Abstract
Lack of understanding of endocrine resistance remains one of the major challenges for breast cancer researchers, clinicians, and patients. Current reductionist approaches to understanding the molecular signaling driving resistance have offered mostly incremental progress over the past 10 years. As the field of systems biology has begun to mature, the approaches and network modeling tools being developed and applied therein offer a different way to think about how molecular signaling and the regulation of critical cellular functions are integrated. To gain novel insights, we first describe some of the key challenges facing network modeling of endocrine resistance, many of which arise from the properties of the data spaces being studied. We then use activation of the unfolded protein response (UPR) following induction of endoplasmic reticulum stress in breast cancer cells by antiestrogens, to illustrate our approaches to computational modeling. Activation of UPR is a key determinant of cell fate decision making and regulation of autophagy and apoptosis. These initial studies provide insight into a small subnetwork topology obtained using differential dependency network analysis and focused on the UPR gene XBP1. The XBP1 subnetwork topology incorporates BCAR3, BCL2, BIK, NFκB, and other genes as nodes; the connecting edges represent the dependency structures amongst these nodes. As data from ongoing cellular and molecular studies become available, we will build detailed mathematical models of this XBP1-UPR network.
Keywords: Antiestrogen, autophagy, apoptosis, breast cancer, cell signaling, endoplasmic reticulum, estrogens, gene networks, unfolded protein response, computational modeling, mathematical modeling, systems biology
Introduction
Despite over 30 years of relatively safe and effective endocrine therapies, from the advent of Tamoxifen and antiestrogens (AE) to the more recent application of third generation aromatase inhibitors (AI), many estrogen receptor-alpha (ER) positive breast cancers either fail to respond (de novo resistance) or eventually recur on or after endocrine therapy (acquired resistance) (1, 2). The major reductions in the risks of recurrence and death that women with an ER+ breast cancer derive from these therapies represent a major achievement. Nonetheless, our lack of understanding of endocrine resistance remains one of the major challenges for breast cancer researchers, clinicians, and patients (3, 4). While resistance to hormonal therapies is an active area of research, and several genes and signal transduction pathways have been implicated in the underlying processes (5–7), our understanding of the fundamental molecular regulatory networks that drive cell survival and proliferation in this phenotype (or phenotypes) is clearly inadequate. Recent advances in the molecular classification of breast cancers (8, 9) have done little to change routine clinical practice for the management of ER+ breast cancers, which represent 70% of all newly diagnosed breast cancer each year. Unfortunately, few effective new strategies to treat advanced, endocrine resistant, ER+ breast cancer have emerged in recent years. Indeed, metastatic breast cancer remains largely an incurable disease.
To create new opportunities for drug discovery and therapeutic interventions, we believe it is essential to acquire first an adequate understanding of the true nature of the molecular interactions responsible for the endocrine resistance phenotype (6). Current approaches to understanding molecular signaling appear limited and have offered somewhat slow and incremental progress over the past 10 years. As the field of systems biology has begun to mature, the approaches and tools being developed therein may provide a different way to think about how molecular signaling and the regulation of critical cellular functions are integrated. One key difference in a systems approach, compared with the more common reductionist approach, is the application of computational and mathematical modeling to represent dynamic system function. These modeling tools are often applied to the high dimensional data sets obtained from microarray, proteomic, and sequencing technologies. However, there are often poorly understood challenges in the analysis of such large data sets that reflect unique properties of high dimensional data spaces (10, 11).
Network modeling and endocrine responsiveness
A primary reductionist focus on individual genes and/or simple signal transduction pathways is likely one limitation of our ability to derive fundamentally new insights into the molecular underpinning of the phenotype (or perhaps phenotypes) that is resistance (and often crossresistance) to AEs and AIs. These types of signaling-based studies are frequently based on hypotheses framed in the context of the limitations of transduction pathways as understood from largely static models, such as those represented in the KEGG or Biocarta databases, or as constructed de novo from modeling tools such as Ingenuity Pathway Analysis or Ariadne Pathway Studio (6). Many of these tools have their uses but they are limited by the frequent inability to account for cellular context and molecular dynamics. Moreover, the true complexity of molecular signaling is probably affected by biological properties, rules, or functions that we do not yet fully understand. For example, the existence and potentially powerful regulatory influences of miRNAs have been only relatively recently discovered. We have long advocated for a more network-based approach (12) but the tools to achieve this have only recently begun to become widely available (6).
Any individual protein or signal transduction pathway exists within a hugely complex and high-dimensional cellular context as defined by the patterns and interactions among all the other proteins, metabolites, RNA, DNA, and cellular functions, operating concurrently and dynamically in the same cell. While each cell likely contains approximately 30,000 genes, estimates of the size of the human interactome vary considerably. Stumpf et al. estimate the human interactome (entire set of protein interactions) to be ~650,000 interactions, a sparse network of only ~0.2% of all pairwise connections (13). However, this estimate does not consider context-specific interactions or the dynamic nature of the system (13). The latter could substantially increase the number of interactions responsible for maintaining cellular function across time and in response to changing extracellular and intracellular environments. The contributions of protein-DNA, protein-RNA, and protein-metabolite/ligand interactions may not be adequately captured in this estimate and these could further increase the dimensionality of the edges in the overall signaling network regulating cellular function.
Understanding the properties of networks of this size and complexity offer remarkable challenges, not least of which are the unique properties of high dimensional spaces (10). For example, in such large networks it is estimated that the shortest distance between any two nodes (usually a gene or protein) is no longer than 6 connections (14, 15); likely a major contributor to the signaling redundancy and degeneracy that can confer apparent plasticity on network topology. Multiple inputs to the human interactome are occurring concurrently and the network is dynamically responding to each of these inputs – many of which modify the function of other regions of the interactome. This level of interconnectivity and dynamism is fundamentally lacking in most current approaches to gene network modeling. Moreover, in all likelihood, we do not yet fully understand the properties of such large networks or their implications for building fully accurate and robust models of their function.
