Abstract
Background
Macrophages (MΦ) predominate among the inflammatory cells in rejecting allografts. These innate immune cells, in addition to allospecific T cells, can damage cardiomyocytes directly.
Methods and Results
We explored if sensitive PET/CT imaging of MΦ-avid nanoparticles detects rejection of heart allografts in mice. In addition, we employed the imaging method to follow the immunomodulatory impact of angiotensin converting enzyme inhibititor therapy (ACEi) on myeloid cells in allografts. Dextran nanoparticles were derivatized with the PET isotope copper-64 and imaged seven days after transplantation. C57/BL6 recipients of BALB/c allografts displayed robust PET signal (standard uptake value allograft, 2.8±0.3; isograft control, 1.7±0.2; p<0.05). Autoradiography and scintillation counting confirmed the in vivo findings. We then imaged the effects of ACEi (5mg/kg Enalapril). ACEi significantly decreased nanoparticle signal (p<0.05). Histology and flow cytometry showed a reduced number of myeloid cells in the graft, blood and lymph nodes, as well as diminished antigen presentation (p<0.05 versus untreated allografts). ACEi also significantly prolonged allograft survival (12 versus 7 days, p<0.0001).
Conclusions
Nanoparticle MΦ PET-CT detects heart transplant rejection and predicts organ survival by reporting on myeloid cells.
Keywords: heart transplantation, macrophages, imaging, PET/CT
Transplantation remains an important option for patients with advanced stages of heart failure. Detection of parenchymal rejection and monitoring immunosuppressive therapy presents an ongoing clinical challenge. The current approach to surveillance for rejection involves performing serial multiple endomyocardial biopsies obtained by invasive transvenous access. Typically, the right ventricle is biopsied in 3–4 locations1. The procedure, which is performed biweekly in the first months after transplantation, causes complications in up to 3% of the cases including valvular insufficiency, arrhythmia, infection and bleeding2. Sampling errors can also confound biopsy surveillance, since the bioptome often misses areas of myocardial inflammation. Moreover, inflamed myocardium beyond the endocardial surface can not be detected with this procedure. There is thus a need for a better, less invasive method for monitoring rejection in transplant recipients.
Prior research has established lymphocytes as central orchestrators of tolerance and rejection. Macrophages and their circulating monocyte precursors, parts of the innate immune system, traditionally received attention as mediators of myocardial damage during rejection. An increasing body of work in models of inflammatory disease suggests that myeloid cells play very active roles in immunity3. Human biopsy studies revealed that macrophages comprise up to 60% of the inflammatory cell population in allografts4. Initiating inflammatory events recruit monocytes into the graft where they differentiate into macrophages, which then amplify inflammation by release of soluble factors such as TNF-α, interleukins, myeloperoxidase, iNOS and reactive oxygen species. Monocytes and macrophages are involved in cell killing, scavenging of debris and phagocytosis of non-self material, and remodel the extracellular matrix through proteolysis, fibrosis and angiogenesis (reviewed in ref3,5). Moreover, monocytes and macrophages (MΦ) can prime allospecific T cells by alloantigen presentation6.
Many of these functions may have important diagnostic and therapeutic implications in transplant rejection. Indeed, a clinical trial concluded that kidney allograft dysfunction correlates more closely with macrophage than T cell content in biopsies7. Based on these considerations, nanoparticle imaging of macrophages emerged as a modality to follow rejection, mostly relying on MRI detection of their iron oxide cores8, 9. Because PET offers the most sensitivity and quantifiability among imaging modalities currently available in the clinic10, 11, we here explored PET/CT imaging of monocyte/macrophage infiltration in cardiac mouse allografts. To this end, we derivatized phagocytosable nanoparticles with the long-lived PET isotope copper-64, hypothesizing that the PET signal would reflect organ rejection and effects of MΦ-modulating therapy.
Methods
Transplantation
Female C57BL/6J (B6 WT, H-2b), BALB/c (H-2d) mice were purchased from the Jackson Laboratory. Animals were enrolled at 6–10 weeks of age (body weight 20–25 g). The studies were approved by the Subcommittee on Animal Research Care at Massachusetts General Hospital (13th Street, Charlestown, MA). In the treatment cohort, Enalapril was administered at a dose of 5 mg/kg daily by gastric gavage starting two days prior to surgery and continuing until grafts were rejected. Ketamine (50 mg/mL, Bioniche Pharma) and xylazine (100 mg/mL, Vedco) were used as anesthetic agents. Vascularized intraabdominal heterotopic heart transplantation was performed using microsurgical techniques as described previously9, 12. Hearts were transplanted into the peritoneal cavity of recipients by establishment of an end-to-side anastomosis of the donor aorta to the recipient aorta, and an end-to-side anastomosis of the donor pulmonary trunk to the recipient inferior vena cava. The total surgery time was approximately 45 min. If myocardial contractions were not palpated through the abdomen immediately postoperatively, the procedure was considered a failure and about 1 in 10 recipients were excluded from the study. We investigated total allo-mismatched grafts (donor mouse, BALB/c, recipient C57/B6, H2d into H2b) and compared those to syngeneic isograft controls (donor C57/BL6, recipient C57/BL6). The use of isograft controls permitted differentiation of alloimmune-mediated injury of parenchymal rejection from background signals arising from ischemic injury (due to harvesting and anastomosis), pericardial inflammation (due to surgery), as well as from the occasional formation of intracavitary thrombi within the vascularized but non-pumping grafts. Cardiac allograft survival was subsequently assessed through daily palpation. Rejection was defined as the complete cessation of cardiac contraction, and was confirmed by direct visualization.
