Abstract
Purpose
Nerve growth factor over expression in the bladder has a role in overactive bladder symptoms via the mediation of functional changes in bladder afferent pathways. We studied whether blocking nerve growth factor over expression in bladder urothelium by a sequence specific gene silencing mechanism would suppress bladder overactivity and chemokine expression induced by acetic acid.
Materials and Methods
Female Sprague-Dawley® rats anesthetized with isoflurane were instilled with 0.5 ml saline, scrambled or TYE™ 563 labeled antisense oligonucleotide targeting nerve growth factor (12 μM) alone or complexed with cationic liposomes for 30 minutes. The efficacy of nerve growth factor antisense treatments for acetic acid induced bladder overactivity was assessed by cystometry. Bladder nerve growth factor expression levels and cellular distribution were quantified by immunofluorescence staining and enzyme-linked immunosorbent assay. Effects on bladder chemokine expression were measured by Luminex® xMAP® analysis.
Results
Liposomes were needed for bladder uptake of oligonucleotide, as seen by the absence of bright red TYE 563 fluorescence in rats instilled with oligonucleotide alone. At 24 hours after liposome-oligonucleotide treatment baseline bladder activity during saline infusion was indistinct in the sham and antisense treated groups with a mean ± SEM intercontraction interval of 348 ± 55 and 390 ± 120 seconds, respectively. Acetic acid induced bladder overactivity was shown by a decrease in the intercontraction interval to a mean of 33.2% ± 4.0% of baseline in sham treated rats. However, the reduction was blunted to a mean of 75.8% ± 3.4% of baseline in rats treated with liposomal antisense oligonucleotide (p <0.05). Acetic acid induced increased nerve growth factor in the urothelium of sham treated rats, which was decreased by antisense treatment, as shown by enzyme-linked immunosorbent assay and reduced nerve growth factor immunoreactivity in the urothelium. Increased nerve growth factor in bladder tissue was associated with sICAM-1, sE-selectin, CXCL-10 and 1, leptin, MCP-1 and vascular endothelial growth factor over expression, which was significantly decreased by nerve growth factor antisense treatment (p <0.01).
Conclusions
Acetic acid induced bladder overactivity is associated with nerve growth factor over expression in the urothelium and with chemokine up-regulation. Treatment with liposomal antisense suppresses bladder overactivity, and nerve growth factor and chemokine expression. Local suppression of nerve growth factor in the bladder could be an attractive approach for overactive bladder. It would avoid the systemic side effects that may be associated with nonspecific blockade of nerve growth factor expression.
Keywords: urinary bladder, overactive, nerve growth factor, urothelium, chemokines, liposomes
Previous studies by various groups demonstrated that NGF over expression in the bladder and bladder afferent pathways is involved in the emergence of hyperexcitability in bladder C-fiber sensory pathways.1,2 C-fiber hyperexcitability is considered responsible for symptoms common to OAB.3 Intrathecal application of NGF antibodies decreased NGF levels in bladder afferent pathways and normalized bladder/urethral function in spinal cord injured rats.4
Recently, exogenous over expression of NGF in the urothelium was shown to cause micturition dysfunction and pelvic hypersensitivity in a transgenic mouse model.2,5 The major role of NGF in lower urinary tract symptoms encouraged the systemic administration of monoclonal human NGF antibodies in patients with mixed results.6 In patients generalized blockade of NGF at sites other than the bladder was associated with the incidence of paresthesia, hypoesthesia and arthralgia.
Therefore, to decrease the intrinsic toxicity of systemic blockade of NGF, we developed what is to our knowledge a novel intravesical therapy for OAB by targeting intracellular synthesis of NGF in the urothelium. We measured the functional efficacy of liposomes complexed with antisense OND targeting NGF, including the effect on the NGF signaling pathway.7-10
METHODS
Reagents
The 18mer phosphorothioated antisense OND was custom made. It had a 5′ tag of TYE 563 (bright red fluorescent dye that is a direct substitute for Cy3™) with the sequence 5′GCCCGAGACGCCTCCCGA3′. A similar length scrambled sequence 5′ACGACCTCGCGACCGGCC3′ was designed using GenScript (https://www.genscript.com/ssl-bin/app/scramble). Cationic liposomes composed of DOTAP were made by the thin film hydration method, in which lipid film was hydrated with nuclease-free water with a final lipid concentration of 7 mM. Lyophilized OND was dispersed in nuclease-free water at a concentration of 12 μM. It was then complexed with liposomes by incubating the 2 entities together at room temperature for 30 minutes. The molar ratio of OND to lipid in the liposomal complex was 1:10.
Animals
The study was performed in 37 female adult Sprague-Dawley rats weighing 225 to 250 gm divided into 6 study groups of 5 to 8 each. We used 2 group for bladder uptake and the remaining 4 for CMG, which also provided tissue for subsequent immunohistochemistry, NGF and chemokine analysis.
