Abstract
Alterations of blood rheology (hemorheology) are important for the early diagnosis, prognosis, and prevention of many diseases, including myocardial infarction, stroke, sickle cell anemia, thromboembolism, trauma, inflammation, and malignancy. However, real-time in vivo monitoring of hemorheological status using multiple parameters over long periods of time has not been reported. Here we describe the capability of label-free photoacoustic (PA) and photothermal (PT) flow cytometry in detection and imaging modes for dynamic monitoring of rheological parameters in circulating blood. We show that this integrated platform can simultaneously measure the main rheological parameters and may improve their diagnostic value. Using phenomenological approaches, we analyze correlations of PT and PA signal characteristics in the dynamic modes with red blood cell (RBC) aggregation, deformability, shape (e.g., as in sickle cells), intracellular hemoglobin distribution, individual cell velocity, flux of RBCs, and likely shear rate. Proof of concept is demonstrated in ex vivo and in vivo tests, including high-speed PT imaging of RBC shape in pathological conditions and identification of sickle cells in a mouse model of human sickle cell disease. These studies revealed the potential of this new platform integrating PT, PA, and conventional optical techniques for translation to use in humans using safe, portable, laser-based medical devices for point-of-care screening of disease progression and therapy efficiency.
INTRODUCTION
Blood rheology (also termed hemorheology) is an integrated branch of physics and medicine studying the relation of the flow and deformation behavior of blood and its internal structures (1,2). Changes of blood rheology are thought to be crucial in the progression of many severe diseases, such as stroke, heart attack, anemia, diabetes, and sickle cell disease (1, 3–5). These changes may also dramatically complicate therapeutic interventions (e.g., infusion of heparin or warfarin). The grand challenge of hemorheology in live organism is the complexity of multiple dynamic relationships. The most important rheological property of blood is its resistance to flow, or viscosity. The major unique flow-affecting determinants of whole-blood viscosity, and thus hemorheology, include (1) hematocrit (Ht), the fraction of the blood volume occupied by red blood cells (RBCs); (2) RBC deformability, the ability of RBCs to undergo deformation in flow, depending on the shape, membrane deformability, and properties (e.g., chemical structure or clustering capability) of hemoglobin (Hb); and (3) RBC aggregation, the formation of reversible (physiological) rouleaux or irreversible (pathological) clumps of RBCs. Blood rheology and viscosity are also determined by shear rates (velocity difference), flux of RBCs (number of RBCs per second), and individual RBC velocity and perfusion (velocity and flux) (1–4). Another rheological determinant, plasma viscosity, usually has little influence on whole-blood viscosity. Indeed, when RBCs are added to plasma, blood viscosity becomes increasingly sensitive to Ht, with an almost exponential relationship between the Ht value and blood viscosity (1). Thus, at moderate to high shear rates, a 1% change in Ht (from 45% to 46%) increases blood viscosity by 4%.
Hemorheology has been intensively studied for decades by many methods ex vivo, including rotational, viscosimetry, micropipette aspiration, sedimentation rate, micropore filtration, and flow cytometry (1–5). However, they are limited by 1) single-parameter measurement (e.g., RBC aggregation or Ht); 2) invasiveness, which may unpredictably alter rheological properties and prevent long-term monitoring in the native biological environment; 3) time-consuming preparation procedures (several hours if not an entire day); 4) discontinuous sampling with limited, discrete time points; and 5) the small blood volume extracted (typically a few milliliters). Furthermore, ex vivo/in vitro tests cannot replicate the flow-affecting and transient character of rheological changes, which thus can only become fully apparent in the natural blood circulation in vivo under control of endothelial, nervous, and humoral stimuli. It is likely that in vitro approaches can use rheological testing for a supplementary diagnosis.
Technological progress in the development of methods for evaluating blood rheology in vivo is promising to extend its clinical significance (2). Among numerous in vivo diagnostic techniques (e.g., ultrasound, magnetic resonance imaging, positron emission tomography, and various optical techniques), photothermal (PT) and photoacoustic (PA) methods have exhibited a high level of sensitivity in the detection of individual cells (6–10) and the monitoring of oxygenation and total Hb content in humans (11–13). Fluorescence techniques can also detect and image individual cells and blood flow (14–18), although the use of fluorescence labeling in vivo raises potential problems for translating this technology to humans because of (1) the cytotoxicity of available fluorescent tags, (2) photobleaching or blinking of tags, (3) undesired immune responses to tags, and (4) the strong influence of scattering light and autofluorescence of the background, which allow the assessment of only superficial microvessels having a slow flow velocity. Our contribution in this field includes the development of PA and PT flow cytometry (PAFC/PTFC) for in vivo real-time detection of individual circulating cells either with intrinsic absorbing markers (e.g., Hb in RBCs or melanin in melanoma) or synthetic gold and other nanoparticles as PT and PA contrast agents (7–10,19–26). PT spectroscopy has demonstrated the capability to identify several Hb chemical subtypes (e.g., sulpha-, met-, CO- or HbS [in sickle cells]) (27). Our contribution also includes Raman and integrated PA-PT-Raman spectroscopy and imaging for in vivo identification with chemical specificity of individual cells and nanoparticles (28–29). However, the application of PT and PA techniques to the study of blood rheology has not yet been reported. Here we analyze the capability of a combined platform integrating high-speed PT, PA, and optical imaging for in vivo real-time monitoring of multiple rheological parameters at the single-blood-cell level.
METHODS
Experimental setup
The two integrated setups were built on the technical platform of an upright and invert Olympus BX51, and IX81 microscopes (Olympus America, Inc.) and two tunable optical parametric oscillators (OPO) (Lotis Ltd. Minsk, Belarus and Opolette HR 355 LD, OPOTEK, Carlsbad, CA, respectively) producing pump laser pulses with the following parameters: wavelength, 420–2,300 nm and 410–2500 nm; pulse width, 8 ns and 5 ns; a repetition rate, 10 Hz and 100 Hz; pulse fluence, 0.01–100 J/cm2 and 0.001–20 J/cm2, respectively, as described elsewhere (21,37–38,43–47). PA signals were detected using two ultrasound transducers: 1) unfocused, model 6528101, 3.5 MHz, 5.5 mm in diameter (Imasonic, Inc.); and 2) focused, model V316-SM, 20 MHz, focal length, 12.5 mm (Panametrics- NDT. The signals after amplifier (model 5662: bandwidth 50 kHz–5 MHz, gain 54 dB; and model 5678: 40 MHz, and gain 60 dB; both from Panametrics- NDT) were recorded with a PC and a Tektronix TDS 3032B oscilloscope, and analyzed with standard and customized software. The transducer was gently attached to the samples and warm water was topically applied for good acoustic coupling.
In one channel PT thermal lens schematic (37), pump laser-induced temperature-dependent variations of the refractive index around absorbing zones caused defocusing of a continuous- wave collinear probe beam from He-Ne laser with wavelength of 633 nm and power of 1.4 mW (model 117A, Spectra-Physics, Inc.;). The subsequent change in the beam’s intensity at its center (referred to as PT signal) is detected after passing through a pinhole by a photodiode with built-in preamplifier (PDA36A, 40 dB amplification, ThorLabs Inc.). PT signals were detected either from a whole cells using relatively large laser beam with diameter of 10–30 um or from localized intracellular zones using focused laser beams with diameter of 300–700 nm.
PT imaging (PTI) was performed in two setups using different modes. In the first one using the microscope, laser-induced the refractive index variations were visualized with a multiplex thermal-lens schematic and a CCD camera (AE-260E, Apogee Inc) using a pump laser pulse from OPO and second, probe pulse from Raman Shifter (wavelength, 639 nm; pulse width, 12 ns; pulse energy, 2 nJ; pulse delay, 0–10 μs) (44). Refractive index variations were obtained by comparison of the sample probe images before and right after the excitation with pump laser pulse (38, 44) with each pixel of the CCD camera acting as an individual photodiode with pinhole. The pump and probe beam diameters were adjustable (range 10–60 μm and 5–50 μm, respectively) by changing condensers and their axial moving. The lateral resolution was 700 nm with a 20× microobjective and ~450 nm with a 60× water-immersion objective. The cells in the standard microscopic slide were navigated by using an automatic scanning microscopic stage (Conix Research, Inc) and in-house written Visual Basic™ software.
