Abstract
We determined whether the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) N-(3-chlorophenyl)-6,7-dimethoxy-4-quinazolinamine (tyrphostin AG-1478) causes hypomagnesemia and cardiac dysfunction in rats. Tyrphostin was administered (3 times per week, intraperitoneal injection, to achieve 21.4 mg·(kg body mass)−1·day−1) to normomagnesemic rats for 5 weeks. Levels of magnesium in the plasma of the tyrphostin-treated rats decreased significantly by the following amount: 17% at week 1, 27% at week 2, and 26%–35% between weeks 3 to 5. Levels of the plasma lipid peroxidation marker 8-isoprostane rose significantly: by 58% at week 1, 168% at week 3, and 113% at week 5. At week 5, blood neutrophils from the tyrphostin-treated group displayed a 2.26-fold higher basal level of generation; the ratio of oxidized glutathione (glutathione disufide; GSSG) to reduced glutathione (GSH) in the red blood cells increased 2.5-fold. At week 5, echocardiography revealed that TKI treatment resulted in significant cardiac systolic dysfunction, with impaired diastolic function and dilated cardiomyopathy. Since hypomagnesemia alone can trigger oxidative stress and cardiac injury, we suggest that inhibition of EGFR–TK caused magnesium wasting, which partly contributed to decreased cardiac contractility.
Keywords: EGFR tyrosine kinase inhibitor, hypomagnesemia, cardiac dysfunction, neutrophil activation, oxidative stress
Introduction
It has been well established that the epidermal growth factor (EGF) pathway, through its receptor (EGFR), plays a key role in carcinogenesis (Gill et al. 1984). Multiple clinical trials have shown enhanced survival of cancer patients treated with antibodies to the EGFR (cetuximab and panitumumab) (Tejpar et al. 2007), as well as inhibitors of the tyrosine kinase (TKI) intracellular portion of the EGFR (Jimeno and Hidalgo 2006). Two TKIs, gefitinide and erlotinib, are in common clinical use; they compete with ATP binding to the TK region of the receptor complex (Jimeno and Hidalgo 2006). The TRPM6 (transient receptor potential melastatin 6) channel expressed in the kidney and colon is the rate-limiting gatekeeper for active Mg2+ re-absorption, and its activity is enhanced by EGFR stimulation (Jimeno and Hidalgo 2006; Tejpar et al. 2007). When cetuximab was used to treat metastatic colorectal cancer patients, it was found that 47% of the patients developed grade 3 and 4 hypomagnesemia (40%– 60% lower blood magnesium) after 6 months of therapy (Fakih et al. 2006). Cisplatin, a known magnesium-wasting drug, is often used in combination therapy with EGFR inhibitors, which could lead to even more pronounced hypomagnesemia (Buckley et al. 1984). However, it is unclear whether EGFR-TK inhibition also causes hypomagnesemia and associated cardiotoxicity. The current study examined whether the TKI tyrphostin AG-1478, a relatively specific inhibitor for ErbB1, caused hypomagnesemia with associated systemic oxidative stress and cardiac dysfunction in a rat model.