Studies
Bladder uptake
Rats were anesthetized with 2% isoflurane. The bladder was catheterized by a 24 gauge angiocatheter (BD™) and washed with saline to instill 0.5 ml fluorescent TYE conjugated OND in saline (6 rats) or complexed with liposomes for 30 minutes (6 rats). A purse-string suture was placed around the urethra to occlude for 30 minutes, which was later withdrawn to restart voiding by awake rats in metabolic cages at the end of instillation. Rats were sacrificed 8 and 24 hours (3 per time point per group) after instillation to harvest bladder tissue for cryosectioning into 8 μm cryosections.
Efficacy
Rats were instilled with 12 μM NGF antisense (6) or scrambled OND complexed with liposome (5), or with saline as sham treatment (8) after the described bladder uptake studies. CMG was done 24 hours later in the 4 groups, including a control group of 6 rats without prior instillation, under anesthesia using urethane (1.0 gm/kg subcutaneously). A polyethylene-50 catheter was connected by a 3-way stopcock to a pressure transducer and to a syringe pump. The catheter was inserted in the bladder through the dome to record intravesical pressure and infuse solutions into the bladder. Intravesical pressure was recorded with Chart™ data acquisition software at a sampling rate of 400 Hz on a computer system equipped with an analog-to-digital converter. Body temperature was maintained in the physiological range with a heat lamp.
Control CMG was performed by filling the bladder with saline at 0.04 ml per minute to elicit repetitive voiding for more than 1 hour. Subsequently, bladder irritation was induced by 0.25% AA infusion for more than 3 hours to induce BO, which is considered similar to the phenotype of OAB observed clinically. The ICI of reflex bladder contractions during saline and AA infusion was determined as the time between 2 continuing contraction cycles. We compared the average of at least 3 ICIs measured for more than 30 minutes during saline infusion and 60 minutes after AA infusion, respectively.
Immunohistochemistry
At the end of CMG, a portion of the bladders (5 preparations) from each group were cryopreserved. Cryosections (8 μm) were washed in PBS and preincubated with PBS containing 20% normal serum (Jackson ImmunoResearch, West Grove, Pennsylvania) and 0.2% Triton™ X-100 for 2 hours at room temperature. Primary polyclonal rabbit H-20 antibody (1:50) (Santa Cruz Biotechnology, Santa Cruz, California) for NGF was applied in PBS containing 5% normal serum and 0.2% Triton X-100 for 16 to 18 hours at 4C. Sections were washed 4 times in PBS containing 0.1% bovine serum albumin and 0.1% Triton X-100 for 5 minutes each at room temperature. They were then incubated for 2 hours at room temperature with secondary donkey anti-rabbit Alexa Fluor® 488 antibody (1:200). Washing was performed 3 times at room temperature in PBS. Sections were mounted with aqueous mounting medium.
Harvested Bladder NGF
At the end of CMG, mucosa containing the urothelium was surgically separated from the detrusor in a portion of rat bladder from each group (5 preparations), as previously described.11 Tissues were homogenized using the RIPA Lysis Buffer System (Santa Cruz Biotechnology) to isolate protein and measure NGF using antigen capture enzyme-linked immunosorbent assay with the Emax® ImmunoAssay System according to manufacturer instructions, as previously described.12 Tissue NGF values are expressed as pg/mg protein. Total RNA was isolated from whole bladders in control and antisense treated rats. It was later transcribed into cDNA to measure NGF mRNA levels by quantitative polymerase chain reaction, as previously described.13
Effect on Downstream Effectors of NGF Signaling Pathway
Tissue lysates prepared from whole bladder (5 preparations) were analyzed for the chemokines sICAM-1,14 sE-selectin, MCP-1,9 VEGF,8 leptin,7 and CXCL-19 and 10 using the Luminex xMAP kit, as previously described.9
Statistical Analysis
Results are shown as the mean ± SEM. Statistical significance between the mean values of different groups was analyzed using 1-way ANOVA, followed by the Tukey post test. The correlation of NGF and chemokine expression was assessed by the Pearson r and Spearman rs correlation tests. In all statistical tests the minimum criterion chosen to discard the null hypothesis was set at p <0.05.
RESULTS
Bladder Uptake
Rat bladders harvested after OND instillation with or without liposomes were cryosectioned for viewing under a LSM 510 META confocal microscope (Carl Zeiss, Jena, Germany). The fluorescent signal was used as a measure of bladder uptake of OND after instillation. The need for liposomes in successful OND delivery was demonstrated by the lack of bright red fluorescence from the TYE 563 tag of OND in the absence of liposomes (fig. 1, A). Figure 1, B and C show the bright field image since there was no fluorescent signal in the images from that group. Liposomes mediated bladder uptake of OND was evident due to intense fluorescence at 8 hours (fig. 1, B). Penetration depth was restricted to the urothelium but it increased from 8 to 24 hours (fig. 1, C). Fluorescence localization in the urothelium revealed successful bladder uptake and retention of OND in target cells.
Figure 1.