Nanoparticle preparation
Copper-64-cross-linked iron oxide-Vivotag 680 (64Cu-CLIO-VT680) was prepared as described previously, resulting in a particle diameter of 20nm13. Briefly, CLIO-47 was incubated with dianhydride diethylenetriamine pentaacetic acid (DTPA; Sigma, St. Louis, MO) for 2 hours at room temperature and then purified with a PD-10 column. DTPA-CLIO-VT680 (100 µg) was labeling with 64CuCl2 (523 ± 44 MBq, n=3) in ammonium acetate buffer (400 mM, pH 6.5) at 95 °C for 1 hour. Filtration (molecular weight cut off 50 kDa) of the reaction mixture provided the labeled nanoparticle (342 ± 148 MBq) in 72.0% decay-corrected radiochemical yield. 64Cu-CLIO-VT680 was prepared for injection by resuspension in phosphate buffered saline.
PET-CT imaging
Mice were imaged with PET-CT using an Inveon small animal scanner (Siemens) 7 days after transplantation (n=8–9 transplant recipients per group). This time point was chosen based on previous flow cytometric studies12. Imaging was done 24 hours after injection of 64Cu-CLIO-VT680.†Mice were anesthetized with isoflurane (1.5%; O2 2 L/minute) for the duration of imaging. After acquisition of 600 million counts, a high resolution Fourier algorithm was used to rebin sinograms, and a filtered back-projection algorithm was then used to reconstruct three-dimensional PET images. Image voxel size was 0.80 × 0.86 × 0.86 mm, for a total of 128 × 128 × 159 voxels. Calibration of the PET signal with a cylindrical phantom containing 64Cu†isotopes was performed prior to scanning. Data are expressed as standard uptake values (SUV), which normalizes activity for body weight and injected activity. Mean injected activity was 2.1±0.15†mCi in 60±10µL saline and the mean weight of mice was 22.8±0.8g. CT images were reconstructed from 360 cone-beam X-ray projections with a power of 80keV and 500µA. The isotropic voxel size of the CT images was 110µm. During CT acquisition, iodine contrast was infused into the tail vein at a rate of 35 µl/minute to enhance intravascular contrast. Projections were acquired at end expiration, which was identified using a BioVet gating system (M2M Imaging, Cleveland, OH). The CT acquisition time was ~10 minutes. Reconstruction of data sets, PET-CT fusion and image analysis were done using Inveon Research Workplace (Siemens). Three-dimensional visualizations were produced using the DICOM viewer OsiriX (The OsiriX foundation, Geneva, Switzerland).
Measurement of ex vivo tissue activity
All mice were placed into a well counter (CRC-127R, Capintec, Ramsey, NJ) following injection of 64Cu-CLIO-VT680 and again after imaging was completed prior to dissection, to record total corporeal activity. After sacrifice, grafts were excised, and radioactivity was measured using a gamma counter (1480 Wizard 3”, PerkinElmer, Boston, MA). Finally, tissues were exposed on phosphor imaging plates that†were then read using a Typhoon 8600 scanner (GE).
Histologic study
Cardiac allografts were harvested 7 days after transplantation and embedded in optimal cutting temperature (OCT) compound (Sakura Finetek). Fresh-frozen 6µm-thick serial sections were examined by hematoxylin-eosin (H&E) and immunohistochemical staining for both CD11b+ myeloid cells (anti-CD11b primary antibody, clone M1/70, BD Biosciences) and MAC-3+ MΦ (anti-Mac3 antibody, clone M3/84 BD Biosciences). Immunoperoxidase staining was performed using a biotinylated secondary antibody and ABC kit (Vector Laboratories Inc.), and the reaction was visualized using a 3-amino-9-ethyl-carbazole substrate (AEC, DAKO California). Slides were scanned using a Nanozoomer 2.0RS (Hamamatsu, Japan), and the percentage positive area was quantitated with IPlab (version 3.9.3; Scanalytics) in 4–5 high power fields (HPF) per slide.
Flow cytometry
Transplanted hearts, para-aortal lymph nodes, spleen, and blood were collected and processed immediately (n=5 per group). Single cell suspensions were produced in flow cytometry (FACS) buffer. Approximately 3 million cells were diluted in 200 microliter of FACS buffer for staining with antibodies against leukocyte lineage markers (B220, CD49b, CD90, Ly6G, NK1.1, and Ter119), CD11b, CD11c, F4/80, Ly6C, and MHC class II (major histocompatibility complex class II; BD Pharmingen and eBioscience). To detect antigen presentation by flow cytometry, we stained the harvested cell suspensions with Y-Ae antibody (1:30, eBioscience), which recognizes Balb/c Ea52–68 peptide presented in context with B6 IAb (MHC class II). The stained cells were then analyzed using a LSRII flow cytometer (BD biosciences). The data were analyzed using flowjo software (Tree Star, Inc.).
Statistics
Results are expressed as means ± SEM. Statistical comparisons between two groups were evaluated using the Student’s t-test and corrected using analysis of variance (ANOVA) for multiple comparisons. Kaplan-Meier survival graphs were constructed, and a logrank comparison of the groups was used to calculate P values. A value of p<0.05 was considered statistically significant. We used Prism 6 (Graphpad) for statistical analysis.
