Abstract
Delineating the relative contributions of B lymphocytes during the course of autoimmune disease has been difficult. Therefore, the effects of depleting all mature B cells using a potent CD20 mAb, or depleting circulating and marginal zone B cells using a ligand-blocking CD22 mAb were compared in NZB/W F1 mice, a model for human systemic lupus erythematosus. Single low-dose mAb treatments depleted B cells efficiently in both NZB/W F1 and C57BL/6 mice. Prophylactic B cell depletion by repeated CD20 mAb treatments prolonged survival during pristane-accelerated lupus in NZB/W F1 mice, while CD22 mAb had little effect. Despite effective B cell depletion, neither mAb treatment prevented autoantibody generation. In addition, CD20, CD22, and control mAb-treated NZB/W F1 mice developed anti-mouse IgG autoantibodies in contrast to parental NZB and NZW strains, which may have reduced the effectiveness of B cell depletion. Despite this, low dose CD20 mAb treatment initiated at 12–28 weeks of age and then every 4 weeks significantly delayed spontaneous disease in NZB/W F1 mice. By contrast, B cell depletion starting at 4 wks of age hastened disease onset, which paralleled depletion of the IL-10-producing regulatory B cell subset called B10 cells. B10 cells were phenotypically similar in NZB/W F1 and C57BL/6 mice, but were expanded significantly in young NZB/W F1 mice. Thus, B cell depletion had significant effects on NZB/W mouse survival that were dependent on the timing of treatment initiation. Thereby, distinct B cell populations may have opposing protective and pathogenic roles during lupus progression.
Keywords: B cells, CD20, CD22, immunotherapy, lupus, regulatory B cells
Introduction
Systemic lupus erythematosus (SLE)4 is a multi-organ autoimmune disease characterized by autoantibody (autoAb) production and immune complex deposition, with subsequent end-organ damage. In addition to the contributions of T cells, B cells also play essential roles in human SLE development and/or pathogenesis (1, 2). Spontaneous lupus-like disease also develops in NZB/NZW (NZB/W) F1 hybrid mice, where immune complex-mediated glomerulonephritis is associated with IgG autoAb production against nuclear Ags, including dsDNA, RNA, chromatin, and histones (3). Polyclonal B cell activation, expansion of the B-1a and marginal zone B cell subsets (4–9), and high serum IgM and IgG levels (10, 11) are prominent in NZB/W F1 mice, even before disease manifestations. Intrinsic B cell defects are also found in MRL/lpr mice that develop a similar lupus-like disease (12). Thereby, aberrant B cell function is thought to be central to the development and/or progression of lupus-like diseases in mice and humans (1, 2).
Although B cells are generally thought to promote lupus and other autoimmune conditions by producing autoAbs, B cells also have critical functions in regulating autoimmune disease pathogenesis that extend beyond Ab production (13). For example, B cells regulate disease development and pathogenesis by promoting pathogenic CD4+ T cell activation through their APC function and cytokine production (14–17). In support of this, mIgM.MRL/lpr mice that express transgenic membrane IgM but do not secrete appreciable serum Ig still develop nephritis and vasculitis in contrast to B cell-deficient MRL/lpr mice that exhibit comparatively less pronounced disease (18, 19). While informative, the use of genetically B cell-deficient mice to study autoimmunity is complicated by their multiple pre-existing immune system alterations (20–25). Furthermore, it is not possible to examine the role of B cells at different time points during disease in congenitally B cell-deficient mice. This is important since B cells have opposing roles during the initiation and progressive stages of inflammation, experimental autoimmune encephalomyelitis (EAE), and potentially other autoimmune diseases (26–28).
B cell depletion using a therapeutic CD20 mAb has shown clinical efficacy in some SLE patients, although the Rituximab anti-human CD20 mAb has not had a significant therapeutic benefit in larger phase II/III and phase III randomized, placebo-controlled clinical trials for lupus nephritis and moderate to severe SLE without active nephritis (29–33). In part, this may be explained by the recent report that autoimmune B cells are inherently resistant to B cell depletion in MRL/lpr mice expressing a human CD20 transgene (34). Despite resistance to therapy, high-dose mouse anti-human CD20 mAb treatments reduced clinical disease and anti-nuclear antibody (ANA) levels, although it is unknown whether B cell depletion or high doses of exogenous IgG ameliorated disease symptoms since disease was not assessed in control IgG-treated mice. For contrast, CD22 represents another B cell-specific target for human and mouse B cell depletion (35, 36). A humanized CD22 mAb, Epratuzumab, reduces disease activity in some patients with SLE and other autoimmune diseases (32). Thus, B cells may be clinically important during SLE and lupus-like diseases, but the therapeutic effectiveness of B cell depletion remains unresolved.
B cell depleting mouse anti-mouse CD20 and CD22 mAbs have provided mechanistic insights into B cell functions in autoimmune mouse models of tight skin fibrosis, EAE, type 1 diabetes, collagen and proteoglycan induced arthritis, and thyroiditis (27, 36–44). The MB20-11 anti-mouse CD20 mAb depletes the vast majority of mature blood, peripheral lymph node, and spleen B cells in C57BL/6 mice through antibody dependent cellular cytotoxicity (ADCC) (37, 45). By contrast, CD22 mAbs selectively deplete blood, mature recirculating bone marrow, and marginal zone B cells in C57BL/6 mice (36). CD22 mAbs such as MB22-11 deplete these B cell subsets by interfering with CD22 ligand binding, an important survival factor for peripheral B cells (36, 46). Since CD20 and CD22 mAbs deplete different B cell populations, and different CD20 mAbs have different B cell depletion efficiencies in vivo (37, 47), we have examined B cell depletion in C57BL/6 and NZB/W F1 mice using low doses of well-characterized CD20 and CD22 mAbs, and determined whether B cell depletion affects lupus-like disease in NZB/W F1 mice (36, 37, 45). Remarkably, B cells exerted pathogenic and protective functions at distinct time points during the course of lupus initiation and progression, which may correlate with the expansion of a recently defined regulatory B cell subset in mice (26–28, 48).
Material and Methods
Mice
C57BL/6 and female NZB/W F1 mice for pristane-accelerated lupus and the analysis of short-term B cell depletion were from The Jackson Laboratory (Bar Harbor, ME). For accelerated lupus, 8 wk-old NZB/W F1 mice were injected i.p. with 200 μl pristane (Sigma, St. Louis). New Zealand Black (NZB) and New Zealand White (NZW) mice were purchased from Japan SLC, Inc. (Shizuoka, Japan) for conventional lupus experiments, with female NZB/W F1 mice generated by mating female NZB and male NZW mice. All mice were housed under specific pathogen free conditions. All procedures were approved by Duke University’s Animal Care and Use Committee and the Animal Committee of The International Medical Center of Japan.
Abs
Mouse anti-mouse CD20 mAb, MB20-11, and mouse anti-mouse CD22 mAb, MB22-11, were as described (36–38). MB20-11 and MB22-11 mAbs were purified using protein A Hi-Trap columns according to the manufacturer’s instructions (Amersham Pharmacia, Piscataway, NJ). MB20-11 and MB22-11 are IgG2c mAbs due to their C57BL/6 origins (49, 50). Nonetheless, IgG2a-specific Ab reagents (Southern Biotechnology Associates, Inc., Birmingham, AL) reacted with IgG2c mAbs. Other reagents included: FITC-, PE-, and PE-cy5-conjugated anti-mouse B220 (CD45R, RA3-6B2), CD5 (53-7.3), CD19 (1D3), CD21/35 (7G6), CD21 (B3B4), CD24 (M1/69), CD43 (S7), and CD93 (AA4.1) mAbs (all from BD Biosciences, San Jose, CA); CD11b (M170) and CD1d (1B1; from eBioscience, San Diego, CA) mAbs; and polyclonal goat anti-IgM and –IgD Abs (Southern Biotechnology Associates). IgG1, IgG2a, and IgG2c mAb isotype standards were either generated in-house or purchased from Southern Biotechnology Associates, Inc.
B cell depletion and phenotype analysis
Sterile MB20-11, MB22-11, or IgG2a isotype control (generated in house) mAbs (10–100 μg in 100–200 μl PBS) were given to NZB/W F1 or C57BL/6 mice. For pristane-accelerated lupus, each mAb (100 μg) was given i.p. 2 wks following pristane treatment, with repeated mAb treatment every 10 days until the mice had received 11 doses. For the conventional lupus model, mAb (10 μg i.v.) was given between 4- to 32-wks of age, with treatment repeated every subsequent 4 wks.
After mAb treatment, blood leukocyte numbers were quantified by hemocytometer following red cell lysis, with cell frequencies determined by immunofluorescence staining with flow cytometry analysis. Single-cell leukocyte suspensions from spleen, bone marrow (bilateral femur), peritoneal lavage, and peripheral lymph nodes (bilateral inguinal) were isolated, with erythrocytes lysed in Tris-buffered 100 mM ammonium chloride solution. Leukocytes were then stained at 4°C using pre-determined optimal concentrations of each Ab for 30 min. For whole blood, erythrocytes were lysed after staining using FACS™ Lysing Solution (Becton Dickinson, San Jose, CA). Ab binding was analyzed on a FACScan flow cytometer (Becton Dickinson) by gating on cells with the forward and side light scatter properties of lymphocytes. Non-reactive, isotype-matched Abs (Caltag/eBioscience/BD Biosciences) were used as controls for background staining.
ELISAs
Total serum IgM, IgG, IgG1, IgG2a, IgG2b, and IgG3 concentrations were determined as described (51). Briefly, 96-well ELISA plates were coated with 2 μg/ml goat anti-mouse Ig (H+L) Abs (Southern Biotechnology Assoc., Inc.) in 0.1 M borate buffered saline and incubated overnight at 4°C. Plates were blocked with TBS containing 1% BSA and 2% gelatin, washed with TBS containing 0.1% Tween, and incubated with serum (diluted 1:10000 for IgM; 1:50000 for IgG; 1:7500 for IgG1; 1:20000 for IgG2a; 1:5000 for IgG2b; and 1:2500 for IgG3 detection) in TBS containing 1% BSA) from NZB/W F1 mice for 1.5 h at room temperature. Washed plates were incubated 1 h at room temperature with AP-conjugated goat anti-mouse IgM, -IgG, -IgG1, -IgG2a, -IgG2b, and -IgG3 Abs (Southern Biotechnology Assoc., Inc), washed, and developed using 1 mg/ml pNPP (Sigma) in 0.1 M diethanolamine. Ab concentrations were determined from standard curves generated using isotype–specific standards (Southern Biotechnology Assoc., Inc). Anti-dsDNA and anti-histone serum IgM, IgG, IgG1, IgG2a, IgG2b, and IgG3 Ab levels were assessed in a similar manner using sera diluted at 1:500, except that the plates were pre-coated with 2 μg/ml calf thymus DNA (Sigma) in 1X SSC or 5 μg/ml type II-AS histone (Sigma) in 0.1 M borate buffered saline.
