to be effective in the urgent treatment of medically refractory and life-threatening movement disorders.
Keywords: deep brain stimulation, globus pallidus, movement disorder, rhabdomyolysis, status dystonicus, functional neurosurgery
Generalized dystonic syndromes may escalate into relentless episodes of devastating, persistent generalized dystonia known as status dystonicus.13 Treatment of this condition is often managed with infusion of antispasmodics and sedatives, such as midazolam and baclofen, frequently necessitating a medically induced coma and mechanical ventilation. Intrathecal baclofen has also been used in this condition.13 Some patients may develop dystonia-induced rhabdomyolysis and/or respiratory compromise, which can be life-threatening. Historically and rarely, pallidotomy has been performed in this acute, critical setting.2,11,12,19 Recently, DBS has been used in a limited number of such cases.1,10,14,19,24 Much has been written on DBS for non–life-threatening generalized dystonias.8,18,20,22,23 Here, we report 3 clinical cases involving children who presented in medically refractory status dystonicus and underwent bilateral globus pallidus internus DBS surgery.
Approval from the Massachusetts General Hospital institutional review board was granted for this investigation as a retrospective study.
Case Reports
Case 1
History and Presentation
This 14-year-old boy with a history of perinatal kernicterus, spastic cerebral palsy, and choreoathetosis was transferred from a referring hospital with a significant worsening of his generalized dystonia following an extensive spinal fusion procedure for scoliosis and subsequent wound infection. At baseline he was developmentally delayed, nonverbal but able to respond to yes/no questions with eye movement, wheel-chair bound, and had reduced trunk tone with minimal movement of the limbs. When excited, anxious, or ill, he would typically display large amplitude choreoathetoid movements of all 4 limbs that were associated with trunk arching and cervical dystonia. In the past, when these movements occurred during illness, he was treated with high doses of baclofen and diazepam. Prior to his spinal fusion surgery, he began treatment with both baclofen and diazepam to control any possible increase in movements. However, during the recovery period, he developed progressive stridor and desaturation episodes related to his dystonia. A variety of medications, such as oral baclofen, diazepam, and cyclobenzaprine, were tried without success. An MR imaging study did not reveal any acute pathology to account for his symptoms. He eventually required mechanical ventilation; treatment with tetrabenazine and an intravenous midazolam and dexmedetomidine infusion was initiated, but the dystonic, choreoathetotic syndrome remained uncontrollable when his medications were lessened. His examination was notable for head rotation to 60° and moderate retrocollis. He had severe choreoathetoid movement of all 4 extremities with spasticity. He required frequent bolus dosing of intravenous midazolam and diazepam, with a continuous infusion of dexmedetomidine. His spinal wound infection was resolved at this point (2 months after the spinal fusion surgery). However, he could not be weaned from ventilatory support due to the large doses of sedatives required to suppress the continuous movements. His movement disorder was severe with ballistic movement that posed a danger to him and others around him. He was urgently evaluated by the movement disorders committee and determined to be a candidate for DBS surgery.
Operation
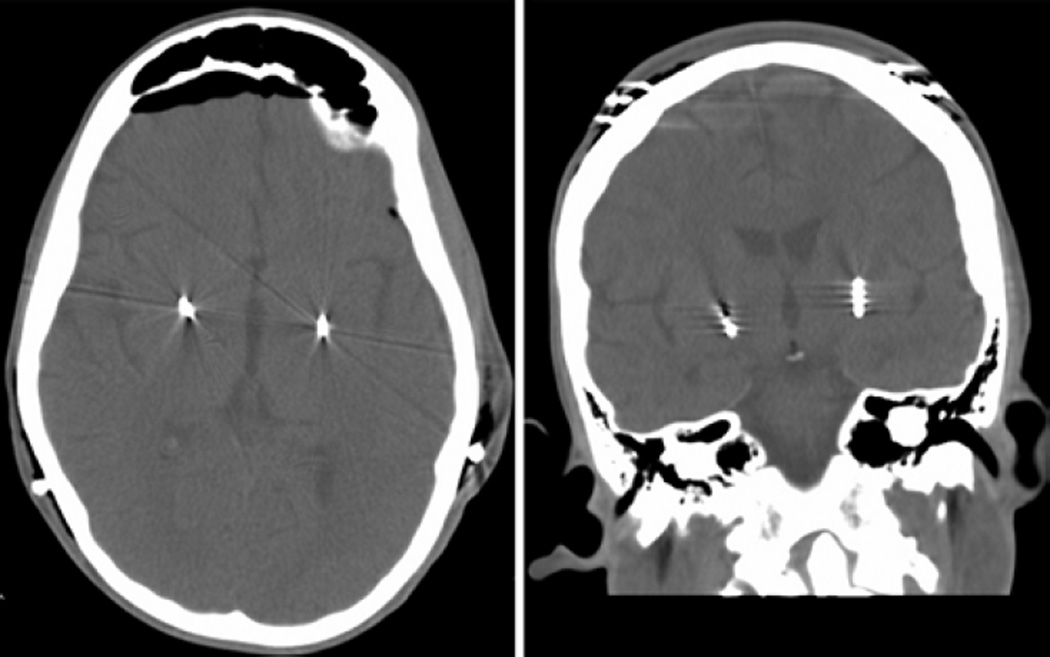
Two months after the spinal fusion surgery, bilateral DBS electrodes (lead model 3387, Medtronic Inc.) were introduced to the globus pallidus internus at a target 2 mm anterior to the midanterior commissural point, 20 mm lateral to midline, and 4 mm below the commissural plane using stereotactic and neurophysiological recording guidance (Fig. 1). The leads were then each attached to an infraclavicular pulse generator (Soletra model 7426, Medtronic Inc.). Subsequently, the stimulation was titrated to an amplitude of 2 V, a pulse width of 150 microseconds, and a frequency of 60 Hz.