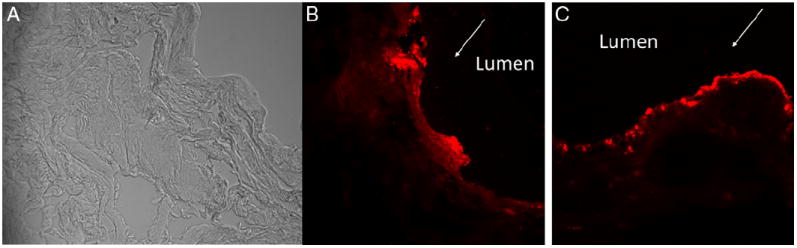
Confocal images show harvested rat bladders instilled with antisense OND with 5′ tag of TYE 563 without liposome (A), and with OND complexed with liposomes at 8 (B) and at 24 (C) hours. Bright red fluorescence demonstrates successful uptake and retention in target cells of OND delivered by liposomes (B and C). Fluorescence was more homogenous in urothelium at 24 hours vs discrete localization to lumen surface at 8 hours. Arrow indicates lumen side. Bright field image is also shown since there was no fluorescent signal for image to be taken for group instilled with antisense OND with 5′ tag of TYE 563 without liposome (A). Absence of red fluorescence reveals liposome need for successful bladder uptake of OND. Reduced from ×40.
Cystometry
Baseline CMG under saline infusion was indistinct between the groups, as evident by the mean ICI of 348 ± 55 and 390 ± 120 seconds in the sham and liposomal antisense treated groups, respectively. AA induced BO was evident in the sham treated group due to the mean percent reduction to 33.2% ± 4.0% of baseline ICI (fig. 2, A). Pretreatment with anti-sense OND complexed with liposomes blocked AA induced BO with the mean percent reduction restored to 75.8% ± 3.4% of baseline (6 preparations) (fig. 2, A). Sequence specificity of NGF antisense was shown by the lack of effect in the group instilled with scrambled OND sequence complexed with liposomes (fig. 2, A). ICI was longer in the liposomal antisense treated group than in the sham treated group. Differences were statistically significant (1-way ANOVA followed by the Tukey post test p <0.05, fig. 2, B).
Figure 2.
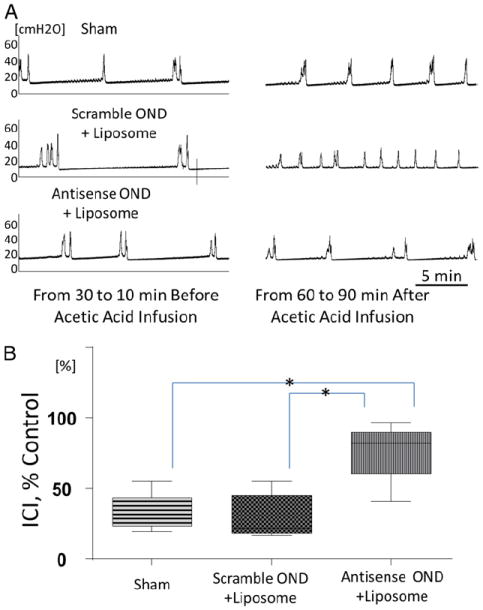
CMG analysis of bladder overactivity in treated groups induced by intravesical application of 0.25% AA. Representative CMG was performed 24 hours after instillation of saline in sham treated group (Sham), liposomal complex of scrambled OND or antisense OND (A). Note CMG traces 30 to 10 minutes before and 60 to 90 minutes after AA application. AA-induced reduction in ICI was seen in sham and scrambled OND treated rats but not in group treated with liposomal NGF antisense complex which demonstrates antisense OND protective effect. CMG parameters in absence of AA did not significantly differ in 3 groups. Changes in ICI after intravesical AA application expressed as percent of control ICI before AA (B). AA induced ICI decrease was significantly smaller in liposome-NGF antisense treated group vs sham and liposome-scrambled OND treated groups. Asterisk indicates p <0.05.
Bladder NGF
AA exposure increased NGF production in the sham treated group relative to controls. Pretreatment with NGF antisense OND significantly blocked AA induced NGF over expression in the urothelium as well as in detrusor lysates (fig. 3, A and B). Changes in NGF protein expression varied in scrambled OND treated rats, resulting in insignificant changes in NGF protein expression. However, it tended to decrease, as shown by the plot of individual values for each group (fig. 3, A and B). Results could be directly inferred from the urothelial uptake of OND facilitated by liposomes (fig. 3, A), while the effect on detrusor NGF levels may be explained by the downstream effect of NGF expressed in the urothelium.2
Figure 3.