Serum IgG reactivity with mAbs used in pristane-accelerated lupus was determined using 96-well ELISA plates coated overnight at 4°C with 5 μg/ml of MB20-11, MB22-11, treatment control mAb, or mouse IgG2a or IgG1 in 0.1 M borate buffered saline. The plates were blocked, incubated with serum (diluted 1:500 in TBS containing 1% BSA) for 1.5 h at room temperature, and washed. Plates coated with IgG2a or IgG2c mAbs, or IgG2a were incubated with a cocktail of AP-conjugated anti-mouse IgG1, -IgG2b, and -IgG3 Abs (Southern Biotechnology Assoc., Inc) for 1 h. Plates coated with IgG1 were incubated with a cocktail of AP-conjugated anti-mouse IgG2a, -IgG2b, and -IgG3 Abs. MB20-11, IgG2a, and IgG2c control mAb reactivity in conventional lupus was also assessed by incubating serum (diluted 1:5 in PBS) with plates coated with goat anti-mouse IgG (H+L) (4 μg/ml; Invitrogen, Carlsbad, CA). The plates were washed, incubated with biotinylated MB20-11 mAb, biotinylated IgG2a, or biotinylated IgG2c (clone 6.3; Southern Biotechnology Assoc., Inc.) mAb (4 μg/ml; biotinylated using EZ-Link sulfo-NHS-Biotin; Thermo Scientific, Rockford, IL) in PBS containing 10% FBS for 1 h at room temperature, washed, and incubated with HRP-conjugated streptavidin (Fisher Scientific, Pittsburgh, PA) for 1 h at room temperature. All plates were washed and developed with TMB substrate (Thermo Scientific), stopped with 1 N H2SO4 before 450 nm wavelength light absorbance values exceeded 2.0. Mean optical densities from three control non-Ab-coated blank wells (<0.05) were subtracted from each determination.
ANA and proteinuria assays
Serum ANA reactivity with human HEp-2 cells (HEp-2 Cell Substrate Slide; MBL, Japan) was examined by incubating sera (diluted 1:100 in PBS) with fixed HEp-2-cell-coated slides for 30 min at room temperature. The slides were then washed three times with PBS, with ANA detected using FITC-conjugated goat anti-mouse IgG (H+L) Ab at optimal concentrations for 30 min at room temperature. The slides were evaluated by fluorescence microscopy at 400x magnification. ANA staining exhibited a homogenous to speckled nuclear pattern, with fluorescence staining above background (secondary Ab alone) levels considered positive. Proteinurea was assessed using Nephrosticks L (BAYER Medical, Tokyo, Japan). NZB/W F1 mice demonstrated glomerulonephritis and immune complex deposition on glomerular basement membranes when their proteinurea values exceeded 3 mg/ml (data not shown). Therefore, mice were considered positive for proteinurea when readings were greater than 3 mg/ml.
B10 cell enumeration and analysis
Intracellular IL-10 production by B cells was examined by flow cytometry as described (26). Briefly, isolated leukocytes or purified cells were resuspended (2 × 106 cells/ml) in complete medium [RPMI 1640 media containing 10% FCS, 200 μg/ml penicillin, 200 U/ml streptomycin, 4 mM L-Glutamine, and 5 × 10−5 M 2-mercaptoethanol (all from Gibco, Carlsbad, CA)] along with LPS (10 μg/ml, Escherichia coli serotype 0111: B4, Sigma), PMA (50 ng/ml; Sigma), ionomycin (500 ng/ml; Sigma), and monensin (2 μM; eBioscience) for 5 h at 37°C. Following cell-surface staining, the cells were washed, fixed and permeabilized using a Cytofix/Cytoperm kit (BD PharMingen) according to the manufacturer’s instructions. Cells were then stained with PE-conjugated mouse anti-IL-10 mAb (JES5-16E3, eBioscience). A PE-conjugated rat IgG2b isotype control mAb was used as a negative control to establish background staining levels. Dead cells were excluded from the analysis based on their forward- and side-light scatter properties and the use of LIVE/DEAD Fixable Dead Cell Stain Kits (Invitrogen-Molecular Probes, Carlsbad, CA). B10pro plus B10 cell numbers were determined as described (48). Briefly, cells were cultured for 48 h with agonistic CD40 mAb (1 μg/ml, clone HM40-3, BD Biosciences), with LPS, PMA, ionomycin and monensin added during the final 5 h of culture. Leukocytes from IL-10−/− mice served as negative controls to verify reagent specificity and to establish background IL-10 staining levels.
Statistical analysis
ANA, proteinurea, and survival data were analyzed using Kaplan-Meier curves and the Log rank test. Unless indicated otherwise, data are shown as mean values (± SEM). Comparisons between groups were made using Steel-Dwass’ analysis or Student’s t-test. A p value less than 0.05 was considered to be statistically significant.
Results
CD20 and CD22 mAb-induced B cell depletion in C57BL/6 and NZB/W F1 mice
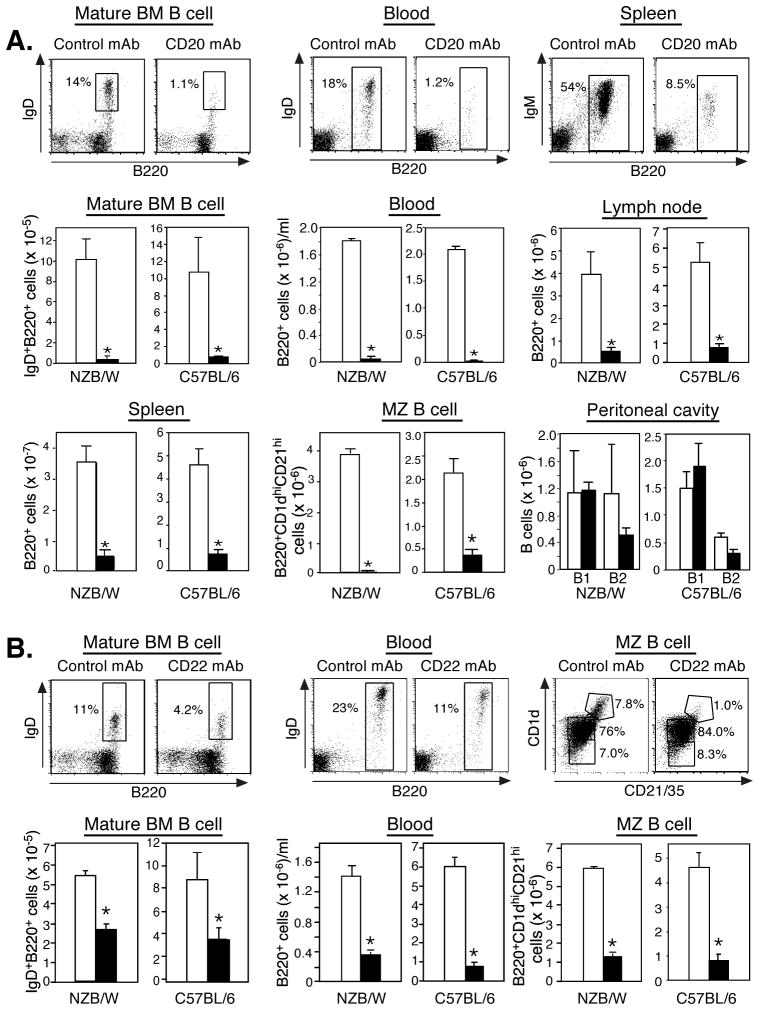
The efficacy of CD20 and CD22 mAb-induced B cell depletion was first assessed in 8- to 10-wk-old female NZB/W F1 mice. CD20 mAb (100 μg) depleted comparable numbers of mature B cells in NZB/W F1 and C57BL/6 mice 7 days following treatment (Fig. 1A). In NZB/W F1 and C57BL/6 mice, CD20 mAb depleted mature B cells from the bone marrow (96 vs. 94%), blood (97 vs. 99%), spleen (90 vs. 84%), spleen marginal zone (99 vs. 83%), and lymph nodes (86 vs. 84%). Thus, significant intrinsic differences in CD20 mAb-induced B cell depletion were not found between NZB/W F1 and C57BL/6 mice.
Figure 1.
B cell depletion by CD20 and CD22 mAbs in NZB/W F1 and C57BL/6 mice. CD20 (A), CD22 (B), or IgG2a control mAb (100 μg) was given i.p. to 8–10 wk-old mice with B cell numbers quantified on day 7. B220+ blood, B220+ spleen, B220+ peripheral lymph node, mature recirculating bone marrow (IgD+B220hi), marginal zone (B220+CD21hiCD1dhi), and peritoneal B1 (B220+IgM+CD11b+) and B2 (B220+IgM+CD11b−) cell numbers were determined by immunofluorescence staining with flow cytometric analysis. Representative dot plots are shown for mAb-treated NZB/W F1 mice with gated B cell percentages indicated. Significant differences between mean (±SEM) values for control mAb-treated (open bars) and CD20 or CD22 mAb-treated (filled bars) mice are indicated; *p<0.05. Data are representative of results obtained from ≥3 mice per mAb treatment.