Precisely because the human protein interactome is dynamic and adaptable, building a model of how it works has many characteristics of a “wicked problem” (16, 17). Amongst several criteria, a wicked problem is one where there is incomplete, and sometimes contradictory information, and the changing nature of the requirements of the network (in the case of a cellular system in response to stress, external signaling that may change the function or differentiation status of the cell, or other factors) that are difficult to recognize. Moreover, there may be more than one solution - what explains how the interactome works for endocrine resistance in ER+ breast cancers may not explain how it works in any other cancer resistance problems. While perhaps not all of the criteria apply, getting the scientific community to engineer an agreed solution could well be a wicked problem in the original social planning sense.
While it might be tempting to assume that these various challenges do not apply to the study of endocrine resistance, it is not immediately clear that this is a reasonable assumption (6). ER- mediated responses can encompass coordinating functions from complex organism-level sexual, aggressive and reproductive behaviors, down to the subcellular level as might be represented by coordinating the subcellular functions that are required to execute the decision of a breast cancer cell to exit G1 and enter S-phase of the cell cycle. Perhaps these functions are provided by very different ER-regulated genes in neurons, for example, than in mammary epithelial cells (the network nodes - and so also the edges - could be very different). However, nature is often parsimonious, and the possibility that many of the same molecular players in breast cells also operate in brain cells cannot be discounted. If this is the case, then it is not so much the nodes (genes/proteins) that are different in brain and breast cells, it is the edges (connections) that link them. At some level, the ER-regulated network could broadly retain its overall topology, adapting primarily (but not exclusively) by locally modifying how some of its nodes are interconnected. The same may be true for the differences between endocrine sensitive and resistant topologies of the ER-regulated network.
The current state of knowledge in biology, mathematics, statistics, and signaling transduction probably limit our ability to fundamentally address modeling of any complex biological network in a single approach. Pragmatically, we must make some general assumptions and work with the acknowledged limitations of current knowledge and existing tools. Thus, we propose that the endocrine resistance phenotype(s) is primarily controlled by a large and complex subnetwork that exists within the context of the much larger human interactome. From this starting point, a simple, linear thought process allows us to derive other reasonable but possibly incomplete assumptions about this subnetwork
In sensitive breast cancer cells, endocrine therapies initially induce a profound G0/G1 cell cycle arrest. Clearly, one of the regulatory functions of our hypothetical subnetwork is the decision to enter or exit the proliferative phases of the cell cycle, and a coordinated regulation of the attendant cellular functions required to provide the energy and nutrients needed to make a duplicate copy of the existing cell. This latter series of events follows where the decision is to remain cycling and so exit G1 and enter S; as would be the case in treating resistant cells, or providing estrogen to estrogen-dependent cells.
Since endocrine therapies can lead to improvements in overall survival, at some point each breast cancer cell will make and then execute a decision to live or die. A further component of our subnetwork must govern the cell survival decision and the attendant functions that allow the cell to survive. Such functions include blocking induction of apoptotic cell death and providing for the integrity of those subcellular functions required for prolonged cell survival, such as maintaining adequate energy levels for basic metabolic functions.
ER can regulate (or at least influence) both cell proliferation and cell survival decisions, and so it must also arrange for coordinating the cellular functions required to execute these decisions. Thus, ER must be a central node in the subnetwork. Indeed, most ER+ breast cancers that acquire a resistant phenotype remain ER+ (18), and siRNA targeting ER in antiestrogen resistant cells is growth inhibitory (19). Much is known about how ER functions and of various growth factors and other signaling molecules that, in the context of endocrine regulation of breast cancer cells, can influence ER functions and endocrine responsiveness (5–7). Thus, we can begin with a simple list of genes that will become initial seed genes (nodes) around which we can build out a more complete network model (20).
Individual modules for the functional execution of the cell cycle decision are well known and these appear to have significant components maintained by evolution across multiple species. One example is the execution network that enables cells to complete a turn of the cell cycle, which was initially modeled in yeast cells (21, 22). Components of the unfolded protein response (UPR) are also conserved across species and these include homologues of X-Box binding protein-1 (XBP1). Thus, we can separate our subnetwork into a series of modules that perform specific functions, and a series of (presumably) interconnected decision signaling networks that make the determination of which execution modules to activate or repress and the timing of these execution/repression decisions. Modules would then include, at the very least, cell cycle, UPR, apoptosis, autophagy.
How we approach construction of the mathematical models and control signaling is described elsewhere (23). Overall, we apply an integrated approach where we use computational modeling tools and high dimensional data to extract local topological information of the relationships among the genes and functions we believe to be of most initial relevance. For the purposes of this review, computational modeling uses tools mostly from the field of computational statistics such as artificial neural networks and support vector machines; these tools are used to learn the key features of the data as they relate to phenotype. By mathematical modeling we mean the process of deriving a mathematical description that captures the relevant mechanistic details of the system and can be simulated to predict how the system evolves in time. Such descriptions may, for example, use differential equations or stochastic reaction networks to model gene expression and protein interactions relevant to the phenotypes being studied. Both computational and mathematical models can generate simulations and make predictions of how the systems they are modeling responds when perturbed.
Once validated experimentally, we integrate this knowledge with preliminary mathematical models for each module and/or control function. In an iterative approach, using both computational and mathematical modeling, we begin to learn how the system may function – mostly from the failure of the initial models to recapitulate experimental data and the subsequent predictions of what functions are required to allow the models to work.
In this review, we will focus on the potential role of one module for regulating key survival functions that we have implicated in acquired endocrine resistance. Specifically, we will review evidence implicating activation of the unfolded protein response as a critical subcellular function and follow through on early computational modeling of what appears to be prosurvival signaling out from the UPR as regulated by controlling the expression and unconventional splicing of XBP1. Of necessity, these initial representations are largely static wiring diagrams. However in the longer term, we will use our experimental data and that available in the literature to guide the construction of initial mathematical models of the UPR and its role in governing prosurvival signaling in the context of endocrine responsiveness in breast cancer.
Endoplasmic reticulum stress and the unfolded protein response in normal and neoplastic breast tissues
The folding of proteins within the endoplasmic reticulum (EnR) is an energy-dependent function, which in the absence of sufficient energy or other nutrient limitations can result in the accumulation of unfolded proteins within the EnR lumen. Normally, these proteins are detected and additional energy is consumed as the cell attempts to fold (or unfold and refold) them into their correct form(s). However, as unfolded proteins accumulate, the cell may have less and less energy available to meet this increased demand, particularly if it is experiencing external stressors and the resources to fold correctly these proteins are inadequate. The accumulation of these unfolded proteins creates a condition known as endoplasmic reticulum stress, which ultimately initiates an attempt to restore balance through several means including lowering energy/nutrient demands by reducing the rates of mRNA transcription and protein translation, and removing for degradation (rather than refolding) inappropriately folded or unfolded proteins. Degradation usually occurs through the endoplasmic reticulum-associated degradation pathway (ERAD) (24). Prolonged EnR stress may activate more substantive prosurvival processes, such as a prosurvival autophagy.