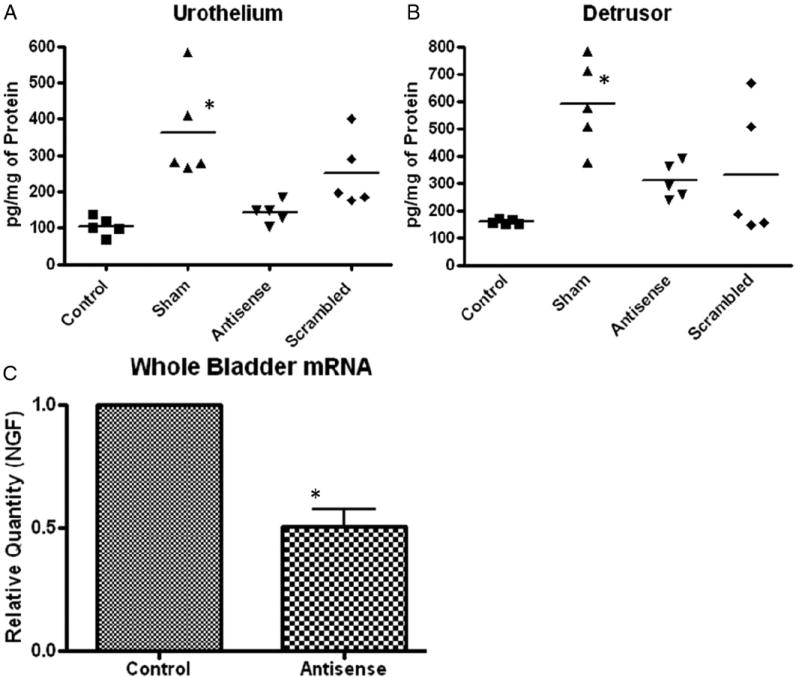
Antisense OND mediated suppression of AA induced NGF protein expression in urothelium (A) and detrusor (B), and NGF mRNA expression in whole bladder (C). AA exposure caused significant increase in NGF in sham treated group (Sham) vs untreated controls. NGF antisense OND significantly blunted NGF increase vs sham treated group. Data points represent NGF values of individual rats around mean (horizontal line) of each group. Significant decrease was noted in whole bladder mRNA levels of rats treated with NGF antisense OND complexed with liposomes vs levels in controls (C). Normalized NGF levels were compared by ANOVA followed by Tukey test. Asterisk indicates p <0.05.
NGF mRNA levels were measured in controls. NGF transcript levels in the urothelium were around 25% higher than in the detrusor (data not shown). Tissue damage from 3-hour exposure to AA during CMG hindered successful isolation of mRNA from the bladder tissue of treated groups, except for the control and antisense OND treated groups. A significant reduction in NGF transcript levels was observed in rats treated with NGF anti-sense OND complexed with liposomes compared to controls (p <0.05, fig. 3, C).
Effect on Downstream Effectors of NGF Signaling Pathway
Measurement of tissue lysates from different groups revealed that AA exposure increased the expression of chemokines activated by NGF, including sICAM-1, sE-selectin, CXCL-1 and 10, leptin, MCP-1 and VEGF (figs. 4 and 5). sE-selectin and sICAM-1 are adhesion molecules expressed by chemokine activated endothelial cells to mediate leukocyte and lymphocyte adhesion. The involvement of NGF in chemokine expression was shown by an increase in chemokines with scrambled OND and a significant reduction with liposomal NGF antisense (ANOVA followed by the Tukey test p <0.05, p <0.01 or p <0.001). Except for the high leptin levels in sham treated rats, the levels of other chemokines were highest for scrambled OND. AA induced over expression of other chemokines, including sICAM-1, sE-selectin, CXCL-1 and 10, VEGF and MCP-1, was further increased in the scrambled OND treated group vs the sham treated group. This did not rule out any nonspecific induction of chemokine expression by scrambled OND.
Figure 4.
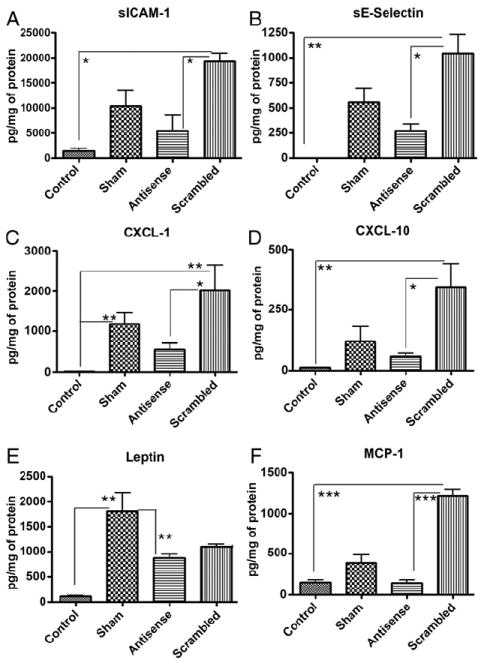
Effect of antisense OND on chemokines activated by NGF signaling pathway. NGF antisense OND significantly reduced chemokine over expression (A to F). Except for leptin, which was highest in sham treated rats (Sham), chemokine levels were highest in rat bladders instilled with scrambled sequence OND complexed with liposomes. Bars represent mean ± SEM. Significance of differences was determined vs sham or scramble treated rats by ANOVA followed by Tukey test. Single asterisk indicates p <0.05. Double asterisks indicate p <0.01. Triple asterisks indicate p <0.001.