Materials and methods
Animals, diet, and drug treatments
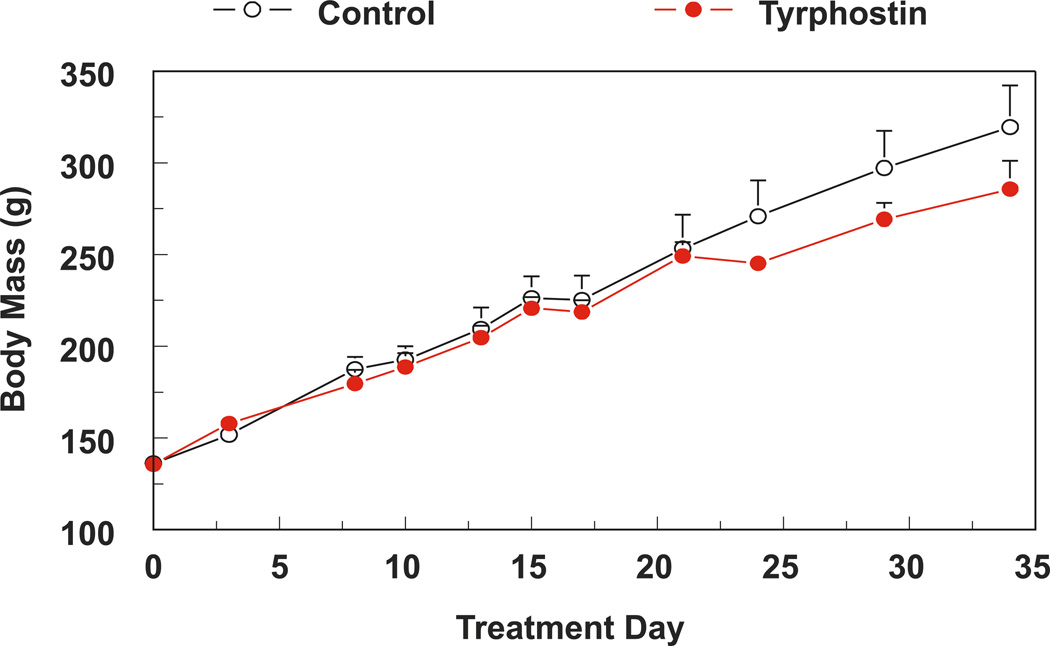
All experiments were guided by the principles of use of laboratory animals recommended by US Department of Health and Human Services and approved by The George Washington University Animal Care and Use Committee. Male Sprague–Dawley rats (200 g) were fed a Mg-sufficient diet (25 mmol magnesium oxide·(kg diet)−1; Harlan Teklad, Madison, Wisconsin, USA). Rats were treated (3 times per week, intraperitoneal injection (i.p.)) with tyrphostin AG-1478 (LC Laboratories, Boston, Massachussetts, USA) to provide a dose of 21.4 mg·(kg body mass)−1·day−1 for up to 5 weeks. In the control group, rats were treated with an equal volume of the vehicle, dimethyl sulfoxide (DMSO). The dose of tyrphostin is comparable with that employed by others (Barrick et al. 2008), and it produced no significant impact on animal mass gain over the entire period of study (Fig. 1).
Fig. 1.
Five weeks of tyrphostin AG-1478 treatment does not significantly alter the rate of animal weight gain. Data are the mean ± SEM of 4–6 rats per group.
Plasma levels of magnesium, calcium, and isoprostane
Blood samples, obtained either by tail bleeding or on the day of sacrifice, were treated with aprotinin (0.016 U·mL−1; proteolysis inhibitor) and heparin (358 U·mL−1), and plasma aliquots were stored at −80 °C. Levels of magnesium and calcium in the plasma were determined by flame emission atomic absorption spectroscopy (Shimadzu AA-6200; Shimadzu, Columbia, Maryland, USA). Levels of 8-isoprostane in the plasma were determined by an enzyme immunoassay (EIA) kit (Cayman Chemical, Michigan, USA) (Mak et al. 2009).
Neutrophil superoxide generation and red blood cell glutathione status
At sacrifice, whole blood was obtained by cardiac puncture. Neutrophils were isolated by a step-gradient centrifugation method (Mak et al. 2009). Superoxide anion production from neutrophils (0.5–0.75 × 106·mL−1) with or without phorbol myristate acetate (PMA; 100 ng·mL−1) was assayed in a phosphate buffer (pH 7.6) containing 5 mmol·L−1 glucose, 1 mmol·L−1 CaCl2, 1 mmol·L−1 MgCl2, and 75 µmol·L−1 cytochrome c ± 50 µg SOD. Superoxide generation was estimated as SOD-inhibitable reduction of cytochrome c using the following extinction coefficient: E550 = 2.1 × 104 mol·cm−1. Red blood cell ((RBC) levels of reduced glutathione (GSH) and oxidized glutathione (glutathione disufide; GSSG) were determined enzymatically by the 5,5′-dithiobis( 2-nitrobenzoic acid)–glutathione disulfide (DTNB–GSSG) reductase method (Mak et al. 2009).