Three forms of autophagy exist: microautophagy, chaperone-mediated autophagy, and macroautophagy (25) (here we use the term “autophagy” to denote macroautophagy). A lysosomal process, autophagy occurs when the cell begins to self-digest its subcellular organelles; these are usually defective organelles, perhaps rendered such by an excessive and unmet total energy/nutrient demand within the entire cell. Autophagy can be either prodeath (autophagic cell death) and act as an alternative cell death pathway to apoptosis (26), or prosurvival when extracellular nutrients or growth factors are limited (27). The primary goal of this prosurvival autophagic process appears to be to recover sufficient energy and nutrients from the unnecessary/damaged organelles to meet the demands of more fundamental cell processes.
Prolonged unresolved EnR stress often causes cell death, which may include an autophagic cell death driven by autophagy cannibalizing subcellular organelles to a point beyond which the cell can no longer survive. Whether the ultimate cell death is a consequence of induction of an energy dependent cell death process such as apoptosis, or one less dependent upon available energy sources such as necrosis, is an area of considerable interest and investigation.
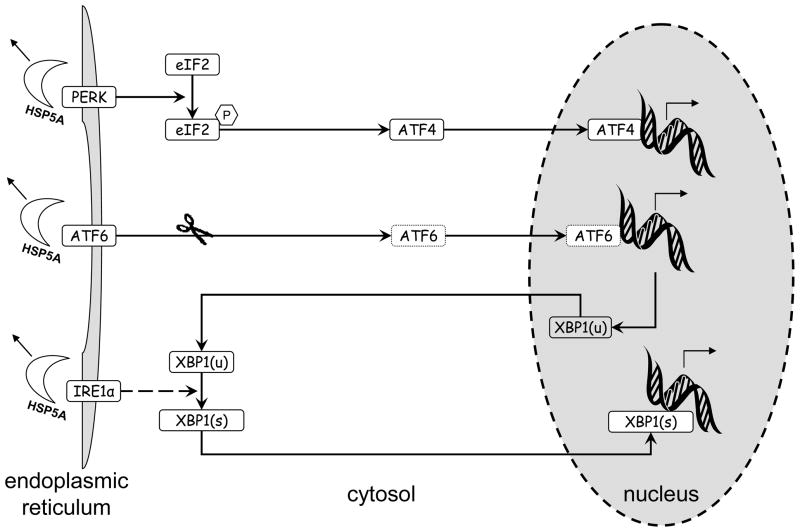
The initial coordinated response to EnR stress is a process called the unfolded protein response (UPR). Since there are several excellent reviews available that describe the UPR in detail (28, 29), we here provide only a brief overview. The UPR has three primary arms, each initiated by a specific sensor, respectively PERK, ATF6, and IRE1α (Figure 1). Under normal conditions, each sensor is maintained in an inactive state through its association with the molecular chaperone HSP5A - also known as glucose-regulated protein 78 (GRP78) or immunoglobulin heavy chain-binding protein (BiP). In the presence of unfolded proteins, HSP5A disassociates from the molecular sensors and binds to the misfolded proteins in an attempt to activate their repair (30), thus activating the sensors.
Figure 1.
Overview of the three arms of the unfolded protein response (UPR). Accumulation of unfolded proteins causes HSP5A to dissociate from (and so activate) the three sensors PERK, ATF6, IRE1α. The activated sensors then initiate their respective signaling arms, each of which results in the regulation of transcription (by ATF4, cleaved ATF6, and spliced XBP1, respectively). The role of XBP1 in the ATF6 arm (induction of XBP1(u) transcription) and IRE1α arm (creation of XBP1(s) by XBP1(u) splicing) is shown.
It seems likely that the normal mammary epithelium has a particularly well-coordinated and active UPR. For example, the prolonged production of substantial amounts of secretory proteins is essential during lactation, when the mammary epithelial cells must balance the need to apply energy resources to translate, fold, and secrete proteins with those of the basic cell survival functions. Furthermore, it would make strong biological sense for the cell to coordinate the fulfillment of its nutrient and energy demands with this protein production requirement, so as not to induce a prolonged and potentially fatal EnR stress. Since the stimuli to regulate milk production are under the regulation of the lactogenic hormones (prolactin, insulin, and the glucocorticoids), it is reasonable to assume that these hormones also assist in initiating and/or maintaining the coordinated functions required to balance a high rate of protein production, and the potentially associated EnR stress, with cell survival. Thus, normal breast epithelial cells are likely well primed to adapt to prolonged EnR stress, and the recruitment of these strategies by neoplastic breast cells as a primary survival mechanism in the face of the stress of endocrine therapy (or other therapies) would seem predictable. Since ER-mediated activities in breast cancer cells appear to regulate multiple functions, including general cellular metabolism and the highly energy/nutrient demanding functions required to execute a decision to enter the cell cycle, it is reasonable to expect activation of ER to play a central role in affecting UPR-associated activities in breast cancer cells.
XBP1 transcription and splicing, and its interactions with ER
The application of stress to cells results in several changes in metabolism and can induce various stress response functions. A reduction in access to adequate oxygen, nutrients, or energy can cause cells to redirect their available resources to perform basic functions in order to survive. Inadequate vascularization places many tumor cells under some level of hypoxic stress and nutrient deprivation, stressors known to induce EnR stress (31). Inhibition of ER activity in estrogen-dependent breast cancers by AEs or AIs likely exacerbates these problems and further activates endoplasmic reticulum stress. Thus, the UPR is a primary candidate for one survival mechanism that, if successfully activated, could allow cells to survive the stress of endocrine therapies and confer a resistance phenotype.