Results
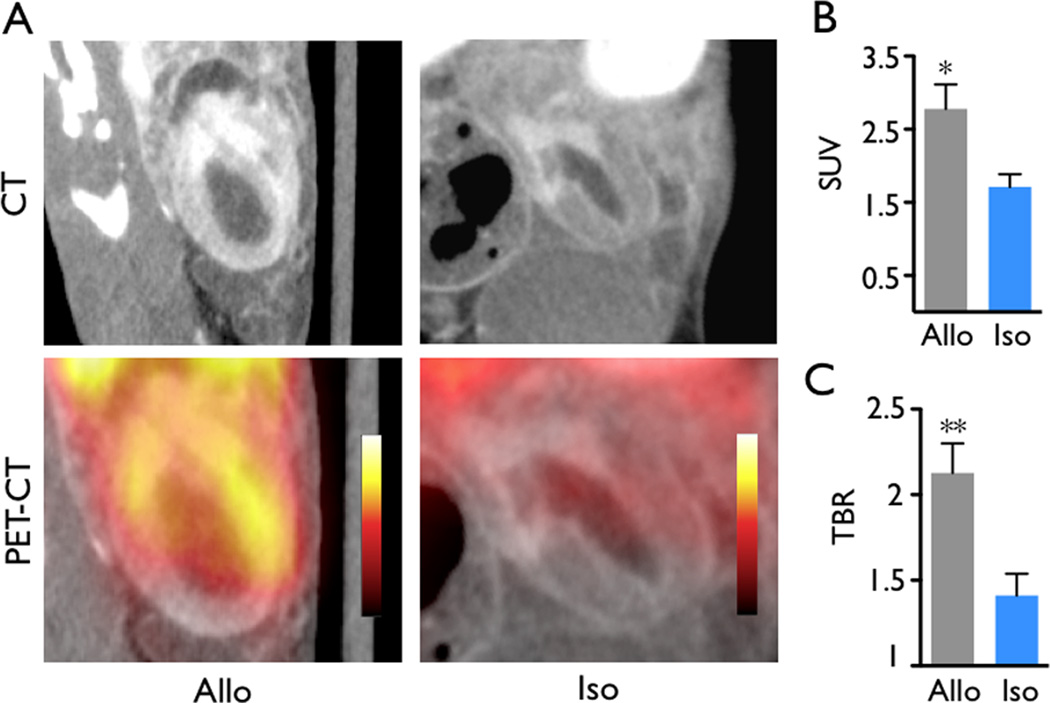
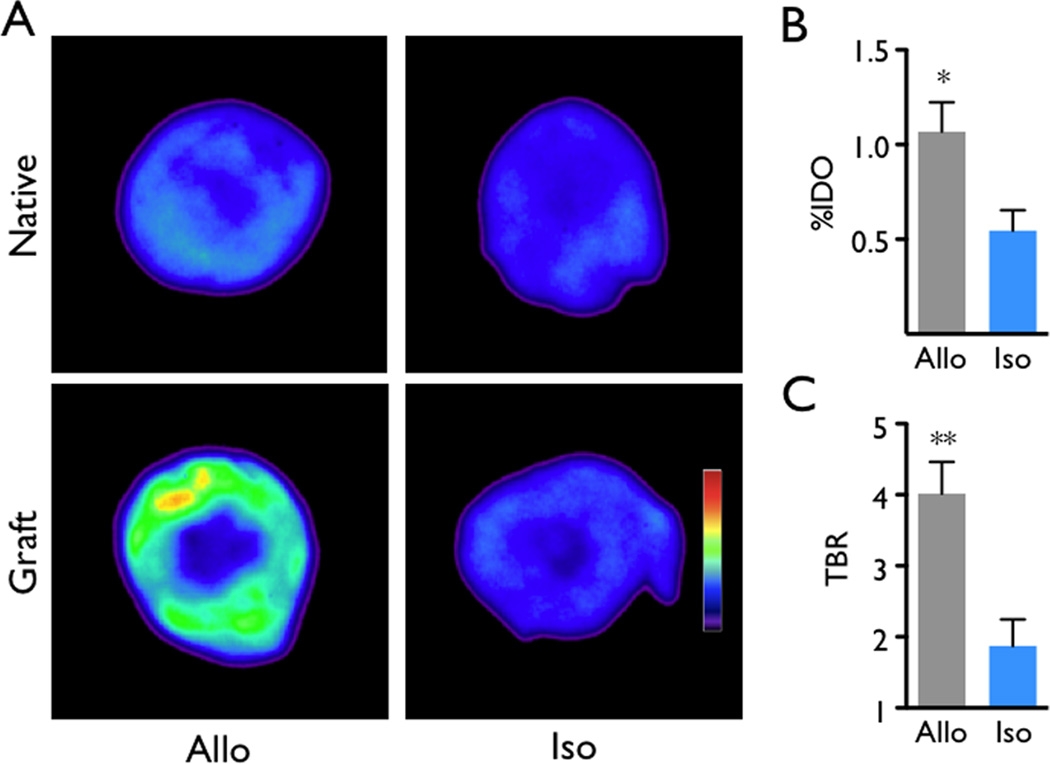
We found that the in vivo nanoparticle PET signal in the allografts was higher than that in isograft controls (standard uptake value, 2.8±0.3 versus 1.7±0.2, p<0.05, Figure 1A, B). The target-to-background ratio, using the native heart for normalization to background radioactivity, was also increased in allograft recipients (Figure 1C, 2.1±0.2 versus 1.4±0.1, p<0.01), reflecting lower uptake of nanoparticles by the native, non-inflamed heart. To validate these in vivo findings, we next harvested the hearts for ex vivo analysis. Scintillation counting of the organ as well as autoradiography both showed increased graft activity (Figure 2, p<0.05). In agreement with previous data9, 12, allografts also exhibited increased levels of CD11b+ myeloid cells and MAC-3+ MΦ (Figure 3A), cells that avidly ingest dextran nanoparticles9, 14–16.
Figure 1.
In vivo nanoparticle PET-CT 7 days after transplantation. (A) Heterotopic grafts in the recipient's abdomen. The allograft (Allo) showed higher PET signal compared to the isograft (Iso). (B) Allografts showed significantly higher standard uptake value (SUV) and (C) target-to-backgound ratios (TBR) than isograft controls. Data are mean ± SEM, *p<0.05, **p<0.01.
Figure 2.
