Abstract
Aim
We have shown hypertrophic cerebrovascular remodeling in the Goto-Kakizaki (GK) rat model of diabetes. This study tested the hypotheses that 1) vascular remodeling develops as the disease progresses and alters myogenic reactivity of resistance vessels important for regulation of cerebral blood flow, and 2) glycemic control prevents cerebrovascular remodeling and myogenic dysfunction.
Methods
Middle cerebral artery lumen diameter, media:lumen (M:L) ratio, cross-sectional area (CSA) and myogenic tone were measured in 10- and 18-week-old control Wistar and diabetic GK rats using pressurized arteriograph (n=8–14/group). Mean arterial blood pressure (MAP) was measured with telemetry (n=5/group). Additional GK rats were treated with metformin (300 mg/kg/day) for glycemic control starting at 7 weeks after the onset of diabetes until 18 weeks (n=9).
Results
In the control group, there was no difference in remodeling indices, myogenic tone, or MAP between ages. 18-week diabetic rats displayed increased M:L ratio and CSA but decreased lumen diameter and myogenic tone compared to 10-week GK or 18-week control rats. MAP increased starting around 10 weeks of age and remained slightly higher in the GK rats. Glycemic control normalized M:L ratio, CSA, lumen diameter and myogenic tone with no effect on blood pressure.
Conclusions
These findings suggest that diabetic rats develop middle cerebral artery (MCA) remodeling as the disease progresses but this is associated with impaired myogenic reactivity which may ultimately affect cerebral blood flow. Our results also provide evidence that glycemic control is an effective therapeutic strategy to prevent cerebrovascular remodeling and dysfunction.
Keywords: Diabetes, middle cerebral artery, myogenic tone, vascular remodeling
Introduction
Diabetes is a rapidly growing global problem that is associated with increased morbidity and mortality mainly due to cardiovascular complications such as stroke (American Diabetes Association, American Heart Association). Cerebral perfusion is related to the fourth power of vessel radius (Paulson et al., 1990); consequently even a small decrease in lumen diameter may disrupt perfusion under normal conditions and more so in ischemia/reperfusion injury. Therefore, a better understanding of diabetes-induced changes in vascular function and structure that ultimately affect lumen diameter is essential for developing better vascular protection strategies before and after a stroke.
Vascular smooth muscle (VSM) myogenic reactivity significantly contributes to autoregulation which is critical for cerebral perfusion (Johansson, 1989, Cipolla et al., 1997a, Coulson et al., 2004, Cipolla et al., 2004, Cipolla et al., 1997b). Both contractile and dilatory aspects of myogenic regulation are important since an increased tone would decrease vessel size and impair CBF whereas loss of myogenic reactivity would result in increased and unregulated intravascular pressure on distal capillary beds, thereby exacerbating blood brain barrier (BBB) disruption and edema and interrupting CBF (Faraci and Heistad, 1990, Tamaki et al., 1984, Heistad, 1984, Koudstaal et al., 1988). This is especially important in acute ischemic injury as the outcome of an ischemic stroke is directly affected by the structure and function of the cerebral vasculature. An earlier study showed that isolated middle cerebral arteries (MCAs) lose myogenic tone when exposed to acute hyperglycemia (Cipolla et al., 1997a). Zimmermann et al. showed a constriction of MCAs in type 1 diabetic rats (Zimmermann et al., 1997) Another study reported enhanced posterior cerebral artery tone in an obese model of diabetes with very high glucose levels (Jarajapu et al., 2008). However, the temporal regulation of cerebrovascular myogenic reactivity and tone and structure in a type 2 diabetes model that mimic blood glucose levels seen in stroke patients are yet to be determined.
Lumen diameter is an important determinant of vascular structure. Since MCAs are commonly affected vessels in human stroke, we previously studied MCA structure in GK rats, a lean and mild model of diabetes. GK rats spontaneously develop hyperglycaemia and insulin resistance around 6 weeks of age with blood glucose ranges between 150–200 mg/dl which closely resembles the range observed in most stroke patients (Johnston et al., 2009, Bruno et al., 2008, Gray et al., 2007). We reported that by 18 weeks of age, GK rats develop hypertrophic remodeling of MCAs with increased wall thickness and wall:lumen (W:L) ratio that is associated with increased matrix metalloprotease (MMP) activity (Harris et al., 2005). Our results also showed that endothelin-1 (ET) antagonism prevents this remodeling response. The important questions related to temporal regulation of MCA structure and relationship to myogenic properties of these vessels remained unanswered. Accordingly, the goals of this study were to test the hypotheses that 1) vascular remodeling develops as the disease progresses and alters myogenic reactivity of resistance vessels important for regulation of cerebral blood flow, and 2) glycemic control prevents cerebrovascular remodeling and myogenic dysfunction.
