Abstract
This article describes the assembly and performance of a simple and inexpensive ultraviolet-flash system suitable for rapid focal photolysis of caged compounds in cultured neurons and brain slices. Advantages and limitations of this system are discussed. Examples are provided illustrating how this system can be used for stimulating neurons and mapping their functional inputs in brain slices.
INTRODUCTION
Use of laser-based scanning focal photolysis has become widespread for rapid and local stimulation of neurons (Callaway and Katz 1993; Pettit et al. 1997; Dantzker and Callaway 2000; Nikolenko et al. 2007). However, for many applications, the high spatial resolution and scanning speed provided by a laser-based system are not required. For these cases, an inexpensive and easy to assemble and operate uncaging system is more desirable. Here, we describe a simple system composed of amercury arc lamp for ultraviolet (UV) light generation and an optical fiber for light delivery, which can be implemented without modifications to the existing microscopy setup. This system has been used to stimulate small dendritic segments of neurons in the hippocampus (via caged glutamate) and in the auditory brain stem (via caged γ-aminobutyric acid [GABA]). In addition, it has been used to map functional connectivity patterns (Kandler et al. 1998; Kim and Kandler 2003). By selectively stimulating presynaptic inhibitory neurons, this approach has been combined with calcium imaging to measure post-synaptic responses in populations of neurons (Kullmann et al. 2002). The system is capable of rapidly photolyzing a variety of caged compounds and is applicable to a diversity of cell types and neuronal systems in slices as well as cultured neurons.
MATERIALS TO ASSEMBLE THE UNCAGING SYSTEM
The light source we use is a 100-W mercury arc lamp with a Series Q lamp housing and a UV grade fused silica condenser (Oriel, Newport Corporation). The power supply is from OPTI QUIP. The electronic shutter used to control light pulse width is a critical component. We use a Uniblitz Model LSG with AlMgF2 coating (Vincent Associates), which can generate light pulses as brief as 5 msec. The shutter is triggered by a pulse generator (Master 8, A.M.P.I.). The uncaging system is mounted onto a breadboard. Required mounting components are a fiber positioner, fiber chucks, and mounting hardware (base plates, mounting rods, and screws) (Newport Corporation, New Focus, and Thorlabs, Inc.). The optical fibers are made from high-OH high UV-transmitting fused silica. We have used fibers with inner diameters ranging from 5 to 50 µm obtained from CeramOptec Industries, Inc. or Polymicro Technologies. Fibers are cut to the required length with a FITEL fiber cleaver from Fiber Instrument Sales, Inc.
