Abstract
Emerging evidence points to a close crosstalk between metabolic organs and innate immunity in the course of metabolic disorders. In particular, cellular and humoral factors of innate immunity are thought to contribute to metabolic dysregulation of the adipose tissue or the liver, as well as to dysfunction of the pancreas; all these conditions are linked to the development of insulin resistance and diabetes mellitus. A central component of innate immunity is the complement system. Interestingly, the classical view of complement as a major system of host defense that copes with infections is changing to that of a multi-functional player in tissue homeostasis, degeneration, and regeneration. In the present review, we will discuss the link between complement and metabolic organs, focusing on the pancreas, adipose tissue, and liver and the diverse effects of complement system on metabolic disorders.
Keywords: Complement, Insulin resistance, Pancreas, Diabetes, Adipose tissue, Liver, Steatosis
1) Introduction: The crosstalk between the immune system and metabolism
Emerging evidence the recent years, points to an important crosstalk between the innate and adaptive immune systems and metabolic disease. Immune cells and inflammation are not only an epiphenomenon of the dysfunction of metabolic and endocrine organs. Immune cells (e.g., macrophages and T cells), cytokines (e.g., TNF and IL-6) and further factors such as the inflammasome system all contribute directly and significantly to the metabolic dysfunction seen in insulin target organs, such as adipose tissue (AT), or in the liver in the course of obesity. In fact, the obesity-associated chronic low-grade inflammation seen in the AT and the liver is unequivocally linked to the development of non-alcoholic fatty liver disease, insulin resistance and type 2 diabetes mellitus (T2DM), and their associated cardiovascular complications. On the other hand, the inflammation in the pancreatic islets, including (but not limited to) the presence of macrophages and IL-1beta-dependent reactions, contributes directly to the islet dysfunction in the course of T2DM pathogenesis. Specifically, pancreatic islet inflammation is thought to be directly associated with apoptosis in the islets. All the aforementioned pathogenetic links between inflammation and metabolic diseases are supported by promising clinical studies that show a benefit for immunomodulatory agents, such as antagonists of IL-1 or salicylates in T2DM [1–9].
Several components of the immune response have been implicated in the crosstalk between the immune system and metabolism. A prominent example is the interaction between toll-like receptor (TLR)-4 and lipid metabolism, based on the interaction of TLR-4 with free fatty acids, either directly or via the free fatty acid carrier, fetuin-A, which triggers NFkappaB activation and pro-inflammatory pathways in immune cells [10, 11]. The TLR-4 / free fatty acid interaction promotes AT inflammation and insulin resistance in diet-induced obesity [10,11].
A major component of human innate immunity is the complement system. The best-studied function of this humoral system, which consists of a cascade of proteases and soluble factors, is in innate immunity and microbial killing. However, for more than a decade now, the complement system has been implicated in a multitude of processes in the course of development, degeneration, and regeneration [12–15]. The complement cascade can be activated through three different pathways: the classical, alternative and lectin pathways. The classical pathway is initiated by the activation of the C1 complex by antigen-antibody complexes recognized by the complement component C1q. The lectin pathway shares similarities to the classical pathway; however, its starting point is the recognition of mannose residues on pathogen surfaces by mannose binding lectin (MBL) and ficolin. Both the classical and lectin pathways then continue with activation of C4 and C2, into C4a and C4b and C2a and C2b, respectively. C4b and C2b form the C3 convertase, resulting in the cleavage of C3 into C3a and C3b. Together with C4bC2b, C3b then forms the C5 convertase. The C3 and C5 conversions are the central reactions in complement activation. C3b is involved in opsonization and phagocytosis, in part via an interaction with the multi-ligand receptor complement receptor-3 (or Mac-1- integrin); C3a and C5a are anaphylatoxins with very potent chemoattractant activity that amplify leukocyte recruitment to the inflamed tissue; the fast conversion of C5a in vivo leads to C5aDesArg, which may also drive local inflammation [16]. C5b, together with C6–C9, forms the terminal “membrane attack complex” (MAC), which is capable of lysing pathogens. When the alternative pathway occurs on microbial surfaces, spontaneous hydrolysis of C3 into C3(H2O) enables the association of factor B (fB), which is then cleaved by factor D (fD) to Ba and Bb. The alternative pathway C3 convertase is stabilized by properdin and can function to activate C3 associated with the surface of pathogens or cells [17–20]. The protection of host from complement activation is conferred by expression of complement regulatory proteins, such as C1 esterase inhibitor, decay accelerating factor (DAF), factor H (fH), CR1, CD46, CD59, factor I (fI), and vitronectin, whereas carboxypeptidases serve to degrade anaphylatoxins into their less active, desarginated (desArg) forms [12, 14, 21]. Intriguingly, at the site of inflammation or wound healing, host proteases such as neutrophil elastase or the hemostatic factors kallikrein or thrombin, can directly cleave C3 and C5, thereby triggering complement activation and anaphylatoxin release without activation of the whole cascade [13, 22–24]; thus, they make the complement system a central player in most thrombo-inflammatory responses and in many homeostatic or pathological processes in the tissue.
The role of complement in metabolism and metabolic disorders has come into the foreground recently and has received increasing scientific attention. The present review will discuss the role of the complement system in the course of metabolic disease, with a special focus on the pancreas/islets and the insulin target organs, AT and liver.
2) The role of complement in physiology and pathology of the pancreas
The pancreas is an organ with a major regulatory role in metabolism, since it is the source of insulin and other hormones regulating glucose homeostasis. The β-cells of the pancreatic islets produce and secrete insulin upon glucose stimulation. Interestingly, the complement degradation product, acylation stimulating protein (ASP), can stimulate glucose-dependent insulin secretion from islets [25]. In contrast, complement fH, which is produced remotely by the liver and also locally in the pancreas, is thought to suppress insulin secretion of β-cells in a rather indirect manner [26].
In T2DM, the dysfunction (i.e., impaired insulin secretion), apoptosis, and loss of β- cells with long-term hyperglycemia [27, 28] involves pro-inflammatory signaling, including local cytokine release and an accumulation of activated macrophages [29, 30]. A further hallmark of the islets in type 2 diabetic patients is the ectopic accumulation of extracellular amyloid fibrils, which is partially mediated by excess free fatty acids (FFA) and exerts cytotoxic effects on β-cells [31, 32]. Intriguingly, amyloid fibrils can trigger local complement activation via C1q [33, 34], thereby facilitating local inflammation and macrophage activation, ultimately promoting β-cell death. Nevertheless, immunohistology studies have shown that there is limited MAC deposition on the islets that colocalizes with amyloid polypeptide fibrils [34]. This limited MAC deposition could be attributed to the fact that amyloid fibrils interact with C4BP and fH, which inhibit complement activation[34]. Thus, amyloid fibrils may also limit complement activity in the islets. These data highlight the ambivalent role of complement factors in the pancreatic islets and their failure in the course of T2DM.
In type 1 diabetes mellitus (T1DM), β-cell destruction is the result of an autoimmune reaction against insulin or islet antigens [35–39]. Interestingly, non-obese diabetic (NOD) mice, which are a model for T1DM, lack C5 because of a 2-base pair deletion in the coding region [40]. In vitro, treatment of a rat pancreatic β-cell line with serum from newly diagnosed T1DM patients inhibited their capacity for insulin secretion [41, 42], a phenomenon that was dependent on C1q and C3, since depletion of these complement components reversed the inhibitory effect of the serum of T1DM patients [41]. Furthermore, complement activation, as assessed by the presence of MAC in the serum, was higher in newly diagnosed patients with T1DM than in control individuals, whereas conditioned medium of isolated rat islet cells treated with sera from T1DM patients displayed increased terminal complement activation when compared to medium from cells treated with control serum [41, 43]. These observations have led to a hypothesis that islet apoptosis in T1DM may be partially mediated by complement activation [41, 43, 44].