Gu et al. first implicated UPR signaling in antiestrogen resistance and estrogen independence, reporting the increased expression of XBP1 and its associated cAMP-response element-driven transcriptional activity and that of other UPR related proteins (NFκB; HSP27) in LCC9 breast cancer cells (32). The functional relevance of the role of XBP1 was established when the full length XBP1 cDNA was overexpressed in both the MCF-7 and T47D human breast cancer cell lines by Gomez et al. (33), data also consistent with the ability of the upstream UPR regulator HSP5A to protect cells from estrogen withdrawal (34). Interestingly, the primary form of XBP1 protein present is the spliced form XBP1(s), indicating that, at least in these cell models, transcriptional regulation of XBP1 may be rate limiting and not the rate of its unconventional splicing by the endonuclease activity of IRE1α. XBP1 splicing is unconventional because it occurs predominantly in the cytosol (35). While IRE1α can splice multiple RNAs, it is the only enzyme known to splice XBP1. Splicing removes a short 25 basepair sequence from XBP1 that deletes a stop codon and creates a longer mRNA reading frame.
Translation of the XBP1(s) RNA template results in the production of a larger protein that can acts as a transcription factor. Regulation of transcription by XBP1(s) is a consequence of its homodimers activating specific cAMP response elements (CREs) with a conserved ACGT core sequence GATGACGTG(T/G) NNN(A/T)T; sometimes called the UPR element (36, 37). In marked contrast, translation of the unspliced XBP(u) generates a shorter protein that cannot act as a transcription factor but can act as an endogenous dominant negative inhibitor of XBP1(s) (38, 39). Thus, consistent with the critical nature of the functions it regulates, control of XBP1 activity is multifactorial, for example, (i) rate of transcription (includes regulation by cleaved ATF6 and ER), (ii) rate of splicing by IRE1α (perhaps not a common mechanism in breast cancer), (iii) ratio of XBP(1u):XBP1(s).
Of particular relevance to breast cancer is the observation that XBP1 is a major estrogen induced gene, being rapidly induced in response to E2-stimulation (40, 41). Expression of XBP1 is a key component in the molecular classification scheme that defines luminal, basal, HER2+, and normal-like breast cancers (8), being associated with the ER+ phenotype (42). Furthermore, XBP1 protein can act as a coactivator of ER, forming ligand-independent XBP1:ER heterodimers that are more effective in driving transcription from an estrogen responsive element (43). These observations suggest that the XBP1-ER interactions may be used to “fine-tune” some critical UPR functions.
Modeling XBP1 signaling in breast cancer cells
The evidence implicating XBP1 expression in acquired resistance (32, 33) and our hypothesis of its potentially central role during lactation, led us to explore possible new predictive models of XBP1 signaling. As a precursor to developing mathematical models, we have begun to develop computational modeling tools and apply these to existing data sets to try to uncover new topological knowledge of XBP1 signaling (20, 44–47). The primary goal is to discover topological features of an XBP1-associated signaling module in the context of endocrine responsiveness, with a particular focus on an initial series of genes we believe are likely to contribute to the regulation and/or execution of proliferation or cell death/survival decisions. Subsequently, we perform wet laboratory experiments to validate and extend these topological features and to explore more fully how signaling flows to affect endocrine responsiveness. Initial models are necessarily simplistic and static in their representations of what is definitively a dynamic and adaptable process. Nonetheless, these representations should allows us to eventually build truly dynamic models that can more accurately predict the most important signaling that affects key subcellular functions relevant to the endocrine resistant phenotype. The dynamic nature of the process is captured by the models allowing changes to be made in the input values for specific nodes or edges. The model will then calculate how the signaling is perturbed as a consequence these changed values, leading to predictions about signal transduction and the altered regulation of the relevant cellular function(s).
In our work to develop new methods for computational network modeling, we have recently developed a powerful new approach called differential dependency network (DDN) analysis (20, 48). DDN was derived specifically to model statistically significant topological changes between two conditions and was initially applied to transcriptome data from gene expression microarrays. Local dependency models decompose the whole network, as represented by the entire data set, into a series of local networks. Rather than look at 2-wise or 3-wise interrelationships, the local dependency models are applied with a Lasso technique (least absolute shrinkage and selection operator; a least squares regression method with an L1 norm constraint) that can select the optimum number of dependent variables and help ease the risk of overfitting (20, 49). To detect statistically significant network topological changes, DDN applies permutation tests under the two conditions and estimates a p-value for each of the local structures. Ultimately, local topological features are represented by a set of conditional probabilities, and each node can be assigned more than one conditional probability distribution. The latter can allow nodes to “belong” to more than one local dependency network and/or acquire multiple edges. Edges in DDN reflect the dependency structures among genes that are learned by the Lasso method. Since DDN characterizes the statistically significant network changes between two biological conditions, the dashed and solid edges in Fig 2 represent the condition-specific dependencies. For instance, if gene A is a good predictor of gene B under condition 1, but shows no such relationship under condition 2, then in the DDN we will expect there is a condition-specific edge between gene A and gene B under condition 1. A key goal of DDN modeling is to find “hot spots”, which are those genes that exhibit statistically significant network changes between two conditions given a predetermined significance level. The assumption with respect to these “hot spots” is that robust topological changes likely reflect important or meaningful biological events. Greater detail on the derivation of this method can be found elsewhere (20, 48).
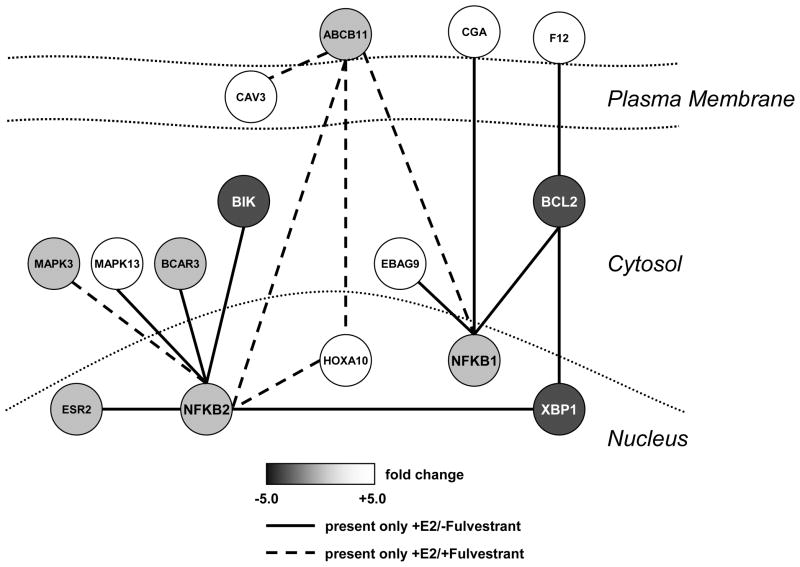
Figure 2.