In the second setup using the inverted microscope, imaging was provided by scanning cells with a two-dimensional (2D; X–Y) translation stage (H117 ProScan II, Prior Scientific, Rockland, MA) having a positioning accuracy of 50 nm (45,46). The intensity of each pixel of PT image represents the average of PT signals from several OPO pulses. The lateral resolution of the PTI level at 0.5–6 μm was determined by the focal spot size of the pump laser beam. This setup was equipped with a high-speed (200 MHz) analog-to-digital converter board (National Instruments Corp., PCI-5152, 12-bit card, 128 MB of memory), specialized software (LabVIEW; National Instruments), and a Dell Precision 690 workstation with a quadcore processor, 4 GB of RAM, and Windows Vista 64-bit operating system.
High-resolution (~300 nm) transmission digital microscopy (TDM) module with a cooled, color CCD camera (DP72, Olympus), a high-speed (up to 40,000 frames per second [fps]) CMOS camera (MV-D1024-160-CL8; Photonfocus AG, Lachen, Switzerland), and high sensitive CCD (Cascade: 512; Photometrics, Roper Scientific, Inc.) camera were used for the navigation of laser beams and cell imaging.
Preparation of samples in vitro
Blood samples were collected from donor mice and rats (protocols were approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee). RBCs were prepared by their isolation through initial centrifugation (200×g for 6 minutes) of whole blood after the removal of plasma and leukocyte layers. Then, RBCs were washed via centrifugation at 1,000×g for 10 minutes at room temperature. The pellet was then res-suspended in PBS to obtain normal RBCs.
Diamide (Sigma), Chlorpromazine and distilled water were added to the RBC solution to form rigid RBCs, stomatocytes or spherocytes, respectively. RBC aggregation was produced by Dextran (MW=500; Sigma). The Hb powder (Sigma) was dissolved in plasma to obtain physiological concentration of total Hb (110–160 g/L).
Each sample (~8 μL) was placed in the individual well (S-24737, Molecular Probes) and assessed with PT/PA imaging and TDM. The well depth of 120 μm allowed keeping the original shape of RBCs and their aggregates.
Animal models
In vivo experiments involved a nude mice (nu/nu), the genetically modified mice expressing human sickle hemoglobin (STOCK Hbatm1Paz Hbbtm1Tow Tg(HBA-HBBs)41Paz/J mice) and rat models (Sprague-Dawley rats), in accordance with protocols approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee. We selected rat mesentery as an almost ideal minimally invasive animal model because it has a thin tissue layer with the clearly distinguished single-blood vessels with individual fast moving cells (36). After anesthesia (ketamine/xylazine, 50/10 mg/kg, i.m.), the animal was laparotomized and a mesentery was exteriorized on a customized, heated (38°C) microscope stage. It was then suffused with warmed Ringer’s solution (38°C, pH 7.4) containing 1% bovine serum albumin.
Experiments with the mice were performed on a mouse ear, with well-distinguished blood microvessels at 50–100 μm depth and 50–150 μm diameter. The anesthetized animal was placed on the heated microscope stage with a topical application of water on the ear for acoustic coupling of the ultrasound transducer and the tissue.
Various alterations of hemorheological status in vivo were produced by intravenous injections of different drugs with well-established effects: saline solution (400μL; 0.9% solution of NaCl) to decrease Ht; Diamide (20 mg/kg) to produce rigid RBCs; distillate water to form swollen spherocytes);and Dextran (2.4 g/kg of blood) for RBC aggregation.
Statistical analysis
A minimum of 3 animals were used for each experiment unless otherwise noted. Results are expressed as a mean ± standard error. Spearman correlations for which P <0.05 were considered statistically significant. MATLAB 7.0.1 (MathWorks, Natick, MA), and LabVIEW (National Instruments) were used for the statistical calculations. Data were summarized as a mean, standard deviation (SD), median, inter-quartile range, and full range. Comparisons of PA and PT data were via scatter plot in conjunction with the Spearman correlation analysis.
RESULTS
Phenomenological models of in vivo blood rheology using PT and PA methods
General approach
Each physical process responsible for PT and PA effects (excitation → nonradiative relaxation → heating → thermal relaxation → acoustic wave) can be sensitive to rheological parameters. Because these effects are also spectrally, spatially, and energy selective, the changes of the laser wavelength, pulse energy, and beam diameter can be used to obtain more information of blood properties. For example, spectrally selective optical excitation may provide information on RBC chemical and composition (27). The quenching of radiative relaxation (leading to increased PT/PA signal amplitude or a shortened rise time) (26) may depend on the RBC/Hb functional state and properties of surrounding proteins. The processes of thermal relaxation depend on RBC size and shape (38). Nonlinear effects at high laser energy levels, such as multiphoton absorption (7), may amplify spectral imaging contrast. Laser-induced nano- and microbubble formation (10,37) around localized overheated zones with high Hb concentrations (31) can be a marker of abnormal Hb clustering within individual RBCs. The PT/PA effects may also be sensitive to the impact of changes in blood rheology due to environmental (e.g., nicotine, alcohol) or therapeutic (e.g., radiation, drugs) agents. On the basis of our previous and current findings, we summarize below the methodological details for in vivo PT and PA measurement of blood rheological parameters.
High-speed imaging of nonlabeled and labeled RBCs and plasma proteins
Because of high endogenous absorption of Hb and low absorption of plasma proteins, label-free, high-speed (up to 10,000 fps), high-resolution (250–300 nm) transmission (30) and PT imaging (31–33) of thin tissue, such as ear and mesentery in animal models, enables time-resolved determination of the shape of single RBCs in a capillary with single-file flow, the size of the capillary, and its velocity profile (30). This can provide information on RBC flux, shear stress, and Ht. Resolution in the z direction (along the light path) can be increased by confocal PT microscopy (35). Pathological cell shapes (e.g., sickle cells) can be identified by high-resolution PT imaging. In addition, multiwavelength PAFC with multicolor dyes can provide measurements of circulating blood volume (34).
PT and PA measurements of individual RBC velocity and blood velocity profiles
Measurement of RBC velocity with high spatial resolution can be obtained by either 1) video-rate analysis (36); 2) label-free, two-beam (pump–probe) PT flow velocimetry, in which a pump laser induces a heated zone in blood flow while the movement of this zone with flow is detected through refraction or defocusing of the probe beam (time-of-flight technique) (19); or 3) monitoring of PA signal width determined by the transit time of RBCs through the laser beam (9,25). In latter case, PA signal width is acquired by the averaging of many PA signals from the same cells with high-pulse-repetition-rate laser. The transit time of cells through the laser beam depends on cell velocity and beam and cell sizes. Fast scanning of a vessel cross-section with a strongly focused laser beam may be used to estimate velocity profiles. Because the behavior of cells in flow (e.g., velocity) depends on their morphological type, PT/PA measurements of velocity allows cell discrimination (e.g., fast moving RBCs in axial flow is distinguished from rolling leukocytes near the vessel wall).
PT monitoring of RBC size and shape
Under the influence of a short laser pulse, the temperature elevation of RBCΔT(t), referred to as PT signals, can be described in the first approximation as follows (37,38):
| (1) |
where ΔTmax is the maximum temperature elevation in the irradiated cells, τRT is the rise time, and τT is the thermal relaxation time. The typical range of τRT is 0.1–10 ns, which depends on the nonradiative relaxation time, the time to the photodetector’s response, the laser pulse width (tp), and spatial averaging of thermal effects from individually heated Hb molecules within the whole RBC (37). If the laser pulse width, tp, is shorter than the cell thermal relaxation time (thermal confinement, tp < τT), for RBCs with two basic geometries—(a) a sphere with radius R, and (b) a planar discoid with radius RD and thickness d(d≪RD)—the thermal relaxation time τT may be estimated, respectively, as follows (37)
| (2) |
| (3) |
where kT is the heat diffusion coefficient. For example, for a discoid RBC with d = 2 μm and a spherical RBC with R = 3 μm, estimations of τT (kT = 1.44×10−3 cm2/s for water parameters) are approximately 4.4 μs and 14.4 μs, respectively. Thus, PT signals demonstrate the standard fast-rising peak associated with rapid RBC heating and a slower trailing edge (referred to as the signal tail) related to the cooling of RBCs, with their shape and size dependent on time (Fig. 1, oscilloscope on bottom). As a result, the transformation of normal RBCs with a standard discoid form to a pathological sphere-like shape can be controlled directly in flow without imaging through the monitoring of the PT signal duration. This approach can also be used to monitor the volume of individual RBCs when they swell, shrink, or aggregate (see below).