Ex vivo validation of rejection imaging by autoradiography of native and transplanted hearts. (A) Representative autoradiographic images showed higher signals in allografts. (B) Scintillation counting of explanted grafts yields higher percent injected dose per organ (%IDO) in allografts. (C) Target to background ratio (TBR) of grafted hearts. The native heart served as background. Data are mean ± SEM, *p<0.05, **p<0.01.
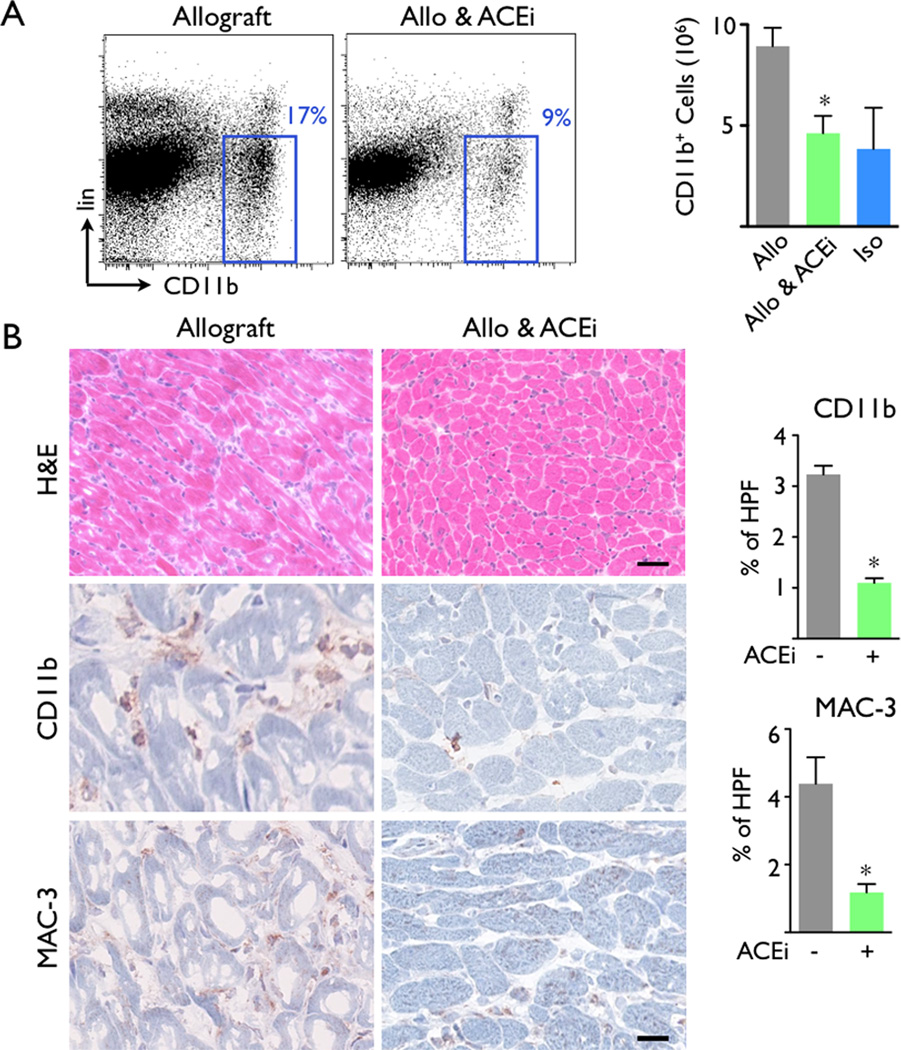
Figure 3.
Angiotensin Enzyme Converting inhibitor treatment (ACEi) decreased the accumulation of CD11b+ cells. (A) H&E and immunohistological stains using CD11b and MAC-3 showed that ACEi treatment decreased inflammation in allografts. Scale bar indicates 50µm. (B) ACEi administration significantly reduced CD11b+ monocyte/macrophage numbers in grafted hearts compared to untreated recipients. Iso: isograft controls. Data are mean ± SEM, *p<0.05.
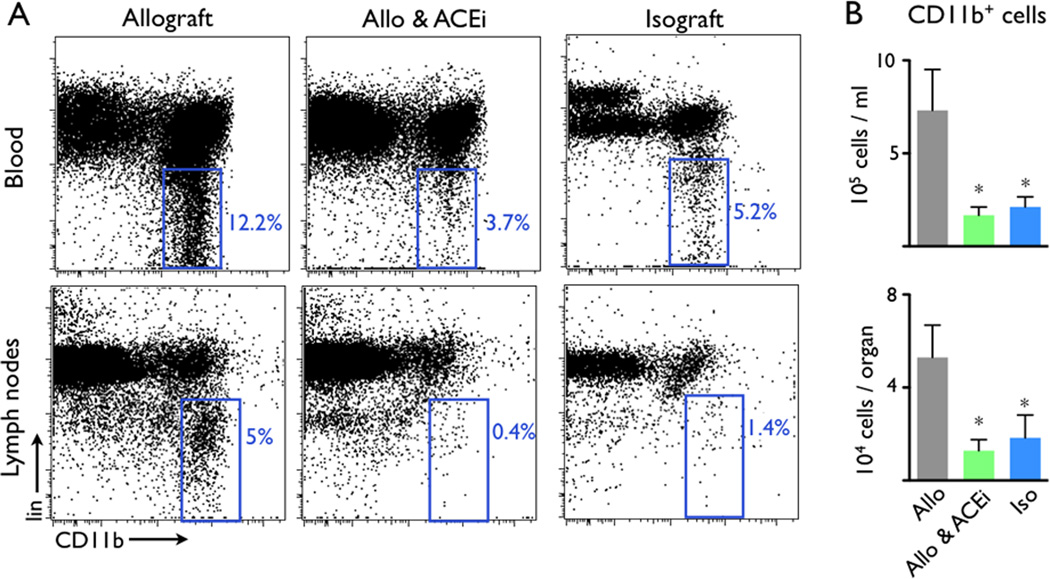
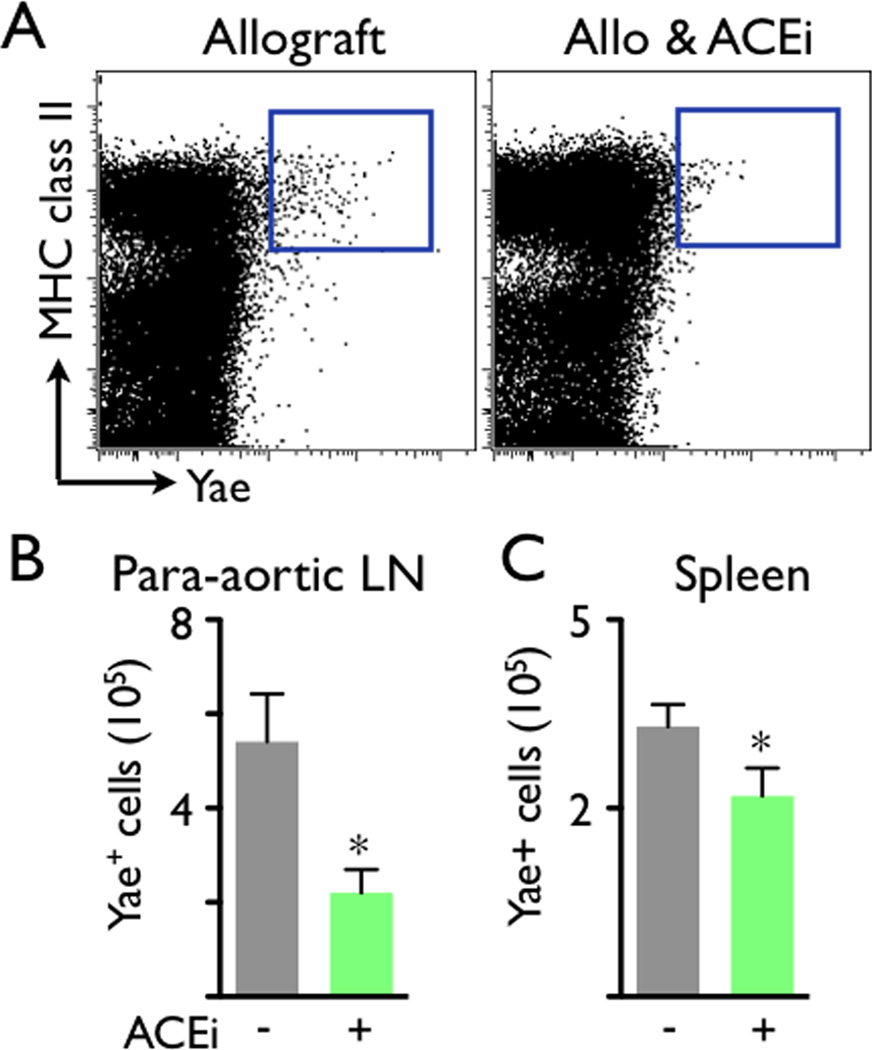
Next, we tested this imaging approach in a trial examining the immunomodulatory actions of ACEi (5mg/kg Enalapril per gavage). Based on our previous finding that angiotensin-II triggers release of inflammatory monocytes into the blood17, 18, we hypothesized that Enalapril treatment would reduce inflammation in mice with allograft rejection by dampening myeloid cell flux into the heart. Indeed, flow cytometry and immunoreactive histology both showed that administration of the ACE inhibitor diminished the presence of CD11b+ myeloid cells and MAC-3+ MΦ in allografts on day 7 after transplantation (Figure 