DDN model showing initial topology (nodes and predicted edges) of a subnetwork featuring XBP1, BCL2, and NFκB. Overexpression of XBP1 in MCF-7 cells results in the upregulation of endogenous BCL2; the BCL2 gene has three XBP1-CREs in its upstream promoter region. Solid edges are those present with E2 treatment; dashed edges are present with 17β-estradiol and Fulvestrant co-treatment. Adapted from figure 3 in reference (20).
In our initial studies, we selected 55 genes associated with antiestrogen responsiveness, including XBP1, and applied DDN to a publicly accessible gene expression microarray data set from T47D human breast cancer cells treated with 17β-estradiol ± Fulvestrant (Faslodex; ICI 182780). Fulvestant is an ER antagonist antiestrogen that does not exhibit partial agonism and normally targets the ER for degradation (50). The study from which the data were obtained was reported in detail by Lin et al. (51), and incorporates time course experimental design of 16 time points over a 24 hr period. Thus, we used DDN to look for topological features in the data set that could reflect “early” estrogen regulated signaling that is perturbed by the antiestrogen.
Initial representation of XBP1-associated signaling
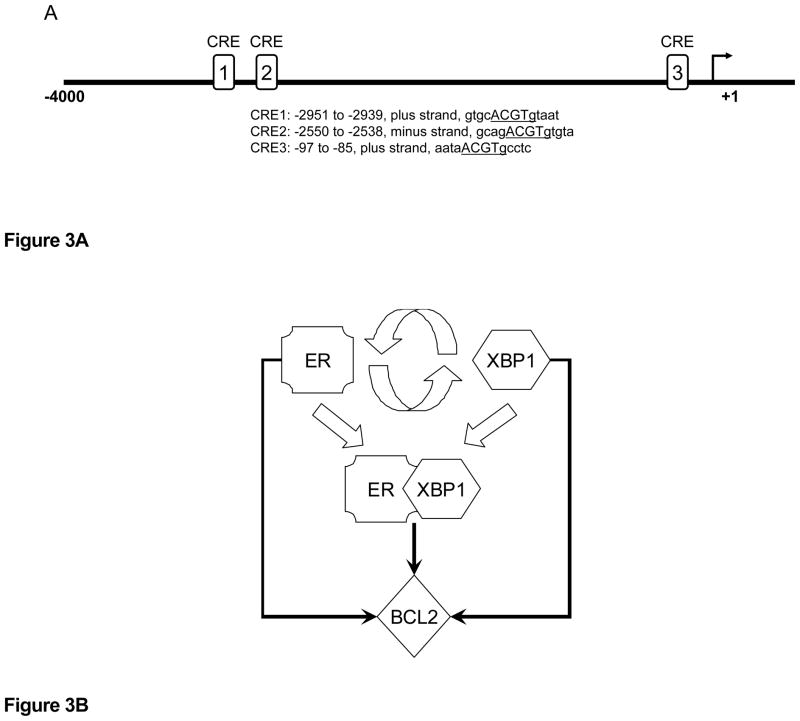
The results of these initial studies using DDN are shown in Figure 2; this is a general representation of one small area of the overall subnetwork regulating the cell fate decision and provides a series of seed nodes and edges for validation in wet laboratory experiments (20, 48). The edges are coded to reflect those present with E2 treatment (solid lines) and those present with estradiol and Fulvestrant co-treatment (dashed lines). Hence, solid lines are implied to disappear when the antiestrogen is added. From the perspective of XBP1, proposed connections with BCL2 and NFkB would be present only with estrogens and lost with the addition of Fulvestrant. BCL2 is a key determinant for maintaining cell survival with the UPR (28), and we now know that BCL2 is overexpressed in antiestrogen resistant cells that also overexpress the endogenous XBP1(s) (52). Estrogenic induction of BCL2 is well known, and we have shown that XBP1 is also a likely regulator of BCL2, which is overexpressed in cells that have been transfected with XBP1 (33). The BCL2 promoter contains at least three XBP1-CRE sites that could drive a direct transcriptional activation of BCL2 by XBP1 (Figure 3A). When considered together, these data strongly suggest that some breast cancer cells may use the cooperation between ER and XBP1 to provide redundant signaling and increase the likelihood of cell survival despite any concurrent EnR stress (Figure 3B). Importantly, antiestrogen resistant cells that overexpress BCL2 are more sensitive to growth inhibition by small molecule inhibitors of BCL2 (52).
Figure 3.
Figure 3A. BCL2 promoter contains at least three of the specific cAMP responsive element sequences regulated by XBP1(s).
Figure 3B. ER and XBP1 interact to induce the prosurvival factor BCL2. ER can induce XBP1, and XBP1 can induce ER. ER and XBP1 can form transcription complexes that are more effective at driving transcription from EREs. Independently, ER and XBP1 (and presumably also ER:XBP1 complexes) can induce BCL2, providing integrated and potentially redundant prosurvival signaling from the UPR.
The DDN model already correctly incorporates known knowledge of the relationship between XBP1 and BCL2. New relationships are predicted including potential roles for ERβ (ESR2), BCAR3, and NFkB. Data implicating each of these genes individually in antiestrogen responsiveness is already available. For example, NFKB2 is associated with estrogen independence and may be selectively activated in breast tumors (53). BCAR3 activity is strongly associated with estrogen independence, and antiestrogen resistance (54–56). This small topological representation includes two MAPK family members (MAPK3; MAK13), suggesting that it also may begin to explain coordinated signaling for the regulation of both proliferation and survival. Most of the edges represented in Figure 2 remain to be experimentally validated, and whether there are intervening latent variables is unknown at this time. Nonetheless, the model provides further evidence implicating UPR associated genes in endocrine responsiveness and offers some novel hypotheses as to how these genes may further interact.