Fig. 1.
Axial (left) and coronal (right) CT scans of the brain demonstrating stimulator lead placement in the bilateral globus pallidus.
Postoperative Course
The patient was weaned from mechanical ventilation over a period of days and all medical therapy was transitioned from intravenous medications to enteral form over a period of 4 weeks. At 1-year follow-up, he was markedly improved and on a medication regimen of diazepam, clonidine, and baclofen. His examination was notable for full motor strength and intermittently increased tone in the upper and lower extremities on the right with athetotic movements triggered by increased neck tone or excitement. He did have intermittent increases in the left arm and leg muscle tone with choreoathetotic movements activated with movement of the right side. Interrogation revealed that the left lower contacts were not functioning properly, consistent with the laterality seen in his physical examination. His pulse generator settings were stabilized at an amplitude of 1.8 V, a pulse width of 90 µsec, and a frequency of 60 Hz after sequential adjustment according to standard departmental adjustment protocol. Since his left-sided implant seems to be only partially working, both on interrogation and based on physical examination findings, he may be a candidate for reoperation in the future.
Case 2
History and Presentation
This 9-year-old girl with a history of generalized dystonia developed progressive dystonic symptoms over a period of 4 months. She began to have severe bilateral leg spasms and flexion of the right upper extremity that led to a persistent dystonic posture. Because of this, she was unable to feed herself or complete any activity of daily living. Medication changes on an outpatient basis, such as titration of oral baclofen and diazepam, resulted in little to no improvement. A trial of carbidopa-levodopa was also ineffective. Eventually, she was directly admitted to the intensive care unit for a midazolam infusion in an attempt to “break” her dystonic crisis. Despite administration of multiple different intravenous antispasmodic and sedative medications, she continued to have painful dystonic spasms and her serum creatine kinase level rose to 2334 U/L (reference range 40–150 U/L), concerning for dystonia-induced rhabdomyolysis. At this point, she was transferred from the referring facility intensive care unit to our institution. She was urgently evaluated by the movement disorders committee who agreed that maximal medical management had been attempted and found her to be a candidate for DBS.
Operation and Postoperative Course
Bilateral DBS electrodes (lead model 3387, Medtronic Inc.) were introduced to the globus pallidus internus and pulse generators were implanted bilaterally, as described in Case 1. Three weeks following surgery, the right infraclavicular pulse generator was removed in the setting of erythema and purulence from the surgical wound. The generator settings prior to removal were as follows: amplitude 2.0 V, pulse width 90 µsec, and frequency 145 Hz. It was suspected that the skin had become compromised due to the lack of adequate overlying soft tissue. A course of antibiotic therapy was administered for the treatment of methicillin-sensitive Staphylococcus aureus infection. The patient’s dystonic symptoms improved on the right side, and she was discharged home 6 weeks after the surgery. Two months later, she developed discharge from the cranial wound on the same side as her previous pulse generator site infection. She underwent explantation of the right-side cranial hardware and a course of organism-targeted intravenous antibiotic therapy. Ultimately, her unilateral improvement allowed her to produce a voluntary movement and control her right arm, whereas her left upper extremity was pronated in a flexed position with dystonic posturing when she activated proximal musculature on that side. She remains at home, and will ultimately have her right-sided stimulator reimplanted in an elective procedure.