Echocardiography
At week 5, cardiac functional/anatomical parameters were assessed in anesthetized (2% inhaled isoflurane mixed with 100% oxygen) rats using the GE VingMed System Five Echocardiogram with a 10 MHz probe (Kramer et al. 2009).
Statistics
Data were reported as the mean ± SEM of 4–6 animals per group. Statistical differences were evaluated by Student’s t test among the groups.
Results
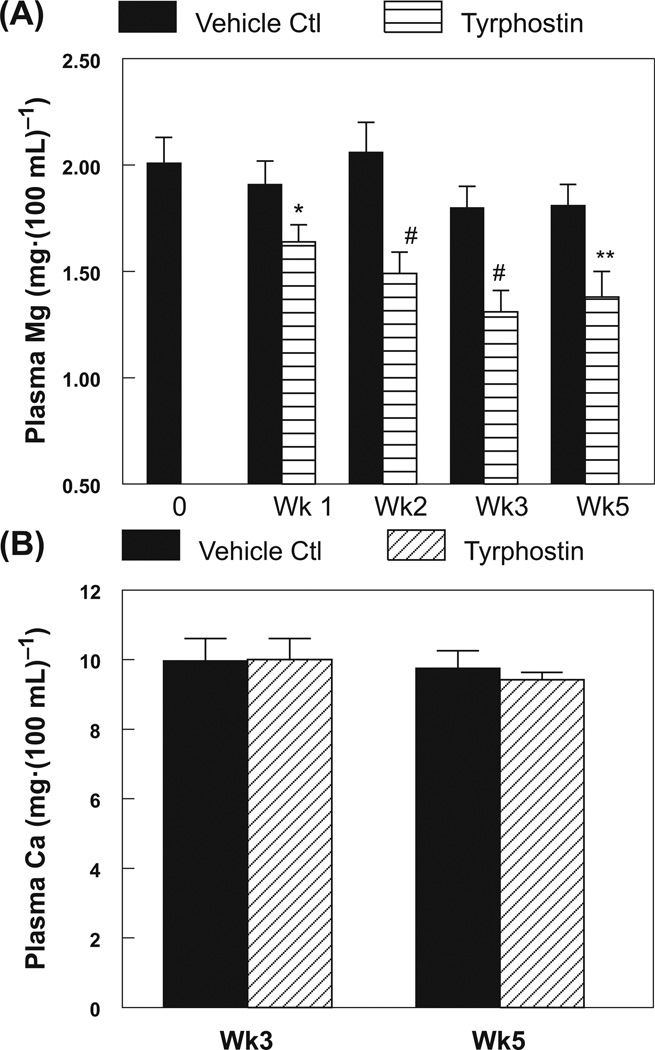
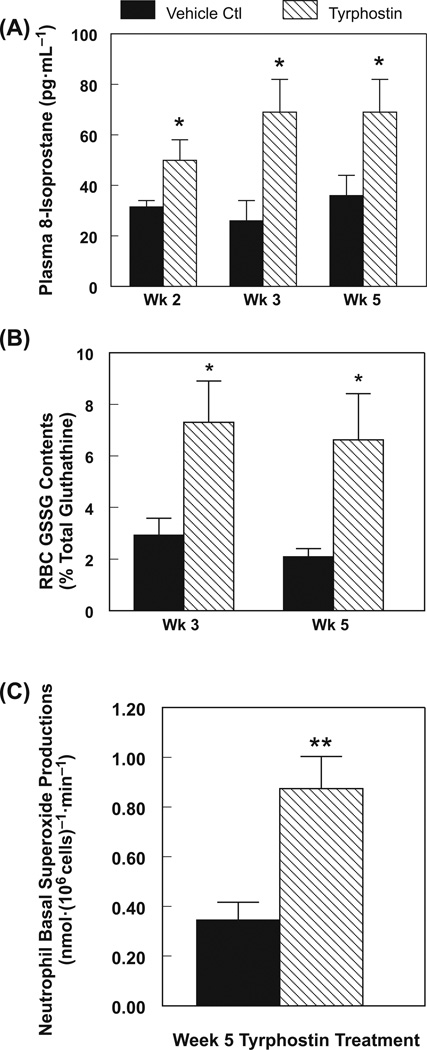
Tyrphostin AG-1478 caused significant hypomagnesemia in rats as early as one week from the start of treatment (17% decrease, p < 0.05), which progressed to moderate severity (26%–35% lower, p < 0.01) with prolonged exposure up to 5 weeks (Fig. 2A). However, TKI had no effect on plasma calcium content for the entire 5 weeks of treatment (Fig. 2B). Significant systemic oxidative stress (p < 0.05), represented by the non-enzymatic lipid peroxidation marker, plasma isoprostane, was evident as early as 2 weeks (58% increase) and worsened with 3–5 weeks of TKI exposure (Fig. 3A: 113%–168% increase); RBC GSSG content rose 2–3-fold in rats treated for 3 and 5 weeks with TKI (Fig. 3B). Neutrophils from the rats treated with TKI for 5 weeks displayed significantly higher (2.26-fold, p < 0.01) basal superoxide anion generating activity (Fig. 3C).