The implication that BCL2 is a key player may represent more than this single gene - the model could also be read as implicating its function; as such, the role of BCL2 in Figure 2 could reflect a role for several members of this family. We have recently shown that the full effect of the small molecule BCL2 inhibitors is mimicked only when both BCL2 and BCL-W are co-inhibited (52). Other interactions also occur but these are not directly reflected in this model. For example, BCL2 and BCL-W can affect cell survival by binding and sequestering BECN1. These interactions prevent the induction of a prodeath autophagy and can contribute to antiestrogen resistance (52). However, these events are further downstream and occur primarily in the proteome, and so might not be reflected in a model based primarily on transcriptome data. This observation identifies one limitation to using such models to try to solve an entire subnetwork topology. However, to understand the transcriptional components of the subnetwork, the application of DDN to gene expression microarray data can uncover known relationships and propose new hypotheses for further study.
How the full subnetwork regulating endocrine resistance is wired remains unknown. Nonetheless, the extraction of topological information supported by experimental biological data in relevant cell systems provides a starting point from which to uncover new nodes and edges and build out the network in an iterative manner (6). As we obtain additional experimental data, we can eventually move towards constructing mathematical representations of the signaling and network function. Ultimately, we will build predictive models that capture how ER-mediated signaling coordinates cell survival and cell proliferation decisions, and the required metabolic and other cellular functions that must be activated or repressed to execute these functions.
Conclusions and future directions
In estrogen dependent cells, estrogen withdrawal (AI) or ER blockade (AE) results in a loss of adequate metabolic activity, likely resulting in low energy production. Inadequate energy depletes exiting stores and eventually fails to meet the needs of the EnR to fold new proteins. This chain of events results in activation of an endoplasmic reticulum stress and induction of the UPR in an attempt to rebalance the energy and nutrient demands the cells need for survival. Those cells best capable of adapting their prosurvival signaling will have the greatest probability of acquiring a stably resistant phenotype. Where this signaling involves upregulation of XBP1(s), the cells will also have a greater likelihood of becoming crossresistant to other endocrine therapies. UPR initiated signaling may also result in an upregulation of autophagy, with surviving cells being those that can adjust this self-digestion to balance the need for energy and nutrients with the risk of activating cell death cascades. A critical signaling integration point for these activities appears to include modulation of the expression of various members of the prosurvival BCL2 family including, but not limited to, BCL2, BCL3, and BCLW (52, 53, 57, 58).
The need to develop a greater understanding of the signaling that regulates endocrine responsiveness is evident. While much is known about the potential contribution of individual genes, and perhaps also some relatively linearly constructed signaling pathways, how this knowledge can be used to build dynamic, predictive models of cell function remains elusive. To create more effective combinatorial therapies it is likely that we must understand the topology of the network with sufficient clarity that we can target only those nodes/edges needed to cause the signaling to collapse, and for the cell to have the least chance to adapt or rewire its signaling to survive. If we are correct, the current practice of treating ER+ breast cancers with single agent endocrine therapies may eventually be replaced with modalities that are more complex. Among the challenges in arriving at this point will be obtaining an adequate understanding of signaling complexity, being able to model the inherent redundancy and degeneracy naturally present within networks that control and execute such fundamental decisions (and that contribute to the apparent plasticity of the phenotypes), and developing safe and effective new drugs for these targets. While this is very probably a wicked problem, current approaches to ease the challenges for this problem include the integration of mathematical and computational tools to help guide the modeling and offer hypotheses for the laboratory experimentalists to test. Data from the hypothesis-testing laboratory experiments provide further insights to adjust iteratively computational and mathematical models. In addition to the need to apply some standard reductionist wet laboratory experiments, at least for the time being, high throughput experimental methods such as the various microarray, proteomic, sequencing, and functional genomics tools now available offer the opportunities to obtain much of the data required to eventually allow building useful models.
Acknowledgments
This work was supported in part by awards from the Department of Defense Breast Cancer Research Program (BC073977) and from the U.S. Department of Health and Human Services (R01-CA131465; U54-CA149147 and 9XS194 In Silico Research Centers of Excellence) to Dr. R. Clarke; R21- CA139246 to Dr. J. Xuan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomized trials. Lancet. 1998;351:1451–67. [PubMed] [Google Scholar]
- 2.Clarke R, Leonessa F, Welch JN, Skaar TC. Cellular and molecular pharmacology of antiestrogen action and resistance. Pharmacol Rev. 2001;53:25–71. [PubMed] [Google Scholar]
- 3.Dowsett M, Goldhirsch A, Hayes DF, Senn HJ, Wood W, Viale G. International Web-based consultation on priorities for translational breast cancer research. Breast Cancer Res. 2007;9:R81. doi: 10.1186/bcr1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson A, Brennan K, Cox A, Gee J, Harcourt D, Harris A, Harvie M, Holen I, Howell A, Nicholson R, Steel M, Streuli C. Evaluation of the current knowledge limitations in breast cancer research: a gap analysis. Breast Cancer Res. 2008;10:R26. doi: 10.1186/bcr1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke R, Liu MC, Bouker KB, Gu Z, Lee RY, Zhu Y, Skaar TC, Gomez B, O’Brien K, Wang Y, Hilakivi-Clarke LA. Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene. 2003;22:7316–39. doi: 10.1038/sj.onc.1206937. [DOI] [PubMed] [Google Scholar]