Mouse anti-mouse CD22 mAb rapidly depletes mature recirculating bone marrow, blood, and marginal zone B cells through ADCC-independent mechanisms, but only ~20% of mature CD22+ follicular B cells in C57BL/6 mice (36). The MB22-11 mouse anti-mouse CD22 mAb (100 μg) depleted mature recirculating bone marrow (49 vs. 58%), blood (75 vs. 84%), and marginal zone (77 vs. 82%) B cells to the same extent in NZB/W F1 and C57BL/6 mice (Fig. 1B). Thus, even though the CD20 and CD22 mAbs deplete different mature B cell subsets through different mechanisms, there do not appear to be intrinsic differences in CD20 or CD22 mAb-induced B cell depletion between NZB/W F1 and C57BL/6 mice.
B cell depletion improves survival during accelerated lupus with minimal effects on Ig and autoAb levels
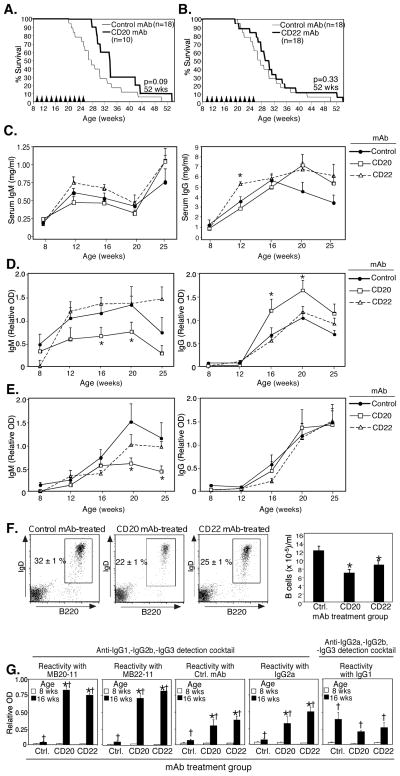
NZB/W F1 mice normally develop ANA by 16 to 24 wks of age, nephritis from 28 to 36 wks, and die around 36 wks of age. However, pristane treatment of NZB/W F1 mice accelerates the course of lupus-like disease (52, 53). Therefore, the role of B cells in pristane-accelerated mortality in 8 wk-old female NZB/W F1 mice was evaluated following low-dose pristane administration with subsequent CD20, CD22, or control mAb (100 μg) treatments every 10 days for 16 wks. From the initiation of mAb treatment up until 34 wks of age, survival was improved in CD20 mAb-treated mice compared to control mAb-treated mice (wks 29–34, p<0.05, Log rank test; Fig. 2A). Median (50%) survival was also increased 23%, from 26 wks in control mAb-treated mice to 32 wks in CD20 mAb-treated mice. However, CD20 mAb-treated mice began to succumb to disease more rapidly following this time point such that differences in overall survival curve comparisons at 52 weeks of age were not found to be significant (p=0.09; Fig. 2A). In contrast to the improved survival during early disease with total mature B cell depletion, survival following CD22 mAb treatment was more comparable to IgG2a control throughout the course of disease (Fig. 2B). Thus, the depletion of most mature B cells improved survival in pristane-accelerated lupus.
Figure 2.
Effects of B cell depletion by CD20 and CD22 mAbs on disease in pristane-accelerated lupus. Eight week-old female NZB/W F1 mice were given 200 μl pristane i.p. Two weeks later, mice were given either CD20 (A), CD22 (B), or control mAb (100 μg) i.p., with the same mAb dose administered every 10 days over a 16-wk period (11 doses total; arrowheads). Kaplan-Meier survival curves are shown with statistical differences assessed at 52 wks of age using the Log-rank test. (C–G) Effects of B cell depletion on serum Ig, autoAb, and anti-treatment Ab levels in pristane-accelerated lupus. Total serum (C), anti-dsDNA (D), and anti-histone (E) Ab levels in 8–25 wk-old mice treated with CD20 (n=10), CD22 (n=18), or control (n=18) mAbs. (F–G), Resistance to B cell depletion in pristane-accelerated lupus in female NZB/W F1. F, Representative plots show blood B220+ B cell depletion 7 days following the 5th dose of CD20, CD22, or control mAb. Bar graphs show mean (±SEM) blood B cell depletion in each mAb treatment group (n≥10). G, Reactivity of serum IgG obtained from mice treated with MB20-11 (CD20 mAb), MB22-11 (CD22 mAb), treatment control mAb, or irrelevant IgG2a and IgG1 mAbs as determined by ELISA. (C–G) Significant differences between mean (±SEM) OD values for control mAb and CD20 or CD22 mAb-treated mice are indicated; *p<0.05. Significant differences between mean (±SEM) OD values for 8 and 16 wk-old mice are indicated; †p<0.05, (n≥10 mice/group).
Prophylactic B cell depletion using CD20 mAb significantly reduces isotype switching and IgG immune responses after Ag challenge, and autoantibody generation in autoimmune prone mice, while therapeutic B cell depletion after autoimmune disease initiation is without effect (39, 40, 54). Similarly, total serum IgM was not dramatically altered by CD20 or CD22 mAb-induced B cell depletion from low-dose pristane-treated NZB/W F1 mice between 8 and 25 wks of age (Fig. 2C). Unexpectedly however, total serum IgG levels increased significantly with age (8 vs. 16 wks, p<0.05) despite CD20 or CD22 mAb treatment when compared to control mAb treated NZB/W F1 littermates. Anti-dsDNA and anti-histone IgM and IgG autoAbs also increased significantly between 8 to 16 wks of age in all treatment groups (p<0.05, Fig. 2D–F). Thus, mature B cell and B cell subset depletion did not dramatically affect serum Ig levels or autoAb production in low-dose pristane-treated NZB/W F1 mice.
Since B cell depletion delayed mortality, but did not cure disease, in pristane-accelerated lupus (Fig. 2), the efficiency of B cell depletion during disease was assessed. First, the ability of CD20 and CD22 mAbs to deplete blood B cells was examined in pristane-treated NZB/W F1 mice given CD20, CD22, or control mAbs during 16 wks of treatment. The level of B cell depletion observed by 16 wks of age for both CD20 and CD22 mAb-treated mice was decreased compared to depletion in 8-wk old mice that had not received pristane (Fig. 2F vs. Fig. 1). Whereas CD20 and CD22 mAbs reduced blood B cell numbers by 75–97% in 8 wk-old mice (Fig. 1), blood B cell numbers were only reduced by 28–43% in 16 wk-old NZB/W F1 mice (Fig 2F). NZB/W F1 mice, like other lupus-prone mice, may generate serum IgM and IgG rheumatoid factors (RF) as disease progression (55, 56). Thus, circulating anti-IgG Ab levels were therefore quantified before and during mAb treatment. All NZB/W F1 mice had significantly higher levels of IgG Abs that were reactive with IgG2c, IgG2a, and IgG1 Abs at 16 wks of age compared to levels at 8 wks of age (Fig. 2G). Sixteen week-old mice given control, CD20, or CD22 mAb four times had ≥2.5-fold higher levels of serum IgG (IgG2a Abs not measured in the assay) reactive with the mAb that they were given when compared to 8-wk old mice. Sixteen week-old mice given CD20 or CD22 mAb had even higher levels of serum IgG (IgG2a Abs not measured in the assay) reactive with IgG2c (MB20-11 and MB22-11) and IgG2a (control and unrelated mAb) compared to 16-wk old mice that had received control mAb. There were no significant differences among age-matched treatment groups in IgG1-reactive serum IgG (IgG1 Abs not measured in the assay) levels. Thus, B cell depletion by CD20 or CD22 mAb treatment did not abrogate the generation of anti-IgG levels in NZB/W F1 mice, which may have contributed to the diminished effectiveness of B cell depletion over time. Moreover, NZB/W F1 mice were unique in that effective B cell depletion (Fig. 1) did not prevent the development of isotype-switched autoAbs (ie., anti-DNA and anti-histone IgG), while IgG autoAbs do not increase in other strains of autoimmune mice after CD20 mAb-induced B cell depletion (39, 40, 42, 54).
Development of a therapy model for B cell depletion in NZB/W F1 mice
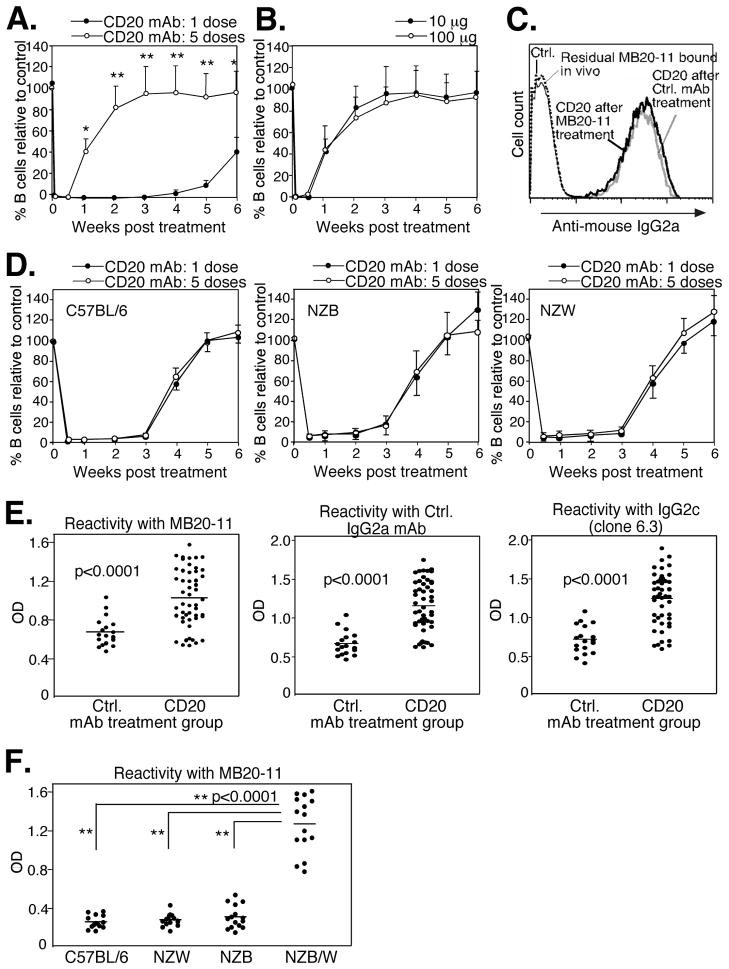
The effects of B cell depletion using lower and less frequent mAb doses in NZB/W F1 mice without pristane pre-treatment was next assessed. Mice were given a 10-fold lower mAb treatment dose at 4-wk intervals. One 10 μg dose of CD20 mAb depleted >95% of blood B cells for ~4 wks in 8 wk-old NZB/W F1 mice (Fig. 3A) as published for C57BL/6 mice (37, 45). However, when 8 wk-old NZB/W F1 mice were given CD20 mAb every 4 wks 5 times, circulating B cells were depleted for <1-wk after the last treatment in these 28-wk-old mice. When mice were repetitively given a higher dosing regimen of CD20 mAb (100 μg/mouse), the period of B cell depletion following the 5th mAb treatment was equivalent to that observed in mice given 10 μg of mAb repetitively (Fig. 3B). Circulating B cells from depletion-resistant NZB/W F1 mice given CD20 mAb 5 times every 4 wks expressed normal levels of CD20 as assessed by CD20 mAb staining in vitro (Fig. 3C). Rather, CD20 mAb was cleared rapidly in vivo since circulating B cells from depletion-resistant NZB/W F1 mice did not have residual CD20 mAb bound to their cell surface as detectable by anti-mouse IgG2a mAb staining (Fig. 3C). Furthermore, serum from depletion-resistant mice contained significantly less circulating CD20 mAb 7 days after treatment than mice that had only been treated with CD20 mAb once as determined using serum from mAb-treated mice to stain normal B cells (data not shown). In contrast to NZB/W F1 mice, no difference was observed in B cell clearance in parental NZB, parental NZW, or C57BL/6 mice given CD20 mAb repeatedly in comparison to mice treated once with mAb (Fig. 3D). Therefore, resistance to mAb-induced B cell depletion was unique to NZB/W F1 mice, developed independent of pristane treatment, and was not mAb dose dependent or due to reduced CD20 expression.