Case 3
History and Presentation
This 9-year old female with a history of spastic quadriparesis and cerebral palsy presented with an acute onset of choreiform movements and 4-extremity ballismus over the course of 2 months following an upper respiratory illness. At first she had trouble grasping items, trouble moving a cup to her mouth, and trouble walking and had twitching movements of her bilateral upper and lower extremities. Over the next month, these movements became much more frequent, to the point where she could no longer bring a cup to her lips or sit without assistance. Her spasms and movements were refractory to medical management with trials of clonazepam, tetrabenazine, valproic acid, midazolam, and diazepam. She was admitted to a local hospital and developed rhabdomyolysis, with a creatine kinase level over 4000 U/L, as a result of the effects of this dystonic and hyperkinetic disorder on her musculature. She was transferred to the intensive care unit for deeper sedation and respiratory support. She was intubated and mechanically ventilated in a midazolam-induced coma in an attempt to suppress her movements and ease her spasms. A pentobarbital infusion was started. At this point, the patient was transferred from the other hospital to our institution. She was urgently evaluated by the movement disorders committee and determined be a candidate for DBS.
Operation and Postoperative Course
Bilateral DBS electrodes (lead model 3387, Medtronic Inc.) were introduced into the globus pallidus internus as described in Case 1. This time, however, in an attempt to provide greater overlying soft tissue coverage in this small child, the pulse generators were placed under the rectus abdominis fascia.
The pulse generator settings started at an amplitude of 0.5 V, a pulse width of 90 µsec, and a frequency of 130 Hz and were subsequently adjusted according to standard departmental protocol. Over the course of 2 weeks, the patient required less sedative medication; specifically, the baseline infusion rates of pentobarbital and midazolam were decreased. Within a month she was weaned entirely off intravenous medication. At 3 months’ follow-up she was alert and nonverbal with occasional crying or happy vocalizations. She was able to follow simple commands and could walk with assistance. Her pulse generator settings were set at an amplitude of 2.2 V, a pulse width of 90 microseconds, and a frequency of 130 Hz. She continues to be followed up for routine interval examinations, device interrogations, and stimulation adjustments.
Discussion
There is a growing body of evidence regarding the efficacy of DBS for dystonic syndromes. The efficacy of the globus pallidus internus target has been demonstrated in several studies, particularly in patients with the DYT1 mutation.4,7 A prospective pilot trial demonstrated the benefit of DBS in dystonia-choreoathetotic conditions for a small cohort.23 The patients reported here were not part of any clinical study or protocol; they were treated “off label”, using existing techniques and devices familiar in a high-volume movement disorders/DBS center, in life-threatening situations. It is well documented that dystonia can lead to rhabdomyolysis16 with resultant renal failure as well as respiratory compromise.3,9,15,17 Given the critical and refractory nature of the cases in our series, we elected to use DBS in an urgent manner, which is different from the typical elective treatment described in prior studies.
The dystonias related to cerebral palsy with basal ganglia dysfunction are mainly secondary to perinatal ischemic events or bilirubin encephalopathy. It is generally thought that these forms of dystonia do not respond as well to neurostimulation.6,8,21 However, this impression is based on reports that involved small patient numbers and in which evaluation was performed without blinding. The only prospective, multicenter study with blinding to assess the effects of pallidal stimulation in these secondary dystonias demonstrated an excellent motor improvement of over 24%.23 In general, the numbers of patients treated in all studies remains small, and the efficacy of neurostimulation, in either primary or secondary dystonias, will need to be further elucidated in larger, prospective, multicenter studies.
Operating on children with status dystonicus is more challenging than treating adult patients with movement disorders and may be associated with a higher risk of complications. Nonetheless, all 3 children in our cases were ultimately weaned from their medically induced coma and mechanical ventilation and were able to go home. Moreover, while unfortunate, the infection in Case 2 and lead failure in Case 1 provided an “imposed control,” by means of which we could assess efficacy of the working implanted device. Both patients had global symptoms and initially underwent implantation of devices in both cerebral hemispheres. In such cases, when a device is nonfunctional or removed, the contralateral body acts as an unblinded control. In Cases 1 and 2, the stimulator effect resulted in a marked asymmetry in left and right symptoms, with much better control of movements on the side with the functional stimulator (such that both patients will be undergoing reimplantation in the near future). While the ability to generalize from the experience is limited, given the lack of blinding and positive controls, these observations suggest that DBS was the critical factor in arresting the status dystonicus and that the improvement seen in these patients was not simply a reflection of the natural history of the disorder.
