Abstract
Angiotensin may promote endothelial dysfunction through iron accumulation. To research this, bovine endothelial cells (ECs) were incubated with iron (30 μmol·L−1) with or without angiotensin II (100 nmol·L−1). After incubation for 6 h, it was observed that the addition of angiotensin enhanced EC iron accumulation by 5.1-fold compared with a 1.8-fold increase for cells incubated with iron only. This enhanced iron uptake was attenuated by losartan (100 nmol·L−1), D-propranolol (10 μmol·L−1), 4-HO-propranolol (5 μmol·L−1), and methylamine, but not by vitamin E or atenolol. After 6 h of incubation, angiotensin plus iron provoked intracellular oxidant formation (2′7′-dichlorofluorescein diacetate (DCF-DA) fluorescence) and elevated oxidized glutathione; significant loss of cell viability occurred at 48 h. Stimulated prostacyclin release decreased by 38% (6 h) and NO synthesis was reduced by 41% (24 h). Both oxidative events and functional impairment were substantially attenuated by losartan or D-propranolol. It is concluded that angiotensin promoted non-transferrin-bound iron uptake via AT-1 receptor activation, leading to EC oxidative functional impairment. The protective effects of D-propranolol and 4-HO-propranolol may be related to their lysosomotropic properties.
Keywords: angiotensin II, endothelial cell iron accumulation, oxidative stress and dysfunction, D-propranolol, lysosomotropic properties
Introduction
An association between increased tissue iron stores and impaired endothelial function is well established in patients with hereditary hemochromatosis (Gaenzer et al. 2002). Experimental studies have suggested that angiotensin II (Ang II) promotes iron (Fe) deposition in several tissues, including the heart and liver (Ishizaka et al. 2002, 2005a, 2005b). In Ang II infused rats simultaneously treated with Fe–dextran, Fe deposition was observed in the aortic endothelial cells (ECs); in association, impairment of aortic relaxation in response to acetylcholine was observed (Ishizaka et al. 2005b). Ang II is known to stimulate NADPH oxidase to generate superoxides in ECs (Zhang et al. 1999), and Fe loading in the cells may further enhance active oxidant (hydroxyl radical) generation causing cell injury and dysfunction.
Ang II may promote Fe accumulation in lysosomal/endosomal structures in vascular cells (Ishizaka et al. 2002). Certain β-antagonists, such as propranolol, are amphiphilic amines that are known to accumulate in various cell types and compartmentalize to the lysosome (Cramb 1986). However, the potential interference of Fe uptake and the biological consequence of this lysosomotropic action of propranolol remain unclear. In this study, we investigated the time-course of Ang II-promoted Fe accumulation and its dependence on AT-1 receptor activation, using cultured ECs. The effects of propranolol analogs and related β-blockers, which display both lysosomotropic (Dickens et al. 2002) and antioxidant activity (Mak and Weglicki 2004), were studied. The effects of Ang II promotion of Fe overload on the cellular functional responses that involve synthesis of prostacyclin (PGI2) and release of NO were examined.
Materials and methods
Chemicals and cell culture
Bovine aortic EC were obtained from the NIA Aging Cell Culture Repository (AG 07684), Coriell Institute for Medical Research, Camden, New Jersey. Most chemicals were purchased from Sigma (St. Louis, Missouri) unless stated otherwise. The cells were cultured in T-25 flasks, in Dulbecco’s modified Eagle’s medium supplemented with 15% calf serum, as described by Dickens et al. (2002) and Mak et al. (2006). Confluent cells (>90%) were loaded with 30 μmol·L−1 Fe–dextran with or without Ang II (100 nmol·L−1) in the presence of 5% calf serum. When the effects of losartan (100 nmol·L−1), D-propranolol, D,L-propranolol, 4-HO-propranolol (US Biologicals, Swampscott, Massachussetts), atenolol, or vitamin E (Trolox, 5–10 μmol·L−1 each) were assessed, the agents were administered 30 min before the introduction of Fe–dextran and Ang II. To determine intracellular oxidant production, 2′ 7′-dichlorofluorescein diacetate (DCF-DA, 30 μmol·L−1) was loaded into the cultured EC in 24-well plates, in serum-free Hank’s balanced salt solution (HBSS) containing 10 mmol·L−1 glucose, pH 7.2, for 30 min. At the end of the labeling, the cells were washed 2× with HBSS to remove residual DCF-DA (Mak et al. 2006), Ang II was reintroduced into the medium, and changes in the endothelial DCF-DA fluorescence signals were followed for up to 60 min by a Synergy HT Multi-Detection Microplate Reader (BioTek Instrument Inc, Highland Park, Vermont).