Materials and Methods
Animals
The Institutional Animal Care and Use Committee (IACUC) of the Medical College of Georgia approved all protocols used in animal work. All experiments were performed on male control Wistar (Harlan, Indianapolis, IN) and diabetic GK (either in-house bred, derived from the Tampa colony or purchased from Taconic Laboratories in Hudson, NY) rats (Standaert et al., 2004). Metformin was titrated to maintain euglycemia in GK rats (~300 mg/kg/day based on blood glucose levels) and was given in drinking water artificially sweetened by non-caloric sweetener starting with the onset of diabetes in GK rats (5–6 weeks of age) until the animals reached 18 weeks. Blood glucose levels were measured from tail vein blood using a glucometer (Freestyle, Alameda, CA). Blood pressure was monitored by telemetry during weeks 6–12 or in a separate set of animals during weeks 14–18 (Elgebaly et al., 2007). At sacrifice, the animals were anesthetized with sodium pentobarbital, ex-sanguinated via cardiac puncture.
Evaluation of MCA vascular structure and myogenic tone
MCAs collected immediately following decapitation were mounted on the pressurized arteriograph (Living Systems Instrumentation, Burlington, VT) to measure wall thickness, as well as lumen and outer diameters with a video dimension analyzer at different pressures ranging from 5–180 mmHg at 20 mmHg pressure increments. Measurements were made 10 min after pressure increase. The system was equilibrated in calcium-free Krebs-HEPES buffer to obtain measurements under passive conditions. Myogenic reactivity was defined as the ability of the vessels to constrict in response to increasing pressure and myogenic tone was defined as the sustained state of contraction in a muscle at a given pressure. Media thickness, lumen and outer diameters, and vessel cross-sectional areas were determined as follows: Media Thickness (MT, µm) = Outer diameter - lumen diameter (i.e., OD - LD); Media/Lumen (M:L) ratio = MT/LD; Cross-Sectional Area (µm2) = Outer vessel area - lumen area. Myogenic tone was calculated as follows: Myogenic Tone (% tone) = (1 - (ODactive/ODpassive)) × 100 (Rigsby et al., 2007).
Vascular structure was also assessed by morphometric analysis of Masson trichrome-stained MCA cross-sections. Vessels were fixed in formalin using the quick-transfer freezing chamber (Living Systems Instrumentation, Burlington, VT), wherein, they were maintained at a constant intraluminal pressure in calcium-free Krebs-HEPES buffer (80 mmHg for 30 min) and frozen immediately. This procedure corrected for variations in vascular structure due to inconsistencies in manual perfusion. Images were captured and wall thickness, lumen and outer diameter measured from Masson-stained cross-sections using SPOT software (Diagnostic Instruments, MI).
Data analysis
Since control rats were not given metformin, the analysis for the effect of metformin on remodeling indices and tone at 80 mmHg pressure in GK rats was performed using a one-way ANOVA (control, GK vehicle, and GK metformin) with post-hoc Tukey test. The analysis for the effects of aging was performed in a 2 Disease (Wistar vs. GK) × 2 Age (10w vs. 18 w) design. Bonferroni correction was used to adjust for multiple comparisons when determining mean differences for significant ANOVA effects. An interaction between disease and treatment would indicate a differential effect of age that is dependent on disease status. Graphpad Prism 5.0 was used for these analyses (Graphpad software, San Diego, CA). Significance was considered at p<0.05. All results are reported as unadjusted mean ± SEM.