When implanting infraclavicular pulse generators in children, avoiding device prominence and wound tension is critically important due to the small size of these patients and their decreased muscle and soft tissue mass— this is particularly important in patients with cerebral palsy. Both growth potential and persistent abnormal movements must be accommodated when placing stimulators. We now use a subfascial implant location in the rectus abdominis, which greatly increases soft tissue coverage over the implant.
It is important to note that despite best efforts DBS implantation is, overall, associated with a hardware complication rate of approximately 15%, including electrode breakage, electrode migration, stimulator migration, erosion and infection, and stimulator malfunction.5 The incidence of DBS complications in pediatric patients with status dystonicus may be even higher considering aspects of soft tissue coverage, suboptimal nutritional status, and prolonged mechanical ventilation inherent in this population.
Conclusions
The lack of a treatment control group and an incomplete understanding of the natural history and pathophysiology of status dystonicus limit this study, but these observations suggest that urgent DBS maybe a life-saving option in these patients. Future investigations will further define the role of DBS in the treatment of these critically ill children.
Abbreviation used in this paper
- DBS
deep brain stimulation.
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: Walcott, Sharma, Eskandar. Acquisition of data: Sharma, Eskandar. Analysis and interpretation of data: all authors. Drafting the article: all authors. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Walcott. Study supervision: Eskandar.
References
- 1.Apetauerova D, Schirmer CM, Shils JL, Zani J, Arle JE. Successful bilateral deep brain stimulation of the globus pallidus internus for persistent status dystonicus and generalized chorea. Report of 2 cases. J Neurosurg. 2010;113:634–638. doi: 10.3171/2010.1.JNS091127. [DOI] [PubMed] [Google Scholar]
- 2.Balas I, Kovacs N, Hollody K. Staged bilateral stereotactic pallidothalamotomy for life-threatening dystonia in a child with Hallervorden-Spatz disease. Mov Disord. 2006;21:82–85. doi: 10.1002/mds.20655. [DOI] [PubMed] [Google Scholar]
- 3.Barach E, Dubin LM, Tomlanovich MC, Kottamasu S. Dystonia presenting as upper airway obstruction. J Emerg Med. 1989;7:237–240. doi: 10.1016/0736-4679(89)90352-1. [DOI] [PubMed] [Google Scholar]
- 4.Bittar RG, Yianni J, Wang S, Liu X, Nandi D, Joint C, et al. Deep brain stimulation for generalised dystonia and spasmodic torticollis. J Clin Neurosci. 2005;12:12–16. doi: 10.1016/j.jocn.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Blomstedt P, Hariz MI. Hardware-related complications of deep brain stimulation: a ten year experience. Acta Neurochir (Wien) 2005;147:1061–1064. doi: 10.1007/s00701-005-0576-5. [DOI] [PubMed] [Google Scholar]
- 6.Cif L, El Fertit H, Vayssiere N, Hemm S, Hardouin E, Gannau A, et al. Treatment of dystonic syndromes by chronic electrical stimulation of the internal globus pallidus. J Neurosurg Sci. 2003;47:52–55. [PubMed] [Google Scholar]
- 7.Coubes P, Vayssiere N, El Fertit H, Hemm S, Cif L, Kienlen J, et al. Deep brain stimulation for dystonia. Surgical technique. Stereotact Funct Neurosurg. 2002;78:183–191. doi: 10.1159/000068962. [DOI] [PubMed] [Google Scholar]
- 8.Eltahawy HA, Saint-Cyr J, Giladi N, Lang AE, Lozano AM. Primary dystonia is more responsive than secondary dystonia to pallidal interventions: outcome after pallidotomy or pallidal deep brain stimulation. Neurosurgery. 2004;54:613–621. doi: 10.1227/01.neu.0000108643.94730.21. [DOI] [PubMed] [Google Scholar]