Determination of endothelial cell glutathione, NO, PGI2, cell viability, and Fe
Total cell glutathione was determined by the cyclic method using a glutathione reductase assay on cells in 6-well plates (Mak et al. 1995, 1996). Oxidized glutathione (GSSG) was determined by the prior masking of reduced glutathione (GSH) with 1% vinyl pyridine. Changes in cell viability and (or) growth were determined by a colorimetric MTT (tetrazolium) assay, for up to 48 h (Mak et al. 1995). PGI2 release after stimulation by human thrombin (0.2 U·mL−1 in 24-well plates; Chronolog Corp., Havertown, Pennsylvania) for 15 min was estimated by measuring its stable product 6-keto-PG-F1α using enzyme immunoassay kits (Assay Designs, Inc., Ann Arbor, Michigan). Total NO released (nitrite + nitrate) over 24 h was determined by the Griess reagent method using Escherichia coli nitrate reductase to convert nitrate to nitrite (Mak et al. 1996). Total EC Fe accumulation was determined by atomic absorption flame emission spectroscopy using a Shimadzu 6200 atomic absorption spectrometer in accordance with the procedure of Kreeftenberg et al. (1984) as described previously (Mak et al. 2006).
Statistics
Results are expressed as the mean ± SD unless otherwise stated. Statistical significance (p < 0.05) of differences between means was determined by Student’s t test.
Results
Time course of Ang II promoted EC Fe uptake
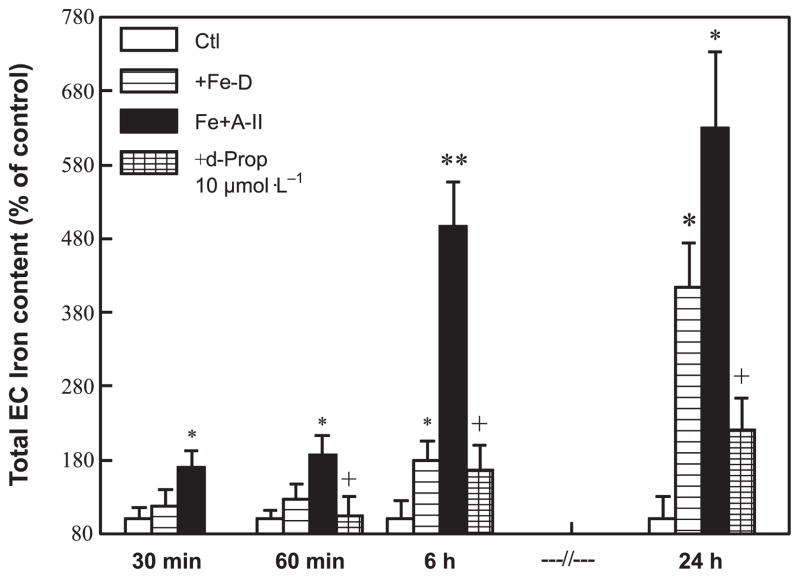
First, the time-dependent accumulation of Fe in the cultured ECs in the presence or absence of Ang II (100 nmol·L−1) was examined. Total Fe content in the cultured ECs was quantified by the atomic absorption flame emission spectroscopy method. As shown in Fig. 1, in the absence of Ang II, EC Fe levels increased slowly in the first 6 h, and 4-fold over 24 h, compared with untreated control cells. However, in the presence of Ang II, the rate of initial Fe accumulation was significantly enhanced; it was 2-fold above normal within 60 min, and by 6 h it was 5.1-fold higher (p < 0.01 compared with the control). By comparison, cells incubated without Ang II accumulated Fe at a rate of only 1.8-fold above the untreated control. After 6 h of incubation, the rate of Fe accumulation (in the presence of Ang II) appeared to progress more slowly. Nevertheless, at the end of 24 h, Ang II stimulated a 6.2-fold increase in Fe content, which was significantly higher than the 4-fold elevation found in the absence of Ang II (p < 0.05). In the same experiment, we found that pretreatment of ECs with 10 μmol·L−1 D-propranolol attenuated the accelerated Fe uptake by 90% at 6 h and inhibited total Fe accumulation by 80% at 24 h.
Fig. 1.