The caged neurotransmitters are supplied by various vendors (Calbiochem, Dojindo Molecular Technologies, Inc., and Invitrogen) or can be synthesized in-house (e.g., Givens et al. 1997; Stensrud et al. 2009).
ASSEMBLY AND OPTIMIZATION
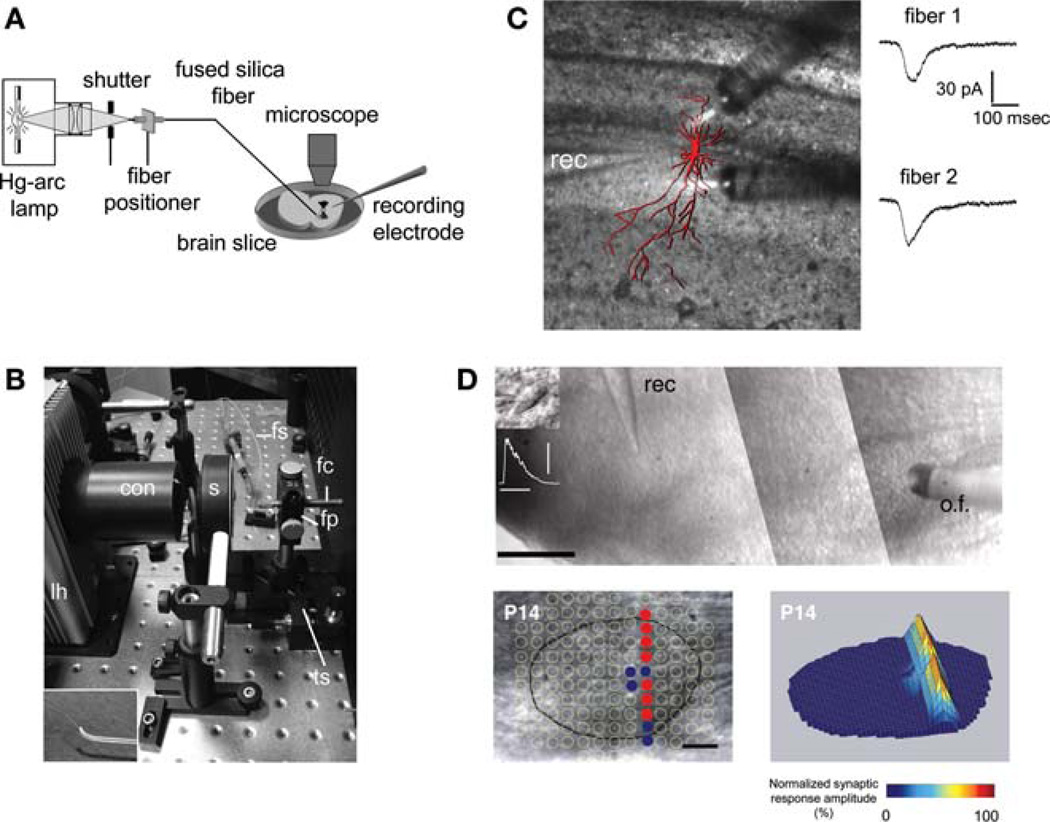
The uncaging system is illustrated in Figure 1A,B. For protection, the optical fiber is placed within small-diameter Tygon tubing. The light-emitting end of the fiber is attached to an aluminum rod that is mounted to a manual micromanipulator. For mapping experiments, we feed the fiber through a bent glass capillary tube (Fig. 1B inset) to ensure that the fiber is approximately perpendicular to the surface of the slice. The position of the optical fiber above the slice is monitored with the microscope. For off-line analysis, a microscope-mounted video camera and a video frame grabber are used to document the exact location of uncaging sites in the slice.
FIGURE 1.
(A) Schematic of the uncaging system. (B) Photograph of lamp housing with optical-fiber mount. Inset (lower left) shows optical fiber protruding from curved glass capillary. con, condenser; fc, fiber chuck; fp, fiber positioner; fs, fiber sleeve; lh, lamp housing; s, shutter; ts, translational stage. (C) Stimulation of two dendritic areas with glutamate uncaging using two optical fibers. Post hoc reconstruction of the recorded hippocampal CA3 pyramidal neuron (red) is overlaid on a photograph of the slice taken during stimulation. Current traces show responses elicited by applying UV flashes (20 msec) through either one of the fibers. (Reprinted, with permission, from Kandler et al. 1998.) (D) Mapping functional connections in an auditory brain-stem slice. The optical fiber (o.f.) is aimed at the medial nucleus of the trapezoid body (MNTB). A neuron in the lateral superior olive (LSO) is recorded in the whole-cell patch-clamp mode (rec). In the lower panel, each uncaging site in and around the MNTB (outlined by the black line) is indicated by a circle. Filled circles mark sites at which uncaging of glutamate elicited synaptic responses in the LSO neuron; open circles mark nonresponse sites. Scale bar, 100 µm. In the three-dimensional plot on the right, stimulation sites are encoded by the peak amplitude of corresponding synaptic responses. (Modified, with permission, from Kim and Kandler 2003.)