Figure 5.
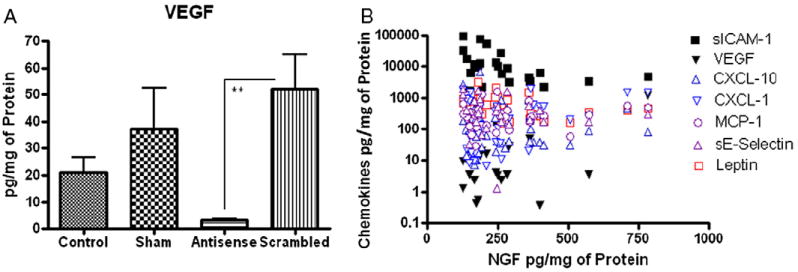
Antisense OND effect on VEGF (A) and NGF plotted with chemokines regardless of treatment group (B). VEGF downstream expression was significantly reduced by antisense OND (A). Decreased VEGF was positively associated with NGF across all treatment groups (Pearson r = 0.75, p <0.001) (B). sICAM-1 was negatively associated with NGF levels regardless of treatment group (Spearman rs = −0.5, p <0.01). When only NGF in scrambled group was plotted against respective chemokines, MCP-1 together with VEGF positively correlated with NGF. Asterisks indicate Pearson r = 0.88, p <0.01 (A).
When NGF levels were plotted against chemokine levels regardless of treatment group, we noted a positive association with VEGF (Pearson r = 0.75, p <0.001) and a negative association with sICAM-1 (Spearman rs = −0.5, p <0.01, fig. 3, A and B). Separate analysis of higher chemokine levels in the scrambled OND group revealed that, apart from VEGF, MCP-1 also positively correlated with NGF (r = 0.88, p <0.01).
NGF Immunostaining
Immunoreactivity for NGF (green staining) was noted in the detrusor region in all groups and it was absent in the apical cells of bladder mucosa containing urothelium untreated with AA (fig. 6, A). NGF immunoreactivity appeared to increase several fold in the detrusor. Its presence was distinctly identified in mucosa containing urothelium after AA infusion in the sham treated group (fig. 6, C). AA induced NGF immunoreactivity in the urothelium was also noted in rats instilled with scrambled OND (fig. 6, B). In contrast, NGF immunoreactivity in the urothelium, including a subpopulation of suburothelial cells, was decreased to levels in controls by pre-treatment with liposomal antisense OND (fig. 6, A and D). Control sections incubated in the absence of primary or secondary antibody were evaluated for specificity or background staining. In the absence of primary antibody, no positive immunoreactivity was observed.
Figure 6.
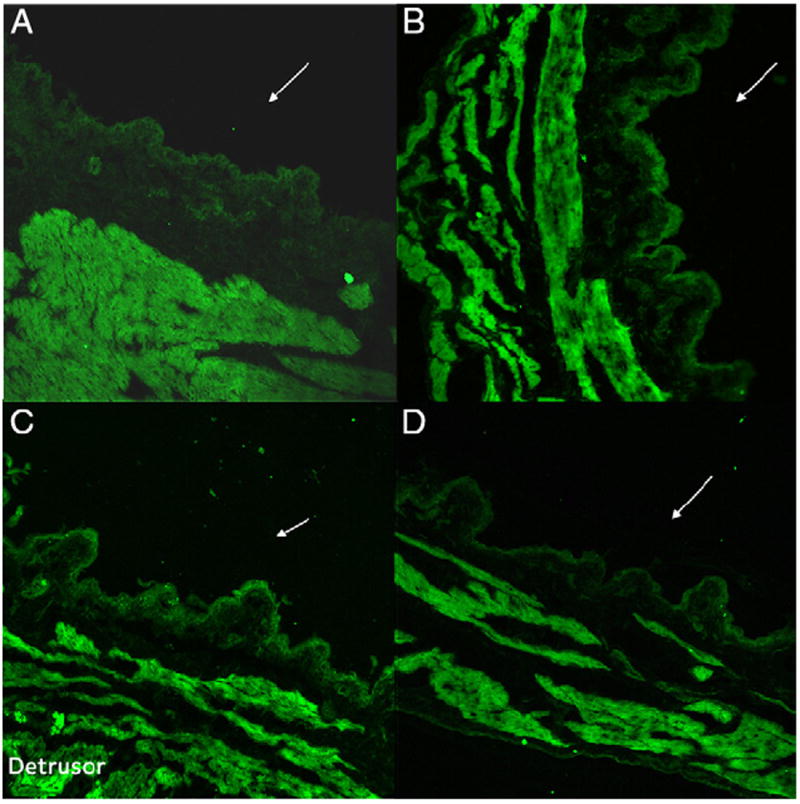
Representative immunofluorescence labeling in rat bladder cross sections reveals that density of NGF immunoreactivity (green areas) was highest in detrusor in all groups. It increased after AA treatment in accordance with NGF expression in muscle. NGF immunoreactivity in bladder mucosa containing urothelium cell layer was absent in rats untreated with AA (A). It was noted in sham (C) and scrambled OND (B) treated rats. NGF immunoreactivity in urothelial cell layer was reduced in rats treated with antisense OND complexed with liposomes (D) to levels comparable to controls (A). Reduced from ×20.