Fig. 2.
Differential effects of tyrphostin AG-1478 treatment on plasma levels of (A) magnesium, and (B) calcium, determined by flame emission atomic absorption spectroscopy. Data are the mean ± SEM of 4–6 animals per group; *, p < 0.05; **, p < 0.01; #, p < 0.001 compared with the time-matched vehicle (DMSO) treated control group.
Fig. 3.
Effects of tyrphostin AG-1478 treatment for 5 weeks on rat (A) plasma 8-isoprostane content, (B) glutathione status in the red blood cells (RBC); and (C) neutrophil basal superoxide generating activity. Data are the mean ± SEM of 4–6 animals per group; *, p < 0.05; **, p < 0.01 compared with the vehicle (DMSO) treated control group.
At 5 weeks, echocardiography revealed that left ventricular (LV) ejection fraction and percent fractional shortening were reduced by 8.9% and 13.8% (Table 1), respectively, compared with the DMSO-treated control, indicating modest, but significant left ventricular (LV) systolic dysfunction, while the mitral valve early diastolic (E) : late atrial (A) wave ratio decreased 15.6% (p = 0.052), suggestive of early LV diastolic dysfunction. Hearts from the TKI-treated rats also exhibited significant (p < 0.05) decreases in the thickness of the interventricular septum and LV posterior wall in diastole; a significant (p < 0.05) increase in LV chamber dimension in diastole; and enhanced LV end-diastolic and -systolic (p < 0.05) blood volumes. Modest, but nonsignificant decreases in aortic pressure and flow velocity maxima (not shown) were also observed.
Table 1.
Effect of chronic tyrphostin treatment on echocardiographic parameters in rats.
| Echocardiographic parameter |
Time-paired control (vehicle) |
Tyrphostin (AG-1478) |
Change (%; ↓↑)* |
P value* |
|---|---|---|---|---|
| LVEF | 0.847±0.018 | 0.772±0.017 | 8.9% ↓ | p < 0.02 |
| LV % FS | 46.3±2.15 | 39.9±1.60 | 13.8% ↓ | p < 0.05 |
| Mitral E/A | 1.54±0.096 | 1.30±0.098 | 15.6% ↓ | p = 0.052 |
| IVSd (mm) | 1.78±0.089 | 1.52±0.132 | 14.6% ↓ | p < 0.05 |
| IVSs (mm) | 3.04±0.236 | 2.76±0.251 | 9.2% ↓ | ns |
| LVDd (mm) | 7.688±0.179 | 8.198±0.180 | 6.6% ↑ | p < 0.05 |
| LVDs (mm) | 4.27±0.139 | 4.81±0.262 | 12.6% ↑ | p = 0.06 |
| LVPWd (mm) | 1.90±0.07 | 1.62±0.10 | 14.7% ↓ | p < 0.05 |
| LVPWs (mm) | 2.79±0.23 | 2.52±0.11 | 9.7% ↓ | ns |
| EDVtz, (mL) | 1.01±0.06 | 1.13±0.09 | 11.9% ↑ | ns |
| ESVtz (mL) | 0.177±0.003 | 0.254±0.030 | 43.5% ↑ | p < 0.05 |
| Ao Pmax (mm Hg) | 6.29±0.86 | 5.41±0.66 | 14.0% ↓ | ns |
Note: Rats treated with tyrphostin AG-1478 (21.4 mg·(kg body mass)−1·day−1) or vehicle (DMSO, control) received echocardiography at 5 weeks. Values are the mean ± SEM of 4–6 rats per group. Values for the DMSO-treated control group were not significantly different from the non-vehicle-treated control group. LVEF, left ventricular ejection fraction; LV % FS, percent fractional shortening; Mitral E/A, mitral valve E: A ratio; IVSd or s, interventricular septum dimension in diastole or systole; LVDd or s, LV chamber dimension in diastole or systole; LVPWd or s, LV posterior wall thickness in diastole or systole; EDVtz or ESVtz, end-diastolic or -systolic volume; and Ao Pmax, aortic pressure maximum.