Figure 3.
NZB/W F1 mice generate anti-IgG Abs and exhibit reduced clearance following multiple treatments with B cell-depleting mAbs in conventional lupus. A–C, Generation of anti-IgG Abs in conventional NZB/W F1 lupus. A, Blood B cell depletion in 8-wk-old female NZB/W F1 mice that were given one 10 μg dose of CD20 or control mAb i.p. or five mAb treatments at 4 week intervals. B220+ blood B cell numbers were analyzed immediately before and after the final CD20 mAb dose at days 1 and 3 and then weekly thereafter. Significant differences in B cell clearance between mice receiving one and five doses of CD20 mAb are indicated *, p<0.05; **, p<0.01, n=25 mice/group. B, Blood B cell depletion in NZB/W F1 mice given CD20 mAb (either 10 μg or 100 μg) every 4 wks five times (n=5 mice/group). Blood B cell numbers were determined after the last dose of CD20 mAb as in (A). C, CD20 expression on B cells from NZB/W F1 mice following five doses of CD20 mAb. Blood was harvested from CD20 or control mAb-treated mice 7 days after the fifth mAb treatment and stained with PE-conjugated B220 mAb and CD20 mAb followed by FITC-conjugated anti-mouse IgG2a Ab. Blood samples were also stained separately with FITC-conjugated anti-mouse IgG2a Ab alone to detect residual surface CD20 mAb that had been bound in vivo. D, Blood B cell depletion in C57BL/6, NZB, and NZW mice given one or 5 doses of CD20 mAb. Eight week-old mice were given 10 μg CD20 mAb either one time or five times at 4 wk intervals with depletion assessed as in (A). E, NZB/W F1 mice given CD20 or control Ab five times produce IgG Abs reactive with both IgG2a and IgG2c Abs. NZB/W F1 mice were given CD20 or control mAb (10 μg) five times as in (A), with serum IgG reactivity with MB20-11 mAb (IgG2c), control treatment mAb (IgG2a), or an irrelevant IgG2c mAb (clone 6.3) measured by ELISA. Mean results (horizontal lines) are significantly different between groups, p<0.0001. F, Only NZB/W F1 mice produce IgG Abs following CD20 mAb (10 μg) treatment five times as in (A). Reactivity of C57BL/6, NZB, NZW, and NZB/W F1 mouse serum with MB20-11 mAb was measured by ELISA. Mean results (horizontal lines) are significantly different between groups as indicated, **, p<0.0001.
To determine whether resistance to mAb-induced B cell depletion was associated with the production of anti-IgG Abs, NZB/W F1 mice were treated with 10 μg doses of control or CD20 mAb at 4-week intervals five times. Subsequently, serum IgG reactive with mouse IgG2a treatment control mAb, CD20 mAb (IgG2c), or irrelevant IgG2c mAb (clone 6.3) was present in the 28-wk old NZB/W F1 mice, although the anti-IgG2a/c autoAb levels were significantly higher in depletion-resistant CD20 mAb-treated mice (Fig. 3E). Importantly, following multiple treatments, autoimmune-prone NZB/W F1 mice had significantly higher levels of anti-MB20-11 Abs than either parental NZB and NZW strains, or C57BL/6 mice (Fig. 3F). Thus, anti-IgG2a/c Abs are generated in both control and CD20 mAb-treated NZB/W F1 mice, but not NZB, NZW, or C57BL/6 mice, with higher levels observed in depletion-resistant NZB/W F1 mice that had received CD20 mAb.
B cell depletion accelerates or delays disease depending on the timing of depletion
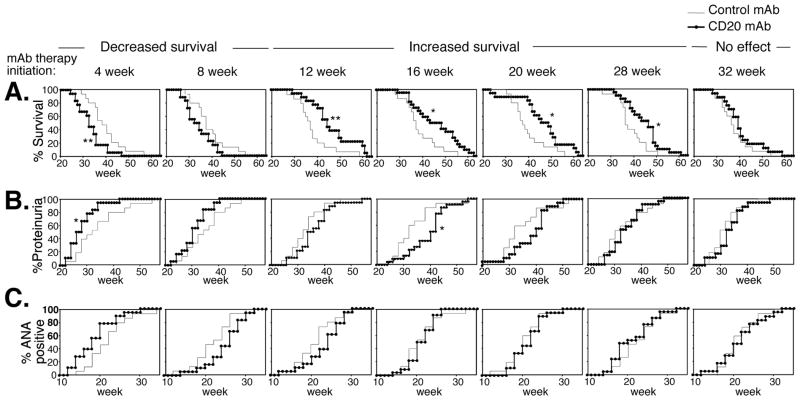
Since total mature B cell depletion improved survival in pristane-accelerated lupus, the efficacy of B cell depletion during conventional (non-accelerated) lupus development was assessed. NZB/W F1 mice were given CD20 mAb (10 μg) starting at different time points, with repeated treatment every subsequent 4 weeks. Unexpectedly, survival was significantly decreased when B cells were depleted beginning at 4 wks of age, in comparison with control mAb treated littermates (Fig. 4A). By contrast, survival was significantly enhanced when B cells were first depleted between 12 and 28 wks of age. CD20 mAb treatment initiated at 32 wks had no effect on survival. The development of proteinuria was also accelerated when treatment was initiated at 4 wks of age, while proteinurea was significantly delayed when CD20 mAb was first given at 16 wks of age (Fig. 4B). CD20 mAb treatment did not significantly alter the frequency of ANA development when compared to control mAb-treated littermates (Fig. 4C). Differences in disease parameters among untreated NZB/W F1 mice in comparison with littermates that had received control IgG2a mAb were not observed (data not shown). Furthermore, differences in disease parameters among NZB/W F1 mice that had received control IgG2a mAb beginning at 4 weeks vs. 32 weeks of age were not observed (Fig. 4). Therefore, B cell depletion had remarkable and yet, opposing effects on disease course in NZB/W F1 mice, with early treatment (begun at 4 wks) exacerbating disease and later treatment (begun at 12 to 28 wks) improving disease.
Figure 4.
Therapeutic B cell depletion by CD20 mAb either accelerates or improves conventional NZB/W F1 lupus depending on the timing of treatment. CD20 or control mAb (10 μg) were first given i.v. from 4 to 32 wks of age as indicated and repeated every 4 wks with (A) survival, (B) proteinuria and (C) ANA production evaluated at least weekly. All data were obtained from ≥15 mice in each treatment group with significant differences indicated. *, p<0.05; **, p<0.01. In (A), Kaplan-Meier survival curves are shown with statistical differences assessed using the Log-rank test.