Assembly of the system requires no special knowledge and can be accomplished in 1–2 days. Attention should be paid to maximizing the amount of light launched into the fiber. To this end, the cut of the fiber has to be flat and smooth and should be checked under a stereomicroscope. A smooth cut is also necessary at the light-emitting end of the fiber. A quick way to check the quality of the cut at this end is to shine the light against a smooth surface: A circular light spot with well-defined edges indicates a good cut. Another crucial step is to correctly align the optical fiber: Both position (the end should be at the focal point of the condenser) and angular orientation are critical. Optimal alignment can be checked by measuring the relative light output power of the fiber with a UV-sensitive photodiode (Thorlabs, Inc.) and a UV-band-pass filter (Oriel, Newport Corporation). We also routinely test for optimal alignment while recording from a neuron using the amplitudes and rise times of membrane currents as sensitive indicators. If uncaging is unsuccessful, checking fiber alignment and the surfaces of the fiber cuts is the starting point for troubleshooting.
EXPERIMENTS UTILIZING THE OPTICAL FIBER UNCAGING SYSTEM
This system has been used to rapidly release glutamate or GABA from several different types of caged compounds (Givens et al. 1997; Kandler et al. 1998; Conrad et al. 2000; Kim and Kandler 2003; Stensrud et al. 2009). The two major neurobiological applications in which this system has been applied are focal dendritic stimulation with glutamate (Fig. 1C) or GABA (Kandler et al. 1998; Kim and Kandler 2003) and construction of functional connectivity maps in a GABA/glycinergic auditory brain-stem circuit (Kim and Kandler 2003). An example of an inhibitory input map for the medial nucleus of the trapezoid body (MNTB) to a single neuron in the lateral superior olive (LSO) is shown in Figure 1D. In these experiments, we uncaged glutamate from p-hydroxyphenacyl glutamate (150–200 µm) (Givens et al. 1997) in and around the MNTB at more than 100 discrete locations, separated by ~50 µm. Synaptic responses in the LSO neuron were elicited from only 12 uncaging locations (filled circles) that formed a sharp dorsoventrally oriented band mirroring the tonotopic organization of the MNTB–LSO pathway.
For correctly interpreting such mapping experiments, it is important to determine the spatial resolution of the uncaging system. This resolution is influenced by numerous factors, such as UV light power and flash duration, concentration of caged glutamate, UV transmittance of the brain area under investigation, and the geometry and physiological properties of the stimulated neurons. In our MNTB–LSO mapping experiments, we defined an effective, spike-eliciting resolution by recording from an MNTB neuron while uncaging glutamate in its vicinity. Under our conditions, action potentials were elicited only if the center of the uncaging spot was <25 µm from the cell body, corresponding to a spatial resolution of 50 µm. Because light is diverging from the optical fiber, light can never be focused to a spot inside the slice and the intensity will decrease exponentially with distance from the fiber. Therefore, axial resolution of the system is expected to be significantly worse than the lateral resolution.
ADVANTAGES AND LIMITATIONS
The major advantages of this uncaging system are its low cost, its ease of use, and the minimal expertise in optics required. The system can be incorporated into any existing in vitro electrophysiology setup without the need for modifications to the existing microscope. The price of the complete system is ~$3000, which is only a small fraction of the cost of a UV laser-based system, the method most commonly used for uncaging (Callaway and Katz 1993; Pettit et al. 1997; Dantzker and Callaway 2000). Additional optical fibers (up to four) can easily be added when using the Series Q arc lamp housing so that one setup can serve multiple electrophysiology rigs or allow for simultaneous multisite stimulation (Fig. 1C). The use of a continuous-wave light source permits a wide range of flash durations and high repetition rates, limited only by the shutter speed.
A limitation of the system described here is that the optical fiber is moved manually between uncaging positions, a rather time-consuming procedure, which requires stable recordings over a considerable period (1–3 h) if large input areas are to be mapped. If such recordings are difficult to obtain, use of amotorized micromanipulator under computer control should be considered. Another limitation arises from the fact that both the fiber and the recording electrode approach the slice from above. Consequently, the area beneath the recording electrode is obstructed, which, in some applications, can result in incomplete maps.