Time course for angiotensin II (A-II; 0.1 μmol·L−1) enhanced iron (Fe) uptake by cultured endothelial cells (EC) incubated with 30 μmol·L−1 Fe–dextran (Fe-D) and the effect of D-propranolol (d-Prop; 10 μmol·L−1). Total EC Fe was determined by atomic absorption flame emission spectroscopy; 100% Fe for the controls = 86 ± 15 ng Fe per 106 cells (n = 6). Data are the mean ± SD of 3–6 separate measurements; *, p < 0.05; and **, p < 0.01 compared with controls; +, p < 0.01 compared with Fe+A-II. Ctl, control.
Effects of losartan and propranolol analogs on EC Fe uptake
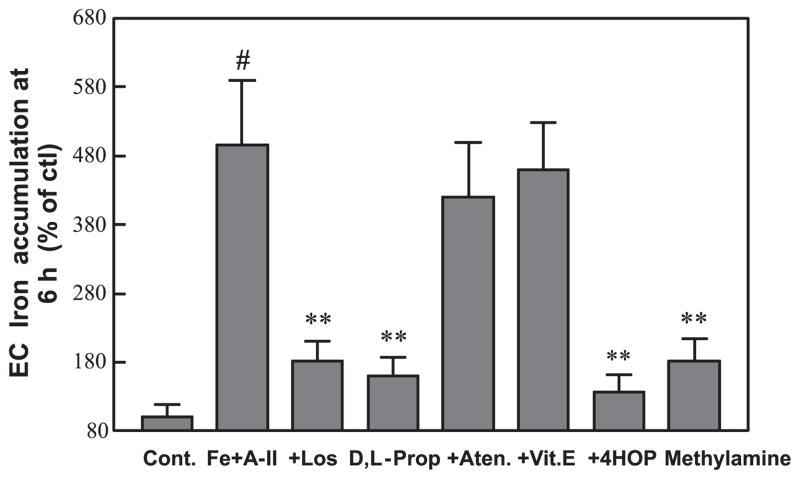
In the next series of experiments, we examined whether the accelerated Fe uptake was receptor dependent. Indeed, it was found that losartan at a relatively low level (100 nmol·L−1) substantially blocked EC Fe uptake (Fig. 2) indicating that the stimulated rate of Fe uptake depended on AT-1 receptor activation. The enhanced uptake of Fe was similarly attenuated by D,L-propranolol (10 μmol·L−1) as with D-propranolol (pharmacologically inactive), suggesting a β-receptor-independent mechanism. We also observed that the propranolol metabolite 4-HO-propranolol (5 μmol·L−1) displayed more potent inhibitory activity against Fe uptake than its parent compound; however, atenolol (10 μmol·L−1, a water soluble β-blocker) and vitamin E (Trolox) were found to have no effect. Interestingly, we found that methylamine (0.1 mmol·L−1), a well-known lysosomotropic weak base (Solheim and Seglen 1983; Cramb 1986), also blocked Fe accumulation.
Fig. 2.
Comparative effects of losartan (Los; 100 nmol·L−1), propranolol and related β-analogs (D,L-Prop; 10 μmol·L−1), vitamin E (Vit. E; 10 μmol·L−1), and methylamine (0.1 mmol·L−1) on angiotensin II (A-II) promoted endothelial cell (EC) iron (Fe) accumulation after incubation for 6 h. Other conditions are as described in Fig. 1. Data are the mean ± SD of 4–6 separate measurements; #, p < 0.001 compared with the control (ctl; Cont.); **, p < 0.01 compared with Fe+A-II. Aten, atenolol (10 μmol·L−1); 4HOP, 4-HO-propranolol (5 μmol·L−1).