Regulatory B10 cells expand in young NZB/W F1 mice and are depleted by CD20 mAb
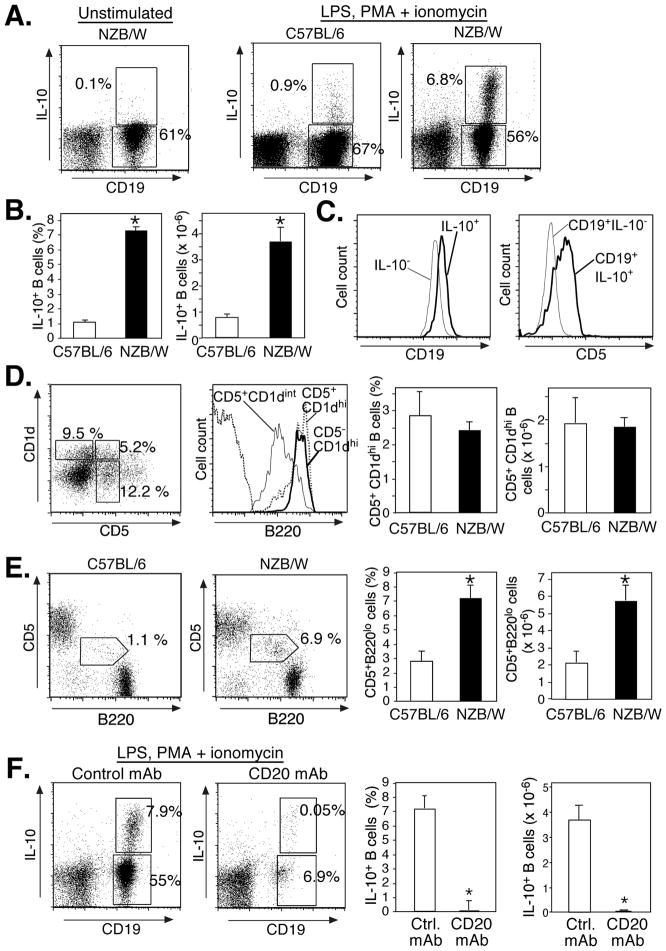
As in NZB/W F1 mice, early B cell depletion by CD20 mAb promotes inflammation (26) and exacerbates EAE (27). This results from the depletion of B10 cells, a unique IL-10-producing regulatory B cell subset found within the spleen CD5+CD1dhi subpopulation (26). Sixteen week-old NZB/W F1 mice have significantly increased B10 cell numbers in comparison with C57BL/6 mice (48). IL-10-producing B cell frequencies and numbers were also increased >4-fold in 10-wk-old NZB/W F1 mice when compared with C57BL/6 mice (Fig. 5A–B). IL-10-producing spleen B cells in NZB/W F1 mice expressed CD5 and higher levels of CD19 in comparison with other B cells (Fig. 5C) as reported for B10 cells in C57BL/6 mice (26). However, NZB/W F1 mice exhibited two splenic CD5+ B cell populations that could be differentiated by their CD1d and B220 expression densities (Fig. 5D). The CD1dhiCD5+ B cell subset expressed high density B220, comparable to that expressed by CD1dhiCD5− B cells. In contrast, the remaining CD5+ B cells expressed intermediate CD1d levels (CD5+CD1dint) and low density B220, characteristics of B-1a cells. The frequencies and numbers CD1dhiCD5+B220+ cell were not expanded in 10 wk-old NZB/W F1 mice relative to C57BL/6 mice (Fig. 5D), although this subset increases significantly by 16 wks of age (48). However, marginal zone (CD21hiCD1dhi) B cell and CD5+B220lo B-1a cell frequencies and numbers were increased in 10 wk-old NZB/W F1 mice relative to age-matched C57BL/6 mice (Fig. 1 and Fig. 5E) as published (4–9). Thus, in addition to MZ and B-1a B cells, the IL-10 competent B10 cell subset was significantly expanded in NZB/W F1 mice prior to disease onset.
Figure 5.
The expanded B10 cell subset in NZB/W F1 mice is depleted by CD20 mAb. A, Representative analysis of IL-10-producing spleen B cell frequencies in NZB/W F1 and C57BL/6 mice. Splenocytes were cultured with LPS, PMA, ionomycin, and monensin for 5 h, stained for cell-surface CD19, CD1d, and CD5, permeabilized before staining for intracellular IL-10, with subsequent multi-color flow cytometry analysis. B, Mean (±SEM) spleen cytoplasmic IL-10+ B (CD19+) cell frequencies and numbers in 10 wk-old NZB/W F1 (control mAb-treated) and C57BL/6 mice (n≥3). C, Relative cell surface CD19 and CD5 expression levels by cytoplasmic IL-10+ and IL-10− spleen B cells from NZB/W F1 mice as assessed in (A). D, Representative B220 expression by CD1dhiCD5+, CD1dhiCD5−, and CD1dintCD5+ spleen B220+ B cell subsets in 10 wk-old NZB/W F1 mice. Bar graphs indicate mean (±SEM) frequencies and numbers of CD1dhiCD5+ spleen B cells in NZB/W F1 and C57BL/6 mice. E, Representative frequencies of spleen CD5+B220lo B cells in 10 wk-old NZB/W F1 and C57BL/6 mice. Bar graphs indicate mean (±SEM) frequencies and numbers of CD5+B220lo B cells. F, Depletion of IL-10-producing spleen B cells in NZB/W F1 mice by CD20 mAb. CD20 or control mAbs (100 μg) were given i.p. with IL-10-producing B cells quantified 7 days later as in (A). Bar graphs indicate mean (±SEM) frequencies and numbers of IL-10-producing CD19+ cells. A–F, All data represent results obtained from ≥3 mice (age 10 weeks) per group, with significant differences between mean (±SEM) values indicated; *p<0.05.
As in C57BL/6 mice, CD20 mAb was able to deplete IL-10-producing B cells in NZB/W F1 mice. Seven days following CD20 or control mAb treatments, mouse splenocytes were cultured with LPS, PMA, ionomycin and monensin, and assessed for cytoplasmic IL-10 production. Remarkably, CD20 mAb treatment reduced both the frequency and number of IL-10-producing B cells in NZB/W F1 mice by 99% (Fig. 5F). Thus, CD20 mAb treatment efficiently depletes both regulatory B10 cells and mature B cells in NZB/W F1 mice.
B10 cell phenotype and development in NZB/W F1 mice
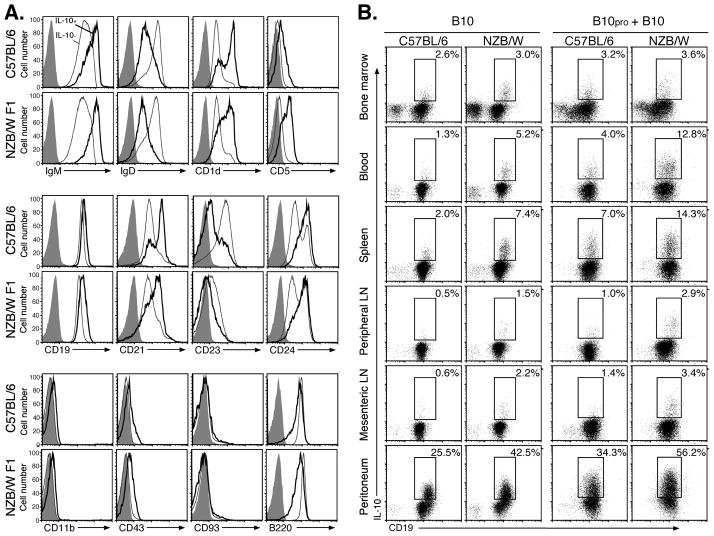
Phenotypically unique spleen B cells that are competent to express cytoplasmic IL-10 following 5 h stimulation with LPS, PMA, and ionomycin are predominantly found within the CD1dhiCD5+ CD19hi subset in wild type C57BL/6 mice (26, 27, 48). We have previously shown that 5 h of LPS, PMA, and ionomycin stimulation does not significantly influence the cell surface phenotype of spleen B cells (26). Therefore, the phenotypes of IL-10+CD19+ B10 cells were characterized in 8 wk-old NZB/W F1 mice in comparison with C57BL/6 mice. IL-10+ B cells were predominantly IgMhi, IgDlo, CD1dhi, CD5hi, CD19hi, CD21int/hi, CD23lo, CD24hi, CD11b+/−, CD43+/−, and CD93− (AA4.1) in both NZB/W F1 and C57BL/6 mice (Fig. 6A). Thereby, spleen B10 cells in NZB/W F1 mice share overlapping phenotypic markers with the B-1a, MZ, and T2-MZ precursor B cell subsets, but are nonetheless phenotypically distinct as observed in C57BL/6 mice (26, 27, 48).
Figure 6.
B10 cell phenotype, development, and distribution in 8-wk-old NZB/W F1 and C57BL/6 mice. A, IL-10-producing B cells were predominantly found within the CD1dhiCD5+ B cell subset in NZB/W F1 and C57BL/6 mice. Splenocytes were cultured with LPS, PMA, ionomycin, and monensin for 5 h, then stained for cell surface molecules before permeabilization and cytoplasmic IL-10 staining. Cell surface staining is shown for IL-10+ (heavy lines) and IL-10− (thin lines) CD19+ cells. Gray histograms represent isotype-matched control mAb staining. Results are representative of those obtained with B cells from ≥3 mice as determined by flow cytometry analysis. B, B10 and B10pro cell distributions. To identify B10 cells, B220+ B cells were isolated from tissues and blood, with in vitro stimulation, staining, and analysis as outlined in (A). To identify B10pro cells, the B cells were cultured with agonistic CD40 mAb for 48 h, with LPS, PMA, ionomycin, and monensin added during the last 5 h of each culture. The cultured cells were isolated, stained with CD19 mAb, permeabilized, and stained using IL-10 mAb with flow cytometry analysis as in (A). Values within representative histograms indicate the percentage of IL-10-producing cells among CD19+ B cells within the gates shown. Results are representative of those obtained from ≥3 mice as determined by flow cytometry analysis.
B10 cell development and numbers in 8 wk-old NZB/W F1 and C57BL/6 mice were also compared. B10 cells were identified by their capacity to express cytoplasmic IL-10 after 5 h of LPS, PMA, and ionomycin stimulation (26, 27). Previous studies have also identified B10 progenitor (B10pro) cells that acquire the capacity to express cytoplasmic IL-10 following 48 h of culture with either CD40 mAb or LPS in vitro, with LPS, PMA, and ionomycin stimulation during the final 5 h of culture (48). NZB/W F1 and C57BL/6 mice had similar frequencies and numbers of B10 cells within bone marrow and peripheral lymph nodes (Fig. 6B, Table I). By contrast, the B10 cell subset was significantly expanded within the blood, spleen, mesenteric lymph nodes and peritoneum of NZB/W F1 mice relative to C57BL/6 mice. This was also true for B10pro plus B10 cells, where 12–13% of blood and spleen B cells in NZB/W F1 mice had the capacity to mature and express cytoplasmic IL-10 with stimulation. Remarkably, almost half of the peritoneal cavity B cells in NZB/W F1 mice had the capacity to mature and express cytoplasmic IL-10 with stimulation. Thus, the B10pro and B10 subsets are rare in both NZB/W F1 and C57BL/6 mice, but these regulatory cells are already significantly expanded in NZB/W F1 mice at the time that B cell depletion exacerbates lupus development (Fig. 4).
Table 1.