DISCUSSION
The bladder is presumed to be the tissue source responsible for increased NGF in the urine of patients with OAB or interstitial cystitis/painful bladder syndrome compared to controls.15 Since NGF over expression in the bladder is implicated as the mediator of symptoms associated with OAB, NGF can be blocked directly by antibodies6 or indirectly by halting the translation of NGF mRNA with sequence specific gene silencing (antisense).
The primary impediment to developing intravesical antisense therapy is inefficient bladder uptake of OND across the urothelium. Bladder uptake of fluorescent OND without liposomes is deficient due to poor intracellular passage of OND across the urothelium (fig. 1, A). Uptake of anionic OND is probably limited by the size of the OND and the charge interaction with the urothelial anionic glycosaminoglycan layer. Our prior studies of liposome interaction with urothelial cells showed liposome adsorption and endocytosis.16 Cationic liposomes were successfully used to deliver siRNA after intravesical administration in the murine bladder.17 The current study supports the use of cationic liposomes as an OND carrier.
The rapid increase in NGF protein levels noted in sham treated rats after AA exposure could be blunted by pretreatment with NGF antisense OND (figs. 3 and 6, C). Down-regulation of NGF mRNA expression is in agreement with decreased protein levels and suppressed NGF-like immunoreactivity in the urothelium (figs. 3, C and 6, D). CMG data, NGF levels and chemokine suppression together support our hypothesis that NGF released from the urothelium is an important chemical mediator responsible for changes in bladder function (figs. 2 to 6).18
Rats pretreated with scrambled OND were devoid of any functional treatment response on CMG or any chemokine suppression (figs. 2, 4 and 5). However, scrambled OND was associated with variable but insignificantly reduced NGF protein compared to sham treated levels (fig. 3, A and B). The disparity in chemokine and NGF levels in the treated groups indicates interaction between chemokine and NGF expression in the bladder after intravesical AA infusion. Increased chemokine expression with scrambled OND indicates aggravated tissue inflammation, which may cause mRNA and protein decay. mRNA degradation may partially explain the inability to perform mRNA analysis in some groups, although it is unclear whether protein degradation had a role in the reduced NGF protein levels in different groups (fig. 3). Future studies in animals that over express the NGF gene may clarify the disparity in NGF and chemokine levels caused by antisense OND.
Increased NGF expression in the urothelium and detrusor combined with dense NGF immunoreactivity in the detrusor confirmed them as 2 major sources of bladder NGF (figs. 3, A and B, and 6).19 Considering our bladder uptake data (fig. 1), we cannot rule out the modulation of detrusor NGF levels by freshly produced NGF in the urothelium.10 NGF immunoreactivity in the absence of AA exposure may be due to receptor bound NGF that is not available to act on the detrusor. The decrease in NGF protein levels and immunoreactivity after antisense treatment is similar to the decreased urinary excretion of NGF protein in patients with OAB after antimuscarinic or Botox® therapy.11,20
It was previously reported that BO caused by exposure to irritants (turpentine), akin to the AA induced BO that we studied, involves a rapid increase in the bladder content of NGF, which occurs within 2 hours of irritant exposure.21 Later studies showed that within 30 minutes of exposure to insults such as lipopolysaccharide, the bladder responds by up-regulating the genes of NGF and sE-selectin, and the receptor of MCP-1.10 These earlier results agree with our findings of the reported increase in the protein levels of NGF, MCP-1, sE-selectin, sICAM-1, leptin, CXCL-1 and 10, and VEGF in bladder tissue after 3-hour AA exposure (figs. 3 to 5).
NGF is a paracrine messenger involved in physiological and pathological signaling2 that activates several downstream effectors to manifest signaling changes.7,8,10,14 Chemokines are one of several downstream effectors activated by NGF.7,8,10,14 Interestingly, chemokine receptors are widely expressed in neural and nonneural elements of the nociceptive pathways responsible for visceral and somatic pain sensation.22 MCP-123 and CXCL-1024 are constitutively expressed in neurons (fig. 4, D and F), where they participate in the excitability of primary afferent neurons via transactivation of transient receptor channels and nociceptor sensitization.24 Chemokine localization in neuron synaptic vesicles is consistent with their ability to act as excitatory neurotransmitters after AA exposure.25
It was recently reported that NGF binding to its high affinity TrkA receptor controls sICAM-1 expression on target cells14 and inhibition of NGF expression significantly down-regulates ICAM-1 expression.14 The negative association of NGF with sICAM-1 in all treatment groups corroborates the reported regulating effect of NGF on sICAM-1 expression (figs. 4, A and 5, B).14 The reported activation of VEGF expression by NGF8 is consistent with the positive association of VEGF and NGF levels in all treatment groups (fig. 5, B).