Discussion
EGFR activation is essential to Mg reabsorption in the kidney and gut; therefore, hypomagnesemia is a major side-effect of some EGFR inhibitors (cetuximab, panitumumab) (Tejpar et al. 2007) along with many commonly-used anti-cancer drugs such as cisplatin (Gill et al. 1984; Jimeno and Hidalgo 2006). Indeed, a related cohort study (Schrag et al. 2005) revealed that most of the patients with colorectal cancer who received anti-EGFR monoclonal antibodies developed hypomagnesemia due to therapy-induced magnesium wasting. It has been recently discovered that physiological EGF–EGFR activation is a critical up-stream event required for renal and intestinal magnesium reabsorption by the TRPM-6 channel (Schrag et al. 2005; Melenhorst et al. 2008). Since co-existing hypomagnesemia from other causes may occur in the elderly, diabetic, and other patient populations (Barbagallo et al. 2009), long-term therapy with EGFR inhibitors that cause additional magnesium wasting, may greatly increase the risk of morbidity, particularly from cardiovascular events.
Chronic inhibition of the EGFR for 3 months by tyrphostin AG-1478 caused a pathological heart condition in a mouse model (Barrick et al. 2008), along with development of cardiac fibrosis, apoptosis, and evidence of impaired contractile function. However, the underlying mechanism was unclear. In the present study, we found that tyrphostin AG-1478 caused the rapid development of hypomagnesemia in rats, and this was associated with significant systemic oxidative stress and cardiac dysfunction by week 5 of treatment. In addition to systolic dysfunction and a trend towards diastolic dysfunction, the TKI-treatment also caused anatomical changes (Table 1), indicating an early progression toward dilated cardiomyopathy. An useful extension of the current study would be to determine whether administration of an Mg-supplemented diet might correct the tyrphostin AG-1478-induced cardiac dysfunction in this animal model.
Our prior studies with the hypomagnesemic rats found that substance-P-dependent neurogenic inflammation caused cardiomyopathy and eventual cardiac dysfunction after only 5 weeks of treatment (Kramer et al. 2009). Our studies also demonstrated that specific substance-P receptor blockade protected against cardiac dysfunction in hypomagnesemic animals (Kramer et al. 2009; Weglicki et al. 2010; Mak et al. 2011). Similar hypomagnesemia-inducing effects for the EGFR-TKI, erlotinib, were recently reported in mice (Dimke et al. 2010). In a related study (Noma et al. 2007), combined use of erlotinib with isoproterenol, a catecholamine known to cause magnesium wasting, led to enhancement of murine cardiac apoptosis and dysfunction (Noma et al. 2007); this latter finding is reminiscent of our previous report demonstrating enhanced isoproterenol-mediated cardiotoxicity in hypomagnesemic rodents (Atrakchi et al. 1992).