- 9.Giménez-Roldán S, Mateo D, Martín M. Life-threatening cranial dystonia following trihexyphenidyl withdrawal. Mov Disord. 1989;4:349–353. doi: 10.1002/mds.870040411. [DOI] [PubMed] [Google Scholar]
- 10.Jech R, Bares M, Urgosík D, Cerná O, Klement P, Adamovicová M, et al. Deep brain stimulation in acute management of status dystonicus. Mov Disord. 2009;24:2291–2292. doi: 10.1002/mds.22764. [DOI] [PubMed] [Google Scholar]
- 11.Justesen CR, Penn RD, Kroin JS, Egel RT. Stereotactic pallidotomy in a child with Hallervorden-Spatz disease. Case report. J Neurosurg. 1999;90:551–554. doi: 10.3171/jns.1999.90.3.0551. [DOI] [PubMed] [Google Scholar]
- 12.Kyriagis M, Grattan-Smith P, Scheinberg A, Teo C, Nakaji N, Waugh M. Status dystonicus and Hallervorden-Spatz disease: treatment with intrathecal baclofen and pallidotomy. J Paediatr Child Health. 2004;40:322–325. doi: 10.1111/j.1440-1754.2004.00374.x. [DOI] [PubMed] [Google Scholar]
- 13.Manji H, Howard RS, Miller DH, Hirsch NP, Carr L, Bhatia K, et al. Status dystonicus: the syndrome and its management. Brain. 1998;121:243–252. doi: 10.1093/brain/121.2.243. (Erratum in Brain 123:419, 2000) [DOI] [PubMed] [Google Scholar]
- 14.Mariotti P, Fasano A, Contarino MF, Della Marca G, Piastra M, Genovese O, et al. Management of status dystonicus: our experience and review of the literature. Mov Disord. 2007;22:963–968. doi: 10.1002/mds.21471. [DOI] [PubMed] [Google Scholar]
- 15.Newton-John H. Acute upper airway obstruction due to supra-glottic dystonia induced by a neuroleptic. BMJ. 1988;297:964–965. doi: 10.1136/bmj.297.6654.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poels PJ, Gabreëls FJ. Rhabdomyolysis: a review of the literature. Clin Neurol Neurosurg. 1993;95:175–192. doi: 10.1016/0303-8467(93)90122-w. [DOI] [PubMed] [Google Scholar]
- 17.Rowley H, Lynch T, Keogh I, Russell J. Tardive dystonia of the larynx in a quadriplegic patient: an unusual cause of stridor. J Laryngol Otol. 2001;115:918–919. doi: 10.1258/0022215011909350. [DOI] [PubMed] [Google Scholar]
- 18.Tagliati M, Krack P, Volkmann J, Aziz T, Krauss JK, Kupsch A, et al. Long-Term management of DBS in dystonia: response to stimulation, adverse events, battery changes, and special considerations. Mov Disord. 2011;26(Suppl 1):S54–S62. doi: 10.1002/mds.23535. [DOI] [PubMed] [Google Scholar]
- 19.Teive HA, Munhoz RP, Souza MM, Antoniuk SA, Santos ML, Teixeira MJ, et al. Status dystonicus: study of five cases. Arq Neuropsiquiatr. 2005;63:26–29. doi: 10.1590/s0004-282x2005000100005. [DOI] [PubMed] [Google Scholar]
- 20.Umemura A, Jaggi JL, Dolinskas CA, Stern MB, Baltuch GH. Pallidal deep brain stimulation for longstanding severe generalized dystonia in Hallervorden-Spatz syndrome. Case report. J Neurosurg. 2004;100:706–709. doi: 10.3171/jns.2004.100.4.0706. [DOI] [PubMed] [Google Scholar]
- 21.Vercueil L, Pollak P, Fraix V, Caputo E, Moro E, Benazzouz A, et al. Deep brain stimulation in the treatment of severe dystonia. J Neurol. 2001;248:695–700. doi: 10.1007/s004150170116. [DOI] [PubMed] [Google Scholar]
- 22.Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Lagrange C, Yelnik J, et al. Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol. 2007;6:223–229. doi: 10.1016/S1474-4422(07)70035-2. [DOI] [PubMed] [Google Scholar]
- 23.Vidailhet M, Yelnik J, Lagrange C, Fraix V, Grabli D, Thobois S, et al. Bilateral pallidal deep brain stimulation for the treatment of patients with dystonia-choreoathetosis cerebral palsy: a prospective pilot study. Lancet Neurol. 2009;8:709–717. doi: 10.1016/S1474-4422(09)70151-6. [DOI] [PubMed] [Google Scholar]
- 24.Zorzi G, Marras C, Nardocci N, Franzini A, Chiapparini L, Maccagnano E, et al. Stimulation of the globus pallidus internus for childhood-onset dystonia. Mov Disord. 2005;20:1194–1200. doi: 10.1002/mds.20510. [DOI] [PubMed] [Google Scholar]