A recent study demonstrated plasma C3 levels to be higher in patients with T1DM than in healthy individuals [45]. Interestingly, the higher C3 levels in T1DM patients were correlated with prolonged clot lysis, which may be the result of an interaction between C3 and fibrin [45]. Also, the levels of MBL, a major player in the lectin pathway, are elevated in patients with T1DM [46, 47]. These observations were recently confirmed in mice with streptozotocin-induced T1DM [48]. However, the exact role of MBL in the development of autoimmune insulitis is not entirely clear, although MBL does contribute to diabetic vascular [49, 50] and renal [48, 51] complications.
Whole-genome transcript analysis of pancreata from patients with T1DM show gene-upregulation for both effector and regulatory/inhibitory components of the complement system [52]. Upregulation of C3 and fB has been confirmed in the pancreata of mice with multiple low-dose streptozotocin-induced diabetes, a model for insulitis and T1DM [53]. Remarkably, C3-deficient mice and mice with hematopoietic cell-specific C3 deficiency are protected from development of insulitis and diabetes [53].
An inhibitory role for complement in the development of insulitis may be exerted by the complement receptor of the immunoglobulin superfamily (CRIg) [54]. CRIg is expressed on tissue-resident macrophages and has been implicated in the phagocytosis of complement-deposited pathogens/cells, suppression of complement activation, and as a regulator of T-cell activation (reviewed in [55]). Interestingly, CRIg expression is negatively correlated with diabetes development in NOD mice [54], whereas injection of a CRIg-Fc chimeric protein in NOD mice reduces the development of diabetes [54]. Whether the protective effect of CRIg+ macrophages on diabetes is a result of their capacity for limiting T-cell proliferation or their activity in promoting phagocytosis needs to be elucidated in further studies.
3) The role of complement in adipose tissue biology
AT biology can be influenced by a variety of complement components. Adipocytes are a major source of adipsin, which is identical to the murine factor D [56, 57] that participates in alternative complement activation, as described above in section one. Interestingly, adipsin contributes to the maturation of preadipocytes into adipocytes [56, 58], suggesting that this complement component has functions over and above its role in innate immunity. Subsequent studies have demonstrated the presence of further components of the alternative pathway, including C3, fB, properdin, fH, and fI, in the AT [57, 59, 60], providing a basis for the hypothesis that local complement activation can influence AT biology.
An interesting role as a regulator of AT biology has been attributed to the C3- degradation product C3adesArg [61], which is identical to the serum-derived ASP [61, 62]. ASP and C3 levels are higher in young obese children [63]. C3 and ASP production can be increased by chylomicrons and insulin [64–66], suggesting that ASP production can be stimulated postprandially. Moreover, murine plasma ASP levels show a positive correlation with plasma levels of FFA and cholesterol [67]. In contrast, no differences have been observed in fasting or postprandial ASP levels in patients with familial combined hyperlipidemia when compared to normal controls [68].
ASP stimulates lipogenesis in adipocytes in a synergistic fashion with insulin through diverse mechanisms, including increased glucose uptake and utilization via enhanced surface translocation of glucose transporters (Glut1, 3 and 4); as well as stimulation of triglyceride (TG) synthesis via elevated diacylglycerol acyltransferase activity [69, 70]. ASP also acts in an anti-lipolytic fashion, inhibiting the release of FFA derived from lipolysis and stimulating the re-esterification of FFA [71]. Thus, ASP promotes enhanced lipid storage in adipocytes. C3-deficient mice and fB-deficient mice (both lacking ASP) display glucose intolerance, delayed TG and FFA clearance, and a rather anti-adipogenic phenotype with decreased TG storage [67, 72]. However, ASP is not the only factor altered in C3- and fB-deficient mice; thus the degree to which the metabolic changes seen in these mice are attributable solely to the absence of ASP is not entirely clear. Furthermore, in C3 deficiency, an increased lipid uptake in the liver and muscle, with increased fatty acid oxidation in these organs, is accompanied by decreased lipid uptake in white and brown AT [73] and reduced leptin levels [72]. However, in another study, no differences were found in the fasting levels of TG, cholesterol, or free fatty acid in C3-deficient mice, as compared to wildtype mice [74]. In addition, the clearance of TG and FFA in response to oral fat loading was not affected by C3 deficiency [74]. These discrepancies in the metabolic phenotype of C3 deficiency can possibly be attributed to the different genetic backgrounds of the mice used in the various studies. Furthermore, ASP administration in C3 deficient mice on a high fat diet increased AT inflammation and insulin resistance [75]. Thus, the metabolic functions and the inflammatory actions of C3 and ASP may both contribute to the role these factors play in obese AT dysfunction and insulin resistance development. Thus, the exact role of ASP and C3 in lipid metabolism and insulin resistance is not entirely clear and requires further studies.
The functional receptor through which ASP signals has not been clearly elucidated [76–79]. C5L2 has been postulated to act as a receptor for ASP [78, 79]. C5L2 is a receptor for anaphylatoxin C5a and its desarginated form C5adesArg; however, whether and to what extent it transmits pro-inflammatory signaling is not settled [76, 77, 80, 81]. The binding of ASP to C5L2 is controversial, given that other reports have not demonstrated a direct interaction between them [76, 77]. Interestingly, C5L2-deficient mice fed a diabetogenic diet display delayed postprandial TG clearance and reduced adipocyte size, as well as higher glucose uptake and lipid deposition in the liver, which are associated with worsened insulin resistance and a rather pro-inflammatory profile [82, 83]. If C5L2 indeed exerts a decoy action for C5a [76, 77], the C5L2 deficiency phenotype could be linked to a higher activity of the C5a-C5aR axis. Indeed the C5a-C5aR axis may promote AT inflammation and insulin resistance [84] (and Phieler et al., unpublished observation). Recent experimental evidence suggests that antagonists of C3aR and C5aR administered to diet-induced obese rats prevent metabolic dysfunction and cause a decrease in body weight [84], which could be ascribed to the effects of C3a and C5a on adipocytes with regard to incorporation of glucose and FFA and inhibition of lipolysis, as well as on macrophages with regard to pro-inflammatory cytokine secretion [84]. These findings are consistent with C5a’s effect in shifting macrophages into a pro-inflammatory phenotype [85]. They are also consistent with the improved insulin sensitivity and reduced diet-induced macrophage accumulation in the AT that are seen in mice deficient in the receptor for anaphylatoxin C3a (C3aR) [86, 87]. Finally, another action of anaphylatoxins that requires further attention is the direct influence they are thought to exert on food intake regulation by mediating prostaglandin levels in the central nervous system [88]. In conclusion, the effects of complement components ASP, C3a, C5a, and of their receptors in the AT are highly varied and clearly far from being completely understood.
4) The role of complement in liver homeostasis and fatty liver disease
The liver and hepatocytes represent the main source of plasma complement proteins, including factors of all three activation pathways (classical, lectin, and alternative) as well as fluid-phase regulators. [89–91]. In addition, parenchymal (hepatocytes) and non-parenchymal cells (Kupffer cells, stellate and sinusoidal endothelial cells) express complement receptors C3aR, C5aR, and C5L2, which can also be upregulated by pro-inflammatory factors and under conditions of stress [79, 92, 93]. Many different roles have been suggested for hepatic complement, including induction of acute-phase responses, glucose release, synthesis of pro-inflammatory factors, and clearance of immune complexes (reviewed in [91]); here we will primarily focus on the roles of complement in alcoholic and non-alcoholic fatty liver disease.