- 6.Clarke R, Shajahan AN, Riggins R, Cho Y, Crawford AC, Xuan J, Wang Y, Zwart A, Nehra N, Liu MC. Gene network signaling in hormone responsiveness modifies apoptosis and autophagy in breast cancer cells. J Steroid Biochem Mol Biol. 2009;114:8–20. doi: 10.1016/j.jsbmb.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–43. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 8.Perou CM, Sorlie T, Eisen MB, Van de RM, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 9.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 10.Clarke R, Ressom HW, Wang A, Xuan J, Liu MC, Gehan EA, Wang Y. The properties of very high dimensional data spaces: implications for exploring gene and protein expression data. Nature Rev Cancer. 2008;8:37–49. doi: 10.1038/nrc2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Miller DJ, Clarke R. Approaches to working in high-dimensional data spaces: gene expression microarrays. Br J Cancer. 2008;98:1023–8. doi: 10.1038/sj.bjc.6604207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke R, Brünner N. Acquired estrogen independence and antiestrogen resistance in breast cancer: estrogen receptor-driven phenotypes? Trends Endocrinol Metab. 1996;7:25–35. doi: 10.1016/s1043-2760(96)00127-0. [DOI] [PubMed] [Google Scholar]
- 13.Stumpf MPH, Thorne T, de Silva E, Stewart R, An HJ, Lappe M, Wiuf C. Estimating the size of the human interactome. Proc Natl Acad Sci. 2008;105:6959–64. doi: 10.1073/pnas.0708078105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milgram S. The small world problem. Psychology Today. 1967;1:60–7. [Google Scholar]
- 15.Albert R, Barabási AL. Statistical mechanics of complex networks. Rev Mod Phys. 2002;74:47–97. [Google Scholar]
- 16.Rittle H, Webber M. Dilemmas in a general theory of planning. Policy Sciences. 1973;4:155–69. [Google Scholar]
- 17.Courtney JF. Decision making and knowledge management in inquiring organizations: towards a new decision-making paradigm for DSS. Dec Supp Sys. 2001;31:17–38. [Google Scholar]
- 18.Nicholson RI, Hutcheson IR, Harper ME, Knowlden JM, Barrow D, McClelland RA, Jones HE, Wakeling AE, Gee JM. Modulation of epidermal growth factor receptor in endocrine-resistant, oestrogen receptor-positive breast cancer. Endocr Relat Cancer. 2001;8:175–82. doi: 10.1677/erc.0.0080175. [DOI] [PubMed] [Google Scholar]
- 19.Kuske B, Naughton C, Moore K, Macleod KG, Miller WR, Clarke R, Langdon SP, Cameron DA. Endocrine therapy resistance can be associated with high estrogen receptor alpha (ERalpha) expression and reduced ERalpha phosphorylation in breast cancer models. Endocr Relat Cancer. 2006;13:1121–33. doi: 10.1677/erc.1.01257. [DOI] [PubMed] [Google Scholar]
- 20.Zhang B, Li H, Riggins R, Zhan M, Xuan J, Zhang Z, Hoffman EP, Clarke R, Wang Y. Differential dependency network analysis to identify condition-specific topological changes in biological networks. Bioinformatics. 2009;25:526–32. doi: 10.1093/bioinformatics/btn660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Csikasz-Nagy A, Battogtokh D, Chen KC, Novak B, Tyson JJ. Analysis of a generic model of eukaryotic cell-cycle regulation. Biophys J. 2006;90:4361–79. doi: 10.1529/biophysj.106.081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tyson JJ, Novak B. Temporal organization of the cell cycle. Curr Biol. 2008;18:R759–R768. doi: 10.1016/j.cub.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyson JJ, Baumann WT, Chen C, Verdugo A, Tavassoly I, Wang Y, Weiner LM, Clarke R. Dynamic models of molecular signaling networks regulating cell proliferation and death. Nat Rev Cancer. 2010 in preparation. [Google Scholar]
- 24.Meusser B, Hirsch C, Jarosch E, Sommer T. ERAD: the long road to destruction. Nat Cell Biol. 2005;7:766–72. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- 25.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–21. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–10. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin S. Autophagy, mitochondrial quality control, and oncogenesis. Autophagy. 2006;2:80–4. doi: 10.4161/auto.2.2.2460. [DOI] [PubMed] [Google Scholar]
- 28.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–5. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heath-Engel HM, Chang NC, Shore GC. The endoplasmic reticulum in apoptosis and autophagy: role of the BCL-2 protein family. Oncogene. 2008;27:6419–33. doi: 10.1038/onc.2008.309. [DOI] [PubMed] [Google Scholar]
- 30.Hur J, Bell DW, Dean KL, Coser KR, Hilario PC, Okimoto RA, Tobey EM, Smith SL, Isselbacher KJ, Shioda T. Regulation of Expression of BIK Proapoptotic Protein in Human Breast Cancer Cells: p53-Dependent Induction of BIK mRNA by Fulvestrant and Proteasomal Degradation of BIK Protein. Cancer Res. 2006;66:10153–61. doi: 10.1158/0008-5472.CAN-05-3696. [DOI] [PubMed] [Google Scholar]
- 31.DuRose JB, Tam AB, Niwa M. Intrinsic capacities of molecular sensors of the unfolded protein response to sense alternate forms of endoplasmic reticulum stress. Mol Biol Cell. 2006;17:3095–107. doi: 10.1091/mbc.E06-01-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu Z, Lee RY, Skaar TC, Bouker KB, Welch JN, Lu J, Liu A, Zhu Y, Davis N, Leonessa F, Brunner N, Wang Y, Clarke R. Association of interferon regulatory factor-1, nucleophosmin, nuclear factor-kappaB, and cyclic AMP response element binding with acquired resistance to faslodex (ICI 182,780) Cancer Res. 2002;62:3428–37. [PubMed] [Google Scholar]
- 33.Gomez BP, Riggins R, Shajahan AN, Klimach U, Wang A, Crawford AC, Zhu Y, Zwart A, Wang M, Clarke R. Human X-Box binding protein-1 confers both estrogen independence and antiestrogen resistance in breast cancer cell lines. FASEB J. 2007;21:4013–27. doi: 10.1096/fj.06-7990com. [DOI] [PubMed] [Google Scholar]
- 34.Fu Y, Li J, Lee AS. GRP78/BiP inhibits endoplasmic reticulum BIK and protects human breast cancer cells against estrogen starvation-induced apoptosis. Cancer Res. 2007;67:3734–40. doi: 10.1158/0008-5472.CAN-06-4594. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida H. Unconventional splicing of XBP-1 mRNA in the unfolded protein response. Antioxid Redox Signal. 2007;9:2323–33. doi: 10.1089/ars.2007.1800. [DOI] [PubMed] [Google Scholar]