3A, B). ACEi treatment also reduced the number of myeloid cells in the blood and draining lymph nodes of allograft recipients (Figure 4). We then examined whether ACEi reduces antigen presentation, a process that initiates and maintains the adaptive immune response to foreign tissue, including transplanted organs. To this end, we used flow cytometric analysis after staining cell suspensions with an Yae antibody. This antibody detects a peptide from the I-Ea chain bound to I-Ab, reporting on MHCII presentation of BALB/c antigen, mice that had served as heart donors. In graft-draining lymph nodes and the spleen, the number of Yae+ cells was significantly lower in recipients that were treated with Enalapril (p<0.05, Figure 5).
Figure 4.
ACEi reduced the numbers of CD11b+ myeloid cells in allograft recipient blood, spleen and para-aortic lymph nodes. (A) Representative flow cytometry dot plots. The blue gates outline lineage (lin)− CD11b+ monocytes/MΦ. (B) ACEi administration significantly reduced the total number of lin− CD11b+ cells in grafted hearts compared to untreated recipients. Iso: isograft controls. Data are mean ± SEM, *p<0.05.
Figure 5.
Antigen presentation, represented by major histocompatibility complex class II (MHC class II+) Yae+ cells, was reduced in the para-aortic lymph node (LN) and spleen of ACEi-treated allograft recipients.. (A) Representative flow cytometry dot plots. The blue gates outline the MHC class II+ Yae+ cells. (B) Total number of Y-Ae+ cells in the para-aortic lymph node (LN) and spleen of ACEi-treated allograft recipients. Data are mean ± SEM, *p<0.05.