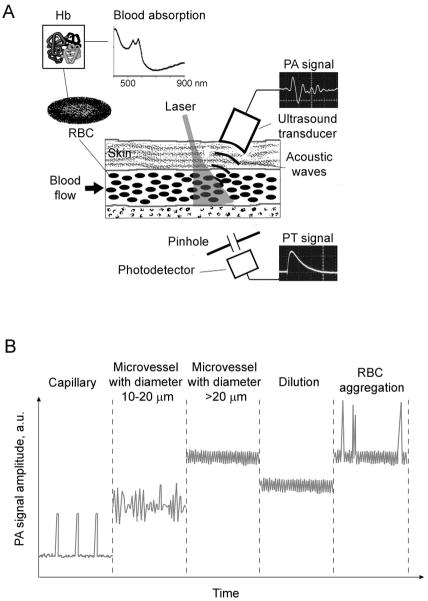
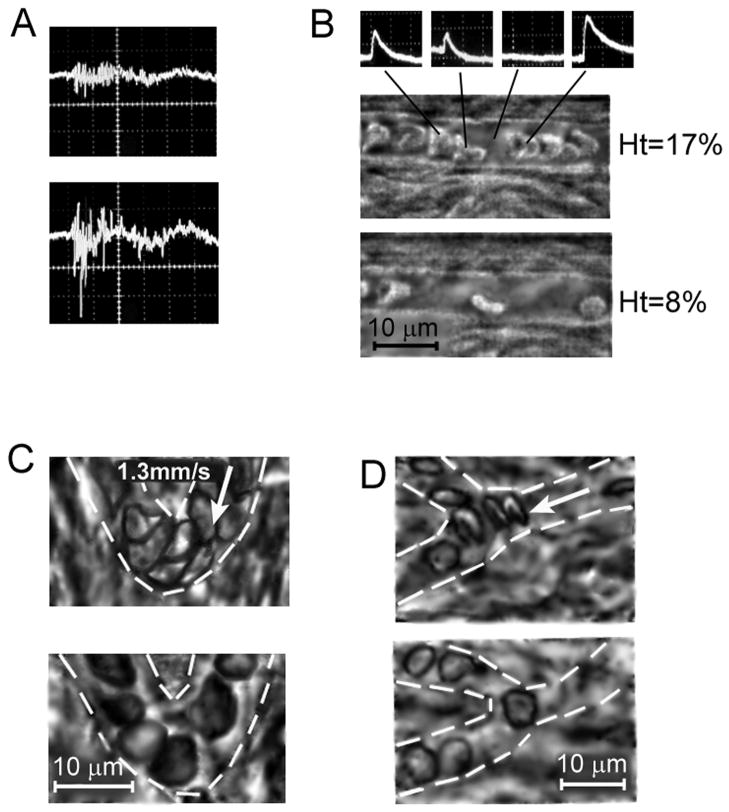
Figure 1.
The principles of in vivo PA and PT blood rheology tests. (A) Schematics. The insets on right shows PA signal (top) and PT signal (bottom) after one laser pulse. (B) Phenomenological model: PA signal trace dynamics in different vessels after averaging many signals generated by a high pulse rate laser.
PA and PT monitoring of hematocrit (Ht)
The acquisition of many PT/PA signals during irradiation of relatively large vessels with high-pulse-repetition-rate lasers creates a constant PT/PA background from RBCs (Fig. 1B, middle). The average level of this background is proportional to the number of RBCs in the irradiated volume, thus allowing the estimation of the Ht after corresponding calibration taking into account blood oxygenation or hemoglobin concentration (11). The fluctuation of the background is associated with changes of RBC number in the irradiated volume. These fluctuations increase as vascular diameter decreases. Ultimately, in a capillary with single-file cell flow, PT/PA signals appear above the noise when individual RBCs sequentially pass through the irradiated volume (Fig. 1B). In arterioles and venules in which several RBCs appear simultaneously in the irradiated volume, the background level increases and its fluctuation decreases. In large vessels with many RBCs (hundreds and thousands) in the irradiated volume, the background continues to rise, but fluctuations are minimized and are already determined by laser energy instability. The dilution of blood with a physiological solution leads to a decrease in the background level that can be used to calculate Ht and diagnose associated disease (52).
Monitoring of RBC aggregation and change of RBC volume
When RBC aggregates with increased localized absorption pass through the irradiated volume of a vessel, they should lead to PA signal fluctuations or transient strong PA peaks because of the many small aggregates or the single large aggregates, respectively, compared to the relatively stable PA background from randomly distributed individual RBCs in the same volume in normal blood (Fig. 2B). RBC aggregates can be detected when they have a higher absorption than the normal RBC background. For example, five RBC aggregates have a volume of ~450 fL (corpuscular volume of a single RBC is 90 fL (40) with a total Hb amount of 150 pg (~30 pg of Hb per one RBC), while the same volume of whole blood with a Ht of 45% contains just two RBCs with 60 pg of Hb.Thus, the expected ratio of PT to PA signals from even small RBC aggregates to blood background is 2.5, which is quite enough for the detection of aggregates. The localized increase in absorption and decrease in gaps between individual RBCs in three-dimensional aggregates lead to a significant increase of PT/PA signal fluctuation as a marker of abnormal RBC aggregation, compared with linear RBC aggregates (rouleaux).
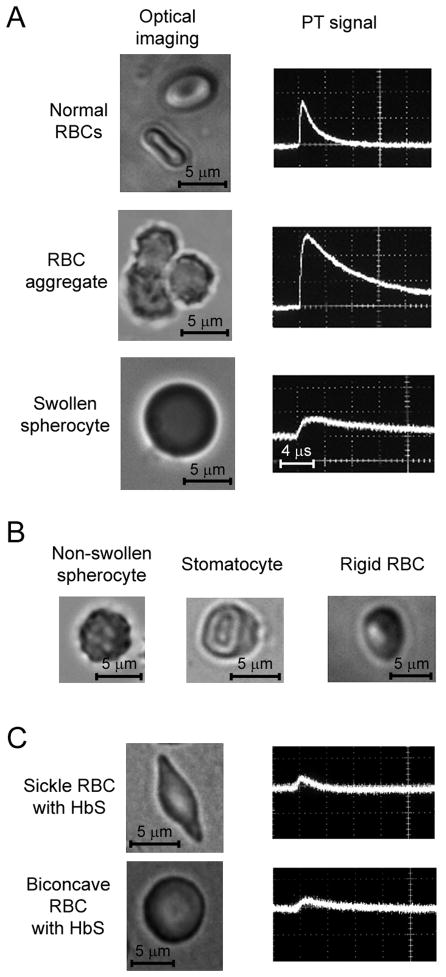
Figure 2.
Ex vivo rheology tests using optical and PT techniques. (A) High-resolution (×100) optical images (left column) and pulsed PT signals (right column) from single normal RBC, RBC aggregates consisting of three RBCs, and low-deformable swollen spherocyte. (B) Typical pathological types of low-deformable RBCs. (C) High-resolution (×100) optical images (left column) and PT signals (right column) from sickle (top) and round (bottom) RBCs with HbS.
The change of a single RBC’s volume leads to a change of the PT/PA signal amplitude, which is strongly dependent on RBC size (as ~1/R3) (41). Therefore, even a small geometric RBC change can lead to an enhanced signal change. In particular, hypo-osmolarity (<300 mOsmol/kg) causes of RBC swelling, while hyperosmolarity (>300 mOsmol/kg) leads to RBC shrinking. These RBC-size variations can be detected through the cell size–dependent behavior of PA signal amplitude (larger size, smaller amplitude) at the same concentration of Hb in RBCs.
Sickle RBCs differ from normal RBCs on the basis of their shape (sickle vs. biconcave), spatial distribution, and chemical structure, as well as the optical properties of Hb (HbS vs. HbA). In particular, the identification of sickle RBCs can be based on differences in PT/PA signal amplitudes, even without monitoring of cell shape.
Blood viscosity
It is well recognized that blood viscosity and its influence on the microcirculation are determined mainly by Ht, RBC aggregation, and shear-induced deformation of RBCs, and much less by plasma viscosity (1). Indeed, whole blood is a non-newtonian fluid with a marked increase in viscosity at a low flow rate, mainly due to RBC aggregation. Another important determinant of blood viscosity is RBC deformability, which depends on intrinsic factors, including cell geometry, membrane flexibly, and internal viscosity. The capability of PT/PA methods to measure both RBC and plasma parameters, RBC deformability (which is influenced by high-shear viscosity), is the key point to estimate blood viscosity.
