Abstract
OBJECTIVES
Signal transducer and activator of transcription 3 (STAT3) has been implicated in the development and progression of various solid tumors. We examined the efficacy of STAT3 inhibition as a novel therapeutic option for head and neck squamous cell carcinoma (HNSCC).
MATERIALS AND METHODS
Activation and expression of STAT3 and hypoxia-inducible factor-1 (HIF-1) in HNSCC cell lines were assessed by immunoblots. The small molecule inhibitor, Stattic, was used to target STAT3 in HNSCC cell lines. MTT assays were performed to determine the effect of STAT3 inhibition on HNSCC cell viability, while clonogenic survival assays were used to assess the ability of Stattic to sensitize HNSCC cells to radiation therapy. We also examined the effect of Stattic on tumor growth and radiosensitivity in vivo using an orthotopic xenograft model of HNSCC.
RESULTS
Stattic effectively inhibited STAT3 activation and expression, resulting in decreased cell survival and proliferation and increased radiosensitivity. STAT3-mediated HIF-1 expression was also reduced in response to Stattic treatment. Oral administration of Stattic significantly reduced the growth of HNSCC tumors in a murine orthotopic xenograft, and analysis of tumor lysates confirmed decreased STAT3 phosphorylation.
CONCLUSION
STAT3 inhibition modulates HIF-1 expression, resulting in decreased tumor growth and possible enhanced radiosensitivity in HNSCC. Our results provide support for further exploration of STAT3 as a novel molecular therapeutic target in HNSCC.
Keywords: Head and neck squamous cell carcinoma, STAT3, HIF-1, Stattic, Radiation Therapy, Radiosensitization
Introduction
Despite continued evolution in surgery, radiation therapy (XRT) and chemotherapy, the treatment of head and neck squamous cell carcinoma (HNSCC) has not significantly improved patient survival over the past the last 50 years and 3–5 year survival rates for patients with advanced-stage (T3 or T4) HNSCC continue to remain low.1 Efforts to improve treatment outcomes have shifted to the identification of alternative targeted therapies that complement conventional treatment strategies.
Recent studies have identified several promising therapeutic targets in HNSCC.2 Of these, signal transducer and activator of transcription 3 (STAT3) is a leading candidate given its increased expression in HNSCC,3,4 which promotes tumor metastasis, angiogenesis, immune evasion and suppression.5–7 Additionally, increased STAT3 activation has been linked to poor prognosis in HNSCC patients.8,9
Hypoxic environments, characterized by an acute or chronic decline in oxygen tension, contribute to malignant behavior such as tumor progression, invasion, and acquisition of resistance to chemotherapy and radiotherapy.10 This is accomplished by activating a specific set of tumor promoting transcription factors, including nuclear factor kappa-B (NFκB), cyclic AMP response element binding protein (CREB), p53, early growth response-1 (Egr-1), nuclear factor for interleukin 6 (NF-IL6), STAT3 and hypoxia-inducible factor 1 (HIF-1).11,12 The transcription factors themselves can act in conjunction to regulate the adaptation to hypoxic conditions, as in the case of STAT3 and HIF-1 which activate the vascular endothelial growth factor (VEGF) gene in response to phosphatidylinosital-3-kinase (PI3K), protein kinase B (Akt) and mammalian target of rapamycin (mTOR) signaling.13
Several strategies have been employed in the development of effective STAT3 inhibitors.3 Virtual screening to identify candidate nonpeptidic small molecules that inhibit STAT3 by binding directly to its Src homology 2 (SH2) domain has brought about a whole new class of inhibitors.14,15 Of these, the commercially available inhibitor Stattic has been shown to selectively inhibit the function of the STAT3 SH2 domain regardless of STAT3 phosphorylation status.16 Stattic potently and selectively inhibits the activation, dimerization, and nuclear translocation of STAT3, resulting in an increase in apoptosis of HNSCC cells.17 Despite an abundance of work in vitro, in vivo studies have not yet been reported. The purpose of this work is to provide an initial assessment of the potential therapeutic utility of STAT3 inhibition by Stattic in vitro and in a murine orthotopic xenograft model of HNSCC.