AA exposure is not known to selectively induce NGF expression and the involvement of other mediators, such as prostaglandins,26 in the BO model tested cannot be ruled out. The role of prostaglandins in AA induced BO may explain the variable expression of chemokines in the different groups. Prostaglandins induce mRNA coding for CXCL-127 and MCP-1,28 while at the same time decreasing leptin mRNA levels (fig. 4, C, E and F).28 NGF dependent leptin expression7 is presumed to emerge from bladder adipocytes associated with afferent neurons.29 Compared to the short biological half-life of prostaglandins, chemokines are long acting downstream effectors and may be better suited to track treatment response in tissue or urine.9,30
Taken together, our data support the hypothesis that increased bladder NGF content after AA irritation can be blocked by local instillation of antisense OND complexed with liposomes. These observations are consistent with the presumed role of NGF in OAB symptoms.20 However, to our knowledge it remains to be determined how NGF expression blockade in the urothelium affects the excitability of bladder afferents leading to BO. Future steps in the drug development of this strategy will be the duration of effect and the effect in other models of bladder irritation and overactivity.
CONCLUSIONS
NGF down-regulation as novel treatment for BO also suppresses the downstream signaling cascades activated by NGF leading to reduced chemokine expression. The intravesical route is the most appropriate choice for anti-NGF therapy since the bladder is the putative source of NGF responsible for increased C-fiber afferent nerve excitability and BO. Liposomes represent a delivery platform for the local delivery of antisense based therapy that may avoid systemic toxicity.
Acknowledgments
OND was made at Integrated DNA Technologies, San Diego, California.
Study received animal care and use committee approval.
Supported by National Institutes of Health DK057267 and DK088836, and Department of Defense W81XWH-11-1-0763 and W81XWH-12-1-0565.
Abbreviations and Acronyms
- AA
acetic acid
- BO
bladder overactivity
- CMG
cystometrogram
- ICI
intercontraction interval
- MCP-1
monocyte chemoattractant protein-1
- NGF
nerve growth factor
- OAB
overactive bladder
- OND
oligonucleotide
- PBS
phosphate buffered saline
- sE-selectin
soluble endothelial adhesion molecule
- sICAM-1
soluble intracellular adhesion molecule
- VEGF
vascular endothelial growth factor
References
- 1.Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999;19:4644. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnegelsberg B, Sun TT, Cain G, et al. over expression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol. 2010;298:R534. doi: 10.1152/ajpregu.00367.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ochodnicky P, Cruz CD, Yoshimura N, et al. Neurotrophins as regulators of urinary bladder function. Nat Rev Urol. 2012;9:628. doi: 10.1038/nrurol.2012.178. [DOI] [PubMed] [Google Scholar]
- 4.Seki S, Sasaki K, Fraser MO, et al. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol. 2002;168:2269. doi: 10.1016/S0022-5347(05)64369-8. [DOI] [PubMed] [Google Scholar]
- 5.Zhang YH, Vasko MR, Nicol GD. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na(+) current and delayed rectifier K(+) current in rat sensory neurons. J Physiol. 2002;544:385. doi: 10.1113/jphysiol.2002.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans RJ, Moldwin RM, Cossons N, et al. Proof of concept trial of tanezumab for the treatment of symptoms associated with interstitial cystitis. J Urol. 2011;185:1716. doi: 10.1016/j.juro.2010.12.088. [DOI] [PubMed] [Google Scholar]
- 7.Cardenas S, Scuri M, Samsell L, et al. Neurotrophic and neuroimmune responses to early-life Pseudomonas aeruginosa infection in rat lungs. Am J Physiol Lung Cell Mol Physiol. 2010;299:L334. doi: 10.1152/ajplung.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura K, Tan F, Li Z, et al. NGF activation of TrkA induces vascular endothelial growth factor expression via induction of hypoxia-inducible factor-1alpha. Mol Cell Neurosci. 2011;46:498. doi: 10.1016/j.mcn.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyagi P, Barclay D, Zamora R, et al. Urine cytokines suggest an inflammatory response in the overactive bladder: a pilot study. Int Urol Nephrol. 2010;42:629. doi: 10.1007/s11255-009-9647-5. [DOI] [PubMed] [Google Scholar]
- 10.Saban MR, Hellmich H, Nguyen NB, et al. Time course of LPS-induced gene expression in a mouse model of genitourinary inflammation. Physiol Genomics. 2001;5:147. doi: 10.1152/physiolgenomics.2001.5.3.147. [DOI] [PubMed] [Google Scholar]