Fatty liver disease is the most common liver dysfunction worldwide and is usually divided into alcoholic-, and non-alcoholic-fatty liver disease (NAFLD) [94, 95]. Both conditions are associated with steatosis and the accumulation of lipids in hepatocytes, which together trigger an inflammatory response that results in progression to steatohepatitis. Steatohepatitis is associated with serious sequelae, including fibrosis, cirrhosis, and even hepatocellular carcinoma [96, 97]. Complement is thought to be a component of the inflammatory response that is linked to both pathologies.
In alcoholic fatty liver disease, excessive ethanol consumption induces an imbalance in lipid metabolism of the liver involving several mechanisms, including increased lipogenesis, reduced lipolysis, reduced AMP-activated protein kinase (AMPK) activity, production of reactive oxygen species (ROS) and pro-inflammatory cytokines, and activation of natural killer (NK) cells [95, 98]. In this context, increased ethanol ingestion in rodents can result in acute and chronic deposition and activation of complement in the liver, including hepatic accumulation of complement factors C1, C3b, C8, and C9 and elevated plasma levels of C3a, accompanied by a reduction in complement inhibitors such as complement receptor 1- related gene/protein-y and CD59 [99–105].
The role of complement in the pathogenesis of alcoholic liver disease has been underscored by studies of mice deficient in complement components. C3-deficient mice have been found to be protected from alcohol-induced steatosis and from microvesicular and macrovesicular hepatic triglyceride accumulation [102, 103]. In addition, C3-deficient mice on an ethanol diet have a decreased expression of lipogenic enzymes, elevated serum and liver adiponectin levels, and a reduced ethanol-mediated induction of serum alanine aminotransferase (ALT) activity [101–103]. Whereas mice deficient in the complement regulatory molecule decay accelerating factor CD55/DAF show enhanced ethanol-induced hepatic steatosis, injury and inflammation, mice deficient in C5 are not protected from steatosis but instead display decreased serum ALT and hepatic inflammation [103]. Thus, C3 and C5 may contribute through different mechanisms to the pathogenesis of alcohol-induced liver disease.
A role for the classical complement pathway in alcoholic liver disease has also been demonstrated, since C1q, C3b, iC3b, and C3c are found to be deposited in the vicinity of apoptotic Kupffer cells in ethanol-fed C1q-proficient but not C1q-deficient mice [104]. Consistently, C1q-deficient mice displayed decreased steatosis and inflammatory markers (TNF and IL-6) after both acute and chronic ethanol treatment [104]. On the other hand, abrogation of the terminal complement cascade in C6-deficient rats leads to a reduced ethanol-induced deposition of other complement factors (C1, C3, C8, and C9) but an increased pro-inflammatory profile, implying a potentially protective role for C6 in alcoholic liver disease progression [100]. It is clear that additional studies about the specific mechanism of each of the complement components are required to fully elucidate the role of complement in the progression of alcohol-induced liver disease.
The role of complement in NAFLD and non-alcoholic steatohepatitis (NASH) is even less well characterized. Patients with NAFLD showed increased C3 deposition and plasma C3 and ASP levels that are correlating with insulin resistance [106–108]. The accumulation of C3 in biopsies of NAFLD patient is associated with higher hepatic mannose-binding lectin and C1q, an enhanced presence of C9 and MAC, as well as higher hepatocyte apoptosis, neutrophil infiltration, and IL-8 and IL-6 expression. All these factors are positively correlated with the degree of steatosis [106]. In addition, patients with a progressive NASH have an elevated expression of the C3 gene in the liver [109]. However, because liver function is decreased in cirrhosis, the serum concentrations of C3 and C4 are reduced in severe cirrhosis [110].
In rodents, Gregoire and colleagues have reported that mice given a high-fat diet showed enhanced hepatic expression of factor D (adipsin), a key component of the alternative pathway, suggesting a possible role for this complement component in the development of NAFLD [111]. This effect was more pronounced in mice deficient for intercellular adhesion molecule-1 (ICAM-1) [111].
Interestingly, liver expresses C5L2 abundantly [79], and this receptor has been associated in vitro [79] and in vivo [82, 83] with triglyceride synthesis. C3−/− mice and C5L2- deficient mice on a high-fat diet are prone to develop enhanced hepatic steatosis as a result of increased hepatic triglyceride content, increased lipogenesis-related gene expression, hepatic glucose uptake, and reduced fatty acid oxidation, as determined by hydroxyacyl-Coenzyme A dehydrogenase activity [83, 101, 102]. Together, these findings, suggest a protective role for C3 and C5L2 in the development of hepatic steatosis. In contrast, no significant increase in high-fat diet-induced hepatic triglyceride accumulation has been observed in C3-deficient mice in other studies [103, 112].
The role of complement factor C3 in regulating hepatic steatosis has been reinforced in a different model, partial hepatectomy-induced liver regeneration. Hepatectomy is a procedure associated with transient triglyceride accumulation in the liver as a result of the induction of lipogenic enzymes [113]. However, C3-deficient mice, unlike C3-proficient mice, develop enhanced steatosis after hepatectomy. The effect of C3 deficiency is likely a result of the absence of ASP, as shown by ASP reconstitution experiments [114]. Intriguingly, in addition to regulating steatosis after partial hepatectomy, complement is also linked to the regulation of the subsequent proliferative response. Mice deficient in either C3 or C5 show an increased lethality and decreased regenerative potential after partial hepatectomy [115–117]. Double deficiency in C3 and C5 results in an aggravated phenotype which can be reversed by concomitant administration of C3a and C5a [115, 116]. Further studies have implicated NFκB and STAT-3 activation; IL-4, IL-6, and TNF production; NKT cell recruitment; and activation of the Akt/PI3K/mTOR and ERK pathways in the complement-dependent regulation of liver regeneration [115, 116, 118, 119]. Finally, a role for C5L2 and ASP in liver regeneration has also been suggested, since administration of ASP in C3−/− mice restores adequate liver regeneration [114]. Given the detrimental actions of complement, and especially C3, as proinflammatory molecules in hepatic ischemia-reperfusion injury [120], a delicate balance must exist between complement-mediated injury and regeneration [114]. In particular, C3 deficiency or C3 inhibition protects mice from hepatic ischemia-reperfusion injury, even in obesity, which normally exacerbates ischemia/reperfusion injury [112]. However, when ischemia-reperfusion injury is combined with hepatectomy, C3 deficiency results in more severe hepatic injury [114].
Taking all these data together, despite the role of C3 in promoting alcohol-induced steatosis, the role of complement in diet-induced steatosis is unclear. On the other hand complement seems to counteract the development of hepatic steatosis associated with partial hepatectomy, and to be required for liver regeneration. Future investigations are required to explain these different actions of complement in the liver.
Both alcoholic- and non-alcoholic liver diseases are associated with a higher risk of liver fibrosis. In a study using inbred mouse strains either susceptible or resistant for liver fibrosis [121], a deletion in the locus encoding C5 in chromosome 2 was found to be responsible for conferring resistance to hepatotoxin-induced fibrosis. Susceptibility to fibrosis could be reconstituted in a fibrosis-resistant strain by introducing a wildtype C5 gene. On the other hand, fibrosis in a susceptible mouse strain was blocked by C5aR antagonists. These results have been confirmed in humans by showing that C5 haplotypes are correlated with the degree of liver fibrosis in chronic hepatitis C-infected patients [121]. However, in another study, no correlation was found between variants and polymorphisms of C5 and chronic hepatitis C-induced hepatic fibrosis or other chronic liver disorders [122].