Tissue distribution of B10 and B10pro cells in 8-wk-old NZB/W F1 and C57BL/6 mice.
| Tissue | B10 cellsa
|
B10pro + B10 cellsb
|
||||||
|---|---|---|---|---|---|---|---|---|
| Percentagec | Numberd | Percentage | Number | |||||
| C57BL/6 | NZB/W | C57BL/6 | NZB/W | C57BL/6 | NZB/W | C57BL/6 | NZB/W | |
| Bone marrow:e | 2.8±0.6c | 3.1±0.2 | 0.19±0.03 | 0.15±0.02 | 3.4±0.2 | 3.7±0.5 | 0.18±0.03 | 0.16±0.03 |
| Blood: | 1.3±0.1 | 5.1±0.4** | 0.03±0.01 | 0.08±0.02* | 3.6±0.6 | 12.1±0.7** | 0.07±0.02 | 0.23±0.03* |
| Spleen: | 2.0±0.2 | 6.5±0.6* | 1.15±0.24 | 3.38±0.38* | 6.4±0.3 | 13.4±1.3* | 3.86±0.20 | 7.16±0.61* |
| PLN: | 0.6±0.1 | 1.3±0.2 | 0.01±0.01 | 0.02±0.002 | 1.4±0.3 | 2.9±0.1* | 0.03±0.01 | 0.04±0.01 |
| MLN: | 0.7±0.1 | 1.9±0.2* | 0.02±0.01 | 0.05±0.01* | 1.5±0.1 | 3.5±0.2** | 0.04±0.01 | 0.09±0.01* |
| Peritoneum: | 24.4±3.0 | 38.2±1.8** | 0.50±0.02 | 1.57±0.05** | 32.9±3.5 | 46.9±5.2 | 0.70±0.13 | 1.91±0.10** |
B10 cell numbers were quantified by flow cytometry after 5 h of culture with LPS, PMA, ionomycin and monensin as in figure 6B with ≥3 mice per value.
B10pro cell numbers were quantified by flow cytometry after culture with agonistic CD40 mAb for 48 h, with LPS, PMA, ionomycin and monensin added during the last 5 h of culture. Numbers include both the B10pro and B10 cell subsets as in figure 6B with ≥3 mice per value.
Values represent the mean percentages of cytoplasmic IL-10+ cells within the B220+ B cell compartment isolated from each tissue. Significant differences between mean values from C57BL/6 versus NZB/W F1 mice are indicated;
p<0.05,
p<0.01.
Values represent the mean number of IL-10+ B220+ B cells (x 10−6) within each tissue, except for blood where values represent 10−6 cells/ml.
Bone marrow leukocytes from both femurs were pooled to calculate IL-10+B220+ cell numbers. Peripheral lymph nodes (PLN) include pooled bilateral axial and inguinal lymph nodes. MLN, mesenteric lymph nodes.
Discussion
This study revealed both protective and pathogenic roles for B cells in murine lupus. Prophylactic depletion of mature B cells in NZB/W F1 mice by CD20 mAb prolonged survival in pristane-accelerated lupus up until ~32 wks of age (Fig. 2). By contrast, CD22 mAb depletion of the mature recirculating bone marrow, blood, and marginal zone B cell subsets resulted in only a slight improvement in survival. Therapeutic low-dose CD20 mAb treatment also successfully prolonged survival and delayed the appearance of proteinuria during spontaneous lupus in NZB/W F1 mice when administered during the onset of disease symptoms between 12 and 20 wks of age (Fig. 4). By contrast, CD20 mAb-induced B cell depletion at 4 wks of age significantly shortened survival time and accelerated proteinuria development. B cell depletion during late stage disease did not affect survival or disease symptoms in NZB/W F1 mice. Thereby, mature B cell depletion by CD20 mAb resulted in strikingly different disease outcomes depending on the timing of B cell depletion, with B cell depletion during disease progression preferentially reducing the pathogenic effects of B cells on disease.
Mature B cell depletion in young NZB/W F1 mice prior to disease symptoms led to accelerated mortality, nephritis, and ANA production (Fig. 4). Similarly, total B cell depletion during contact hypersensitivity induction or EAE initiation dramatically exacerbates inflammation and disease, whereas B cell depletion during EAE progression significantly inhibits disease manifestations (26, 27). Early B cell depletion exacerbates both hypersensitivity responses and EAE due to the depletion of splenic B10 cells, a recently-characterized IL-10-producing regulatory B cell subset (26–28, 57). IL-10-producing B10 cells and potentially other regulatory B cell subsets also have critical suppressive roles in arthritis, colitis, and autoimmune diabetes in mice (40, 42, 58–60). B10 cells in NZB/W F1 mice were phenotypically similar, if not identical to their IL-10 competent B cell counterparts in C57BL/6 mice (Fig. 6). However, B10 and B10pro cell frequencies and numbers were significantly expanded along with the marginal zone and B1a subsets in 8–10 wk-old NZB/W F1 mice (Figs. 1, 5 and 6, Table I), a time point prior to disease onset (4–9). Importantly, CD20 mAb treatment depleted 99% of IL-10-producing B cells in both NZB/W F1 and C57BL/6 mice (Fig. 5F). IL-10-producing regulatory B cell elimination by early CD20 mAb treatment may thereby explain exacerbated lupus-like disease in young NZB/W F1 mice. Proof of this concept awaits the production of CD20-deficient NZB/W F1 mice that will allow the adoptive transfer of IL-10-producing CD1dhiCD5+ B cells from CD20−/− NZB/W F1 mice into CD20 mAb-treated NZB/W F1 mice. The generation of B cell-specific IL-10−/− NZB/W F1 mice will also determine whether exacerbated disease is due exclusively to B cell IL-10 production. Nonetheless, as currently demonstrated in the companion manuscript, the pathologic manifestations of nephritis appear significantly earlier and survival is significantly reduced in NZB/W F1 mice that lack B10 cells due to constitutive CD19-deficiency (Watanabe, R., N. Ishiura, H. Nakashima, Y. Kuwano, H. Okochi, K. Tamaki, S. Sato, T. F. Tedder, M. Fujimoto. Regulatory cells (B10 cells) have a suppressive role in murine lupus, submitted). Moreover, the transfer of splenic CD1dhiCD5+ B cells from wild type NZB/W F1 mice into CD19−/− NZB/W F1 recipients significantly prolongs their survival. Thus, B10 cells in NZB/W F1 mice appear to be functionally similar to their phenotypically identical counterparts in C57BL/6 mice (Fig. 6), where they are known to regulate acute inflammation. Thereby, both early and late stage lupus represent a balance between regulatory B10 cells and pathogenic B cell functions in combination with regulatory and pathogenic T cells.
B cell depletion during autoimmunity in NZB/W F1 mice is likely to have beneficial effects beyond reducing autoAb production (13). Specifically, B cell depletion had protective effects in NZB/W F1 mice, but did not substantially reduce anti-histone or dsDNA autoAb production, isotype switching to pathogenic IgG subclasses (Fig. 2, data not shown), or ANA generation (Fig. 4C). B cell depletion using the MB20-11 CD20 mAb normally blocks humoral immune responses and the serum Ig and autoAb increases typically found in aging autoimmune mice (39, 40, 54). However, CD20 mAb-induced B cell depletion does not have significant effects on pre-existing (CD20-negative) long-lived plasma cells and produces only small reductions in serum Ig or autoAb levels in adult mice (54). AutoAb production and increases in total serum Ig in NZB/W F1 mice may thereby reflect the inability of even potent CD20 mAbs to deplete long-lived plasma cells that could have been generated prior to mAb treatment and/or disease onset (54). Furthermore, B1a and conventional B cells in the peritoneal cavity are depleted at slower rates, which may provide a niche in NZB/W F1 mice that is protected from ADCC (45). Importantly, successful CD20 mAb treatment in SLE patients does not always correlate with reduced autoAb levels (30, 33, 61–63), consistent with pathogenic B cells contributing to autoimmunity via mechanisms independent of autoAb production. Nonetheless, CD20 mAb treatment near the onset of disease initiation successfully enhanced survival in NZB/W F1 mice in both pristane-accelerated and conventional lupus.
Consistent with autoAb and potential RF production by autoimmune NZB/W F1 mice (55, 56), serum IgG Abs reactive with IgG2a-, IgG2c-, and IgG1 were present in control, CD20, and CD22 mAb-treated NZB/W F1 mice at 16 wks of age. Autoimmune patients treated with Rituxan (anti-human CD20) or other mAb therapies frequently produce anti-therapeutic mAb Ab responses and this is often correlated with decreased treatment efficacy (64–66). Anti-IgG Ab production was likely to also contribute to the reduced effectiveness of B cell depletion in older NZB/W F1 mice (Figs. 2F and 4), particularly in mice given CD20 or CD22 mAbs multiple times. It is possible that CD20 or CD22 mAbs bound to the surface of B cells form immune complexes that enhance the production of anti-mAb treatment Abs. It is possible that the therapeutic CD20 and CD22 mAbs or their idiotypes are recognized as foreign proteins since the NZB and NZW mouse strains express IgG2a e and n haplotypes, respectively, while the MB20-11 and BALB/c-derived IgG2a isotype control mAbs are IgG2c (a.k.a., IgG2ab) and IgG2a (a haplotype), respectively. Thus, it will be necessary to repeat and validate the current results in this study using properly isotype-matched IgG2a e or n haplotype mAbs to determine whether the current control and treatment mAbs had any effects beyond B cell depletion that may have ultimately contributed to the development of resistance to therapy in mice that received multiple mAb doses. However, it is interesting that in contrast to NZB/W F1 mice, parental NZB, NZW, and C57BL/6 mice did not develop measurable resistance to CD20 (MB20-11) mAb depletion or generate anti-CD20 Ab responses after repeated treatments (Fig. 3). Anti-therapeutic Ab production and their consequential negative effects on B cell depletion were therefore unique to NZB/W F1 mice. Undoubtedly, the production of anti-therapy Ab and increased Ig and autoAb production in NZB/W F1 mice following B cell depletion reflects the effects of their potent autoimmune susceptibility genes, which may contribute to the inability of total B cell depletion to provide effective long-term disease treatment.