Our findings indicate that Stattic, through inhibition of STAT3 activation and HIF-1 expression, reduces the growth of HNSCC orthotopic xenograft tumors and may sensitize HNSCC to radiation therapy. This work identifies Stattic as a potential targeted therapy that may radiosensitize cells prior to conventional radiation therapy, thus providing more effective treatment for HNSCC patients.
Materials and Methods
Cell lines and maintenance
The HNSCC cell lines UM-SCC-17B, OSC-19, Cal33, and UM-SCC-22B were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) at 37°C and 5% CO2. OSC-19 was acquired from the Japan Health Sciences Foundation, Health Science Research Resources Bank (Osaka, Japan). Cal33 cells were obtained from Centre Antoine Lacassagne (Nice, France). UM-SCC-17B and UM-SCC-22B cells were kindly provided by Dr. Thomas Carey of the Department of Otolaryngology/Head and Neck Surgery at the University of Michigan (Ann Arbor, Michigan, USA). To achieve 1% O2 and 5% CO2 to mimic hypoxic environments, the Ruskin Lifesciences In Vivo 300 hypoxia workstation (Bridgend, UK) was used.
Western blot analysis
Cells were plated at sub-confluent levels and incubated at 37°C for 24 hrs prior to Stattic (EMD Millipore Corporation, Billerica, MA, USA) treatment. The final DMSO concentration in the medium was 0.02% or less. Cells were incubated in normoxia for 1 hr after Stattic treatment, before being placed in the hypoxia chamber overnight or the indicated time periods. For Western blot analysis, cell lysates (30 g) were solubilized in Laemmli sample buffer and then subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. Primary antibodies at 1:1000 dilution were incubated overnight at 4°C with gentle shaking: phospho-STAT3 (pSTAT3; Cell Signaling Technology, Inc., Beverly, MA, USA), STAT3 (Cell Signaling Technology, Inc.), and HIF-1 (BD Transduction Laboratories, San Jose, CA, USA). Secondary antibodies at 1:3000 dilution were incubated for 2 hrs at room temperature and Western Blotting Luminol Reagent (Santa Cruz Biotechnology, CA, USA) was used to detect proteins.
MTT cytotoxicity assay
Cell viability was measured with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT; Amresco LLC, Solon, OH, USA) cytotoxicity assay as previously described.18 Briefly, cells were plated in 96-well plates at 5 000–10 000 cells/well, treated with 0–100 M Stattic and cultured for 24 hrs in normoxic (21% O2 and 5% CO2) or hypoxic (1% O2 and 5% CO2) conditions. MTT solution was diluted in fresh media to a final concentration of 5 mg/ml and incubated for 4 hrs. The supernatant was discarded, DMSO added and the absorbance (570 nm) measured using a standard plate reader (BMG Labtech, Cary, NC, USA).
Clonogenic assay
Cells were treated with the indicated concentrations of Stattic overnight, in normoxic or hypoxic conditions, and irradiated (0, 2, 4, or 6 Gy). Two hours later, the cells were trypsinized, counted, diluted to the appropriate concentrations and plated in 6-well plates before incubation at 37°C, 21% O2, 5% CO2 for 7–14 days. Colonies were stained with 0.05% crystal violet (Sigma, MO, USA) and counted. Only colonies of ≥50 cells were scored. Surviving fraction (SF) was calculated as (mean number of colonies)/(number of cells inoculated×plating efficiency). The sensitizer enhancement ratio (SER) was calculated as (SF at 2 Gy for radiation alone)/(SF at 2 Gy for radiation plus Stattic).