Results
Metabolic parameters
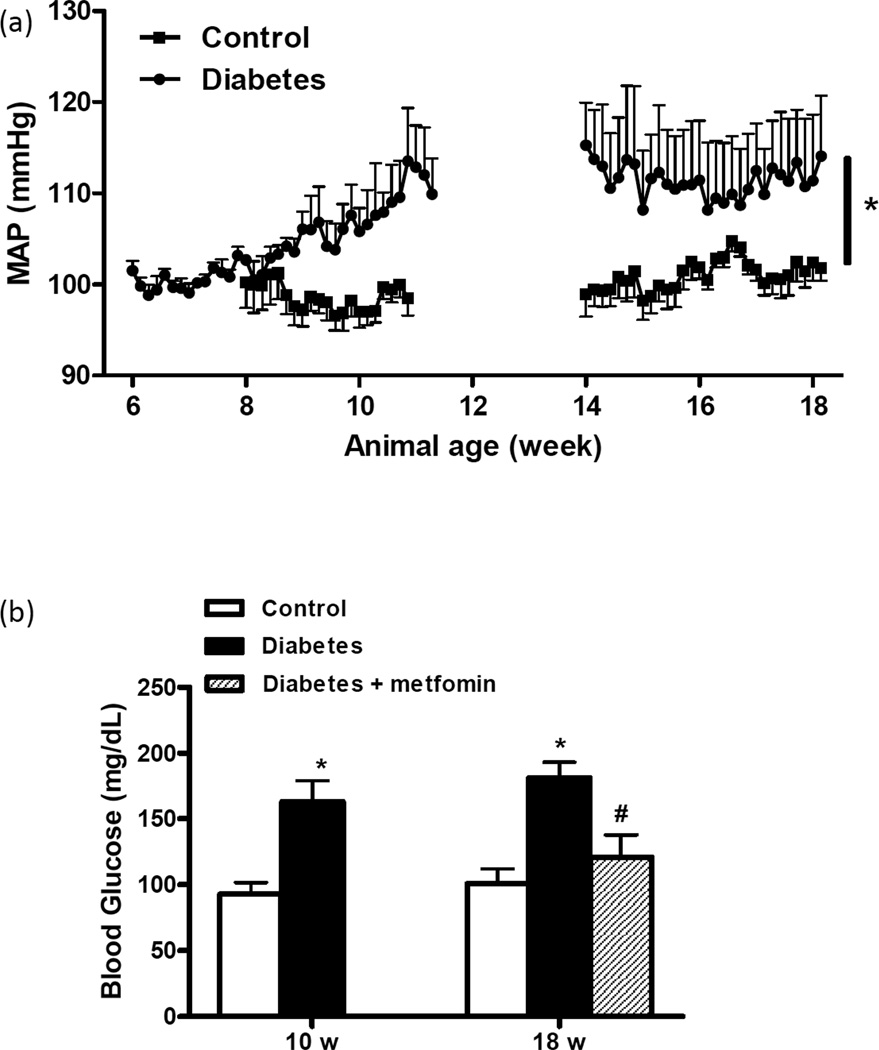
Blood pressure was continuously monitored during weeks 6–12 or 14–18 and MAP over time is shown in Fig. 1A. Starting around 9 weeks, MAP started increasing in diabetic rats, reached a plateau at 12 weeks and remained stable thereafter. Blood pressure was steady in control rats. Average systolic and diastolic pressures for 10 and 18 w animals are shown in Table 1. Metformin treatment did not have an effect on blood pressure in diabetic rats (Table 1). Blood glucose was significantly higher in GK rats at 10 or 18 weeks and metformin treatment normalized blood glucose in 18 w group (Fig. 1B).
Figure 1.
(a) Mean arterial blood pressure (MAP) was monitored by telemetry in two sets of control and diabetic animals between the ages 6–12 and 14–18 weeks. (b) Blood glucose levels were measured in control and diabetic GK rats weekly starting at 6 weeks and average glucose level over the study period is reported. Mean ± SEM, n=5 for MAP and n=7–14 for blood glucose measurements, *p<0.05 vs control, p<0.05 vs 18-w diabetes.
Table 1.