White blood cell (WBC) rheology
WBCs, whose normal concentration in blood is relatively low concentration, may increase blood viscosity only in rare cases of hyperleukocytic leukemia (1). However, we believe that WBCs’ role in blood rheology needs to be reevaluated. Whereas flexible RBCs represent simple membrane capsules enclosing Hb, WBCs are much more rigid, with a level of viscosity three orders of magnitude higher than that of RBCs (1), owing to their rigid nuclei, granular cytoplasm, and active contractile elements. Because WBCs have larger diameters than RBCs (10–12 μm vs. 5–7 μm) and a round shape, they have twice the volume of RBCs. The capacity of PT and PA techniques to detect WBCs in circulation (42) can shed light on this important, still unstudied question. Detection can be based on significant differences in the absorption spectra of WBCs and RBCs owing to the presence of cytochromes and Hb as intrinsic cell-specific markers, respectively (22), as well as to the strong dependence of PT/PA signal amplitude on cell size.
Summary
Even these simple phenomenological models show the high potential of PT and PA methods to assess rheological parameters. The diagnostic principles are based on the high sensitivity of time-resolved PT/PA parameters to dynamic changes of Hb properties (e.g., clustering, concentration, spatial distribution, chemical subtypes) that are specifically associated with alterations of the main hemorheological determinants (RBC rheology, hematocrit, or likely plasma viscosity). For example, fluctuations of PA signal amplitude can be related to pathological aggregation of RBCs, while decreasing PA signal amplitude can be associated with a low Ht. The rheological parameters (e.g., aggregation or viscosity) in the progression of some diseases can change significantly quickly (just a few hours) (1). Thus, real-time in vivo multiparameter monitoring of hermorheological parameters may be crucial for patient survival.
PT tests of hemorheological parameters ex vivo
The phenomenological models were first verified in ex vivo studies. We explored whether a new technical platform was capable of differentiating single RBCs, RBC aggregates, and Hb in solution (Fig. 2A). High-resolution TDM imaging was used to control cell and laser-beam position. When both pump and probe beams at a diameter of 10–20 μm covered an entire normal or abnormal single RBC or small RBC aggregates (10–15 μm), PT signals from single RBCs and RBC aggregates (Fig. 2A, right) exhibited standard signal shapes for all samples: a fast-rising peak and a slower tail. However, the PT signals had a different amplitude and tail duration, depending on individual RBC sizes or number of RBCs in aggregates (Fig. 2A, right). TDM images revealed a relatively homogenous size and shape distribution for normal RBCs that correlated with data in the literature (47,48). PT signal amplitude from individual rare RBCs in suspension also exhibited a low (3–5%) level of heterogeneity at wavelengths of 530 to 580 nm corresponding to strong absorption bands in the visible-spectral range. The duration of the tail was 4–6 μs, which correlated with our estimation for discoid RBCs (see above). The irradiation of whole blood with a physiological Ht value (45%) in a 120-μm-thick well (i.e., ~50 RBCs with 1,500 pg of Hb in the irradiated volume) led to a dramatic increase in the PT signal amplitude; however, the tail duration was similar to those for single RBCs (Fig. 2A, right, top), suggesting that cooling rates were mostly determined by the cooling of individual RBCs with an average distance between them larger to heat diffusion length at a typical Ht of 20–40%. Nevertheless, we cannot exclude the dynamic fluctuation of cooling time in blood flow due to the interaction of thermal fields from individual RBCs at a higher Ht or due to frequent RBC collisions that may create random temporal RBC complexes (rouleaux) having an increased PT amplitude and a longer cooling time.
For comparison, the PT signal amplitude from 1,500 pg of a relatively homogeneous Hb solution in animal plasma was lower than that from 50 RBCs with a similar total Hb concentration (see above), while the PT signal tail was much longer. This can be explained by the larger signal amplitudes from localized zones with high Hb concentrations in RBCs (or the influence of nonlinear amplification of PT signals from overheated localized intracellular zones with Hb clusters) and a larger irradiated zone in the Hb solution, respectively.
These conclusions are in agreement with our experimental results related to increases in the PT signal amplitudes and tail durations when RBCs form aggregates (Fig. 2A, right, middle). The amplitude of PT signals significantly correlated with the size of an aggregate controlled by TDM (i.e., larger aggregates, higher amplitude and longer tails). Large aggregates (>10 RBCs) produced nonlinear signals at a relatively high laser energy level due to bubble formation around overheated local zones (38,49). As expected, the signals from RBC aggregates exceeded those from background blood 2–5-fold.
We then focused on studying RBC deformability, which can be accompanied by changes in cell shape, size, and membrane rigidity, as well as Hb distribution within a cell. The high-resolution TDM imaging was used to verify the size and shape of RBCs. Distillate water (hypo-osmolar), chlorpromazine, or Diamide induced a well documented transformation of biconcave RBCs: “swollen” spherocytes (Fig. 2B, left) with a diameter increase of 42 ± 12% (Fig. 2A, bottom row), stomatocytes with shape likes a uniconcave cup (Fig. 2B, middle), and rigid RBCs (Fig. 2B, right), respectively. All of these low-deformable types of RBCs have increased volume compared to normal cells. In turn, the concentration of Hb molecules per a unit of RBC volume decreased when accompanied by a decrease in PT signal amplitudes (41). For example, the mean amplitude of the PT signal from a 7-μm spherocyte and a “swollen” spherocyte, whose volume increased ~2–4-fold, decreased PT signals from RBCs by 50% and 75%, respectively (e.g., Fig. 2A, right, bottom row) compared to normal discoid RBCs (Fig. 2A, left, top row).
Next, we tested PT signals from sickle RBCs containing human HbS. Under TDM control, we found that single sickle RBCs provided PT signals with lower amplitudes (2.5–4-fold) than PT signals from normal RBCs (Fig. 2A, C). Furthermore, round and biconcave RBCs with HbS exhibited low-amplitude signals, compared to round and biconcave RBCs with HbA (Fig. 2C, bottom row). The shape of PT signals from RBCs with HbS was also characterized by a longer tail.
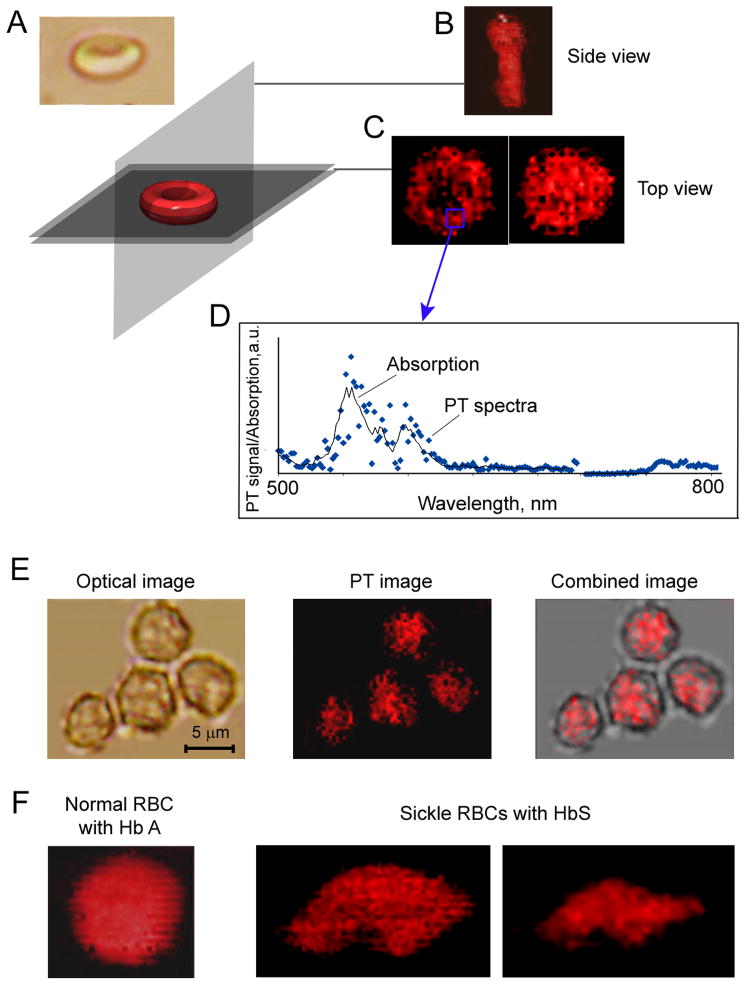
To obtain high-resolution images of normal biconcave RBCs and spherocytes, we applied PT confocal scanning microscopy (35) (Fig. 3). High-amplitude PT signals (coded by red in the Fig. 3B and E) at 530 nm were associated with Hb because the PT spectrum from a red spot corresponded in the first approximation to the absorption spectrum of Hb (Fig. 3D). Because Hb fills the entire RBC volume, the PT signals from Hb replicated the shape of the RBCs. In particular, normal biconcave RBCs exhibited low amounts of Hb in their central thin parts with increased concentrations in the peripheral thick ring (Fig. 3C, left). The high sensitivity of PT imaging was also able to distinguish the internal spatial heterogeneity of Hb distribution in RBCs. Thus, these data confirm our previous finding (31, 33) of the high spatial heterogeneity of PT signals within both normal individual RBCs and spherocytes as indicators of an uneven spatial distribution of Hb molecules and, probably, Hb clusters (Fig. 3B,C). High-resolution optical images of the same cells could not distinguish these highly localized phenomena due to low absorption sensitivity (Fig. 3A, left). Using the method of time-resolved PT microscopy beyond the diffraction limit based on size-dependent PT signal shape (38), we roughly estimated that the sizes of localized zones with presumably high local Hb concentrations lay in the broad range of 50 nm to 300 nm.