Conclusion
Since hypomagnesemia is rather common in hospitalized cardiac patients, as well as in the elderly (Altura et al. 1994; Barbagallo et al. 2009), our animal studies suggest that magnesium-wasting drugs used to treat these patients may induce deleterious cardiovascular side effects. This concept may be relevant to the recent statement by Hasinoff (2010) that “It may not be necessary for heart tissue kinases to be inhibited to cause cardiotoxicity, if the cardiotoxicity is a result of a secondary pathology”. We believe that one “secondary pathology” may be significant hypomagnesemia resulting from the combined effects of several magnesium-wasting therapies or pre-existing conditions. Increasingly, erlotinib is combined with cisplatin in lung cancer clinical trials (Tejpar et al. 2007). It is likely that these 2 magnesium-wasting drugs may cause severe hypomagnesemia during prolonged therapy, and a cascade of events leading to deleterious cardiac side effects. Our results, implicating the potential for significant cardiac side effects when EGFR-TKI drugs are combined with other magnesium wasting drugs, point to the importance of screening patients for hypomagnesemia prior to beginning chemotherapy and the need to correct this electrolyte imbalance prior to and during treatment.
Acknowledgements
This study was supported by United States Public Health Service grants NIH RO1-HL-62282-09 and 1R21NR012649–01.
Contributor Information
William B. Weglicki, Department of Biochemistry & Molecular Biology, Division of Experimental Medicine, The George Washington University, 2300 Eye Street, North-West, Ross Hall, Washington, DC 20037, USA; Department of Medicine, The George Washington University, Washington, DC 20037, USA.
Jay H. Kramer, Department of Biochemistry & Molecular Biology, Division of Experimental Medicine, The George Washington University, 2300 Eye Street, North-West, Ross Hall, Washington, DC 20037, USA.
Christopher F. Spurney, Division of Cardiology, Children’s National Medical Center, Washington, DC 20010, USA.
Joanna J. Chmielinska, Department of Biochemistry & Molecular Biology, Division of Experimental Medicine, The George Washington University, 2300 Eye Street, North-West, Ross Hall, Washington, DC 20037, USA.
I. Tong Mak, Department of Biochemistry & Molecular Biology, Division of Experimental Medicine, The George Washington University, 2300 Eye Street, North-West, Ross Hall, Washington, DC 20037, USA..
References
- Altura BT, Wilimzig C, Trnovec T, Nyulassy S, Altura BM. Comparative effects of a Mg-enriched diet and different orally administered magnesium oxide preparations on ionized Mg, Mg metabolism and electrolytes in serum of human volunteers. J. Am. Coll. Nutr. 1994;13(5):447–454. doi: 10.1080/07315724.1994.10718433. PMID: 7836622. [DOI] [PubMed] [Google Scholar]
- Atrakchi AH, Bloom S, Dickens BF, Mak IT, Weglicki WB. Hypomagnesemia and isoproterenol cardiomyopathies: protection by probucol. J. Cardiovasc. Pathol. 1992;1(2):155–160. doi: 10.1016/1054-8807(92)90019-K. [DOI] [PubMed] [Google Scholar]
- Barbagallo M, Belvedere M, Dominguez LJ. Magnesium homeostasis and aging. Magnes. Res. 2009;22(4):235–246. doi: 10.1684/mrh.2009.0187. PMID: 20228001. [DOI] [PubMed] [Google Scholar]
- Barrick CJ, Yu M, Chao H-H, Threadgill DW. Chronic pharmacologic inhibition of EGFR leads to cardiac dysfunction in C57BL/6J mice. Toxicol. Appl. Pharmacol. 2008;228(3):315–325. doi: 10.1016/j.taap.2007.12.012. PMID: 18313710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JE, Clark VL, Meyer TJ, Pearlman NW. Hypomagnesemia after cisplatin combination chemotherapy. Arch. Intern. Med. 1984;144(12):2347–2348. PMID: 6542342. [PubMed] [Google Scholar]