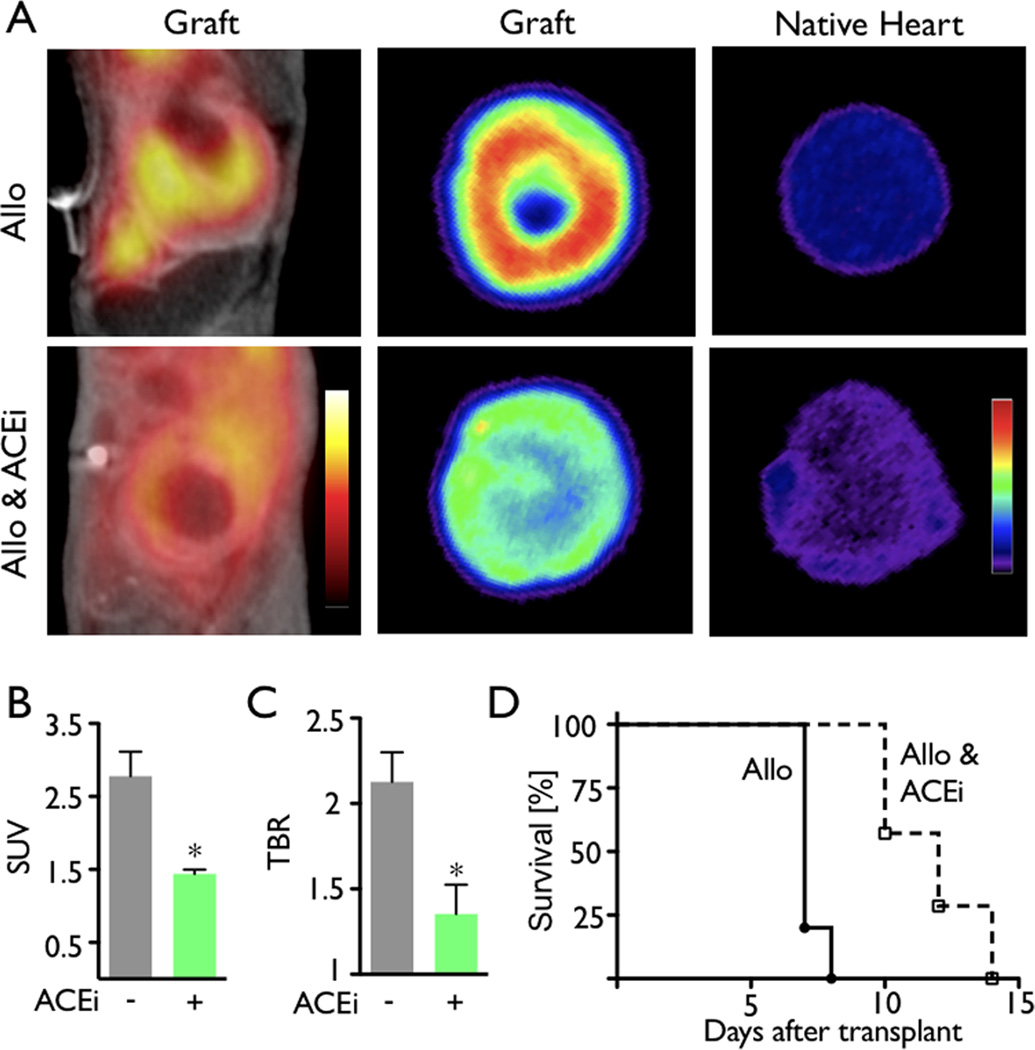
We subsequently studied the anti-inflammatory actions of ACEi in vivo using MΦ-targeted nanoparticle PET-CT. Enalapril treatment was found to significantly reduce the mean PET SUV and target-to-background ratio (Figure 6A–C. Interestingly, the mean allograft survival time increased from seven days in the control group to twelve days in recipients treated with the ACE inhibitor (n=7 per group, Figure 6D, p<0.0001).
Figure 6.
PET–CT detects decreased nanoparticle uptake in allografts following ACEi treatment. (A) In vivo PET-CT and ex vivo autoradiography images of representative allografts in untreated (top) and treated (bottom) groups. (B) In vivo PET signal in allografts displayed as standard uptake value (SUV). (C) Ex vivo target-to-background ratio (TBR) in allografts calculated from autoradiography images. Native hearts served as the background. Data are mean±SEM, **p<0.01, ***p<0.001. (D) Treatment with ACEi resulted in significant prolongation of allograft survival in mice (p<0.0001).
Discussion
Upon receiving a heart allograft, patients are treated with life-long immunosuppressive therapy to suppress rejection of the organ, which is recognized as foreign by the host's immune system. These agents, however, have profound side effects and are thus given at the lowest doses possible. In cases where inflammation of the organ occurs, immunosuppressive therapy must be intensified, which consequently necessitates close monitoring of the patient and the graft. Unfortunately, serial heart biopsy, which is the method recommended by current guidelines1, is associated with a number of shortcomings including complications19, 20 as well as discomfort to the patient. There is thus an urgent need for alternative monitoring strategies, such as leukocyte imaging8, 9, 12, 21 or, possibly more cost-effective in routine surveillance, by following circulating biomarkers22. This study used sensitive MΦ-targeted nanoparticle PET imaging to detect transplant rejection in mice. Copper-64 derivatized dextran nanoparticles were able to sense monocytes/MΦ in rejected heart grafts, and could thus be used to monitor therapeutic immunomodulation in mice with total allo-mismatched hearts.
PET imaging has significant advantages in terms of sensitivity. 18F-FDG, a clinically predominant PET agent, reports on glucose uptake by cells, and can identify sites where MΦ have accumulated and been activated to increase glucose transport. High glucose uptake by cardiac myocytes, however, renders 18F-FDG less suitable for imaging inflammation in the heart, particularly where the target is interstitial inflammation as it is in transplant rejection. In contrast, while nanoparticles undergo avid uptake by myeloid cells, they undergo neglible uptake by other cardiac cells. We therefore employed dextran nanoparticles, which closely resemble nanoparticles in current clinical use23, to image cardiac graft rejection. The particles used in this study have a blood half life of several hours. We thus chose a PET isotope with relatively slow radioactive decay (copper-64, t1/2 12.7 hours) to match the pharmacokinetics of these particles. In doing so, imaging could be delayed sufficiently as to allow nanoparticles not taken up by MΦ to exit the blood stream. Future studies will now explore whether smaller-sized nanoparticles (possibly approaching the 5nm-diameter renal clearence cutoff) accelerate particle excretion such that shorter injection-imaging sequences become feasible; in turn, this would enable use of the more widely available radionuclide fluorine-18 (t1/2 110 minutes).