5) Conclusion
Increasing evidence points to multiple functions of the complement system beyond pathogen killing. Interestingly, the effects of complement seem to be context- and organ-dependent. Here we have focused on the role of complement in metabolic organs such as pancreas, AT, and the liver in metabolic diseases (Fig. 1). Complement components C3 and C5 and their derivatives C3a, C3adesArg (ASP), and C5a are central players influencing the physiology and pathology of these metabolic organs. Basal levels of complement activation have rather beneficial metabolic effects, ranging from stimulation of insulin secretion in pancreatic β-cells (ASP, fH) [25, 26] to insulin-like actions with regard to adipocyte maturation and energy regulation (adipsin, ASP, C3a, C5a) [56, 69, 70, 84]. In contrast, increased complement action can contribute to metabolic pathology. In the pancreas, complement activation can contribute to β-cell apoptosis in T1DM [41, 43, 44, 53] and T2DM [33, 34]. In obesity, anaphylatoxins promote leukocyte recruitment to the AT, thereby facilitating inflammation and the associated insulin resistance [83, 84, 86].
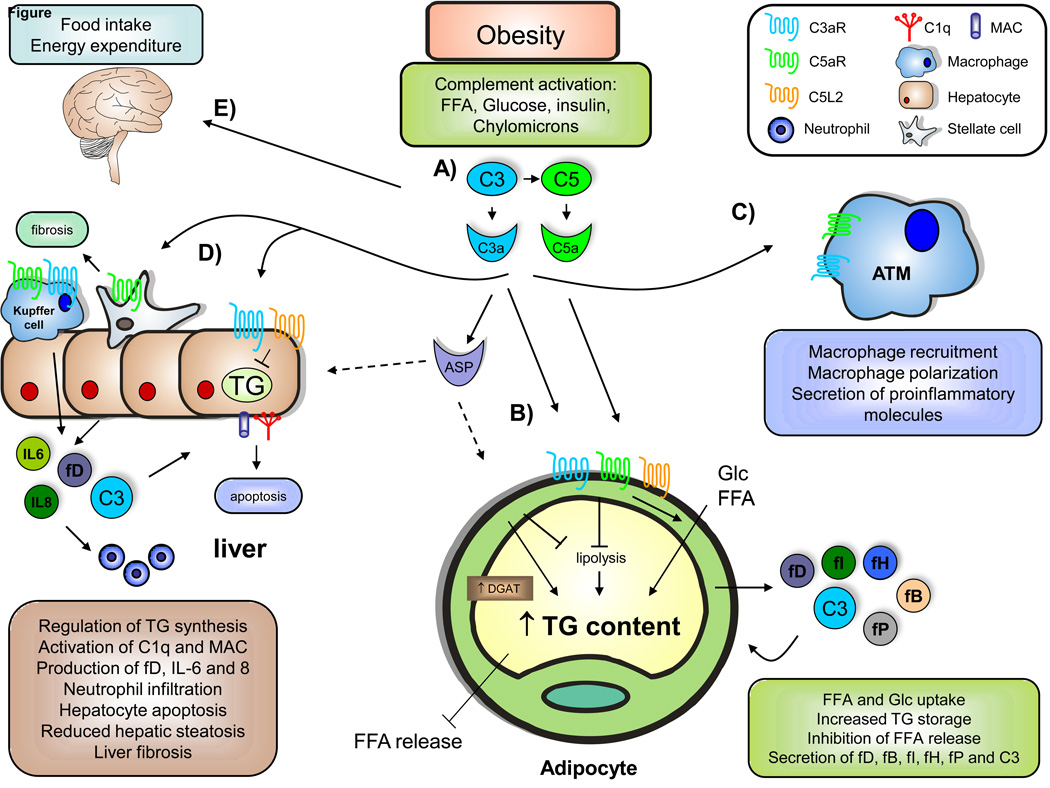
Fig. 1. The role of complement in obesity-related metabolic disorders.
A) During obesity, increased plasma and tissue concentrations of free fatty acids (FFA), glucose (Glc), insulin, and chylomicrons are found. These factors can promote complement activation, resulting in the production of elevated levels of anaphylotoxins C3a and C5a as well as C3adesArg (ASP), both locally and systemically. B) By the binding of C3a and C5a to their respective receptors C3aR and C5aR, complement components could promote triglyceride (TG) formation through the inhibition of lipolysis, enhanced Glc and FFA uptake, and indirect reduction in FFA release. In addition, C5L2 has been shown to promote lipid incorporation into TG via diglyceride acyltransferase (DGAT) activation. Adipocytes in the obese AT are able to produce several complement factors, including factor D (adipsin), fP (properdin), factor B, factor H, and C3. C) Anaphylotoxin C3a, and potentially C5a, can promote adipose tissue macrophage (ATM) recruitment, macrophage polarization to a pro-inflammatory phenotype, and secretion of pro-inflammatory factors, contributing to obesityinduced insulin resistance. D) C3a can potentially regulate TG accumulation in hepatocytes and hepatic steatosis. C3 has also been linked to C1q and membrane attack complex (MAC) deposition as well as hepatocyte apoptosis, although the exact mechanisms remain unclear. In addition, hepatocytes can promote neutrophil recruitment via pro-inflammatory factors such as IL-6 and IL-8, as well as complement activation. Furthermore, C5a has been implicated in liver fibrosis development. E) Complement has also been shown to impair food intake and energy expenditure by acting on the central nervous system.
Interestingly, the complement system contributes to both liver homeostasis and disease. Depending on the context, complement factors act in either a beneficial or detrimental manner. Exacerbated complement activation can aggravate the course of liver ischemia/ reperfusion injury [114]. The effects of complement activation during alcoholic liver disease and NAFLD are highly diverse. For instance, while C3 inhibition results in reduced steatosis in alcoholic liver disease [102, 103], it did not change or rather enhanced the steatosis associated with high-fat diet [101, 102, 112] and liver regeneration [114]. Similarly, C5 inhibition, on the one hand, compromises liver regeneration [115, 116], whereas it prevents alcoholic ingestionmediated inflammation [103] as well as hepatotoxin-induced fibrosis [121].
Complement’s ubiquitous presence throughout the body’s tissues and its diverse activation mechanisms render this system a versatile player not only in host defense but also in complex metabolic and regenerative functions. Deepening our understanding of the divergent actions of the complement system may result in novel, promising treatment alternatives for a multitude of diseases, including metabolic disorders.
Highlights.