Figure 3.
High-resolution PT imaging of Hb in live RBCs. (A) Direction of spatial PT scanning of RBCs. (B) PT image of biconcave RBCs (A, top) obtained with the vertical scanning; (C) PT image of RBCs with a ring or round shape obtained with horizontal scanning. (D) Spectral identification of Hb based on correlation of PT spectra (blue points) from the indicated area on the RBC image with the absorption spectrum of Hb (solid black line). (E) Distribution of Hb in nonswollen spherocytes. (F) Comparison of the heterogeneity in PT images of intact (left) and sickle (middle and right) RBCs.
High-sensitivity PT imaging allowed distinguishing sickle RBCs on the basis of both their specific shape and intracellular PT patterns (Fig. 3F). Indeed, sickle RBCs had more profound spatial heterogeneity (Fig. 3F middle and right) than normal RBCs. This may be associated with increased clustering of HbS compared to HbA.
PT imaging of RBCs in blood flow
We applied PT image cytometry/microscopy (19,31,32,34), using an advanced PT multiplex thermal-lens imaging mode (44) to visualize individual RBCs in the microcirculation of thin (~250 μm) nude-mouse ear (Fig. 4A) with the use of a two-beam (pump–probe) technique (38). The experiments were performed on capillaries with diameters of 5–7 μm and on relatively small microvessels (arterioles, venules) with diameters of 8–12 μm and flow velocities of 0.5–2 mm/s, as well as on relatively large microvessels with diameters of 20–50 μm and flow velocities of 3–8 mm/s (Fig. 4,B,C,D, respectively).
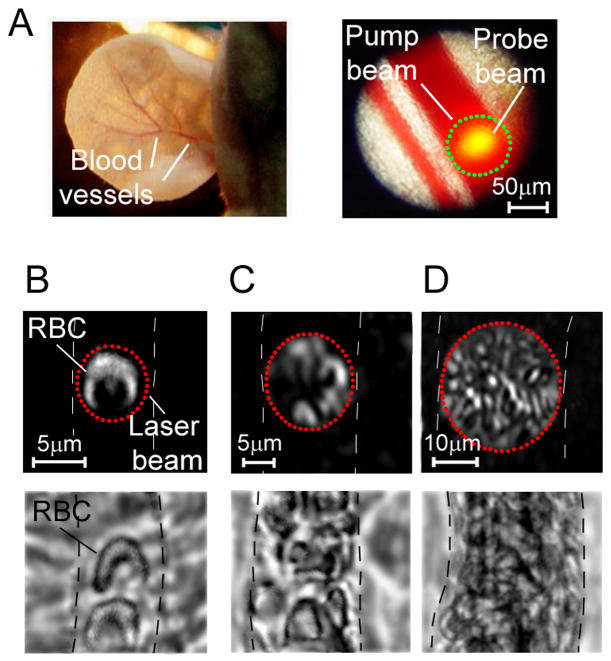
Figure 4.
PT multiplex thermal lens imaging of blood flow. (A) Optical images of animal model: mouse ear. (B) Single RBC in small capillary; magnification 60×. (C) Several RBCs in large capillary, magnification 20×. (D) Multi file RBC flow in microvessels; magnification 10×. Dash lines show the boundary of blood vessels. Top row: PT images of ear blood vessels; bottom row: optical images of mesentery blood vessel.
Both pump and probe laser beams had a circular geometry with diameters comparable to vessel diameters. We obtained high-resolution (300–500 nm) images of individual RBCs in capillaries and microvessels. It is well known that RBCs are highly deformable and are able to move through narrow capillaries, transforming from their basic biconcave-disk shape to a parachute-like shape (30,47). PT imagining allowed the monitoring of this dynamic shape deformation with high image contrast (Fig. 4B, top).
In both capillaries with single-file flow and small venules accommodating the simultaneous passage of several RBCs, we imaged the parachute-like shape of moving RBCs (Fig. 4C, top). The RBC deformation index, representing the ratio of length to width was found to be around 1.5–2. In large microvessels with fast multifile flow (e.g., 40–50-μm arterioles), we observed shapes that were less parachute-like and more elongated (stick-like), likely due to frequent cell collisions (Fig. 4D, top). Despite the overlapping of images from individual RBCs, PT image flow cytometry enabled not only distinguishing single RBCs in vivo but also imaging of local structures associated with the spatial distribution of Hb in RBCs. These structures could hardly be seen on TDM because of their low absorption sensitivity. We also again observed tiny, localized absorbing heterogeneities in PT images of RBCs, which are consistent with our previous findings (31) and with in vitro data above. We hypothesize that these structures could be associated either with the rough RBC membrane surface or with nanoscale Hb clusters with increased localized absorption. If we further exclude any possible artifacts and confirm this hypothesis, PT imaging should be powerful tool for in vivo monitoring of intracellular Hb distribution at the single-RBC level under normal and pathological conditions, including the presence of HbS in sickle RBCs.
Label-free PA monitoring of RBC aggregation in vivo
In vivo real-time PAFC monitoring of mesenteric arteries (41 ± 3 μm in diameter) and veins (45 ± 4 μm) in healthy rats demonstrated relatively small fluctuations of PA signal amplitude levels that are likely related to formation of physiologically reversible RBC aggregates (rouleaux). Specifically, at a laser pulse energy instability of 1–2 %, these fluctuations ranged from 1% to 5% in the arteries and increased to 7–10% in the veins and to 10–12% in bifurcation zones, where hydrodynamic conditions intensify rouleaux formation.
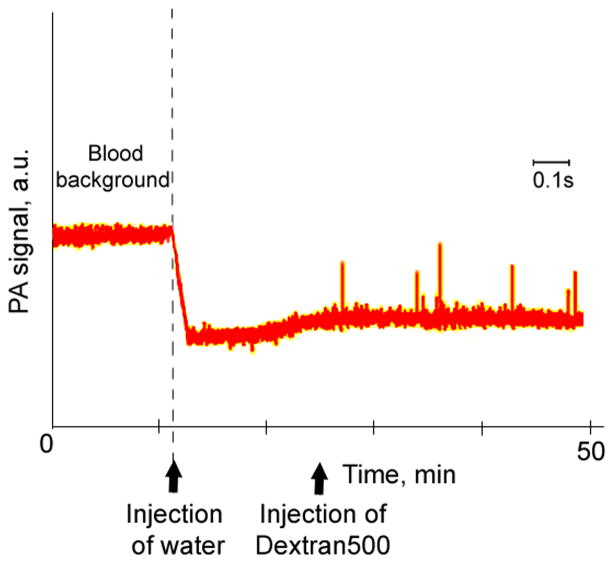
Intravenous introduction of Dextran500 produced well known pathological irreversible RBC aggregates that led to the appearance of strong fluctuations of PA signal amplitude (Fig. 5A, bottom) that were significantly higher than fluctuations from intact blood before injection (Fig. 5A, top). These fluctuations started 1–2 minutes after the Dextran500 injection. For comparison, in an ear blood vessel with a mean diameter of 40 ± 4 μm, the fluctuation started 1.8 min after the Dextran500 injection into the tail vein achieves 55–60% compared to average blood background. These data suggest the potential for PAFC to monitor RBC aggregation by analyzing the fluctuation of PA backgrounds (and probably frequency spectra) from blood in various vessels.