EC oxidative stress
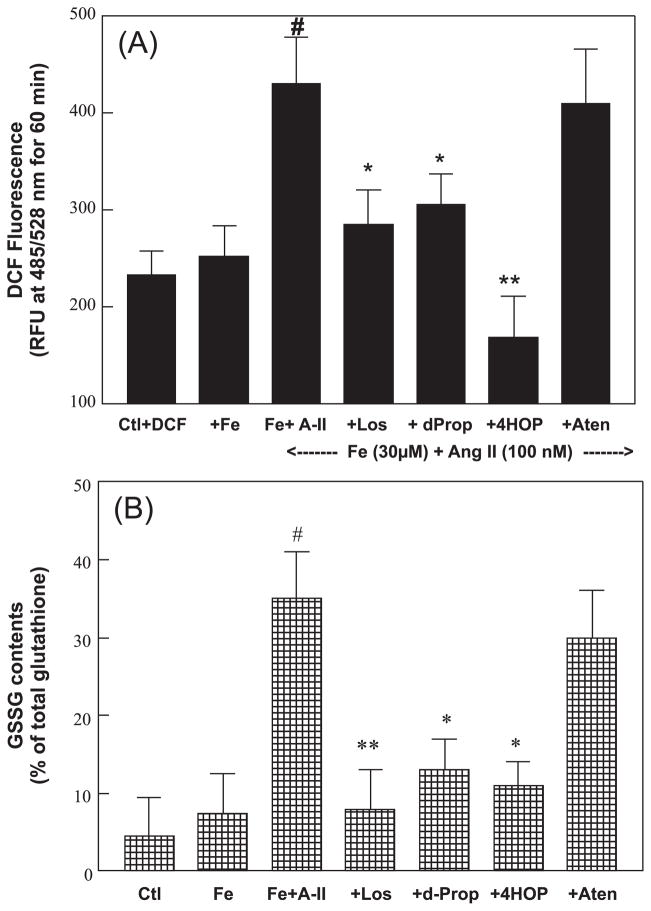
To determine whether Ang II + Fe loading promoted increased cellular oxidative stress, we used DCF-DA as an intracellular probe. DCF-DA would be taken up by the ECs and deacetylated to the nonfluorescent DCF, which reacts with various oxidants to generate fluorescent 2′,7′-dichloro-fluorescein. DCF fluorescence was monitored after dosing with Ang II (100 nmol·L−1) for up to 120 min; the increase in fluorescence was linear up to 60 min. Figure 3A shows the net increases in relative DCF fluorescence units (RFU) over the 60 min period. Fe (30 μmol·L−1 Fe–dextran) alone generated modest and insignificant increases in RFU compared with control samples (DCF-DA-loaded). However, Fe loading + Ang II resulted in significantly higher levels of DCF RFU intensity. In the same study, D-propranolol (10 μmol·L−1), 4-HO-propranolol (5 μmol·L−1), and losartan substantially diminished the elevated RFU compared with the Fe+ Ang II samples; however, atenolol (10 μmol·L−1) only had a minimal effect.
Fig. 3.
Angiotensin II (A-II) plus iron (Fe) (A) enhances endothelial oxidant formation, and (B) increases oxidized glutathione (GSSG) levels after incubation for 6 h. Effects of losartan (Los; 100 nmol·L−1) and selected β-blockers are shown. At 6 h, the cells were loaded with 2′7′-dichlorofluorescein diacetate (DCF; 30 μmol·L−1) for 30 min. After washing with balanced salt buffer, a new dose of A-II was added to the medium and changes in DCF fluorescence were monitored for 60 min. (B) A separate set of cells was used to determine cellular reduced glutathione and GSSG levels after 6 h of incubation. Data are the mean ± SD of 3–6 separate measurements. (A) #, p < 0.001 compared with the control (Ctl); *, p < 0.05; and **, p < 0.01 compared with Fe + A-II. (B) **, p < 0.01 compared with Ctl; +, p < 0.05 compared with Fe+A-II. dProp/d-Prop, D-propranolol (10 μmol·L−1); Aten, atenolol (10 μmol·L−1); and 4HOP, 4-HO-propranolol (5 μmol·L−1); RFU, relative DCF fluorescence units.
In a separate set of experiments to assess the altered redox status of cells, changes in total glutathione and GSSG levels were examined (Fig. 3B). Fe-loading + Ang II (6 h) resulted in a significantly higher increase of GSSG content, although the total glutathione remained unchanged (data not shown). These results indicated that the rise in the GSSG content was substantially (p < 0.05) attenuated by losartan, D-propranolol, and 4-HO-propranolol, but not by atenolol.