Complement plays a major role in metabolic organs and in metabolic diseases
Complement components affect insulin secretion and adipocyte maturation
Complement contributes to the pathogenesis of T1DM and T2DM
Complement regulates liver inflammation and steatosis
Acknowledgments
Supported by grants from the Deutsche Forschungsgemeinschaft (CH279/5-1) (to TC), the Else-Kröner-Fresenius Stiftung (to TC) and the German Center for Diabetes Research (to TC) and NIH grants AI003040, AI068730, AI072106, EY020633, and DE021685 (to JDL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhan YT, An W. Roles of liver innate immune cells in nonalcoholic fatty liver disease. World J Gastroenterol. 2010;16:4652–4660. doi: 10.3748/wjg.v16.i37.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 4.Donath MY, Boni-Schnetzler M, Ellingsgaard H, Ehses JA. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda) 2009;24:325–331. doi: 10.1152/physiol.00032.2009. [DOI] [PubMed] [Google Scholar]
- 5.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatzigeorgiou A, Karalis KP, Bornstein SR, Chavakis T. Lymphocytes in obesity-related adipose tissue inflammation. Diabetologia. 2012;55:2583–2592. doi: 10.1007/s00125-012-2607-0. [DOI] [PubMed] [Google Scholar]
- 8.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012 doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 12.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171:715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 15.Wagner E, Frank MM. Therapeutic potential of complement modulation. Nat Rev Drug Discov. 2010;9:43–56. doi: 10.1038/nrd3011. [DOI] [PubMed] [Google Scholar]
- 16.Reis ES, Chen H, Sfyroera G, Monk PN, Kohl J, Ricklin D, et al. C5a receptor-dependent cell activation by physiological concentrations of desarginated C5a: insights from a novel label-free cellular assay. J Immunol. 2012;189:4797–4805. doi: 10.4049/jimmunol.1200834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaboriaud C, Thielens NM, Gregory LA, Rossi V, Fontecilla-Camps JC, Arlaud GJ. Structure and activation of the C1 complex of complement: unraveling the puzzle. Trends Immunol. 2004;25:368–373. doi: 10.1016/j.it.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Wallis R, Mitchell DA, Schmid R, Schwaeble WJ, Keeble AH. Paths reunited: Initiation of the classical and lectin pathways of complement activation. Immunobiology. 2010;215:1–11. doi: 10.1016/j.imbio.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bexborn F, Andersson PO, Chen H, Nilsson B, Ekdahl KN. The tick-over theory revisited: formation and regulation of the soluble alternative complement C3 convertase (C3(H2O)Bb) Mol Immunol. 2008;45:2370–2379. doi: 10.1016/j.molimm.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pangburn MK, Schreiber RD, Muller-Eberhard HJ. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J Exp Med. 1981;154:856–867. doi: 10.1084/jem.154.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim DD, Song WC. Membrane complement regulatory proteins. Clin Immunol. 2006;118:127–136. doi: 10.1016/j.clim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Vogt W, Damerau B, Luhmann B, Hesse D, Haller Y. Complement activation in human lymph: modulation by the contact activation system and by leukocytes. Int Arch Allergy Appl Immunol. 1986;79:423–433. doi: 10.1159/000234013. [DOI] [PubMed] [Google Scholar]
- 23.Wiggins RC, Giclas PC, Henson PM. Chemotactic activity generated from the fifth component of complement by plasma kallikrein of the rabbit. J Exp Med. 1981;153:1391–1404. doi: 10.1084/jem.153.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 25.Ahren B, Havel PJ, Pacini G, Cianflone K. Acylation stimulating protein stimulates insulin secretion. Int J Obes Relat Metab Disord. 2003;27:1037–1043. doi: 10.1038/sj.ijo.0802369. [DOI] [PubMed] [Google Scholar]
- 26.Martinez A, Pio R, Lopez J, Cuttitta F. Expression of the adrenomedullin binding protein, complement factor H, in the pancreas and its physiological impact on insulin secretion. J Endocrinol. 2001;170:503–511. doi: 10.1677/joe.0.1700503. [DOI] [PubMed] [Google Scholar]
- 27.Chick WL, Like AA. Studies in the diabetic mutant mouse. 3. Physiological factors associated with alterations in beta cell proliferation. Diabetologia. 1970;6:243–251. doi: 10.1007/BF01212233. [DOI] [PubMed] [Google Scholar]
- 28.Maedler K, Spinas GA, Lehmann R, Sergeev P, Weber M, Fontana A, et al. Glucose induces beta-cell apoptosis via upregulation of the Fas receptor in human islets. Diabetes. 2001;50:1683–1690. doi: 10.2337/diabetes.50.8.1683. [DOI] [PubMed] [Google Scholar]
- 29.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Homo-Delarche F, Calderari S, Irminger JC, Gangnerau MN, Coulaud J, Rickenbach K, et al. Islet inflammation and fibrosis in a spontaneous model of type 2 diabetes, the GK rat. Diabetes. 2006;55:1625–1633. doi: 10.2337/db05-1526. [DOI] [PubMed] [Google Scholar]
- 31.Hull RL, Westermark GT, Westermark P, Kahn SE. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3629–3643. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- 32.Qi D, Cai K, Wang O, Li Z, Chen J, Deng B, et al. Fatty acids induce amylin expression and secretion by pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2010;298:E99–E107. doi: 10.1152/ajpendo.00242.2009. [DOI] [PubMed] [Google Scholar]
- 33.Klegeris A, McGeer PL. Complement activation by islet amyloid polypeptide (IAPP) and alpha-synuclein 112. Biochem Biophys Res Commun. 2007;357:1096–1099. doi: 10.1016/j.bbrc.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 34.Sjolander J, Westermark GT, Renstrom E, Blom AM. Islet amyloid polypeptide triggers limited complement activation and binds complement inhibitor C4b-binding protein, which enhances fibril formation. J Biol Chem. 2011;287:10824–10833. doi: 10.1074/jbc.M111.244285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 36.Shizuru JA, Taylor-Edwards C, Banks BA, Gregory AK, Fathman CG. Immunotherapy of the nonobese diabetic mouse: treatment with an antibody to T-helper lymphocytes. Science. 1988;240:659–662. doi: 10.1126/science.2966437. [DOI] [PubMed] [Google Scholar]
- 37.Martin AP, Rankin S, Pitchford S, Charo IF, Furtado GC, Lira SA. Increased expression of CCL2 in insulin-producing cells of transgenic mice promotes mobilization of myeloid cells from the bone marrow, marked insulitis, and diabetes. Diabetes. 2008;57:3025–3033. doi: 10.2337/db08-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kendall PL, Case JB, Sullivan AM, Holderness JS, Wells KS, Liu E, et al. Tolerant anti-insulin B cells are effective APCs. J Immunol. 2013;190:2519–2526. doi: 10.4049/jimmunol.1202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heninger AK, Monti P, Wilhelm C, Schwaiger P, Kuehn D, Ziegler AG, et al. Activation of islet autoreactive naive T cells in infants is influenced by homeostatic mechanisms and antigen presenting capacity. Diabetes. 2013 doi: 10.2337/db12-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baxter AG, Cooke A. Complement lytic activity has no role in the pathogenesis of autoimmune diabetes in NOD mice. Diabetes. 1993;42:1574–1578. doi: 10.2337/diab.42.11.1574. [DOI] [PubMed] [Google Scholar]