Figure 5.
Integration of PT flow cytometry and high speed (1,000–5,000 fpame per second) high resolution transmission imaging of circulating blood in vivo. (A) Dynamic PT signals from blood flow before (top) and after (bottom) formation of circulating RBC aggregates. (B) PT signals (top) from the individual RBCs (middle, bottom)) moving with velocity ~1 mm/sec in small mesenteric vessel with diameter of 10 μm (used for estimation of Ht). Optical monitoring of alterations of RBC deformability under chemical impacts of Diamide (C) and Chlorpromazine (D).
Real-time dynamic monitoring of hematocrit and RBC deformability
The PTFC) was conducted in microvessels of rat mesentery with one-file flow and a mean diameter of 6.5±2 μm. The appearance of a RBC in the small irradiated volume was accompanied by PT signals of different amplitudes and tails (Fig. 5B, top). These differences were attributed with random changes of RBC shape, distances between individual RBCs, and their attachments at a different RBC velocity. High-speed optical imaging (1,000–5,000 fps) with the resolution at the one-cell level provided independent measurements of RBC number, dynamic shape, and linear velocity that allowed to verify PT data (Fig. 5B, middle and bottom). For example, during PTFC monitoring of vein with the diameter (D) of 8-μm and RBC velocity (Vrbc) of 1 mm/sec, we detected ~55 signals per one minute associated with RBC number. This means that RBCs occupied 4,950 fL (4.95×10−7 mL) of bulk blood volume passed through the investigated vessel per minute. The bulk volumetric flow rate (Qbulk) of 29.44×10−7 mL/min was calculated from , where as Vbulk=Vrbc/1.6 and A= πD2/4 (50). Thus, for the particular experimental data we found Ht=16.8%. In addition, based on a Newtonian definition (8Vbulk/D), the wall shear rate was estimated as approximately 500 sec−1. Real-time PTFC during one hour demonstrated that the Ht value was varied from 8 to 17%. This estimation was correlated with optical image data, in particular, with maximal and minimal Ht values corresponding to Figure 5B (middle and bottom).
Real-time dynamic optical imaging of RBC deformability
In another set of experiments, we obtained the images of blood flow in microvessels after RBC deformability was altered with chemicals. High-resolution, high-speed video monitoring showed the presence of low-deformable RBCs after an injection of Diamide or chlorpromazine (Fig. 5C,D, bottom) compared to control measurement before injection (Fig. 5C and D, top). This effect was profound in a specific localized area in curved vessels (Fig. 5C) where maximum centrifugal forces were acting on cells, and in bifurcation zones (Fig. 5D) with higher RBC acceleration.
PAFC of blood circulation in mice with human sickle cell disease
PAFC monitoring of normal mice and genetically modified mice with sickle cell disease revealed a slightly lower (20–30%) PA background from ear microvessels and more profound PA signal amplitude fluctuations in mice with sickle cells. These data are consistent with in vitro results above demonstrating lower PT signal amplitudes from sickle cells. Photodamage thresholds associated with bubble formation were also lower for sickle cells, suggesting that laser-induced local temperature in these cells was lower than that in normal RBCs. Indeed, at a high energy level, the threshold for nonlinear PA signal fluctuations was 2–4-fold lower than in mice with sickle cells. These data demonstrate that both PT and PA signals from sickle cells have decreased amplitudes that can be used, after additional verification, as a marker of sickle cell disease.
In vivo noninvasive PA monitoring of complex changes in blood rheology
In this application, noninvasive PAFC was tested with the use of mouse ear blood vessels having a mean diameter of 5 0± 7 μm. Dilution of blood by intravenous injection of 400 μL of physiological solution led to a gradual decrease in PA signals of 20% at 580 nm, which corresponded to a decrease in the systemic Ht value of ~ 7%. The complex alterations of systemic blood rheology were created by subsequent intravenous injections first of distillate water, which produced both spherocytosis and reduced Ht, and, then of Dextran500, which induced RBC aggregation. PAFC monitoring of mouse ear vessels at 580 nm revealed a PA signal amplitude decrease of 42% after the first injection due to blood dilution and formation of “swollen” spherocytes, leading to a decrease in both Ht and average intracellular Hb concentration in large, round RBCs, and hence in PA signals. The second injection of Dextran500 with a lower blood background signal resulted in high-amplitude fluctuations of PA signals that reflected the presence of RBC aggregates in the blood circulation.
DISCUSSION
Here we describe a platform integrating PT and PA techniques with optical imaging for real-time, noninvasive hemorheological studies. This platform, verified in an animal model, represents a further extension of our previous approach in which we applied PT/PA techniques to the in vivo investigation of lymph rheology, permitting the assessment of the “lymphocrit,” cell deformability in lymph flow, and lymph viscosity (22,36). The diagnostic principles are based on the sensitivity of PT/PA signal parameters (e.g., amplitude, temporal profile, and dynamic behavior) to the local concentration and spatial distribution of Hb in RBCs, to RBCs in flow, and in turn to dynamic changes in the hemorheological properties of blood flow.
This technology can provide dynamic multiparameter monitoring and imaging of RBC aggregation, alterations of RBC deformability, and Ht. We also demonstrated in vivo high-speed (up to 10,000 fps), high-resolution (up to 300 nm) imaging for the continuous monitoring of transient RBC deformability in fast blood flow. The relationship between cellular functions and mechanical properties suggests that cell deformability can be used as a marker of early or latent stages of different pathological processes. In particular, we demonstrated that the integration of PAFC, PTFC and PT imaging enabled obtaining in vivo the real-time rheological status of circulating blood in microvessels through dynamic measurements followed by calculations of several parameters simultaneously, including Ht, wall shear rate, dynamic shape of moving RBCs, RBC deformability, and intracellular distribution of Hb. We also found for the first time that this technology could, noninvasively and without labeling, assess relatively large vessels and detect changes in two important rheological parameters, Ht and RBC aggregation, together. Finally the capability of the PT/PA technical platform was tested ex vivo and in vivo in a mouse model of human sickle cell disease. We believe that as an addition to in vivo blood testing and conventional blood testing in vitro, rapid (5–10 min) PT imaging cytometry (31–32) of RBCs ex vivo using a small drop of blood can add valuable information on the spatial distribution of Hb in individual RBCs.
Although we demonstrated proof of this concept using the visible-spectral range, where the absorption of Hb is at its maximum, our previous finding suggests that individual RBCs can produce detectable PA signals that are still detectable in near-infrared (NIR) range, in which light achieves maximum penetration into biotissue. The PT and optical imaging that we have developed further increases the speed and resolution of PA imaging (6, 8) and could provide accurate measurement of blood vessel sizes for calculation of the Ht.
Along with measuring the set of main determinants of hemorheology that we demonstrated here, we are confident that the capability of PTFC and PAFC can be significantly extended to measure many more rheological parameters. In particular, it should be blood viscosity as a complex parameter that depends on Ht, RBC deformability, RBC aggregation, and concentrations of plasma proteins with high molecular weights. PT/PA monitoring of plasma proteins can be performed in far NIR, where they provide readable absorption in the absence of background Hb absorption, particularly in capillaries. The influence of plasma viscosity on whole-blood viscosity is likely important in specific cases only (e.g., lymphedema), while in general this influence is relatively small (1).
Previously, we and others demonstrated that PT and PA techniques have the capability to measure blood flow velocity (9,19,25, 51), which could be the basis for calculating the velocity profile and shear rate of the vascular wall. Because we found that PT and PA signals from an Hb solution and RBCs under the same conditions are significantly different, PAFC and PTFC can be likely used to diagnose pathological intravascular hemolysis in vivo. Such spatially resolved measurements of wall shear rates have the potential to answer long-standing questions of how blood shear forces exerted on endothelial cells contribute to physiological and pathological processes, such as atherogenesis and angiogenesis (52). For example, it is well known that endothelial cells’ sensing of the shear stress gradient promotes the activation of genes responsible for cell migration, vasoregulation, and proliferation. Previously, there were no methods for measuring the shear stress gradient in vivo.