Changes in EC functional activities
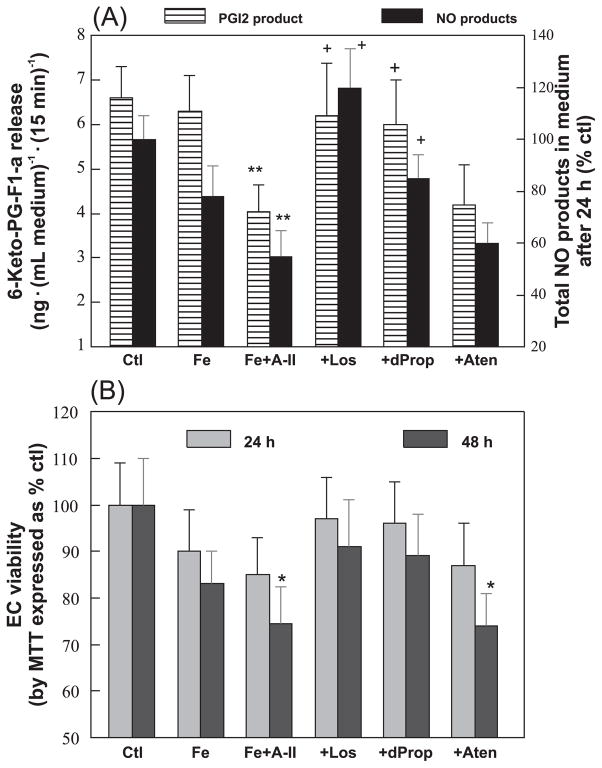
We also studied whether the endothelial functional activity of synthesizing PGI2 and releasing NO may be affected after 6 h of Fe-overload. Figure 4A shows the levels of thrombin-stimulated 6-keto-PG-F1α released into the medium for 15 min; 6-keto-PG-F1α is the stable breakdown metabolite of PGI2. The samples receiving Ang II alone (without Fe over-load) actually generated a higher level (35% ± 5% increase, p < 0.05) of PGI2-derived product. However, the metabolite level was significantly lowered by 38% ± 6% under the condition of Ang II + Fe (Fig. 4A). The depressed release was attenuated by both losartan and D-propranolol (Fig. 4A). We also observed that Ang II + Fe decreased the NO product levels (nitrite + nitrate) by 41% ± 5% (p < 0.05) at 24 h. These lower levels of NO products were restored to within normal range by losartan and D-propranolol. Atenolol had no effects on both functional products (Fig. 4A). 4-HO-propranolol (5 μmol·L−1) also restored the NO product levels back to 88% of controls (data not shown).
Fig. 4.
Effects of angiotensin II (A-II) plus iron (Fe), D-propranolol (dProp), atenolol (Aten) (10 μmol·L−1), or losartan (Los; 0.1 μmol·L−1) on (A) thrombin-stimulated PGI2 release and NO synthesis and (B) on cell viability. At 6 h, cells with different treatments were washed with balanced salt buffer and 0.2 U·mL−1 human thrombin was added; 15 min later, the cell medium was removed and assayed for 6-keto-PG-F1α. In a different set of cells (after 24 h of incubation), cell media were removed for total nitrate + nitrite determination, as described in the Materials and methods. The control level of total nitrate + nitrite was 2.85 ± 4.1 nmol·mL−1 medium. Cell viability was determined at 24 and 48 h by the MTT assay. Data are the mean ± SD of 4–6 separate measurements. *, p < 0.05 and **, p < 0.01 compared with untreated control (Ctl); +, p < 0.05 compared with Fe+A-II.
Changes in EC viability
In an effort to determine whether any of the impaired endothelial function may be secondary to cell death, we determined cell viability after different treatments by the MTT assay (Mak et al. 1995, 2006). No loss of viability was noted after incubation for 6 h. At 24 h, we observed a minor, statistically insignificant decrease in viability for the Ang II + Fe group (−15% ± 8%, p > 0.05). All other drug-treated groups were unchanged. However, at 48 h, modest but statistically significant decreases were noted for the Ang II + Fe (−24% ± 5%, p < 0.05) and Ang II + Fe + atenolol (−26% ± 7%, p < 0.05) groups. All the other treatments groups remained unchanged compared with the controls (Fig. 4B).
Discussion
In a previous study, Ishizaka et al. (2005b) found that treatment with Ang II resulted in Fe deposition in rat aortas. Such deposition was observed both in the adventitial cells and ECs; in association, transferrin receptor expression was enhanced >3-fold, implicating transferrin-dependent enhanced Fe-uptake in vivo. However, during chronic Fe-overload disorders, such as hemochromatosis and transfusional Fe overload, substantial levels of non-transferrin bound Fe (NTBI) exist in the plasma (Grootveld et al. 1989; Scheiber-Mojdehkar et al. 2004; Brissot et al. 2011). In an earlier report (Grootveld et al. 1989), the level of NTBI in the plasma samples from patients with idiopathic hemochromatosis was measured to be in the range of 8 to 22 μmol·L−1. In hemodialysis patients receiving Fe by intravenous infusion, the serum NTBI concentration could reach levels up to 100 μmol·L−1 and remain high for hours (Scheiber-Mojdehkar et al. 2004). As reviewed recently by Brissot et al. (2011), NTBI will be readily taken up by several tissues, including the liver and heart, through the carrier protein dimetal transporter-1 (DMT-1) system. Since transferrin is absent in our cultured EC system, it is presumed that Fe uptake was mediated by a metal carrier system similar to the DMT-1 system that involves an endocytosis step coupled with a (iron) reduction process to facilitate the NTBI assimilation (Brissot et al. 2011). The results of our study support the notion that activation of vesicular Fe/DMT-1 endocytosis may be a downstream event subsequent to Ang II stimulation. This Ang II effect is AT-1 receptor dependent as revealed by inhibition by losartan (Fig. 2). Losartan may have direct metal chelating activity (Miyata et al. 2002). Since the effective concentration of losartan used here was in the nanomolar concentration compared with the micromolar level of Fe in the cell medium, significant chelation was unlikely.