Blood pressure in control and diabetic animals at 10 and 18 weeks.
| 10 w | 18 w | ||||
|---|---|---|---|---|---|
| Control | Diabetes | Control | Diabetes | Diabetes + Metformin |
|
| Systolic BP | 118.7 ± 1.3 | 126.4 ± 2.2* | 120.7 ± 1.7 | 132.3 ± 5.7* | 127.9 ± 3.3 |
| Diastolic BP | 81.2 ± 2.3 | 84.1 ± 1.1 | 84.9 ± 2.0 | 89.8 ± 6.3 | 90.8 ± 2.3 |
p<0.05 vs control
Myogenic reactivity and tone
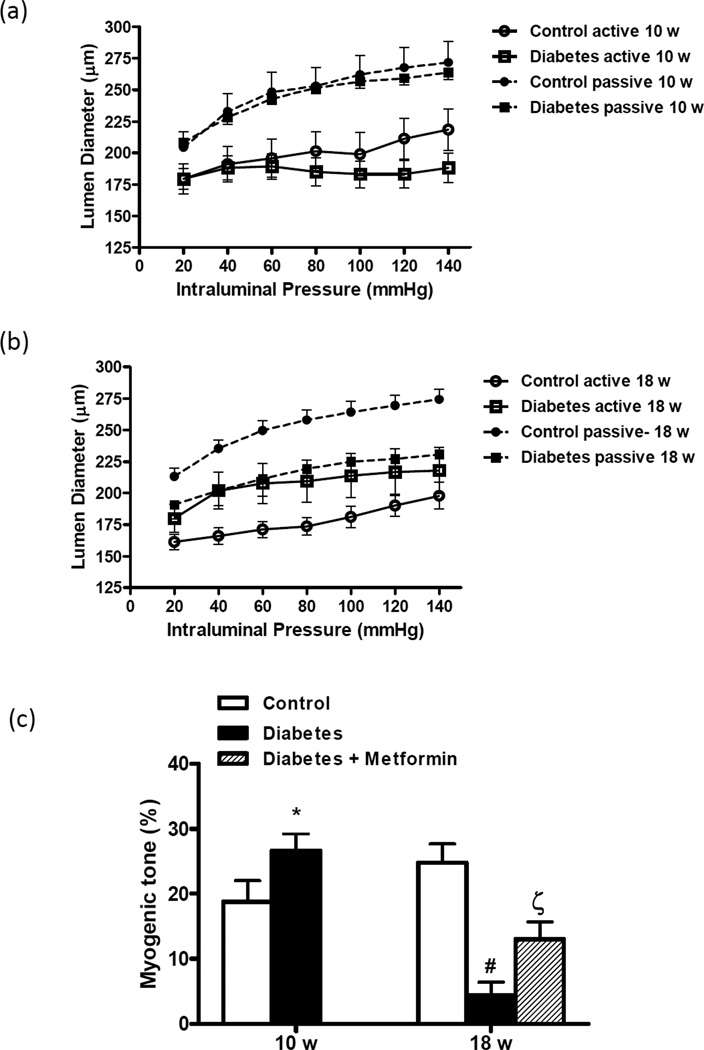
Myogenic reactivity was preserved in both control and diabetic rats at 10 weeks (Fig 2A and B). However, diabetic rats at 10 weeks were able to respond to pressure up to 140 mmHg whereas control rats showed passive dilatation after 100 mmHg. In 18-week animals, control vessels were able to constrict to increasing pressure but the active lumen diameter in GK rats was similar to those obtained under passive conditions indicating that myogenic reactivity is blunted and vessels are not able to generate tone. Indeed, % myogenic tone was significantly lower in 18-week old diabetic animals than in controls (Fig 2C).
Figure 2.
Effect of age and diabetes on myogenic reactivity of isolated MCAs over the pressure range. (a) Vessels from 10-week-old animals constricted in response to increasing intraluminal pressure displaying normal myogenic reactivity and under passive conditions, there was full relaxation with no difference between control and diabetic animals. (b) While 18-week control animals maintained this ability, diabetic animals showed impaired pressure-induced reactivity. (c) There was a disease and age interaction that MCA myogenic tone at 80 mmHg was increased in 10-week diabetic animals but significantly reduced at 18 weeks. Metformin treatment improved tone development. Mean ± SEM, n=7–14, * p<0.05 vs control, **p<0.001 vs 10-week diabetes, ***p<0.05 vs diabetes.