- 36.Clauss IM, Chu M, Zhao JL, Glimcher LH. The basic domain/leucine zipper protein hXBP-1 preferentially binds to and transactivates CRE-like sequences containing an ACGT core. Nucleic Acids Res. 1996;24:1855–64. doi: 10.1093/nar/24.10.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldman DE, Chauhan V, Koong AC. The unfolded protein response: a novel component of the hypoxic stress response in tumors. Mol Cancer Res. 2005;3:597–605. doi: 10.1158/1541-7786.MCR-05-0221. [DOI] [PubMed] [Google Scholar]
- 38.Sriburi R, Jackowski S, Mori K, Brewer JW. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol. 2004;167:35–41. doi: 10.1083/jcb.200406136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc Natl Acad Sci. 2003;100:9946–51. doi: 10.1073/pnas.1334037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang DY, Fulthorpe R, Liss SN, Edwards EA. Identification of estrogen-responsive genes by complementary deoxyribonucleic acid microarray and characterization of a novel early estrogen-induced gene: EEIG1. Mol Endocrinol. 2004;18:402–11. doi: 10.1210/me.2003-0202. [DOI] [PubMed] [Google Scholar]
- 41.Tozlu S, Girault I, Vacher S, Vendrell J, Andrieu C, Spyratos F, Cohen P, Lidereau R, Bieche I. Identification of novel genes that co-cluster with estrogen receptor alpha in breast tumor biopsy specimens, using a large-scale real-time reverse transcription-PCR approach. Endocr Relat Cancer. 2006;13:1109–20. doi: 10.1677/erc.1.01120. [DOI] [PubMed] [Google Scholar]
- 42.Scriven P, Coulson S, Haines R, Balasubramanian S, Cross S, Wyld L. Activation and clinical significance of the unfolded protein response in breast cancer. Br J Cancer. 2009;101:1692–8. doi: 10.1038/sj.bjc.6605365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding L, Yan J, Zhu J, Zhong H, Lu Q, Wang Z, Huang C, Ye Q. Ligand-independent activation of estrogen receptor alpha by XBP-1. Nucleic Acids Res. 2003;31:5266–74. doi: 10.1093/nar/gkg731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Xuan J, de Los Reyes BG, Clarke R, Ressom HW. Reverse engineering module networks by PSO-RNN hybrid modeling. BMC Genomics. 2009;10 (Suppl 1):S15. doi: 10.1186/1471-2164-10-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gong T, Xuan J, Wang C, Li H, Hoffman E, Clarke R, Wang Y. Gene module identification from microarray data using nonnegative independent component analysis. Gene Reg Sys Biol. 2008;1:349–63. [PMC free article] [PubMed] [Google Scholar]
- 46.Chen L, Xuan J, Riggins RB, Wang Y, Hoffman EP, Clarke R. Multilevel support vector regression analysis to identify condition-specific regulatory networks. Bioinformatics. 2010;26:1416–22. doi: 10.1093/bioinformatics/btq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Xuan J, de Los Reyes BG, Clarke R, Ressom HW. Reconstruction of gene regulatory modules in cancer cell cycle by multi-source data integration. PLoS ONE. 2010;5:e10268. doi: 10.1371/journal.pone.0010268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang B, Li H, Clarke R, Hilakivi-Clarke LA, Wang Y. Differential dependency network analysis to identify topological changes in biological networks. In: Emmert-Streib F, Dehmer M, editors. Medical Biostatistics for Complex Diseases. Weinheim: Wiley-VCH Verlag GmbH & Co; 2010. pp. 185–205. [Google Scholar]
- 49.Tibshirani R. Regression shrinkage and selection via Lasso. J Royal Stat Soc B. 1996;58:267–88. [Google Scholar]
- 50.Dauvois S, Danielian PS, White R, Parker MG. Antiestrogen ICI 164,384 reduces cellular estrogen receptor content by increasing its turnover. Proc Natl Acad Sci USA. 1992;89:4037–41. doi: 10.1073/pnas.89.9.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin CY, Strom A, Vega VB, Kong SL, Yeo AL, Thomsen JS, Chan WC, Doray B, Bangarusamy DK, Ramasamy A, Vergara LA, Tang S, Chong A, Bajic VB, Miller LD, Gustafsson JA, Liu ET. Discovery of estrogen receptor alpha target genes and response elements in breast tumor cells. Genome Biol. 2004;5:R66. doi: 10.1186/gb-2004-5-9-r66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crawford AC, Riggins RB, Shajahan AN, Zwart A, Clarke R. Co-inhibition of BCL-W and BCL2 restores antiestrogen sensitivity through BECN1 and promotes an autophagy-associated necrosis. PLoS ONE. 2010;5:e8604. doi: 10.1371/journal.pone.0008604. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pratt MAC, Bishop TE, White D, Yasvinski G, Menard M, Niu MY, Clarke R. Estrogen withdrawal-induced NF-kappaB activity and bcl-3 expression in breast cancer cells: roles in growth and hormone independence. Mol Cell Biol. 2003;23:6887–900. doi: 10.1128/MCB.23.19.6887-6900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai D, Iyer A, Felekkis KN, Near RI, Luo Z, Chernoff J, Albanese C, Pestell RG, Lerner A. AND-34/BCAR3, a GDP exchange factor whose overexpression confers antiestrogen resistance, activates Rac, PAK1, and the cyclin D1 promoter. Cancer Res. 2003;63:6802–8. [PubMed] [Google Scholar]
- 55.Riggins RB, Quilliam LA, Bouton AH. Synergistic promotion of c-Src activation and cell migration by Cas and AND-34/BCAR3. J Biol Chem. 2003;278:28264–73. doi: 10.1074/jbc.M303535200. [DOI] [PubMed] [Google Scholar]
- 56.van Agthoven T, van Agthoven TL, Dekker A, van der Spek PJ, Vreede L, Dorssers LC. Identification of BCAR3 by a random search for genes involved in antiestrogen resistance of human breast cancer cells. EMBO J. 1998;17:2799–808. doi: 10.1093/emboj/17.10.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nehra R, Riggins RB, Shajahan AN, Zwart A, Crawford AC, Clarke R. BCL2 and CASP8 regulation by NFkB differentially affect mitochondrial function and cell fate in antiestrogen senstiive and resistant breast cancer cells. FASEB J. 2010;24:2039–54. doi: 10.1096/fj.09-138305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ning Y, Riggins RB, Mulla JE, Chung H, Zwart A, Clarke R. Interferon gamma restores breast cancer sensitivity to Fulvestrant by regulating STAT1, IRF1, NFkB, BCL2 family members and sigaling to a caspase-dependent apoptosis. Mol Cancer Ther. 2010;9:1274–85. doi: 10.1158/1535-7163.MCT-09-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]