Orthotopic mouse model of HNSCC
UM-SCC-17B cells (1–5×106) were injected into the tongues of athymic, 8–10 week old, male, nude mice (NCI, Frederick, MD, USA). Once tumors were established, mice (40 total; 10/group) were randomized into four treatment groups: (1) control; (2) Stattic; (3) radiation therapy; (4) Stattic and radiation therapy. Stattic was administered by oral gavage 5 days a week for 4 weeks at a dose of 50 mg/kg in 1% Tween 80 PBS. Radiation was delivered at 2 Gy/day for the first 3 days (total 6 Gy) of treatment using a small-animal irradiator. For the fourth group, Stattic was given 1 hr before the single dose of radiation. Tumor size was measured twice a week and tumor volume was calculated as length*width*height*π/6. Animals were euthanatized when they lost more than 20% body weight or at the completion of the experiment. Mouse tumors were analyzed by Western blotting for pSTAT3 and STAT3 as described above.
The facilities at The University of Texas MD Anderson Cancer Center were approved by the AAALAC and are regulated by the US Department of Agriculture, the U.S. Department of Health and Human Services, and the NIH. Animal manipulation protocols were approved by the Institutional Animal Care Use Committee.
TUNEL assay
Apoptosis was measured with the terminal deoxynucleotidyl transferase mediated dUTP nick-end labeling (TUNEL; Promega Corporation, Madison, WI, USA) kit according to the manufacturer's protocol, as previously described.18 Briefly, slides were washed with PBS, counterstained with DAPI, and mounted using Vectashield Mounting Medium (Vector Laboratories, Inc., Burlingame, CA, USA). Immunofluorescence microscopy was carried out using a DMLA microscope (Leica Microsystems, Buffalo Grove, IL, USA) equipped with a 100-W HBO mercury bulb and filter set (Chroma, Inc., Bellows Falls, VT, USA) that captures blue and green fluorescent images individually. Images were captured using a cooled charge-coupled device Hamamatsu 5810 camera (Hamamatsu Corp., Bridgewater, NJ, USA) and Image Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD, USA). Color images were captured using the same microscope equipped with a 3-chip charge-coupled device color camera (model DXC-990; Sony Corp., Woodcliff Lake, NJ, USA). Three slides from each group were selected and apoptotic endothelial cells quantified as the average ratio of apoptotic cells to total cells in a 0.04-mm2 field of each slide (200× magnification).
Statistical analysis
All experiments were performed in triplicate. Data are expressed as means ± standard deviation or means ± standard error of mean. Differences among groups were analyzed using Student's t-test. Statistical significance was considered as p < 0.05.
Results
IC50 and GI50 for Stattic in HNSCC cells
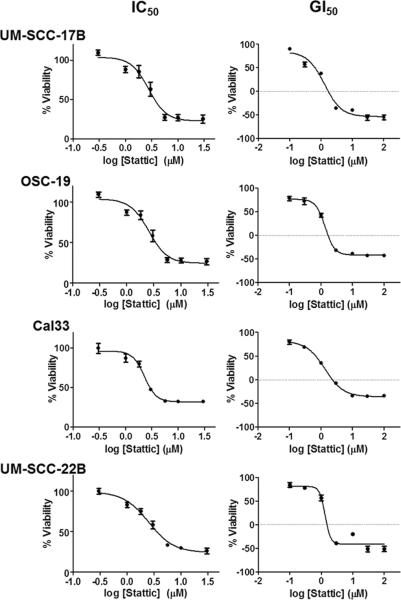
Dose-response curves for Stattic (0–100 M) were created from cell viability measurements with MTT in HNSCC cell lines (Fig. 1). Inhibitory concentration (IC50) and growth inhibition (GI50) values were extrapolated for each cell line (Table 1). Of the four cell lines used, OSC-19 was the most resistant with a GI50 value of 1.37 ± 0.77 M, whereas UM-SCC-17B proved to be most sensitive with a GI50 value of 1.28 ± 0.19 M. Overall, the HNSCC cell lines had similar growth inhibition response rates, suggesting that the effect of Stattic was consistent among this group. The micromolar values of GI50 observed in the cell lines confirmed the feasibility of using Stattic in a preclinical setting.