Vascular remodeling indices
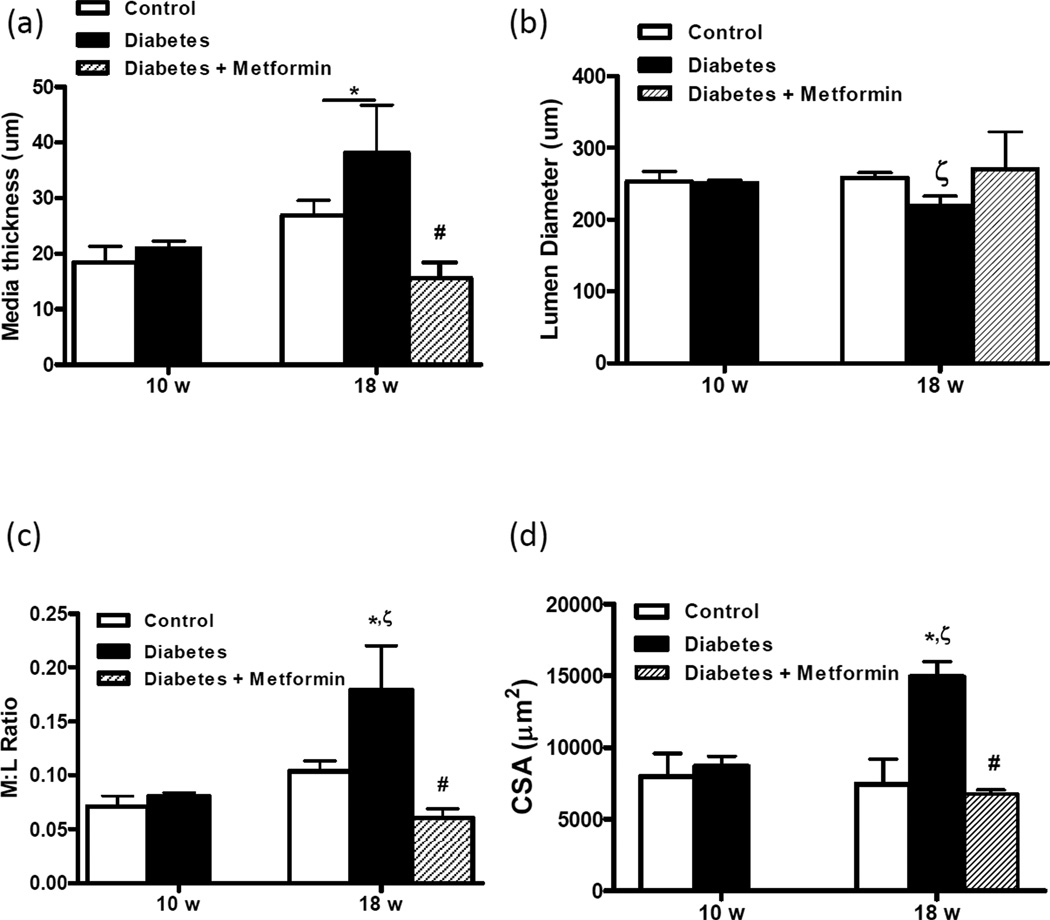
Vessel wall and lumen diameter thickness were measured by pressurized arteriography under passive conditions—which allows evaluation of these indices when arteries are fully relaxed—as well as by morphometry of vessels fixed at 80 mmHg pressure. Arteriograph results indicated that wall thickness increased in both control and diabetic rats with age (Fig 3A) but lumen diameter (Fig 3B) got smaller, rendering an increased M:L ratio (Fig 3C) in diabetes. CSA was significantly increased in 18-week-old diabetic rats as compared to 18-week controls or 10-week-old diabetic rats (Fig 3D). Media thickness and M:L ratio calculated from fixed MCA sections yielded similar results to those obtained with arteriograph (not shown).
Figure 3.
Diabetic rats develop vascular remodeling over time. Wall thickness (a), lumen diameter (b), W:L ratio (c) and CSA (d) were measured in MCAs under passive conditions in the fully dilated state. While there was no difference in these structural indices between control and diabetic rats at 10 weeks, by 18 weeks all indices were increased in diabetes. Metformin treatment starting at the onset of diabetes prevented vascular remodeling. Mean ± SEM, n=7–14, *p<0.05 vs 10-week control, **p<0.05 vs 18-week diabetes, *** p<0.05 vs 18-week control,
Effect of glycemic control
Treatment of diabetic rats with oral metformin starting at the onset of diabetes prevented myogenic dysfunction in diabetic rats and restored myogenic tone to control levels in 18-week-old animals (Fig 2B). Chronic glycemic control also prevented vascular remodeling in diabetes (Figs 3A–D).