- 41.Conroy SJ, Abdel-Wahab YH, Caraher EM, Byrne PM, Murphy E, Nolan J, et al. Evidence for complement-dependent and -independent inhibition of insulin secretion from clonal beta-cells incubated in the presence of sera of newly diagnosed IDDM patients. J Endocrinol. 2000;164:139–147. doi: 10.1677/joe.0.1640139. [DOI] [PubMed] [Google Scholar]
- 42.Conroy SJ, Green I, Dixon G, Byrne PM, Nolan J, Abdel-Wahab YH, et al. Evidence for a sustained increase in clonal beta-cell basal intracellular Ca2+ levels after incubation in the presence of newly diagnosed Typ-1 diabetic patient sera. Possible role in serum-induced inhibition of insulin secretion. J Endocrinol. 2002;173:53–62. doi: 10.1677/joe.0.1730053. [DOI] [PubMed] [Google Scholar]
- 43.Caraher EM, Conroy SJ, Newsholme P. Evidence for enhanced rates of complement activation in serum from patients with newly diagnosed insulin-dependent diabetes mellitus exposed to rat islet cells and complement-dependent induction of islet cell apoptosis. J Endocrinol. 1999;162:143–153. doi: 10.1677/joe.0.1620143. [DOI] [PubMed] [Google Scholar]
- 44.Radillo O, Nocera A, Leprini A, Barocci S, Mollnes TE, Pocecco M, et al. Complement-fixing islet cell antibodies in typ-1 diabetes can trigger the assembly of the terminal complement complex on human islet cells and are potentially cytotoxic. Clin Immunol Immunopathol. 1996;79:217–223. doi: 10.1006/clin.1996.0071. [DOI] [PubMed] [Google Scholar]
- 45.Hess K, Alzahrani SH, Mathai M, Schroeder V, Carter AM, Howell G, et al. A novel mechanism for hypofibrinolysis in diabetes: the role of complement C3. Diabetologia. 2012;55:1103–1113. doi: 10.1007/s00125-011-2301-7. [DOI] [PubMed] [Google Scholar]
- 46.Bouwman LH, Eerligh P, Terpstra OT, Daha MR, de Knijff P, Ballieux BE, et al. Elevated levels of mannose-binding lectin at clinical manifestation of type 1 diabetes in juveniles. Diabetes. 2005;54:3002–3006. doi: 10.2337/diabetes.54.10.3002. [DOI] [PubMed] [Google Scholar]
- 47.Hansen TK, Thiel S, Knudsen ST, Gravholt CH, Christiansen JS, Mogensen CE, et al. Elevated levels of mannan-binding lectin in patients with type 1 diabetes. J Clin Endocrinol Metab. 2003;88:4857–4861. doi: 10.1210/jc.2003-030742. [DOI] [PubMed] [Google Scholar]
- 48.Ostergaard JA, Bjerre M, Dagnaes-Hansen F, Hansen TK, Thiel S, Flyvbjerg A. Diabetes-induced changes in mannan-binding lectin levels and complement activation in a mouse model of type 1 diabetes. Scand J Immunol. 2012 doi: 10.1111/sji.12027. [DOI] [PubMed] [Google Scholar]
- 49.Hansen TK. Mannose-binding lectin (MBL) and vascular complications in diabetes. Horm Metab Res. 2005;37(Suppl 1):95–98. doi: 10.1055/s-2005-861372. [DOI] [PubMed] [Google Scholar]
- 50.Pavlov VI, La Bonte LR, Baldwin WM, Markiewski MM, Lambris JD, Stahl GL. Absence of mannose-binding lectin prevents hyperglycemic cardiovascular complications. Am J Pathol. 2012;180:104–112. doi: 10.1016/j.ajpath.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ostergaard J, Thiel S, Gadjeva M, Hansen TK, Rasch R, Flyvbjerg A. Mannose-binding lectin deficiency attenuates renal changes in a streptozotocin-induced model of type 1 diabetes in mice. Diabetologia. 2007;50:1541–1549. doi: 10.1007/s00125-007-0686-0. [DOI] [PubMed] [Google Scholar]
- 52.Planas R, Carrillo J, Sanchez A, de Villa MC, Nunez F, Verdaguer J, et al. Gene expression profiles for the human pancreas and purified islets in type 1 diabetes: new findings at clinical onset and in long-standing diabetes. Clin Exp Immunol. 2010;159:23–44. doi: 10.1111/j.1365-2249.2009.04053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin M, Yin N, Murphy B, Medof ME, Segerer S, Heeger PS, et al. Immune cell-derived c3 is required for autoimmune diabetes induced by multiple low doses of streptozotocin. Diabetes. 2010;59:2247–2252. doi: 10.2337/db10-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fu W, Wojtkiewicz G, Weissleder R, Benoist C, Mathis D. Early window of diabetes determinism in NOD mice, dependent on the complement receptor CRIg, identified by noninvasive imaging. Nat Immunol. 2012;13:361–368. doi: 10.1038/ni.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He JQ, Wiesmann C, van Lookeren Campagne M. A role of macrophage complement receptor CRIg in immune clearance and inflammation. Mol Immunol. 2008;45:4041–4047. doi: 10.1016/j.molimm.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 56.Cook KS, Groves DL, Min HY, Spiegelman BM. A developmentally regulated mRNA from 3T3 adipocytes encodes a novel serine protease homologue. Proc Natl Acad Sci U S A. 1985;82:6480–6484. doi: 10.1073/pnas.82.19.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choy LN, Rosen BS, Spiegelman BM. Adipsin and an endogenous pathway of complement from adipose cells. J Biol Chem. 1992;267:12736–12741. [PubMed] [Google Scholar]
- 58.Wilkison WO, Min HY, Claffey KP, Satterberg BL, Spiegelman BM. Control of the adipsin gene in adipocyte differentiation. Identification of distinct nuclear factors binding to single- and double-stranded DNA. J Biol Chem. 1990;265:477–482. [PubMed] [Google Scholar]
- 59.Choy LN, Spiegelman BM. Regulation of alternative pathway activation and C3a production by adipose cells. Obes Res. 1996;4:521–532. doi: 10.1002/j.1550-8528.1996.tb00266.x. [DOI] [PubMed] [Google Scholar]
- 60.Peake PW, O'Grady S, Pussell BA, Charlesworth JA. Detection and quantification of the control proteins of the alternative pathway of complement in 3T3-L1 adipocytes. Eur J Clin Invest. 1997;27:922–927. doi: 10.1046/j.1365-2362.1997.2090759.x. [DOI] [PubMed] [Google Scholar]
- 61.Baldo A, Sniderman AD, St-Luce S, Avramoglu RK, Maslowska M, Hoang B, et al. The adipsin-acylation stimulating protein system and regulation of intracellular triglyceride synthesis. J Clin Invest. 1993;92:1543–1547. doi: 10.1172/JCI116733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cianflone KM, Sniderman AD, Walsh MJ, Vu HT, Gagnon J, Rodriguez MA. Purification and characterization of acylation stimulating protein. J Biol Chem. 1989;264:426–430. [PubMed] [Google Scholar]
- 63.Cianflone K, Lu H, Smith J, Yu W, Wang H. Adiponectin, acylation stimulating protein and complement C3 are altered in obesity in very young children. Clin Endocrinol (Oxf) 2005;62:567–572. doi: 10.1111/j.1365-2265.2005.02260.x. [DOI] [PubMed] [Google Scholar]
- 64.Scantlebury T, Maslowska M, Cianflone K. Chylomicron-specific enhancement of acylation stimulating protein and precursor protein C3 production in differentiated human adipocytes. J Biol Chem. 1998;273:20903–20909. doi: 10.1074/jbc.273.33.20903. [DOI] [PubMed] [Google Scholar]
- 65.Halkes CJ, van Dijk H, de Jaegere PP, Plokker HW, van Der Helm Y, Erkelens DW, et al. Postprandial increase of complement component 3 in normolipidemic patients with coronary artery disease: effects of expanded-dose simvastatin. Arterioscler Thromb Vasc Biol. 2001;21:1526–1530. doi: 10.1161/hq0901.095276. [DOI] [PubMed] [Google Scholar]