A novel diagnostic platform using safe laser parameters and a label-free, noninvasive approach can be quickly translated to use in humans. Indeed, compared to other optical modalities, noninvasive PA methods offer higher resolution, sensitivity, and penetration depth (up to 3 cm), while the minimally invasive delivery of laser radiation through tiny fibers (9) may allow the assessment of potentially any site in the human body. The clinical prototype could be in the form of a portable fiber-based device (e.g., see prototype in the supplementary information for ref. 9) that is placed over the different vessels ranging from capillaries in the nail or eye to large vessels in the hand or neck area. Patient management may be improved though ultrasensitive point-of-care monitoring of blood rheology in vivo, instead of existing invasive time-consuming in vitro testing (see Introduction).
The testing that we have described can be useful for the early, sensitive diagnosis of a broad spectrum of diseases related to alterations of multiple rheological parameters in the acute stage of critically ill patients, as well as in cardiovascular dysfunction, cancer, sickle cell disease, infections, and intoxications. Potential applications include use in emergency departments and intensive care units, which are associated with various shocks and crisis, trauma, anemia, hemorrhagic disorder/diathesis, renal insufficiency, neurological deficit, or congestive heart failure.
This would also enable physicians to individualize therapy and assess the therapeutic efficacy of existing and novel compounds. The PA diagnostic tree may integrate new PT/PA rheological testing with techniques that we have recently developed including 1) PT/PA Raman flow cytometry (28–29), providing additional diagnostic parameters of blood chemistry through the differences in PA Raman vibrational contrast from blood components; 2) identification of distinguishing spectral signatures of the internal chemical structures of different forms of Hb (e.g., sulpha-, met-, CO-, or HbS [in sickle cells]) in the NIR region in the background of reduced and oxygenated Hb (27); 3) high-speed spectral imaging for measurement of oxygenation and Hb at the single-RBC level compared to bulk integrated parameters (11–13); 4) PA detection of circulating clots; and 5) monitoring of changes in the volume of circulating blood. Hypothetically, many standard blood parameters that are currently measured by conventional in vitro blood testing can be measured in real-time in vivo more quickly (a few minutes vs. Several hours) and with greater sensitivity.
Figure 6.
Noninvasive real-time PA monitoring of blood rheology in mouse ear vessels. (A) PA signals from blood after injection of distillate water and Dextran producing complex rheological alterations of RBC deformability, Ht and RBC aggregation.
Acknowledgments
This work was supported in part by the National Institutes of Health grant numbers R01CA131164, R01EB009230, R01EB000873, and R21CA139373; the National Science Foundation, grant number DBI-0852737; and the Department of Defense, grant numbers W88XWH-10-2-0130, W81XWH-10-BCRP-CA, and W81XWH-11-1-0129. We would like to thank Evgeny Shashkov, Dmitry Nedosekin, Mustafa Sarimollaoglu, and Scott Fergusson for their help in experiments; and the Office of Grants and Scientific Publications at UAMS for editorial assistance with the preparation of this manuscript.
References
- 1.Lowe GD. Blood rheology in vitro and in vivo. Baillieres Clin Haematol. 1987;1:597–636. doi: 10.1016/s0950-3536(87)80018-5. [DOI] [PubMed] [Google Scholar]
- 2.Lipowsky HH. Microvascular rheology and hemodynamics. Microcirculation. 2005;12:5–15. doi: 10.1080/10739680590894966. [DOI] [PubMed] [Google Scholar]
- 3.Dintenfass L. Blood rheology in cardio-vascular diseases. Nature. 1963;199:813–815. doi: 10.1038/199813a0. [DOI] [PubMed] [Google Scholar]
- 4.Schmid-Schönbein H. Blood rheology and physiology of microcirculation. Ric Clin Lab. 1981;11 (Suppl 1):13–33. [PubMed] [Google Scholar]
- 5.Reggiori G, Occhipinti G, De Gasperi A, Vincent JL, Piagnerelli M. Early alterations of red blood cell rheology in critically ill patients. Crit Care Med. 2009;37:3041–3046. doi: 10.1097/CCM.0b013e3181b02b3f. [DOI] [PubMed] [Google Scholar]
- 6.Wang LV, editor. Photoacoustic Imaging and Spectroscopy. CRC; 2009. [Google Scholar]
- 7.Zharov VP, Letokhov VS. Laser Optoacoustic Spectroscopy. Berlin Heidelberg New York: Springer-Verlag; 1986. [Google Scholar]
- 8.Kim JW, Galanzha EI, Shashkov EV, Moon HM, Zharov VP. Golden carbon nanotubes as multimodal photoacoustic and photothermal high-contrast molecular agents. Nat Nanotechnol. 2009;4:688–694. doi: 10.1038/nnano.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galanzha EI, Shashkov EV, Spring PM, Suen JY, Zharov VP. In vivo, noninvasive, label-free detection and eradication of circulating metastatic melanoma cells using two-color photoacoustic flow cytometry with a diode laser. Cancer Res. 2009;69:7926–7934. doi: 10.1158/0008-5472.CAN-08-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zharov VP. Ultrasharp nonlinear photothermal and photoacoustic resonances and holes beyond the spectral limit. Nat Photon. 2011;5:110–116. doi: 10.1038/nphoton.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrova IY, Esenaliev RO, Petrov YY, Brecht HPE, Svensen CH, Olsson J, Deyo DJ, Prough DS. Optoacoustic monitoring of blood hemoglobin concentration: a pilot clinical study. Opt Lett. 2005;30:1677–1679. doi: 10.1364/ol.30.001677. [DOI] [PubMed] [Google Scholar]
- 12.Brecht HP, Prough DS, Petrov YY, Patrikeev I, Petrova IY, Deyo DJ, Cicenaite I, Esenaliev RO. In vivo monitoring of blood oxygenation in large veins with a triple-wavelength optoacoustic system. Opt Express. 2007;15:16261–16269. doi: 10.1364/oe.15.016261. [DOI] [PubMed] [Google Scholar]
- 13.Hu S, Maslov K, Wang LV. Noninvasive label-free imaging of microhemodynamics by optical-resolution photoacoustic microscopy. Opt Express. 2009;17:7688–7693. doi: 10.1364/oe.17.007688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novak J, Georgakoudi I, Wei X, Prossin A, Lin CP. In vivo flow cytometer for real-time detection and quantification of circulating cells. Opt Lett. 2004;29:77–79. doi: 10.1364/ol.29.000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgakoudi I, Solban N, Novak J, Rice WL, Wei XB, Hasan T, Lin CP. In vivo flow cytometry: A new method for enumerating circulating cancer cells. Cancer Res. 2004;64:5044–5047. doi: 10.1158/0008-5472.CAN-04-1058. [DOI] [PubMed] [Google Scholar]
- 16.Boutrus S, Greiner C, Hwu D, Chan M, Kuperwasser C, Lin CP, Georgakoudi I. Portable two-color in vivo flow cytometer for real-time detection of fluorescently-labeled circulating cells. J Biomed Opt. 2007;12:020507. doi: 10.1117/1.2722733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He W, Wang HF, Hartmann LC, Cheng JX, Low PS. In vivo quantitation of rare circulating tumor cells by multiphoton intravital flow cytometry. Proc Natl Acad Sci USA. 2007;104:11760–11765. doi: 10.1073/pnas.0703875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tkaczyk ER, Zhong CF, Ye JY, Myc A, Thomas T, Cao Z, Duran-Struuck R, Luker KE, Luker GD, Norris TB, Baker JR. In vivo monitoring of multiple circulating cell populations using two-photon flow cytometry. Opt. Commun. 2008;281:888–894. doi: 10.1016/j.optcom.2007.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zharov VP, Galanzha EI, Tuchin VV. Photothermal imaging of moving cells in lymph and blood flow in vivo. Proc SPIE. 2004;5320:256–263. [Google Scholar]
- 20.Zharov VP, Galanzha EI, Shashkov EV, Khlebtsov NG, Tuchin VV. In vivo photoacoustic flow cytometry for monitoring of circulating single cancer cells and contrast agents. Opt Lett. 2006;31:3623–3625. doi: 10.1364/ol.31.003623. [DOI] [PubMed] [Google Scholar]