Discussion
The current study was designed to address the following important questions: 1) What are the structural and functional changes that take place over time in cerebral blood vessels from control and diabetic animals?, 2) Are changes in myogenic reactivity associated with structural alterations?, and 3) Does glycemic control prevent myogenic dysfunction and remodeling of cerebral arteries? Our results demonstrate that diabetes increases myogenic tone of MCAs in the absence of vascular remodeling at 10 weeks. As animals age, there is significant vascular remodeling characterized by increased M:L ratio and CSA in diabetic animals as compared to their age-matched controls. However, this remodeling is associated with impaired myogenic reactivity and decreased tone. Glycemic control with metformin prevents remodeling and improves myogenic function.
We have previously reported that 18-week-old diabetic GK rats have thicker walls and extracellular matrix deposition in MCAs as compared to age-matched controls (Harris et al., 2005). These differences were associated with differential regulation of MMPs. ETA receptor antagonism started at 12 weeks after the onset of diabetes prevented this effect suggesting that vascular remodeling develops as a result of diabetes and not inherent to the GK model. However, the temporal progression of remodeling and whether glycemic control prevents these vascular changes remained unknown. The current study provides evidence that at 10 weeks, shortly after the onset of diabetes in GK rats, there was no difference in MCA lumen and wall size between control and diabetic animals. Over a time of 8 weeks, these indices remained the same in control animals. Diabetic animals, on the other hand, showed increased wall thickness and decreased lumen diameter resulting in enhanced M:L ratio and CSA confirming the development of hypertrophic remodeling in our model. Numerous studies have reported that increased blood pressure negatively influences cerebrovasculature and causes eutrophic vascular remodeling (Faraci and Heistad, 1990, Rigsby et al., 2007). GK rats are not hypertensive per se but start developing slightly higher blood pressure beginning around 9–10 weeks of age. In our previous study we found that chronic ETA receptor antagonism given during 14–18 weeks prevented remodeling. This treatment also lowered the blood pressure and therefore whether the prevention of remodeling was due to lower blood pressure or direct effect of ET-1 on vasculature was unclear. In the present study we provide evidence that metformin treatment prevented the remodeling in the absence of any changes in blood pressure suggesting that these vascular responses are independent of slightly higher pressures in GK rats.
Cerebral circulation is able to regulate blood flow within a wide pressure range to maintain the nutrient and oxygen supply to the brain. The myogenic reactivity of VSMC contributes to this autoregulatory capacity (Cipolla et al., 1997a, Coulson et al., 2004, Coulson et al., 2002). A previous study reported that a failure of cerebral blood vessels to remodel in response to increased pressure contributes to development of eclampsia in pregnancy suggesting that remodeling may be an adaptive and not a pathological response to regulate cerebrovascular pressure. Therefore, we wanted to study the effects of diabetes on this important functional feature of cerebral vessels. Previous studies reported different myogenic reactivity in different diabetes models. An earlier study showed decreased MCA tone when isolated vessels were exposed to acute hyperglycemia ex vivo (Cipolla et al., 1997b). Zimmermann et al reported increased MCA tone in type 1 diabetes (Zimmermann et al., 1997). A more recent study investigated the time-course of cerebrovascular dysfunction and showed increased tone in the obese BBZDR/Wor model of type 2 diabetes with relatively high (>450 mg/dl) glucose levels (Jarajapu et al., 2008). Since cerebrovascular changes may be very relevant to increased risk and poor outcome of stroke, the current study used a type 2 model of diabetes that closely resembles the blood glucose levels observed in most stroke patients (Johnston et al., 2009, Bruno et al., 2008, Gray et al., 2007). In the current study, the myogenic reactivity across the normal pressure range (40–120 mmHg) was extended up to 140 mmHg in 10-week-old diabetic rats whereas control rats showed forced dilatation after 100 mmHg. This resulted in a greater tone in diabetic animals. Interestingly, at 18 weeks, diabetic rats lost the ability to autoregulate and developed a much lower tone when compared to 10-week animals or 18-week controls. Taken together with the vascular remodeling studies, these findings suggest that as the MCAs remodel and matrix deposition occurs in diabetes, VSMCs lose the ability to generate tone. While underlying mechanisms of these differential responses in younger vs older diabetic animals remain to be determined, it is a likely possibility that longer duration of hyperglycemia modifies contractile proteins and oxidative stress may play an important role in this response.