- 66.Maslowska M, Scantlebury T, Germinario R, Cianflone K. Acute in vitro production of acylation stimulating protein in differentiated human adipocytes. J Lipid Res. 1997;38:1–11. [PubMed] [Google Scholar]
- 67.Paglialunga S, Fisette A, Yan Y, Deshaies Y, Brouillette JF, Pekna M, et al. Acylation-stimulating protein deficiency and altered adipose tissue in alternative complement pathway knockout mice. Am J Physiol Endocrinol Metab. 2008;294:E521–E529. doi: 10.1152/ajpendo.00590.2007. [DOI] [PubMed] [Google Scholar]
- 68.Ylitalo K, Pajukanta P, Meri S, Cantor RM, Mero-Matikainen N, Vakkilainen J, et al. Serum C3 but not plasma acylation-stimulating protein is elevated in Finnish patients with familial combined hyperlipidemia. Arterioscler Thromb Vasc Biol. 2001;21:838–843. doi: 10.1161/01.atv.21.5.838. [DOI] [PubMed] [Google Scholar]
- 69.Faraj M, Sniderman AD, Cianflone K. ASP enhances in situ lipoprotein lipase activity by increasing fatty acid trapping in adipocytes. J Lipid Res. 2004;45:657–666. doi: 10.1194/jlr.M300299-JLR200. [DOI] [PubMed] [Google Scholar]
- 70.Yasruel Z, Cianflone K, Sniderman AD, Rosenbloom M, Walsh M, Rodriguez MA. Effect of acylation stimulating protein on the triacylglycerol synthetic pathway of human adipose tissue. Lipids. 1991;26:495–499. doi: 10.1007/BF02536592. [DOI] [PubMed] [Google Scholar]
- 71.Van Harmelen V, Reynisdottir S, Cianflone K, Degerman E, Hoffstedt J, Nilsell K, et al. Mechanisms involved in the regulation of free fatty acid release from isolated human fat cells by acylation-stimulating protein and insulin. J Biol Chem. 1999;274:18243–18251. doi: 10.1074/jbc.274.26.18243. [DOI] [PubMed] [Google Scholar]
- 72.Murray I, Sniderman AD, Havel PJ, Cianflone K. Acylation stimulating protein (ASP) deficiency alters postprandial and adipose tissue metabolism in male mice. J Biol Chem. 1999;274:36219–36225. doi: 10.1074/jbc.274.51.36219. [DOI] [PubMed] [Google Scholar]
- 73.Xia Z, Stanhope KL, Digitale E, Simion OM, Chen L, Havel P, et al. Acylation-stimulating protein (ASP)/complement C3adesArg deficiency results in increased energy expenditure in mice. J Biol Chem. 2004;279:4051–4057. doi: 10.1074/jbc.M311319200. [DOI] [PubMed] [Google Scholar]
- 74.Wetsel RA, Kildsgaard J, Zsigmond E, Liao W, Chan L. Genetic deficiency of acylation stimulating protein (ASP(C3ades-Arg)) does not cause hyperapobetalipoproteinemia in mice. J Biol Chem. 1999;274:19429–19433. doi: 10.1074/jbc.274.27.19429. [DOI] [PubMed] [Google Scholar]
- 75.Munkonda MN, Lapointe M, Miegueu P, Roy C, Gauvreau D, Richard D, et al. Recombinant acylation stimulating protein administration to C3−/ − mice increases insulin resistance via adipocyte inflammatory mechanisms. PLoS One. 2012;7:e46883. doi: 10.1371/journal.pone.0046883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johswich K, Martin M, Thalmann J, Rheinheimer C, Monk PN, Klos A. Ligand specificity of the anaphylatoxin C5L2 receptor and its regulation on myeloid and epithelial cell lines. J Biol Chem. 2006;281:39088–39095. doi: 10.1074/jbc.M609734200. [DOI] [PubMed] [Google Scholar]
- 77.Scola AM, Johswich KO, Morgan BP, Klos A, Monk PN. The human complement fragment receptor, C5L2, is a recycling decoy receptor. Mol Immunol. 2009;46:1149–1162. doi: 10.1016/j.molimm.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kalant D, Cain SA, Maslowska M, Sniderman AD, Cianflone K, Monk PN. The chemoattractant receptor-like protein C5L2 binds the C3a des-Arg77/Acylation-stimulating protein. J Biol Chem. 2003;278:11123–11129. doi: 10.1074/jbc.M206169200. [DOI] [PubMed] [Google Scholar]
- 79.Kalant D, MacLaren R, Cui W, Samanta R, Monk PN, Laporte SA, et al. C5L2 is a functional receptor for acylation-stimulating protein. J Biol Chem. 2005;280:23936–23944. doi: 10.1074/jbc.M406921200. [DOI] [PubMed] [Google Scholar]
- 80.Cain SA, Monk PN. The orphan receptor C5L2 has high affinity binding sites for complement fragments C5a and C5a des-Arg(74) J Biol Chem. 2002;277:7165–7169. doi: 10.1074/jbc.C100714200. [DOI] [PubMed] [Google Scholar]
- 81.Ward PA. Functions of C5a receptors. J Mol Med (Berl) 2009;87:375–378. doi: 10.1007/s00109-009-0442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paglialunga S, Schrauwen P, Roy C, Moonen-Kornips E, Lu H, Hesselink MK, et al. Reduced adipose tissue triglyceride synthesis and increased muscle fatty acid oxidation in C5L2 knockout mice. J Endocrinol. 2007;194:293–304. doi: 10.1677/JOE-07-0205. [DOI] [PubMed] [Google Scholar]
- 83.Fisette A, Munkonda MN, Oikonomopoulou K, Paglialunga S, Lambris JD, Cianflone K. C5L2 receptor disruption enhances the development of diet-induced insulin resistance in mice. Immunobiology. 2013 doi: 10.1016/j.imbio.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 84.Lim J, Iyer A, Suen JY, Seow V, Reid RC, Brown L, et al. C5aR and C3aR antagonists each inhibit diet-induced obesity, metabolic dysfunction, and adipocyte and macrophage signaling. Faseb J. 2013;27:822–831. doi: 10.1096/fj.12-220582. [DOI] [PubMed] [Google Scholar]
- 85.Langer HF, Chung KJ, Orlova VV, Choi EY, Kaul S, Kruhlak MJ, et al. Complement-mediated inhibition of neovascularization reveals a point of convergence between innate immunity and angiogenesis. Blood. 2010;116:4395–4403. doi: 10.1182/blood-2010-01-261503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mamane Y, Chung Chan C, Lavallee G, Morin N, Xu LJ, Huang J, et al. The C3a anaphylatoxin receptor is a key mediator of insulin resistance and functions by modulating adipose tissue macrophage infiltration and activation. Diabetes. 2009;58:2006–2017. doi: 10.2337/db09-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schadt EE, Lamb J, Yang X, Zhu J, Edwards S, Guhathakurta D, et al. An integrative genomics approach to infer causal associations between gene expression and disease. Nat Genet. 2005;37:710–717. doi: 10.1038/ng1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ohinata K, Yoshikawa M. Food intake regulation by central complement system. Adv Exp Med Biol. 2008;632:35–46. [PubMed] [Google Scholar]
- 89.Alper CA, Johnson AM, Birtch AG, Moore FD. Human C'3: evidence for the liver as the primary site of synthesis. Science. 1969;163:286–288. doi: 10.1126/science.163.3864.286. [DOI] [PubMed] [Google Scholar]
- 90.Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 2004;41:1089–1098. doi: 10.1016/j.molimm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 91.Qin X, Gao B. The complement system in liver diseases. Cell Mol Immunol. 2006;3:333–340. [PubMed] [Google Scholar]
- 92.Mack C, Jungermann K, Gotze O, Schieferdecker HL. Anaphylatoxin C5a actions in rat liver: synergistic enhancement by C5a of lipopolysaccharide-dependent alpha(2)-macroglobulin gene expression in hepatocytes via IL-6 release from Kupffer cells. J Immunol. 2001;167:3972–3979. doi: 10.4049/jimmunol.167.7.3972. [DOI] [PubMed] [Google Scholar]
- 93.Schieferdecker HL, Schlaf G, Koleva M, Gotze O, Jungermann K. Induction of functional anaphylatoxin C5a receptors on hepatocytes by in vivo treatment of rats with IL-6. J Immunol. 2000;164:5453–5458. doi: 10.4049/jimmunol.164.10.5453. [DOI] [PubMed] [Google Scholar]