Figure 1. Growth inhibition of HNSCC cell lines following Stattic treatment.
UM-SCC-17B, OSC-19, Cal33, and UM-SCC-22B cells were treated with 0–100 M Stattic for 24 hrs before cell viability was measured with MTT. IC50 and GI50 values were calculated from these curves. Each data point is the mean of three independent experiments ± SD.
Table 1.
IC50 and GI50 values for Stattic in HNSCC cell lines
| Stattic |
||
|---|---|---|
| IC50 | GI50 | |
| UM-SCC-17B | 2.562 ± 0.409 | 1.279 ± 0.194 |
| OSC-19 | 3.481 ± 0.953 | 1.366 ± 0.770 |
| Cal33 | 2.282 ± 0.423 | 1.349 ± 0.363 |
| UM-SCC-22B | 2.648 ± 0.542 | 1.320 ± 0.204 |
Dose and time dependence of Stattic treatment
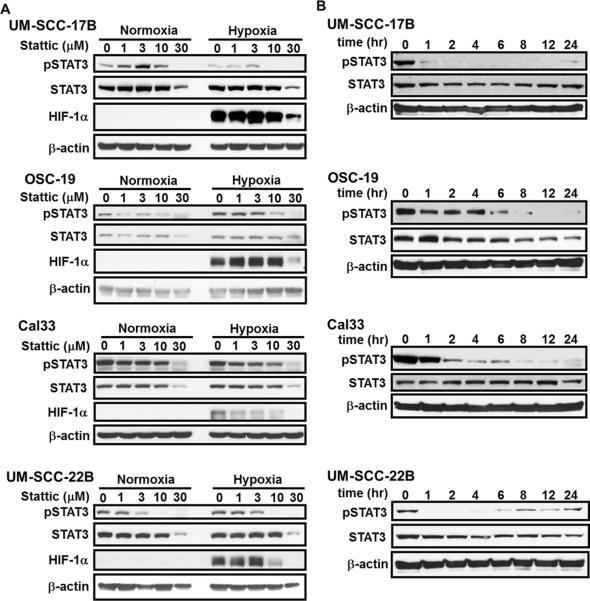
To determine the optimal dose for effective STAT3 inhibition in vitro, UM-SCC-17B, OSC-19, Cal33, and UM-SCC-22B cells were treated with Stattic (1–30 M) for 24 hrs in hypoxic and normoxic conditions (Fig. 2A). Inhibition of STAT3 phosphorylation (Y705) was achieved with as low a concentration of Stattic as 1 M, whereas 10 M proved to be most effective in all cells. With 30 M Stattic treatment, a decrease in total STAT3 was observed suggesting that beyond this dose the effects of the inhibitor might be cytotoxic. Our panel of HNSCC cells does not contain mutations of the regulatory mechanism responsible for constitutive HIF-1 expression, as confirmed by the presence of HIF-1 only in hypoxia. As with pSTAT3, the expression of HIF-1 was reduced in a dose-dependent manner, providing additional evidence for the cross-talk between STAT3 and HIF-1 To optimize the dosing schedule, we analyzed the ability of Stattic to sustain pSTAT3 inhibition over time (Fig. 2B). As expected, pSTAT3 was gradually inhibited with a maximal effect at 4–6 hrs and a sustained effect for 24 hrs. We observed some return of STAT3 phosphorylation around 24 hrs, indicating that Stattic may need to be administered daily for maximal effect.
Figure 2. Stattic inhibition of STAT3 activation was dose and time dependent.