Propranolol is a potent lysosomotropic agent because of the presence of the ethanolamine side chain and its lipophilicity (Cramb 1986). Propranolol accumulates intracellularly in various cell types and approaches levels that are 1000-fold higher than that in the incubation medium (Cramb 1986). Our earlier study found that fluorescently labeled propranolol (acridine–propranolol) accumulated quickly (within 15 min of incubation) into endothelial lysosomal/endosomal structures (Dickens et al. 2002); in association, the lysosomal pH was rapidly alkalinized within 10 min. In the present study, we found that Ang II promoted Fe accumulation that was completely blocked by D-propranolol or D,L-propranolol, whereas atenolol, although it possesses the same ethanolamine-side chain, was found ineffective. Clearly the effect is not related to a β-receptor mechanism. Instead, we suggest that the lysosomotropic properties of the lipophilic β-blocking agents play a dominant role. Since the process of endocytosis is pH dependent, it is further suggested that D-propranolol might alkalinize the endosomal pH to inhibit this process and thus prevent the Ang-II-promoted endocytotic uptake of Fe. Interestingly, we found that the propranolol metabolite 4-HO-propranolol was also very effective in preventing Fe uptake. Since 4-HO-propranolol is also lipophilic (Oatis et al. 1981; Mak and Weglicki 2004), this presumably would confer its lysosomotropic effect. It has been suggested that NTBI is more likely to be accumulated as redox-active labile Fe and thus may be more prone to contribute to intracellular oxidative stress (Tajima et al. 2010; Brissot et al. 2011). Indeed, our study showed that upon exposure to Ang II and Fe, ECs displayed enhanced oxidant levels; increased oxidative stress was also confirmed by elevated GSSG levels (Fig. 3). When Fe uptake was attenuated by losartan, D-propropranol, and 4-HO-propranolol, the increased oxidant levels were reduced and the GSSG levels were normalized. As a consequence of this increased oxidative stress (in the absence of overt loss of cell viability), ECs showed impaired PGI2 synthesis and NO release, which were attenuated by D-propranolol and losartan, reflecting the drugs’ effect to prevent Ang-II-stimulated NTBI uptake. It is presumed that decreased NO release may be related to elevated superoxide production that caused degradation of NO. We concluded that these protective mechanisms are only indirectly due to their inhibitory effects on oxidant formation. However, the molecular events leading to decreased PGI2 production are less obvious. Ang II alone actually modestly stimulated the level of PGI2 produced in the absence of Fe. We speculate that Ang II alone may generate moderate levels of reactive oxygen species that could upregulate COX-2 expression leading to a higher level of PGI2 generation (Jaimes et al. 2010). However, in the presence of increased Fe, a significant decrease of PGI2 was observed, possibly because of decreased PGI2 synthase activity, which is known to be sensitive to potent oxidants such as peroxynitrite (Hein et al. 2009). The reduced NO and PGI2 levels were attenuated by D-propranolol, likely through inhibition of GSSG and (or) reactive oxygen species/reactive nitrogen species formation. Interestingly, significant losses of EC viability occurred only at 48 h. It is presumed that cell death was mediated by a mitochondria-dependent apoptotic process that was reported to be delayed 48–72 h after Fe-loading (Salvador and Oteiza 2011).
Conclusion
In summary, 2 new findings are presented in this brief report. (i) Ang II can promote NTBI (as represented by Fe–dextran) uptake into ECs. This promotion appears to depend on AT-1 receptor activation and possibly endocytotic sequestration of Fe into endosomes/lysosomes. The consequence of such increased NTBI accumulation may result in enhanced oxidative stress and endothelial dysfunction. (ii) Lipophilic lysosomotropic agents, such as D-propropranol and its main metabolite 4-HO-propranolol, can prevent these endothelial oxidative events possibly because of blockade of Ang-II-promoted endocytosis of the Fe carrier; subsequently, key endothelial functions are preserved. As a final note of clinical interest, since deferoxamine, the commonly used Fe chelator, is relatively ineffective in removing NTBI from plasma (Porter et al. 2005), our observation supports the notion that D-propranolol may be useful as an effective adjunct therapeutic agent to protect against vascular Fe accumulation and toxicity especially when elevated Ang II is present.