Heterotopic tranplantation into the abdominal cavity of recipient mice has limitations; namely, it results in a beating but non-working heart and also it has contact with the peritoneum. Translating results from this model to heart transplantation therefore requires caution. In previous preclinical studies, it was demonstrated that MRI could be used to detect heart transplant rejection in rodents8, 9, 24. In a recent clinical trial, it was likewise demonstrated that nanoparticle MRI could detect monocytes/MΦ in the heart of patients with acute myocardial infarction23. This suggests that a similar approach may also work for imaging heart transplant rejection. However, the number of myeloid cells per gram myocardium (or imaging voxel) is particularly high in acute myocardial infarction25, and likely exceeds leukocyte levels in early-stage organ rejection by orders of magnitude. The higher sensitivity of PET versus MRI should facilitate imaging of allograft rejection, particularly in its early stages. Moreover, PET is more readily quantifiable and allows serial data to be compared. This would be particularly useful in clinical trials that employ PET biomarkers as imaging endpoints.
We used PET detection of monocytes/macrophages in heart grafts to study the effects of administration of an ACE inhibitor on organ rejection. In mice treated with Enalapril, the PET signal fell significantly, and allografts survived twice as long when compared to untreated allograft controls. While ACE inhibitor treatment did not abolish rejection (all hearts ceased beating by day 14), our data suggest this therapy could serve as an adjunct to standard immunosuppresive regimen. The rationale for investigating ACE inhibition was based on our previous finding that angiotensin-II triggers release of proinflammatory monocytes from a splenic reservoir into the blood stream17, 18. Indeed, threatment with enalapril reduced the number of circulating monocytes in allograft recepients in the current study, and consequently fewer of these cells accumulated in the graft as assessed by imaging, histology and flow cytometry. Previous clinical reports described prolonged graft survival in patients taking an ACE inhibitor or angitensin-II receptor blocker in kidney transplants26 and heart allografts27 while others did not28. A prospective trial of ACE inhibitor administration in patients after heart transplantation could provide clarity in this regard.
In conclusion, our study shows that MΦ-targeted nanoparticle PET imaging may offer a quantitative and non-invasive alternative to the myocardial biopsies performed after heart transplantation. The method was able to detect both myeloid cells in allografts, as well as their diminution following therapeutic intervention. By optimizing the nanoparticles further, possibly enhancing their pharmacokinetics but still maintaining their phagocyte targeting, it is possible that their clinical translation will be accelerated. Ultimately, our goal is develop a detection method that will eliminate the need for endomyocardial biopsies in allograft recipients.
Acknowledgments
Funding Sources
This work was funded by grants from the NHLBI (R01HL095629, R01HL114477, Contract No. HHSN268201000044C). T.U. is a recipient of the American Heart Association Scientist Development Grant (11SDG5150000). P.D. is a recipient of the American Society of Transplantation Basic Science Fellowship Grant (AST-129).
Footnotes
Disclosures
None.
References
- 1.Miniati DN, Robbins RC. Heart transplantation: a thirty-year perspective. Annu Rev Med. 2002;53:189–205. doi: 10.1146/annurev.med.53.082901.104050. [DOI] [PubMed] [Google Scholar]
- 2.Gradek WQ, D’Amico C, Smith AL, Vega D, Book WM. Routine surveillance endomyocardial biopsy continues to detect significant rejection late after heart transplantation. J Heart Lung Transplant. 2001;20:497–502. doi: 10.1016/s1053-2498(01)00236-4. [DOI] [PubMed] [Google Scholar]
- 3.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hancock WW, Thomson NM, Atkins RC. Composition of interstitial cellular infiltrate identified by monoclonal antibodies in renal biopsies of rejecting human renal allografts. Transplantation. 1983;35:458–463. doi: 10.1097/00007890-198305000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Wyburn KR, Jose MD, Wu H, Atkins RC, Chadban SJ. The role of macrophages in allograft rejection. Transplantation. 2005;80:1641–1647. doi: 10.1097/01.tp.0000173903.26886.20. [DOI] [PubMed] [Google Scholar]
- 6.Garcia MR, Ledgerwood L, Yang Y, Xu J, Lal G, Burrell B, Ma G, Hashimoto D, Li Y, Boros P, Grisotto M, van Rooijen N, Matesanz R, Tacke F, Ginhoux F, Ding Y, Chen SH, Randolph G, Merad M, Bromberg JS, Ochando JC. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J Clin Invest. 2010;120:2486–2496. doi: 10.1172/JCI41628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girlanda R, Kleiner DE, Duan Z, Ford EA, Wright EC, Mannon RB, Kirk AD. Monocyte infiltration and kidney allograft dysfunction during acute rejection. Am J Transplant. 2008;8:600–607. doi: 10.1111/j.1600-6143.2007.02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye Q, Wu YL, Foley LM, Hitchens TK, Eytan DF, Shirwan H, Ho C. Longitudinal tracking of recipient macrophages in a rat chronic cardiac allograft rejection model with noninvasive magnetic resonance imaging using micrometer-sized paramagnetic iron oxide particles. Circulation. 