(A) HNSCC cells were incubated with 0–30 M Stattic under normoxic (21% O2, 5% CO2) or hypoxic (1% O2, 5% CO2) conditions for 24 hrs. Concurrent pSTAT3 and HIF-1 inhibition is observed at doses above 3 M, whereas STAT3 is not reduced until the highest dose. (B) Time course of 10 M Stattic treatment in normoxia indicates the progressive reduction in pSTAT3 activation and subsequent recovery after 24 hrs.
Stattic is an effective radiosensitizer of HNSCC cells in vitro
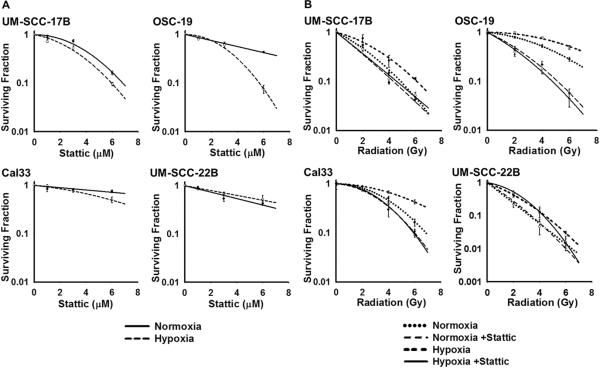
Stattic treatment alone reduced clone formation in our panel of HNSCC cell lines, suggesting that inhibition of STAT3 phosphorylation has a negative effect on adaptation to hypoxic environment via HIF-1. Interestingly, the effect of Stattic was more pronounced in hypoxia as compared to normoxia for UM-SCC-17B, OSC-19, and Cal33 cells, whereas in UM-SCC-22B cells the reverse was true (Fig. 3A).
Figure 3. Stattic radiosensitized HNSCC cell lines in vitro.
(A) UM-SCC-17B (3 M), OSC-19 (3 M), Cal33 (6 M), and UM-SCC-22B (3 M) were treated with Stattic alone for 24 hrs in normoxic or hypoxic conditions prior to plating for colony-formation assays. Stattic treatment alone was able to decrease colony formation in HNSCC. Colonies were counted after 7–14 days and each data point represents the mean of three independent experiments ± SD. (B) Cells were treated with Stattic as described above and irradiated with 0–6 Gy before plating for clonogenic assays. Stattic pre-treatment 24 hrs before irradiation effectively sensitized the HNSCC cells, more so in hypoxia than normoxia. UM-SCC-22B was the exception, showing a slight increase in radioresistance with Stattic treatment. Colonies were counted after 7–14 days and each data point represents the mean of three independent experiments ± SD.
Clonogenic assays with external beam radiation (0–6 Gy) treatment, confirmed the innate radioresistance of the HNSCC cells (Fig. 3B). When combined with radiation, Stattic pre-treatment sensitized UM-SCC-17B, OSC-19, and Cal33 cells and successfully enhanced the effects of radiotherapy. As with Stattic treatment alone, this effect was more prominent in hypoxia versus normoxia, with SERs ranging from 1.16–2.10 versus 1.12–1.62 (Table 2). UM-SCC-22B cells did not conform to this pattern as no sensitization was detected under either the normoxic or hypoxic conditions.
Table 2.
Radiosensitizing effects of Stattic: SF and SER values.