That B cells are inherently resistant to B cell depletion in autoimmune mice expressing a human CD20 transgene (34) has provided a potential explanation for the small therapeutic benefit from Rituximab in recent clinical trials for lupus nephritis and SLE (29–33). However, CD20 and CD22 mAbs efficiently depleted blood and tissue B cells or B cell subsets, respectively, equally in both NZB/W F1 and C57BL/6 mice (Fig. 1). One 10 or 100 μg dose of CD20 mAb (MB20-11) cleared ≥84% of spleen B cells in NZB/W F1 and C57BL/6 mice, and low doses of CD20 mAb in NZB/W F1 mice significantly improved survival and delayed proteinuria. These results contrast with those of Ahuja et al., where B cells from autoimmune strains, including NZB/W F1 and MRL/lpr mice, were found to be inherently refractive to high dose (3 mg/week) CD20 (18B12, IgG1) mAb-induced depletion compared with non-autoimmune-prone BALB/c mice in which ~65% of spleen B cells were depleted (34). The reason for the striking discrepancies in B cell clearance between studies may be due in part to the particular CD20 mAbs or mAb isotypes used, as individual CD20 mAbs do not elicit comparable B cell depletion, and IgG2c CD20 mAbs are superior to IgG1 mAbs in eliciting B cell depletion by ADCC in vivo (37, 47, 67). While B cell depletion using 10–100 μg of MB20-11 CD20 mAb did not significantly decrease total ANA, IgG autoAb levels, or serum IgG in the current study (Figs. 2, 4), Ahuja et al. demonstrated that treatment of human CD20 transgenic-MRL/lpr mice with high doses of anti-human CD20 mAb (10 mg/mouse/week given twice weekly for 7- to 10-wks) reduced disease manifestations including proteinuria, nephritis, and ANA levels, although survival was not assessed. It is unknown whether these high doses of exogenous IgG or B cell depletion ameliorated disease symptoms since disease in mice treated with control IgG was not reported (34). Alternatively, differences in CD20 mAb-mediated alterations in serum Ig, autoAb and ANA levels between our study and the Ahuja et al. study may be related to differences in disease between NZB/W F1 mice versus human CD20Tg-MRL/lpr mice.
In summary, B cells from NZB/W F1 mice were efficiently depleted at the onset of CD20 and CD22 mAb treatment, with total B cell depletion effectively improving survival without eliminating IgG autoAbs in NZB/W F1 mice. Thereby, B cells in NZB/W F1 mice may contribute significantly to autoimmunity through their significant role during CD4+ T cell activation in response to autoantigens and low dose Ag challenge (16). B cell depleting therapies, such as CD20 and CD22 mAbs, may also elicit effects that extend beyond depleting B cells. Although premature B cell depletion in NZB/W F1 mice had deleterious effects, the results of this study suggest that mature B cell depletion may be an effective treatment for patients suffering from SLE when initiated shortly after disease onset. Thereby, while Rituximab has variable effects on autoAb levels in human SLE patients, treatment may improve the clinical manifestations of SLE for some patients in the absence of significant changes in serum anti-dsDNA Ab levels (30, 33, 61–63). This suggests that pathogenic B cells have non-Ab producing effector functions in human SLE as is observed for murine lupus. The development of resistance to B cell depletion therapy may also be a major contributing factor to decreased drug efficacy in patients with SLE, as the degree of blood B cell depletion associates with the extent of clinical improvement, while peripheral blood B cell reconstitution precedes relapse in the majority of patients (30, 62). These findings therefore suggest the combinatory usage of immunosuppressive therapies to inhibit anti-IgG Ab development that may antagonize B cell depletion during CD20 mAb treatment as well as inhibit T cell activation in human autoimmune disease. Since the effects of Rituximab and other B cell depleting therapies on human regulatory B10 cells are unknown, caution should be used when administering B cell depletion therapies, as regulatory B10 cell depletion may potentially initiate and/or accelerate the development of autoimmunity in some cases.
Acknowledgments
We thank Ms. Jacquelyn Bryant for help with the statistical analysis of these results.
Footnotes
This work was supported by grants from the National Institutes of Health (CA96547, CA105001 and AI56363), and a Grant-in-Aid from the Ministry of Education, Science, and Culture of Japan. KMH was supported in part by a Career Development Fellowship Award from the Leukemia and Lymphoma Society. TFT is a shareholder and consultant for Angelica Therapeutics, Inc, and consultant for MedImmune, Inc.
Abbreviations used: ADCC, Ab dependent cellular cytotoxicity; ANA, anti-nuclear Ab; autoAb, autoantibody: EAE, experimental autoimmune encephalomyelitis; RF, rheumatoid factor; SLE, systemic lupus erythematosus.
References
- 1.Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol. 2001;2:764–766. doi: 10.1038/ni0901-764. [DOI] [PubMed] [Google Scholar]
- 2.Grammer AC, Slota R, Fischer R, Gur H, Girschick H, Yarboro C, Illei GG, Lipsky PE. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154-CD40 interactions. J Clin Invest. 2003;112:1506–1520. doi: 10.1172/JCI19301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hahn BH, Singh RR. In: Animal Models of Systemic Lupus Erythematosus in Dubois’ lupus erythematosus. 7. Wallace DJ, Hahn B, Dubois EL, editors. Lippincott Williams & Wilkins; Philadelphia, PA: 2006. pp. 299–355. [Google Scholar]
- 4.Wither JE, Roy V, Brennan LA. Activated B cells express increased levels of costimulatory molecules in young autoimmune NZB and (NZB x NZW)F1 mice. Clin Immunol. 2000;94:51–63. doi: 10.1006/clim.1999.4806. [DOI] [PubMed] [Google Scholar]
- 5.Schuster H, Martin T, Marcellin L, Garaud JC, Pasquali JL, Korganow AS. Expansion of marginal zone B cells is not sufficient for the development of renal disease in NZBxNZW F1 mice. Lupus. 2002;11:277–286. doi: 10.1191/0961203302lu191oa. [DOI] [PubMed] [Google Scholar]
- 6.Atencio S, Amano H, Izui S, Kotzin BL. Separation of the New Zealand Black genetic contribution to lupus from New Zealand Black determined expansions of marginal zone B and B1a cells. J Immunol. 2004;172:4159–4166. doi: 10.4049/jimmunol.172.7.4159. [DOI] [PubMed] [Google Scholar]
- 7.Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. The Journal of experimental medicine. 1983;157:202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wofsy D, Chiang NY. Proliferation of Ly-1 B cells in autoimmune NZB and (NZB x NZW)F1 mice. Eur J Immunol. 1987;17:809–814. doi: 10.1002/eji.1830170612. [DOI] [PubMed] [Google Scholar]
- 9.Xu Z, Butfiloski EJ, Sobel ES, Morel L. Mechanisms of peritoneal B-1a cells accumulation induced by murine lupus susceptibility locus Sle2. J Immunol. 2004;173:6050–6058. doi: 10.4049/jimmunol.173.10.6050. [DOI] [PubMed] [Google Scholar]
- 10.Izui S, McConahey PJ, Dixon FJ. Increased spontaneous polyclonal activation of B lymphocytes in mice with spontaneous autoimmune disease. J Immunol. 1978;121:2213–2219. [PubMed] [Google Scholar]
- 11.Vyse TJ, Halterman RK, Rozzo SJ, Izui S, Kotzin BL. Control of separate pathogenic autoantibody responses marks MHC gene contributions to murine lupus. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8098–8103. doi: 10.1073/pnas.96.14.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan OT, Madaio MP, Shlomchik MJ. The central and multiple roles of B cells in lupus pathogenesis. Immunol Rev. 1999;169:107–121. doi: 10.1111/j.1600-065x.1999.tb01310.x. [DOI] [PubMed] [Google Scholar]
- 13.Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–299. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 14.Chan O, Shlomchik MJ. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J Immunol. 1998;160:51–59. [PubMed] [Google Scholar]
- 15.O’Neill SK, Shlomchik MJ, Glant TT, Cao Y, Doodes PD, Finnegan A. Antigen-specific B cells are required as APCs and autoantibody-producing cells for induction of severe autoimmune arthritis. J Immunol. 2005;174:3781–3788. doi: 10.4049/jimmunol.174.6.3781. [DOI] [PubMed] [Google Scholar]
- 16.Bouaziz JD, Yanaba K, Venturi GM, Wang Y, Tisch RM, Poe JC, Tedder TF. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20882–20887. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ronchese F, Hausmann B. B lymphocytes in vivo fail to prime naive T cells but can stimulate antigen-experienced T lymphocytes. The Journal of experimental medicine. 1993;177:679–690. doi: 10.1084/jem.177.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. The Journal of experimental medicine. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shlomchik MJ, Madaio MP, Ni D, Trounstine M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. The Journal of experimental medicine. 1994;180:1295–1306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joao C, Ogle BM, Gay-Rabinstein C, Platt JL, Cascalho M. B cell-dependent TCR diversification. J Immunol. 2004;172:4709–4716. doi: 10.4049/jimmunol.172.8.4709. [DOI] [PubMed] [Google Scholar]
- 21.AbuAttieh M, Rebrovich M, Wettstein PJ, Vuk-Pavlovic Z, Limper AH, Platt JL, Cascalho M. Fitness of cell-mediated immunity independent of repertoire diversity. J Immunol. 2007;178:2950–2960. doi: 10.4049/jimmunol.178.5.2950. [DOI] [PubMed] [Google Scholar]
- 22.Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. The Journal of experimental medicine. 2000;192:475–482. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ngo VN, Cornall RJ, Cyster JG. Splenic T zone development is B cell dependent. The Journal of experimental medicine. 2001;194:1649–1660. doi: 10.1084/jem.194.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golovkina TV, Shlomchik M, Hannum L, Chervonsky A. Organogenic role of B lymphocytes in mucosal immunity. Science (New York, NY. 1999;286:1965–1968. doi: 10.1126/science.286.5446.1965. [DOI] [PubMed] [Google Scholar]