- Dimke H, Van der Wijst J, Alexander TR, Meijer IMJ, Mulder GM, Van Goor H, et al. Effects of the EGFR inhibitor erlotinib on magnesium handling. J. Am. Soc. Nephrol. 2010;21(8):1309–1316. doi: 10.1681/ASN.2009111153. PMID: 20595681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakih MG, Wilding G, Lombardo J. Cetuximab-induced hypomagnesemia in patients with colorectal cancer. Clin. Colorectal Cancer. 2006;6(2):152–156. doi: 10.3816/CCC.2006.n.033. PMID: 16945172. [DOI] [PubMed] [Google Scholar]
- Gill GN, Kawamoto T, Cochet C, Le A, Sato JD, Masui H, et al. Monoclonal anti-epidermal growth factor receptor antibodies which are inhibitors of epidermal growth factor binding and antagonists of epidermal growth factor-stimulated tyrosine protein kinase activity. J. Biol. Chem. 1984;259(12):7755–7760. PMID: 6330079. [PubMed] [Google Scholar]
- Hasinoff BB. The cardiotoxicity and myocyte damage caused by small molecule anticancer tyrosine kinase inhibitors is correlated with lack of target specificity. Toxicol. Appl. Pharmacol. 2010;244(2):190–195. doi: 10.1016/j.taap.2009.12.032. PMID: 20045709. [DOI] [PubMed] [Google Scholar]
- Jimeno A, Hidalgo M. Pharmacogenomics of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors. Biochim. Biophys. Acta. 2006;1766(2):217–229. doi: 10.1016/j.bbcan.2006.08.008. PMID: 17045403. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Spurney C, Iantorno M, Tziros C, Mak I-T, Tejero-Taldo I, et al. Neurogenic inflammation and cardiac dysfunction due to hypomagnesemia. Am. J. Med. Sci. 2009;338(1):22–27. doi: 10.1097/MAJ.0b013e3181aaee4d. PMID: 19593099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak IT, Chmielinska JJ, Kramer JH, Weglicki WB. AZT-induced oxidative cardiovascular toxicity: attenuation by Mg-supplementation. Cardiovasc. Toxicol. 2009;9(2):78–85. doi: 10.1007/s12012-009-9040-8. PMID: 19484392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak IT, Chmielinska JJ, Kramer JH, Spurney CF, Weglicki WB. Loss of neutral endopeptidase activity contributes to neutrophil activation and cardiac dysfunction during chronic hypomagnesemia: protection by substance P receptor blockade. Exp. Clin. Cardiol. 2011;16(4):121–124. PMID: 22131854. [PMC free article] [PubMed] [Google Scholar]
- Melenhorst WBWH, Mulder GM, Xi Q, Hoenderop JGJ, Kimura K, Eguchi S, van Goor H. Epidermal growth factor receptor signaling in the kidney: Key role in physiology and disease. Hypertension. 2008;52(6):987–993. doi: 10.1161/HYPERTENSIONAHA.108.113860. PMID: 18981331. [DOI] [PubMed] [Google Scholar]
- Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, et al. Beta-arrestin-mediated beta 1-adrenergic receptor transactivation of the EGFR confers cardio-protection. J. Clin. Invest. 2007;117(9):2445–2458. doi: 10.1172/JCI31901. PMID: 17786238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag D, Chung KY, Flombaum C, Saltz L. Cetuximab therapy and symptomatic hypomagnesemia. J. Natl. Cancer Inst. 2005;97(16):1221–1224. doi: 10.1093/jnci/dji242. PMID: 16106027. [DOI] [PubMed] [Google Scholar]
- Tejpar S, Piessevaux H, Claes K, Piront P, Hoenderop JGJ, Verslype C, Van Cutsem E. Magnesium wasting associated with epidermal-growth-factor receptor-targeting antibodies in colorectal cancer: a prospective study. Lancet Oncol. 2007;8(5):387–394. doi: 10.1016/S1470-2045(07)70108-0. PMID: 17466895. [DOI] [PubMed] [Google Scholar]
- Weglicki WB, Mak IT, Chmielinska JJ, Tejero-Taldo MI, Komarov A, Kramer JH. The role of magnesium deficiency in cardiovascular and intestinal inflammation. Magnes. Res. 2010;23(4):1–8. doi: 10.1684/mrh.2010.0218. PMID: 20971697. [DOI] [PMC free article] [PubMed] [Google Scholar]