- 11.Ha US, Park EY, Kim JC. Effect of botulinum toxin on expression of nerve growth factor and transient receptor potential vanilloid 1 in urothelium and detrusor muscle of rats with bladder outlet obstruction-induced detrusor overactivity. Urology. 2011;78:721 e1. doi: 10.1016/j.urology.2011.03.070. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs BL, Smaldone MC, Tyagi V, et al. Increased nerve growth factor in neurogenic overactive bladder and interstitial cystitis patients. Can J Urol. 2010;17:4989. [PubMed] [Google Scholar]
- 13.Tyagi S, Tyagi P, Van-le S, et al. Qualitative and quantitative expression profile of muscarinic receptors in human urothelium and detrusor. J Urol. 2006;176:1673. doi: 10.1016/j.juro.2006.06.088. [DOI] [PubMed] [Google Scholar]
- 14.Othumpangat S, Regier M, Piedimonte G. Nerve growth factor modulates human rhinovirus infection in airway epithelial cells by controlling ICAM-1 expression. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1057. doi: 10.1152/ajplung.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu HT, Tyagi P, Chancellor MB, et al. Urinary nerve growth factor but not prostaglandin E2 increases in patients with interstitial cystitis/bladder pain syndrome and detrusor overactivity. BJU Int. 2010;106:1681. doi: 10.1111/j.1464-410X.2009.08851.x. [DOI] [PubMed] [Google Scholar]
- 16.Nirmal J, Tyagi P, Dang L, et al. Endocytosis uptake of liposomes in urothelium cells detected by transmission electron microscopy. J Urol. 2012;187:e15. [Google Scholar]
- 17.Nogawa M, Yuasa T, Kimura S, et al. Intravesical administration of small interfering RNA targeting PLK-1 successfully prevents the growth of bladder cancer. J Clin Invest. 2005;115:978. doi: 10.1172/JCI23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupont MC, Spitsbergen JM, Kim KB, et al. Histological and neurotrophic changes triggered by varying models of bladder inflammation. J Urol. 2001;166:1111. [PubMed] [Google Scholar]
- 19.Tanner R, Chambers P, Khadra MH, et al. The production of nerve growth factor by human bladder smooth muscle cells in vivo and in vitro. BJU Int. 2000;85:1115. doi: 10.1046/j.1464-410x.2000.00562.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu HT, Chancellor MB, Kuo HC. Decrease of urinary nerve growth factor levels after antimuscarinic therapy in patients with overactive bladder. BJU Int. 2009;103:1668. doi: 10.1111/j.1464-410X.2009.08380.x. [DOI] [PubMed] [Google Scholar]
- 21.Oddiah D, Anand P, McMahon SB, et al. Rapid increase of NGF, BDNF and NT-3 mRNAs in inflamed bladder. Neuroreport. 1998;9:1455. doi: 10.1097/00001756-199805110-00038. [DOI] [PubMed] [Google Scholar]
- 22.White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci U S A. 2007;104:20151. doi: 10.1073/pnas.0709250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banisadr G, Gosselin RD, Mechighel P, et al. Constitutive neuronal expression of CCR2 chemokine receptor and its colocalization with neurotransmitters in normal rat brain: functional effect of MCP-1/CCL2 on calcium mobilization in primary cultured neurons. J Comp Neurol. 2005;492:178. doi: 10.1002/cne.20729. [DOI] [PubMed] [Google Scholar]
- 24.Bhangoo S, Ren D, Miller RJ, et al. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: a mechanism for the development of chronic sensitization of peripheral nociceptors. Mol Pain. 2007;3:38. doi: 10.1186/1744-8069-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung H, Toth PT, White FA, et al. Monocyte chemoattractant protein-1 functions as a neuro-modulator in dorsal root ganglia neurons. J Neurochem. 2008;104:254. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chidiac JJ, Al-Asmar B, Rifai K, et al. Inflammatory mediators released following application of irritants on the rat injured incisors. The effect of treatment with anti-inflammatory drugs. Cytokine. 2009;46:194. doi: 10.1016/j.cyto.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Wang H, Brown J, et al. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J Exp Med. 2006;203:941. doi: 10.1084/jem.20052124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peeraully MR, Sievert H, Bulló M, et al. Prostaglandin D2 and J2-series (PGJ2, Delta12-PGJ2) prostaglandins stimulate IL-6 and MCP-1, but inhibit leptin, expression and secretion by 3T3-L1 adipocytes. Pflugers Arch. 2006;453:177. doi: 10.1007/s00424-006-0118-x. [DOI] [PubMed] [Google Scholar]
- 29.Maeda T, Kiguchi N, Kobayashi Y, et al. Leptin derived from adipocytes in injured peripheral nerves facilitates development of neuropathic pain via macrophage stimulation. Proc Natl Acad Sci U S A. 2009;106:13076. doi: 10.1073/pnas.0903524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tyagi P, Killinger K, Tyagi V, et al. Urinary chemokines as noninvasive predictors of ulcerative interstitial cystitis. J Urol. 2012;187:2243. doi: 10.1016/j.juro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]