- 25.Crowley MT, Reilly CR, Lo D. Influence of lymphocytes on the presence and organization of dendritic cell subsets in the spleen. J Immunol. 1999;163:4894–4900. [PubMed] [Google Scholar]
- 26.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 29.Anolik JH, Barnard J, Cappione A, Pugh-Bernard AE, Felgar RE, Looney RJ, Sanz I. Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum. 2004;50:3580–3590. doi: 10.1002/art.20592. [DOI] [PubMed] [Google Scholar]
- 30.Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, Sloand JA, Rosenblatt J, Sanz I. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum. 2004;50:2580–2589. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 31.Gottenberg JE, Guillevin L, Lambotte O, Combe B, Allanore Y, Cantagrel A, Larroche C, Soubrier M, Bouillet L, Dougados M, Fain O, Farge D, Kyndt X, Lortholary O, Masson C, Moura B, Remy P, Thomas T, Wendling D, Anaya JM, Sibilia J, Mariette X. Tolerance and short term efficacy of rituximab in 43 patients with systemic autoimmune diseases. Annals of the rheumatic diseases. 2005;64:913–920. doi: 10.1136/ard.2004.029694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding C, Foote S, Jones G. B-cell-targeted therapy for systemic lupus erythematosus: an update. BioDrugs. 2008;22:239–249. doi: 10.2165/00063030-200822040-00003. [DOI] [PubMed] [Google Scholar]
- 33.Karim MY, Pisoni CN, Khamashta MA. Update on immunotherapy for systemic lupus erythematosus--what’s hot and what’s not! Rheumatology (Oxford) 2009;48:332–341. doi: 10.1093/rheumatology/ken476. [DOI] [PubMed] [Google Scholar]
- 34.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol. 2007;179:3351–3361. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- 35.StClair WE, Tedder TF. New prospects for autoimmune disease therapy: B cells on deathwatch. Arthritis Rheum. 2006;54:1–9. doi: 10.1002/art.21525. [DOI] [PubMed] [Google Scholar]
- 36.Haas KM, Sen S, Sanford IG, Miller AS, Poe JC, Tedder TF. CD22 ligand binding regulates normal and malignant B lymphocyte survival in vivo. J Immunol. 2006;177:3063–3073. doi: 10.4049/jimmunol.177.5.3063. [DOI] [PubMed] [Google Scholar]
- 37.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, Tedder TF. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. The Journal of experimental medicine. 2004;199:1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uchida J, Lee Y, Hasegawa M, Liang Y, Bradney A, Oliver JA, Bowen K, Steeber DA, Haas KM, Poe JC, Tedder TF. Mouse CD20 expression and function. Int Immunol. 2004;16:119–129. doi: 10.1093/intimm/dxh009. [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa M, Hamaguchi Y, Yanaba K, Bouaziz JD, Uchida J, Fujimoto M, Matsushita T, Matsushita Y, Horikawa M, Komura K, Takehara K, Sato S, Tedder TF. B-lymphocyte depletion reduces skin fibrosis and autoimmunity in the tight-skin mouse model for systemic sclerosis. Am J Pathol. 2006;169:954–966. doi: 10.2353/ajpath.2006.060205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yanaba K, Hamaguchi Y, Venturi GM, Steeber DA, StClair EW, Tedder TF. B cell depletion delays collagen-induced arthritis in mice: arthritis induction requires synergy between humoral and cell-mediated immunity. J Immunol. 2007;179:1369–1380. doi: 10.4049/jimmunol.179.2.1369. [DOI] [PubMed] [Google Scholar]
- 41.Hu CY, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, Shlomchik MJ, Wen L. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117:3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiu Y, Wong CP, Hamaguchi Y, Wang Y, Pop S, Tisch RM, Tedder TF. B lymphocytes depletion by CD20 monoclonal antibody prevents diabetes in NOD mice despite isotype-specific differences in Fc R effector functions. J Immunol. 2008;180:2863–2875. doi: 10.4049/jimmunol.180.5.2863. [DOI] [PubMed] [Google Scholar]
- 43.Yu S, Dunn R, Kehry MR, Braley-Mullen H. B cell depletion inhibits spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Immunol. 2008;180:7706–7713. doi: 10.4049/jimmunol.180.11.7706. [DOI] [PubMed] [Google Scholar]
- 44.Hamel K, Doodes P, Cao Y, Wang Y, Martinson J, Dunn R, Kehry MR, Farkas B, Finnegan A. Suppression of proteoglycan-induced arthritis by anti-CD20 B cell depletion therapy is mediated by reduction in autoantibodies and CD4+ T cell reactivity. J Immunol. 2008;180:4994–5003. doi: 10.4049/jimmunol.180.7.4994. [DOI] [PubMed] [Google Scholar]
- 45.Hamaguchi Y, Uchida J, Cain DW, Venturi GM, Poe JC, Haas KM, Tedder TF. The peritoneal cavity provides a protective niche for B1 and conventional B lymphocytes during anti-CD20 immunotherapy in mice. J Immunol. 2005;174:4389–4399. doi: 10.4049/jimmunol.174.7.4389. [DOI] [PubMed] [Google Scholar]
- 46.Poe JC, Fujimoto Y, Hasegawa M, Haas KM, Miller AS, Sanford IG, Bock CB, Fujimoto M, Tedder TF. CD22 regulates B lymphocyte function in vivo through both ligand-dependent and ligand-independent mechanisms. Nat Immunol. 2004;5:1078–1087. doi: 10.1038/ni1121. [DOI] [PubMed] [Google Scholar]
- 47.Hamaguchi Y, Xiu Y, Komura K, Nimmerjahn F, Tedder TF. Antibody isotype-specific engagement of Fc receptors regulates B lymphocyte depletion during CD20 immunotherapy. The Journal of experimental medicine. 2006;203:743–753. doi: 10.1084/jem.20052283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yanaba K, Bouaziz JD, Matsushita T, Tasubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin RM, Brady JL, Lew AM. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J Immunol Methods. 1998;212:187–192. doi: 10.1016/s0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 50.Schreier PH, Bothwell AL, Mueller-Hill B, Baltimore D. Multiple differences between the nucleic acid sequences of the IgG2aa and IgG2ab alleles of the mouse. Proc Natl Acad Sci U S A. 1981;78:4495–4499. doi: 10.1073/pnas.78.7.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haas KM, Hasegawa M, Steeber DA, Poe JC, Zabel MD, Bock CB, Karp DR, Briles DE, Weis JH, Tedder TF. Complement receptors CD21/35 link innate and protective immunity during Streptococcus pneumoniae infection by regulating IgG3 antibody responses. Immunity. 2002;17:713–723. doi: 10.1016/s1074-7613(02)00483-1. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida H, Satoh M, Behney KM, Lee CG, Richards HB, Shaheen VM, Yang JQ, Singh RR, Reeves WH. Effect of an exogenous trigger on the pathogenesis of lupus in (NZB x NZW)F1 mice. Arthritis Rheum. 2002;46:2235–2244. doi: 10.1002/art.10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee PY, Kumagai Y, Li Y, Takeuchi O, Yoshida H, Weinstein J, Kellner ES, Nacionales D, Barker T, Kelly-Scumpia K, van Rooijen N, Kumar H, Kawai T, Satoh M, Akira S, Reeves WH. TLR7-dependent and Fc R-independent production of type I interferon in experimental mouse lupus. The Journal of experimental medicine. 2008;205:2995–3006. doi: 10.1084/jem.20080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DiLillo DJ, Hamaguchi Y, Ueda Y, Yang K, Uchida J, Haas KM, Kelsoe G, Tedder TF. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. 2008;180:361–371. doi: 10.4049/jimmunol.180.1.361. [DOI] [PubMed] [Google Scholar]
- 55.Van Snick JL, Stassin V, de Lestre B. Isotypic and allotypic specificity of mouse rheumatoid factors. The Journal of experimental medicine. 1983;157:1006–1019. doi: 10.1084/jem.157.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugisaki T, Takase S. Composition of immune deposits present in glomeruli of NZB/W F1 mice. Clin Immunol Immunopathol. 1991;61:296–308. doi: 10.1016/s0090-1229(05)80002-9. [DOI] [PubMed] [Google Scholar]
- 57.DiLillo DJ, Matsushita T, Tedder TF. B10 and regulatory B cells balance immune responses during inflammation, autoimmunity and cancer. Annals New York Acad Sci. 2009 doi: 10.1111/j.1749-6632.2009.05137.x. (in press) [DOI] [PubMed] [Google Scholar]
- 58.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 59.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. The Journal of experimental medicine. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 61.Leandro MJ, Cambridge G, Edwards JC, Ehrenstein MR, Isenberg DA. B-cell depletion in the treatment of patients with systemic lupus erythematosus: a longitudinal analysis of 24 patients. Rheumatology (Oxford) 2005;44:1542–1545. doi: 10.1093/rheumatology/kei080. [DOI] [PubMed] [Google Scholar]
- 62.Smith KG, Jones RB, Burns SM, Jayne DR. Long-term comparison of rituximab treatment for refractory systemic lupus erythematosus and vasculitis: Remission, relapse, and re-treatment. Arthritis Rheum. 2006;54:2970–2982. doi: 10.1002/art.22046. [DOI] [PubMed] [Google Scholar]
- 63.Cambridge G, Leandro MJ, Teodorescu M, Manson J, Rahman A, Isenberg DA, Edwards JC. B cell depletion therapy in systemic lupus erythematosus: effect on autoantibody and antimicrobial antibody profiles. Arthritis Rheum. 2006;54:3612–3622. doi: 10.1002/art.22211. [DOI] [PubMed] [Google Scholar]
- 64.Emi Aikawa N, de Carvalho JF, Artur Almeida Silva C, Bonfa E. Immunogenicity of anti-TNF-a agents in autoimmune aiseases. Clin Rev Allergy Immunol. 2009 doi: 10.1007/s12016-009-8140-3. (in press) [DOI] [PubMed] [Google Scholar]
- 65.Thurlings RM, Teng O, Vos K, Gerlag DM, Aarden L, Stapel SO, van Laar JM, Tak PP, Wolbink GJ. Clinical response, pharmacokinetics, development of human anti-chimeric antibodies, and synovial tissue response to rituximab treatment in patients with rheumatoid arthritis. Annals of the rheumatic diseases. 2009 doi: 10.1136/ard.2009.109041. (in press) [DOI] [PubMed] [Google Scholar]
- 66.Schmidt E, Hennig K, Mengede C, Zillikens D, Kromminga A. Immunogenicity of rituximab in patients with severe pemphigus. Clin Immunol. 2009;132:334–341. doi: 10.1016/j.clim.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 67.Beers SA, Chan CH, James S, French RR, Attfield KE, Brennan CM, Ahuja A, Shlomchik MJ, Cragg MS, Glennie MJ. Type II (tositumomab) anti-CD20 monoclonal antibody out performs type I (rituximab-like) reagents in B-cell depletion regardless of complement activation. Blood. 2008;112:4170–4177. doi: 10.1182/blood-2008-08-172999. [DOI] [PMC free article] [PubMed] [Google Scholar]