Glycemic control in diabetes is believed to be an important strategy to prevent and/or reduce micro and macrovascular complications of diabetes. It is well established that early and good glycemic control reduces the microvascular complications in both types of diabetes. On the other hand, the relation between glycemic control and macrovascular events prevention has been only proven in type 1 diabetes (Akalin et al., 2009). The most recent major prospective randomized controlled clinical trial ACCORD was prematurely stopped due to the increased macrovascular events, and there is not a definitive evidence for the effect of glycemic control on cardiovascular outcomes in type 2 diabetes. However, the latest consensus statement by the American Diabetes Association highlights that early glycemic control is associated with long term reduction in macrovascular events and supports the overall benefits of glycemic control in reducing vascular events in addition to the importance of controlling other comorbidities (Skyler et al., 2009). We have previously shown that glycemic control prevents microvascular remodeling in mesenteric bed (Sachidanandam et al., 2009). The current study extends these findings to show that glycemic control prevents MCA remodeling and myogenic dysfunction independent of changes in blood pressure.
In conclusion, cerebrovascular remodeling develops over time in diabetes and is associated with myogenic dysfunction. It appears that slightly higher blood pressure is not responsible for this remodeling. Glycemic control not only prevents structural changes but also improves myogenic function. Collectively, this study emphasizes the importance of studying vascular complications of diabetes at various time points during the disease course.
Acknowledgments
This study was supported by NIH R01DK074385, NIH ARRA Supplement R01DK074385-05S1, American Heart Association Established Investigator Award (0740002N), and Department of Veterans Affairs Merit Awards to Adviye Ergul.
Footnotes
Conflict of interest
The authors have no conflict of interest for this study.
References
- Akalin S, Berntorp K, Ceriello A, Das AK, Kilpatrick ES, Koblik T, Munichoodappa CS, Pan CY, Rosenthall W, Shestakova M, Wolnik B, Woo V, Yang WY, Yilmaz MT. Intensive glucose therapy and clinical implications of recent data: a consensus statement from the Global Task Force on Glycemic Control. Int J Clin Pract. 2009;63:1421–1425. doi: 10.1111/j.1742-1241.2009.02165.x. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. National diabetes fact sheet. 2010 http://www.diabetes.org/diabetes-statistics.jsp.
- American Heart Association. Heart and stroke statistical update. 2010 www.americanheart.org.
- Bruno A, Kent TA, Coull BM, Shankar RR, Saha C, Becker KJ, Kissela BM, Williams LS. Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke. 2008;39:384–389. doi: 10.1161/STROKEAHA.107.493544. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Li R, Vitullo L. Perivascular innervation of penetrating brain parenchymal arterioles. J Cardiovasc Pharmacol. 2004;44:1–8. doi: 10.1097/00005344-200407000-00001. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, McCall AL, Lessov N, Porter JM. Reperfusion decreases myogenic reactivity and alters middle cerebral artery function after focal cerebral ischemia in rats. Stroke. 1997a;28:176–180. doi: 10.1161/01.str.28.1.176. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Porter JM, Osol G. High glucose concentrations dilate cerebral arteries and diminish myogenic tone through an endothelial mechanism. Stroke. 1997b;28:405–410. doi: 10.1161/01.str.28.2.405. discussion 410-1. [DOI] [PubMed] [Google Scholar]
- Coulson RJ, Chesler NC, Vitullo L, Cipolla MJ. Effects of ischemia and myogenic activity on active and passive mechanical properties of rat cerebral arteries. Am J Physiol. 2002;283:H2268–H2275. doi: 10.1152/ajpheart.00542.2002. [DOI] [PubMed] [Google Scholar]