Acknowledgments
This study was supported by NIH grant R01-HL-66226 extension. We appreciate the excellent technical assistance of Dean R. Brostowin.
Contributor Information
I. Tong Mak, Department of Biochemistry and Molecular Biology, Division of Experimental Medicine, The George Washington University, 2300 Eye Street, N.W. Ross Hall, Washington, DC 20037, USA.
Kenneth M. Landgraf, Department of Biochemistry and Molecular Biology, Division of Experimental Medicine, The George Washington University, 2300 Eye Street, N.W. Ross Hall, Washington, DC 20037, USA
Joanna J. Chmielinska, Department of Biochemistry and Molecular Biology, Division of Experimental Medicine, The George Washington University, 2300 Eye Street, N.W. Ross Hall, Washington, DC 20037, USA
William B. Weglicki, Department of Biochemistry and Molecular Biology, Division of Experimental Medicine, The George Washington University, 2300 Eye Street, N.W. Ross Hall, Washington, DC 20037, USA; Department of Medicine, The George Washington University, 2300 Eye Street, N.W. Ross Hall 4th floor, Washington, DC 20037, USA
References
- Brissot P, Ropert M, Le Lan C, Loreal O. Non-transferrin bound iron: A key role in iron overload and iron toxicity. Biochim Biophys Acta. 2011;1820(3):403–410. doi: 10.1016/j.bbagen.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Cramb G. Selective lysosomal uptake and accumulation of the β-adrenergic antagonist propranolol in cultured and isolated cell systems. Biochem Pharmacol. 1986;35(8):1365–1372. doi: 10.1016/0006-2952(86)90283-2. [DOI] [PubMed] [Google Scholar]
- Dickens BF, Weglicki WB, Boehme PA, Mak IT. Antioxidant and lysosomotropic properties of acridine-propranolol: protection against oxidative endothelial cell injury. J Mol Cell Cardiol. 2002;34(2):129–137. doi: 10.1006/jmcc.2001.1495. [DOI] [PubMed] [Google Scholar]
- Gaenzer H, Marschang P, Sturm W, Neumayr G, Vogel W, Patsch J, Weiss G. Association between increased iron stores and impaired endothelial function in patients with hereditary hemochromatosis. J Am Coll Cardiol. 2002;40(12):2189–2194. doi: 10.1016/S0735-1097(02)02611-6. [DOI] [PubMed] [Google Scholar]
- Grootveld M, Bell JD, Halliwell B, Aruoma OI, Bomford A, Sadler PJ. Non-transferrin-bound iron in plasma or serum from patients with idiopathic hemochromatosis. Characterization by high performance liquid chromatography and nuclear magnetic resonance spectroscopy. J Biol Chem. 1989;264(8):4417–4422. [PubMed] [Google Scholar]
- Hein TW, Qamirani E, Ren Y, Kuo L. C-reactive protein impairs coronary arteriolar dilation to prostacyclin synthase activation: role of peroxynitrite. J Mol Cell Cardiol. 2009;47(2):196–202. doi: 10.1016/j.yjmcc.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka N, Saito K, Mitani H, Yamazaki I, Sata M, Usui S, et al. Iron overload augments angiotensin II-induced cardiac fibrosis and promotes neointima formation. Circulation. 2002;106(14):1840–1846. doi: 10.1161/01.CIR.0000031161.77536.02. [DOI] [PubMed] [Google Scholar]
- Ishizaka N, Saito K, Noiri E, Sata M, Ikeda H, Ohno M, Nagai R. Administration of Ang II induces iron deposition and upregulation of TGF-β1 mRNA in the rat liver. Am J Physiol Regul Integr Comp Physiol. 2005a;288(4):R1063–R1070. doi: 10.1152/ajpregu.00281.2004. [DOI] [PubMed] [Google Scholar]
- Ishizaka N, Saito K, Mori I, Matsuzaki G, Ohno M, Nagai R. Iron chelation suppresses ferritin upregulation and attenuates vascular dysfunction in the aorta of angiotensin II-infused rats. Arterioscler Thromb Vasc Biol. 2005b;25(11):2282–2288. doi: 10.1161/01.ATV.0000181763.57495.2b. [DOI] [PubMed] [Google Scholar]