In principle, UV light from the arc lamp can be delivered to the microscope objective using lenses and mirrors. However, this would result in much greater light loss than guiding with small optical fibers, and, thus, would require longer flash durations, more powerful light sources, and/or higher concentrations of expensive caged compounds. With optical fibers, the uncaging spot size on the slice ultimately is restricted by the smallest diameter of the fiber core, which is 3–5 µm. Therefore, this system is less suitable for experiments that require extremely small uncaging areas as is necessary for mapping the subcellular distribution of receptors (Pettit et al. 1997; Frick et al. 2001) or for stimulating single spines (Matsuzaki et al. 2001; Smith et al. 2003).
ACKNOWLEDGMENTS
Work in our laboratory has been supported by the National Institute of Deafness and Other Communication Disorders (KK), the Center for Neural Basis of Cognition (TN, KK), and the National Institutes of Health grant GM72910 (RG).
REFERENCES
- Callaway EM, Katz LC. Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc Natl Acad Sci. 1993;90:7661–7665. doi: 10.1073/pnas.90.16.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad PG, Givens RS, Weber JF, Kandler K. New phototriggers: Extending the p-hydroxyphenacyl p-p* absorption range. Org Lett. 2000;2:1545–1547. doi: 10.1021/ol005856n. [DOI] [PubMed] [Google Scholar]
- Dantzker JL, Callaway EM. Laminar sources of synaptic input to cortical inhibitory interneurons and pyramidal neurons. Nat Neurosci. 2000;3:701–707. doi: 10.1038/76656. [DOI] [PubMed] [Google Scholar]
- Frick A, Zieglgansberger W, Dodt HU. Glutamate receptors form hot spots on apical dendrites of neocortical pyramidal neurons. J Neurophysiol. 2001;86:1412–1421. doi: 10.1152/jn.2001.86.3.1412. [DOI] [PubMed] [Google Scholar]
- Givens RS, Jung A, Park CH, Weber J, Bartlett W. New photoactivated protecting groups. 7. p-hydroxyphenacyl: A phototrigger for excitatory amino acids and peptides. J Am Chem Soc. 1997;119:8369–8370. [Google Scholar]
- Kandler K, Katz LC, Kauer JA. Focal photolysis of caged glutamate produces long-term depression of hippocampal glutamate receptors. Nat Neurosci. 1998;1:119–123. doi: 10.1038/368. [DOI] [PubMed] [Google Scholar]
- Kim G, Kandler K. Elimination and strengthening of glycinergic/ GABAergic connections during tonotopic map formation. Nat Neurosci. 2003;6:282–290. doi: 10.1038/nn1015. [DOI] [PubMed] [Google Scholar]
- Kullmann PH, Ene FA, Kandler K. Glycinergic and GABAergic calcium responses in the developing lateral superior olive. Eur J Neurosci. 2002;15:1093–1104. doi: 10.1046/j.1460-9568.2002.01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolenko V, Poskanzer KE, Yuste R. Two-photon photostimulation and imaging of neural circuits. Nat Methods. 2007;4:943–950. doi: 10.1038/nmeth1105. [DOI] [PubMed] [Google Scholar]
- Pettit DL, Wang SS, Gee KR, Augustine GJ. Chemical two-photon uncaging: A novel approach to mapping glutamate receptors. Neuron. 1997;19:465–471. doi: 10.1016/s0896-6273(00)80361-x. [DOI] [PubMed] [Google Scholar]
- Smith MA, Ellis-Davies GC, Magee JC. Mechanism of the distancedependent scaling of Schaffer collateral synapses in rat CA1 pyramidal neurons. J Physiol. 2003;548:245–258. doi: 10.1113/jphysiol.2002.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensrud K, Noh J, Kandler K, Wirz J, Heger D, Givens RS. Competing pathways in the photo-Favorskii rearrangement and release of esters: Studies on fluorinated p-hydroxyphenacyl-caged GABA and glutamate phototriggers. J Org Chem. 2009;74:5219–5227. doi: 10.1021/jo900139h. [DOI] [PMC free article] [PubMed] [Google Scholar]