- 94.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Byun JS, Jeong WI. Involvement of hepatic innate immunity in alcoholic liver disease. Immune Netw. 2010;10:181–187. doi: 10.4110/in.2010.10.6.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 97.Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis. 2012;32:3–13. doi: 10.1055/s-0032-1306421. [DOI] [PubMed] [Google Scholar]
- 98.Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in alcoholic liver disease. Annu Rev Nutr. 2012;32:343–368. doi: 10.1146/annurev-nutr-072610-145138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jarvelainen HA, Vakeva A, Lindros KO, Meri S. Activation of complement components and reduced regulator expression in alcohol-induced liver injury in the rat. Clin Immunol. 2002;105:57–63. doi: 10.1006/clim.2002.5267. [DOI] [PubMed] [Google Scholar]
- 100.Bykov IL, Vakeva A, Jarvelainen HA, Meri S, Lindros KO. Protective function of complement against alcohol-induced rat liver damage. Int Immunopharmacol. 2004;4:1445–1454. doi: 10.1016/j.intimp.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 101.Bykov I, Jauhiainen M, Olkkonen VM, Saarikoski ST, Ehnholm C, Junnikkala S, et al. Hepatic gene expression and lipid parameters in complement C3(−/ −) mice that do not develop ethanol-induced steatosis. J Hepatol. 2007;46:907–914. doi: 10.1016/j.jhep.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 102.Bykov I, Junnikkala S, Pekna M, Lindros KO, Meri S. Complement C3 contributes to ethanol-induced liver steatosis in mice. Ann Med. 2006;38:280–286. doi: 10.1080/07853890600664608. [DOI] [PubMed] [Google Scholar]
- 103.Pritchard MT, McMullen MR, Stavitsky AB, Cohen JI, Lin F, Medof ME, et al. Differential contributions of C3, C5, and decay-accelerating factor to ethanol-induced fatty liver in mice. Gastroenterology. 2007;132:1117–1126. doi: 10.1053/j.gastro.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cohen JI, Roychowdhury S, McMullen MR, Stavitsky AB, Nagy LE. Complement and alcoholic liver disease: role of C1q in the pathogenesis of ethanol-induced liver injury in mice. Gastroenterology. 2010;139:664–674. doi: 10.1053/j.gastro.2010.04.041. 674 e661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roychowdhury S, McMullen MR, Pritchard MT, Hise AG, van Rooijen N, Medof ME, et al. An early complement-dependent and TLR-4-independent phase in the pathogenesis of ethanol-induced liver injury in mice. Hepatology. 2009;49:1326–1334. doi: 10.1002/hep.22776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rensen SS, Slaats Y, Driessen A, Peutz-Kootstra CJ, Nijhuis J, Steffensen R, et al. Activation of the complement system in human nonalcoholic fatty liver disease. Hepatology. 2009;50:1809–1817. doi: 10.1002/hep.23228. [DOI] [PubMed] [Google Scholar]
- 107.van Greevenbroek MM, Jacobs M, van der Kallen CJ, Vermeulen VM, Jansen EH, Schalkwijk CG, et al. The cross-sectional association between insulin resistance and circulating complement C3 is partly explained by plasma alanine aminotransferase, independent of central obesity and general inflammation (the CODAM study) Eur J Clin Invest. 2011;41:372–379. doi: 10.1111/j.1365-2362.2010.02418.x. [DOI] [PubMed] [Google Scholar]
- 108.Yesilova Z, Ozata M, Oktenli C, Bagci S, Ozcan A, Sanisoglu SY, et al. Increased acylation stimulating protein concentrations in nonalcoholic fatty liver disease are associated with insulin resistance. Am J Gastroenterol. 2005;100:842–849. doi: 10.1111/j.1572-0241.2005.40838.x. [DOI] [PubMed] [Google Scholar]
- 109.Sreekumar R, Rosado B, Rasmussen D, Charlton M. Hepatic gene expression in histologically progressive nonalcoholic steatohepatitis. Hepatology. 2003;38:244–251. doi: 10.1053/jhep.2003.50290. [DOI] [PubMed] [Google Scholar]
- 110.Baumann M, Witzke O, Canbay A, Patschan S, Treichel U, Gerken G, et al. Serum C3 complement concentrations correlate with liver function in patients with liver cirrhosis. Hepatogastroenterology. 2004;51:1451–1453. [PubMed] [Google Scholar]
- 111.Gregoire FM, Zhang Q, Smith SJ, Tong C, Ross D, Lopez H, et al. Diet-induced obesity and hepatic gene expression alterations in C57BL/6J and ICAM-1-deficient mice. Am J Physiol Endocrinol Metab. 2002;282:E703–E713. doi: 10.1152/ajpendo.00072.2001. [DOI] [PubMed] [Google Scholar]
- 112.He S, Atkinson C, Evans Z, Ellett JD, Southwood M, Elvington A, et al. A role for complement in the enhanced susceptibility of steatotic livers to ischemia and reperfusion injury. J Immunol. 2009;183:4764–4772. doi: 10.4049/jimmunol.0900550. [DOI] [PubMed] [Google Scholar]
- 113.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 114.He S, Atkinson C, Qiao F, Cianflone K, Chen X, Tomlinson S. A complementdependent balance between hepatic ischemia/reperfusion injury and liver regeneration in mice. J Clin Invest. 2009;119:2304–2316. doi: 10.1172/JCI38289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Strey CW, Markiewski M, Mastellos D, Tudoran R, Spruce LA, Greenbaum LE, et al. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J Exp Med. 2003;198:913–923. doi: 10.1084/jem.20030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Markiewski MM, DeAngelis RA, Strey CW, Foukas PG, Gerard C, Gerard N, et al. The regulation of liver cell survival by complement. J Immunol. 2009;182:5412–5418. doi: 10.4049/jimmunol.0804179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Clark A, Weymann A, Hartman E, Turmelle Y, Carroll M, Thurman JM, et al. Evidence for non-traditional activation of complement factor C3 during murine liver regeneration. Mol Immunol. 2008;45:3125–3132. doi: 10.1016/j.molimm.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Daveau M, Benard M, Scotte M, Schouft MT, Hiron M, Francois A, et al. Expression of a functional C5a receptor in regenerating hepatocytes and its involvement in a proliferative signaling pathway in rat. J Immunol. 2004;173:3418–3424. doi: 10.4049/jimmunol.173.5.3418. [DOI] [PubMed] [Google Scholar]
- 119.DeAngelis RA, Markiewski MM, Kourtzelis I, Rafail S, Syriga M, Sandor A, et al. A complement-IL-4 regulatory circuit controls liver regeneration. J Immunol. 2012;188:641–648. doi: 10.4049/jimmunol.1101925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Abu-Amara M, Yang SY, Tapuria N, Fuller B, Davidson B, Seifalian A. Liver ischemia/reperfusion injury: processes in inflammatory networks--a review. Liver Transpl. 2010;16:1016–1032. doi: 10.1002/lt.22117. [DOI] [PubMed] [Google Scholar]
- 121.Hillebrandt S, Wasmuth HE, Weiskirchen R, Hellerbrand C, Keppeler H, Werth A, et al. Complement factor 5 is a quantitative trait gene that modifies liver fibrogenesis in mice and humans. Nat Genet. 2005;37:835–843. doi: 10.1038/ng1599. [DOI] [PubMed] [Google Scholar]
- 122.Halangk J, Sarrazin C, Neumann K, Puhl G, Mueller T, Teuber G, et al. Evaluation of complement factor 5 variants as genetic risk factors for the development of advanced fibrosis in chronic hepatitis C infection. J Hepatol. 2008;49:339–345. doi: 10.1016/j.jhep.2008.05.021. [DOI] [PubMed] [Google Scholar]