- 21.Zharov VP, Galanzha EI, Shashkov EV, Kim JW, Khlebtsov NG, Tuchin VV. Photoacoustic flow cytometry: principle and application for real-time detection of circulating single nanoparticles, pathogens, and contrast dyes in vivo. J Biomed Opt. 2007;12:051503. doi: 10.1117/1.2793746. [DOI] [PubMed] [Google Scholar]
- 22.Galanzha EI, Shashkov EV, Tuchin VV, Zharov VP. In vivo multispectral, multiparameter, photoacoustic lymph flow cytometry with natural cell focusing, label-free detection and multicolor nanoparticle probes. Cytometry Part A. 2008;73A:884–894. doi: 10.1002/cyto.a.20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galanzha EI, Shashkov EV, Kelly T, Kim JW, Yang LL, Zharov VP. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells. Nat Nanotechnol. 2009;4:855–860. doi: 10.1038/nnano.2009.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galanzha EI, Kim JW, Zharov VP. Nanotechnology-based molecular photoacoustic and photothermal flow cytometry platform for in vivo detection and killing circulating cancer stem cells. J Biophotonics. 2009;1:725–735. doi: 10.1002/jbio.200910078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nedosekin DA, Sarimollaoglu M, Shashkov EV, Galanzha EI, Zharov VP. Ultra-fast photoacoustic flow cytometry with a 0.5 MHz pulse repetition rate nanosecond laser. Opt Express. 2010;18:8605–8620. doi: 10.1364/OE.18.008605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shashkov EV, Everts M, Galanzha EI, Zharov VP. Quantum dots as multimodal photoacoustic and photothermal contrast agents. Nano Lett. 2008;8:3953–3958. doi: 10.1021/nl802442x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brusnichkin AV, Nedosekin DA, Ryndina ES, Proskurnin MA, Gleb EYu, Lapotko DO, Vladimirov YuA, Zharov VP. Determination of various haemoglobin species with thermal-lens spectrometry, Moscow University Chemistry Bulletin. English translation of Vestnik Moskovskogo Universiteta. Vol. 63. Khimiya: Allerton Press Inc; 2008. [Google Scholar]
- 28.Biris AS, Galanzha EI, Li ZR, Mahmood M, Xu Y, Zharov VP. In vivo Raman flow cytometry for real-time detection of carbon nanotube kinetics in lymph, blood, and tissues. J Biomed Opt. 2009;14:021006. doi: 10.1117/1.3119145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shashkov EV, Galanzha EI, Zharov VP. Photothermal and photoacoustic Raman cytometry in vitro and in vivo. Opt Express. 2010;18:6929–6944. doi: 10.1364/OE.18.006929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zharov VP, Galanzha EI, Menyaev YA, Tuchin VV. In vivo high-speed imaging of individual cells in fast blood flow. J Biomed Opt. 2006;11:054034. doi: 10.1117/1.2355666. [DOI] [PubMed] [Google Scholar]
- 31.Zharov VP, Galanzha EI, Tuchin VV. Photothermal flow cytometry in vitro for detection and imaging of individual moving cells. Cytometry Part A. 2007;71A:191–206. doi: 10.1002/cyto.a.20384. [DOI] [PubMed] [Google Scholar]
- 32.Zharov VP, Galanzha EI, Tuchin VV. Photothermal image flow cytometry in vivo. Opt Lett. 2005;30:628–630. doi: 10.1364/ol.30.000628. [DOI] [PubMed] [Google Scholar]
- 33.Zharov VP, Galanzha EI, Tuchin VV. In vivo photothermal flow cytometry: imaging and detection of individual cells in blood and lymph flow. J Cell Biochem. 2006;97:916–932. doi: 10.1002/jcb.20766. [DOI] [PubMed] [Google Scholar]
- 34.Proskurnin MA, Zhidkova TV, Volkov DS, Sarimollaoglu M, Galanzha EI, Mock D, Zharov VP. In vivo photoacoustic flow cytometry with multicolor dyes: a potential for real-time assessment of circulation, dye-cell interaction, and blood volume. Cytometry Part A. 2011 doi: 10.1002/cyto.a.21127. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zharov VP, Galanzha EI, Ferguson S, Tuchin VV. Confocal photothermal flow cytometry in vivo. Proc SPIE. 2005;5697:1167–176. [Google Scholar]
- 36.Galanzha EI, Tuchin VV, Zharov VP. Advances in small animal mesentery models for in vivo flow cytometry, dynamic microscopy, and drug screening (review) World J Gastroenterol. 2007;13:192–218. doi: 10.3748/wjg.v13.i2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zharov VP, Lapotko DO. Photothermal imaging of nanoparticles and cells (review) IEEE J Sel Topics Quant Electron. 2005;11:733–751. [Google Scholar]
- 38.Zharov VP. Far-field photothermal microscopy beyond the diffraction limit. Opt Lett. 2003;28:1314–1316. doi: 10.1364/ol.28.001314. [DOI] [PubMed] [Google Scholar]
- 39.Kirveskari J, Vesaluoma MH, Moilanen JA, Tervo TM, Petroll MW, Linnolahti E, Renkonen R. A novel non-invasive, in vivo technique for the quantification of leukocyte rolling and extravasation at sites of inflammation in human patients. Nat Med. 2001;7:376–379. doi: 10.1038/85538. [DOI] [PubMed] [Google Scholar]
- 40.Stoltz JF. Red blood cell microrheology (clinical and pharmacological applications) Ric Clin Lab. 1983;13 (Suppl 3):53–70. [PubMed] [Google Scholar]
- 41.Zharov VP, Galitovsky V, Chowdhury P. Nanocluster model of photothermal assay: application for high-sensitive monitoring of nicotine–induced changes in metabolism, apoptosis and necrosis at a cellular Level. J Biomed. 2005;10:354–363. doi: 10.1117/1.1990200. [DOI] [PubMed] [Google Scholar]
- 42.Zharov VP, Galanzha EI, Tuchin VV. Integrated photothermal flow cytometry in vivo. J Biomed Opt. 2005;10:51502. doi: 10.1117/1.2070167. [DOI] [PubMed] [Google Scholar]
- 43.Brusnichkin AV, Nedosekin DA, Galanzha EI, Vladimirov YA, Shevtsova EF, Proskurnin MA, Zharov VP. Ultrasensitive label-free photothermal imaging, spectral identification, and quantification of cytochrome c in mitochondria, live cells, and solutions. J Biophotonics. 2010;3:791–806. doi: 10.1002/jbio.201000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zharov VP, Galitovskiy V, Lyle CS, Chambers TC. Super high-sensitive photothermal monitoring of individual cell response to antitumor drug. J Biomed Opt. 2006;11:064034. doi: 10.1117/1.2405349. [DOI] [PubMed] [Google Scholar]
- 45.Nedosekin DA, Shashkov EV, Galanzha EI, Hennings L, Zharov VP. Photothermal multispectral image cytometry for quantitative histology of nanoparticles and micrometastasis in intact, stained and selectively burned tissues. Cytometry Part A. 2010;77A:1049–1058. doi: 10.1002/cyto.a.20977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khodakovskaya MV, de Silva K, Nedosekin DA, Dervishi E, Biris AS, Shashkov EV, Galanzha EI, Zharov VP. Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proc Natl Acad Sci USA. 2011;108:1028–1033. doi: 10.1073/pnas.1008856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skalak R, Branemark PI. Deformation of red blood cells in capillaries. Science. 1969;164:717–719. doi: 10.1126/science.164.3880.717. [DOI] [PubMed] [Google Scholar]
- 48.Roggan A, Friebel M, Doerschel K, Hahn A, Mueller GJ. Optical properties of circulating human blood in the wavelength range 400–2500 nm. J Biomed Opt. 1999;4:36–46. doi: 10.1117/1.429919. [DOI] [PubMed] [Google Scholar]
- 49.Lapotko DO, Zharov VP. Spectral evaluation of laser-induced cell damage with photothermal microscopy. Lasers Surg Med. 2005;36:22–30. doi: 10.1002/lsm.20119. [DOI] [PubMed] [Google Scholar]
- 50.Lipowsky HH, Usami S, Chien S. In vivo measurements of “apparent viscosity” and microvessel hematocrit in the mesentery of the cat. Microvasc Res. 1980;19:297–319. doi: 10.1016/0026-2862(80)90050-3. [DOI] [PubMed] [Google Scholar]
- 51.Yao J, Maslov KI, Shi Y, Taber LA, Wang LV. In vivo photoacoustic imaging of transverse blood flow by using Doppler broadening of bandwidth. Opt Lett. 2010;35:1419–1421. doi: 10.1364/OL.35.001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamoun WS, Chae SS, Lacorre DA, Tyrrell JA, Mitre M, Gillissen MA, Fukumura D, Jain RK, Munn LL. Simultaneous measurement of RBC velocity, flux, hematocrit and shear rate in vascular networks. Nat Methods. 2010;7:655–660. doi: 10.1038/nmeth.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]