- Coulson RJ, Cipolla MJ, Vitullo L, Chesler NC. Mechanical properties of rat middle cerebral arteries with and without myogenic tone. J Biomech Eng. 2004;126:76–81. doi: 10.1115/1.1645525. [DOI] [PubMed] [Google Scholar]
- Elgebaly MM, Portik-Dobos V, Sachidanandam K, Rychly D, Malcom D, Johnson MH, Ergul A. Differential effects of ET(A) and ET(B) receptor antagonism on oxidative stress in type 2 diabetes. Vascul Pharmacol. 2007;47:125–130. doi: 10.1016/j.vph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- Gray CS, Hildreth AJ, Sandercock PA, O'Connell JE, Johnston DE, Cartlidge NE, Bamford JM, James OF, Alberti KG. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK) Lancet Neurol. 2007;6:397–406. doi: 10.1016/S1474-4422(07)70080-7. [DOI] [PubMed] [Google Scholar]
- Harris AK, Hutchinson JR, Sachidanandam K, Johnson MH, Dorrance AM, Stepp DW, Fagan SC, Ergul A. Type 2 diabetes causes remodeling of cerebrovasculature via differential regulation of matrix metalloproteinases and collagen synthesis: role of endothelin-1. Diabetes. 2005;54:2638–2644. doi: 10.2337/diabetes.54.9.2638. [DOI] [PubMed] [Google Scholar]
- Heistad DD. Protection of the blood-brain barrier during acute and chronic hypertension. Fed Proc. 1984;43:205–209. [PubMed] [Google Scholar]
- Jarajapu YP, Guberski DL, Grant MB, Knot HJ. Myogenic tone and reactivity of cerebral arteries in type II diabetic BBZDR/Wor rat. Eur J Pharmacol. 2008;579:298–307. doi: 10.1016/j.ejphar.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B. Myogenic tone and reactivity: definitions based on muscle physiology. J Hypertens Suppl. 1989;7:S5–S8. discussion S9. [PubMed] [Google Scholar]
- Johnston KC, Hall CE, Kissela BM, Bleck TP, Conaway MR. Glucose Regulation in Acute Stroke Patients (GRASP) Trial. A Randomized Pilot Trial. Stroke. 2009 doi: 10.1161/STROKEAHA.109.561498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koudstaal PJ, Stibbe J, Vermeulen M. Fatal ischaemic brain oedema after early thrombolysis with tissue plasminogen activator in acute stroke. Bmj. 1988;297:1571–1574. doi: 10.1136/bmj.297.6663.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- Rigsby CS, Pollock DM, Dorrance AM. Spironolactone improves structure and increases tone in the cerebral vasculature of male spontaneously hypertensive stroke-prone rats. Microvasc Res. 2007;73:198–205. doi: 10.1016/j.mvr.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachidanandam K, Hutchinson JR, Elgebaly MM, Mezzetti EM, Dorrance AM, Motamed K, Ergul A. Glycemic Control Prevents Microvascular Remodeling and Increased Tone in Type 2 Diabetes: Link to Endothelin-1. Am J Physiol. 2009;296:R952–R959. doi: 10.1152/ajpregu.90537.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, Howard BV, Kirkman MS, Kosiborod M, Reaven P, Sherwin RS. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32:187–192. doi: 10.2337/dc08-9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert ML, Sajan MP, Miura A, Kanoh Y, Chen HC, Farese RV, Jr, Farese RV. Insulin-induced activation of atypical protein kinase C, but not protein kinase B, is maintained in diabetic (ob/ob and Goto-Kakazaki) liver. Contrasting insulin signaling patterns in liver versus muscle define phenotypes of type 2 diabetic and high fat-induced insulin-resistant states. J Biol Chem. 2004;279:24929–24934. doi: 10.1074/jbc.M402440200. [DOI] [PubMed] [Google Scholar]
- Tamaki K, Sadoshima S, Baumbach GL, Iadecola C, Reis DJ, Heistad DD. Evidence that disruption of the blood-brain barrier precedes reduction in cerebral blood flow in hypertensive encephalopathy. Hypertension. 1984;6:I75–I81. doi: 10.1161/01.hyp.6.2_pt_2.i75. [DOI] [PubMed] [Google Scholar]
- Zimmermann PA, Knot HJ, Stevenson AS, Nelson MT. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ Res. 1997;81:996–1004. doi: 10.1161/01.res.81.6.996. [DOI] [PubMed] [Google Scholar]