- Jaimes EA, Hua P, Tian RX, Raij L. Human glomerular endothelium: interplay amongglucose, free fatty acids, Angiotensin II, and oxidative stress. Am J Physiol Renal Physiol. 2010;298(1):F125–F132. doi: 10.1152/ajprenal.00248.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreeftenberg HG, Koopman BJ, Huizenga JR, van Vilsteren T, Wolthers BG, Gips CH. Measurement of iron in biopsies — a comparison of three analytical methods. Clin Chim Acta. 1984;144(2–3):255–262. doi: 10.1016/0009-8981(84)90061-5. [DOI] [PubMed] [Google Scholar]
- Mak IT, Weglicki WB. Potent antioxidant properties of 4-hydroxyl-propranolol. J Pharmacol Exp Ther. 2004;308(1):85–90. doi: 10.1124/jpet.103.058032. [DOI] [PubMed] [Google Scholar]
- Mak IT, Boehme PA, Weglicki WB. Protective effects of calcium channel blockers against free radical-impaired endothelial cell proliferation. Biochem Pharmacol. 1995;50(9):1531–1534. doi: 10.1016/0006-2952(95)02039-X. [DOI] [PubMed] [Google Scholar]
- Mak IT, Komarov AM, Wagner TL, Stafford RE, Dickens BF, Weglicki WB. Enhanced NO production during Mg deficiency and its role in mediating red blood cell glutathione loss. Am J Physiol. 1996;271(1):C385–C390. doi: 10.1152/ajpcell.1996.271.1.C385. [DOI] [PubMed] [Google Scholar]
- Mak IT, Chmielinska JJ, Nedelec L, Torres A, Weglicki WB. D-Propranolol attenuates lysosomal iron accumulation and oxidative injury in endothelial cells. J Pharmacol Exp Ther. 2006;317(2):522–528. doi: 10.1124/jpet.105.097709. [DOI] [PubMed] [Google Scholar]
- Miyata T, van Ypersele de Strihou C, Ueda Y, Ichimori K, Inagi R, Onogi H, et al. Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: Biochemical mechanisms. J Am Soc Nephrol. 2002;13(10):2478–2487. doi: 10.1097/01.ASN.0000032418.67267.F2. [DOI] [PubMed] [Google Scholar]
- Oatis JE, Jr, Russell MP, Knapp DR, Walle T. Ring-hydroxylated propranolol: synthesis and beta-receptor antagonist and vasodilating activities of the seven isomers. J Med Chem. 1981;24(3):309–314. doi: 10.1021/jm00135a014. [DOI] [PubMed] [Google Scholar]
- Porter JB, Rafique R, Srichairatanakool S, Davis BA, Shah FT, Hair T, Evans P. Recent insights into interactions of deferoxamine with cellular and plasma iron pools: implications for clinical use. Ann N Y Acad Sci. 2005;1054(1):155–168. doi: 10.1196/annals.1345.018. [DOI] [PubMed] [Google Scholar]
- Salvador GA, Oteiza PI. Iron overload triggers redox-sensitive signals in human IMR-32 neuroblastoma cells. Neurotoxicology. 2011;32(1):75–82. doi: 10.1016/j.neuro.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Scheiber-Mojdehkar B, Lutzky B, Schaufler R, Sturm B, Goldenberg H. Non-transferin-bound iron in the serum of hemodialysis patients who receive ferric saccharate: No correlation to peroxide generation. J Am Soc Nephrol. 2004;15(6):1648–1655. doi: 10.1097/01.ASN.0000130149.18412.56. [DOI] [PubMed] [Google Scholar]
- Solheim AE, Seglen PO. Cellular and lysosomal uptake of methylamine in isolated rathepatocytes. Biochem J. 1983;210(3):929–936. doi: 10.1042/bj2100929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima S, Tsuchiya K, Horinouchi Y, Ishizawa K, Ikeda Y, Kihira Y, et al. Effect of angiotensin II on iron-transporting protein expression and subsequent intracellular labile iron concentration in human glomerular endothelial cells. Hypertens Res. 2010;33(7):713–721. doi: 10.1038/hr.2010.63. [DOI] [PubMed] [Google Scholar]
- Zhang H, Schmeißer A, Garlichs CD, Plötze K, Damme U, Mügge A, et al. Angiotensin II-induced superoxide anion generation in human vascular endothelial cells: Role of membrane-bound NADH/NADPH-oxidase. Cardiovasc Res. 1999;44(1):215–222. doi: 10.1016/S0008-6363(99)00183-2. [DOI] [PubMed] [Google Scholar]