2008;118:149–156. doi: 10.1161/CIRCULATIONAHA.107.746354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christen T, Nahrendorf M, Wildgruber M, Swirski FK, Aikawa E, Waterman P, Shimizu K, Weissleder R, Libby P. Molecular imaging of innate immune cell function in transplant rejection. Circulation. 2009;119:1925–1932. doi: 10.1161/CIRCULATIONAHA.108.796888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buxton DB, Antman M, Danthi N, Dilsizian V, Fayad ZA, Garcia MJ, Jaff MR, Klimas M, Libby P, Nahrendorf M, Sinusas AJ, Wickline SA, Wu JC, Bonow RO, Weissleder R. Report of the National Heart, Lung, and Blood Institute working group on the translation of cardiovascular molecular imaging. Circulation. 2011;123:2157–2163. doi: 10.1161/CIRCULATIONAHA.110.000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nahrendorf M, Sosnovik DE, French BA, Swirski FK, Bengel F, Sadeghi MM, Lindner JR, Wu JC, Kraitchman DL, Fayad ZA, Sinusas AJ. Multimodality cardiovascular molecular imaging, Part II. Circ Cardiovasc Imaging. 2009;2:56–70. doi: 10.1161/CIRCIMAGING.108.839092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swirski FK, Wildgruber M, Ueno T, Figueiredo JL, Panizzi P, Iwamoto Y, Zhang E, Stone JR, Rodriguez E, Chen JW, Pittet MJ, Weissleder R, Nahrendorf M. Myeloperoxidase-rich Ly-6C+ myeloid cells infiltrate allografts and contribute to an imaging signature of organ rejection in mice. J Clin Invest. 2010;120:2627–2634. doi: 10.1172/JCI42304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nahrendorf M, Keliher E, Marinelli B, Waterman P, Feruglio PF, Fexon L, Pivovarov M, Swirski FK, Pittet MJ, Vinegoni C, Weissleder R. Hybrid PET-optical imaging using targeted probes. Proc Natl Acad Sci U S A. 2010;107:7910–7915. doi: 10.1073/pnas.0915163107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nahrendorf M, Keliher E, Marinelli B, Leuschner F, Robbins CS, Gerszten RE, Pittet MJ, Swirski FK, Weissleder R. Detection of macrophages in aortic aneurysms by nanoparticle positron emission tomography-computed tomography. Arterioscler Thromb Vasc Biol. 2011;31:750–757. doi: 10.1161/ATVBAHA.110.221499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leuschner F, Panizzi P, Chico-Calero I, Lee WW, Ueno T, Cortez-Retamozo V, Waterman P, Gorbatov R, Marinelli B, Iwamoto Y, Chudnovskiy A, Figueiredo JL, Sosnovik DE, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res. 2010;107:1364–1373. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gradek WQ, D’Amico C, Smith AL, Vega D, Book WM. Routine surveillance endomyocardial biopsy continues to detect significant rejection late after heart transplantation. J Heart Lung Transplant. 2001;20:497–502. doi: 10.1016/s1053-2498(01)00236-4. [DOI] [PubMed] [Google Scholar]
- 20.Tucker PAn, Jin BS, Gaos CM, Radovancevic B, Frazier OH, Wilansky S. Flail tricuspid leaflet after multiple biopsies following orthotopic heart transplantation: echocardiographic and hemodynamic correlation. J Heart Lung Transplant. 1994;13:466–472. [PubMed] [Google Scholar]
- 21.Wu YL, Ye Q, Foley LM, Hitchens TK, Sato K, Williams JB, Ho C. In situ labeling of immune cells with iron oxide particles: an approach to detect organ rejection by cellular MRI. Proc Natl Acad Sci U S A. 2006;103:1852–1857. doi: 10.1073/pnas.0507198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarwal M, Chua MS, Kambham N, Hsieh SC, Satterwhite T, Masek M, Salvatierra O. Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med. 2003;349:125–138. doi: 10.1056/NEJMoa035588. [DOI] [PubMed] [Google Scholar]
- 23.Alam SR, Shah AS, Richards J, Lang NN, Barnes G, Joshi N, Macgillivray T, McKillop G, Mirsadraee S, Payne J, Fox KA, Henriksen P, Newby DE, Semple SI. Ultrasmall superparamagnetic particles of iron oxide in patients with acute myocardial infarction: early clinical experience. Circ Cardiovasc Imaging. 2012;5:559–565. doi: 10.1161/CIRCIMAGING.112.974907. [DOI] [PubMed] [Google Scholar]
- 24.Hitchens TK, Ye Q, Eytan DF, Janjic JM, Ahrens ET, Ho C. 19F MRI detection of acute allograft rejection with in vivo perfluorocarbon labeling of immune cells. Magn Reson Med. 2011;65:1144–1153. doi: 10.1002/mrm.22702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinze G, Mitterbauer C, Regele H, Kramar R, Winkelmayer WC, Curhan GC, Oberbauer R. Angiotensin-converting enzyme inhibitor or angiotensin II type 1 receptor antagonist therapy is associated with prolonged patient and graft survival after renal transplantation. J Am Soc Nephrol. 2006;17:889–899. doi: 10.1681/ASN.2005090955. [DOI] [PubMed] [Google Scholar]
- 27.Lubitz SA, Baran DA, Alwarshetty MM, Zucker MJ, Arroyo LH, Chan M, Courtney MC, Correa R, Spielvogel D, Lansman SL, Gass AL. Improved survival with statins, angiotensin receptor blockers, and steroid weaning after heart transplantation. Transplant Proc. 2006;38:1501–1506. doi: 10.1016/j.transproceed.2006.02.131. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez D, Muriel A, Abraira V, Perez G, Porrini E, Marrero D, Zamora J, Gonzalez-Posada JM, Delgado P, Rufino M, Torres A. Renin-angiotensin system blockade and kidney transplantation: a longitudinal cohort study. Nephrol Dial Transplant. 2012;27:417–422. doi: 10.1093/ndt/gfr276. [DOI] [PubMed] [Google Scholar]