| UM-SCC-17B | OSC-19 | Cal33 | UM-SCC-22B | ||
|---|---|---|---|---|---|
| SF2: Survival Fraction at 2 Gy | |||||
|
| |||||
| Normoxia | XRT | 0.475 ± 0.005 | 0.797 ± 0.043 | 0.822 ± 0.008 | 0.242 ± 0.005 |
| XRT+Stattic | 0.358 ± 0.022 | 0.491 ± 0.009 | 0.735 ± 0.018 | 0.279 ± 0.024 | |
|
| |||||
| Hypoxia | XRT | 0.633 ± 0.012 | 0.929 ± 0.019 | 0.888 ± 0.022 | 0.429 ± 0.009 |
| XRT+Stattic | 0.372 ± 0.015 | 0.443 ± 0.004 | 0.769 ± 0.002 | 0.529 ± 0.023 | |
|
| |||||
| SER: Sensitizer Enhancement Ratio | |||||
|
| |||||
| Normoxia | 1.292 ± 0.072 | 1.624 ± 0.108 | 1.119 ± 0.033 | 0.868 ± 0.044 | |
| Hypoxia | 1.599 ± 0.054 | 2.095 ± 0.015 | 1.155 ± 0.024 | 0.810 ± 0.075 | |
Stattic inhibits tumor growth and induces apoptosis in an orthotopic xenograft model of HNSCC
UM-SCC-17B tumors were established in the tongues of athymic nude mice and treated with 50 mg/kg Stattic, alone or in combination with 6 Gy radiotherapy, given at the beginning of the experiment for 3 days in 2 Gy fractions, as outlined in Fig. 4A. Western blotting analysis of the tumors confirmed the decrease of STAT3 and pSTAT3 levels within 12 hrs of treatment and subsequent recovery after 24 hrs (Fig. 4B), similar to the in vitro time course (Fig. 2B). This effect was sustained in the presence of radiation therapy and was more prominent on pSTAT3 than STAT3.
Figure 4. Stattic and radiation therapy decrease tumor growth in an orthotopic xenograft model of HNSCC.
(A) Graphic representation of the treatment schedule for Stattic and radiation in HNSCC tumor-bearing mice; XRT = radiotherapy. (B) Homogenized tumor lysates were analyzed by Western blotting for expression of pSTAT3 and STAT3. pSTAT3 inhibition confirmed the activity of Stattic in vivo. (C) UM-SCC-17B tumors in athymic, male mice were treated with 50 mg/kg Stattic in 1% Tween-80 PBS by oral gavage, 5 days a week for 4 weeks. Tumor growth was significantly inhibited in mice treated with Stattic, radiation therapy and the combination of the two; *, p < 0.05. Each data point represents the mean tumor volume for each group ± SEM, normalized to the first day of treatment; XRT = radiotherapy. (D) Scatter plot of tumor volume following treatment. Individual tumor volumes and the mean ± SEM are displayed for each group.
The mice treated with Stattic had significantly (p < 0.05) smaller tumors than the control group (Fig. 4C). Although there was a difference in the tumor size of the Stattic + XRT group as compared to Stattic group (p < 0.05), there was only a small difference in tumor volume between the XRT and Stattic + XRT groups (Fig. 4D). By inhibiting pSTAT3 and subsequently HIF-1 in vivo, Stattic may sensitize tumors to radiation therapy, resulting in increased tumor growth inhibition in the group receiving the combination therapy. However, in order to maximize the radiosensitization effect, we may need to optimize the delivery regimen for Stattic relative to radiation therapy.
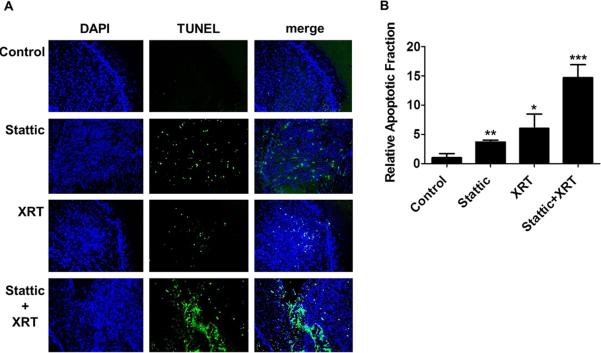
To examine the mechanism behind tumor growth inhibition and potential radiosensitizing effects of Stattic, apoptosis was quantified by TUNEL staining of tumor sections (Fig. 5A). Stattic alone induced a 3.67 ± 0.34 fold increase in apoptosis compared to controls, whereas the addition of radiation dramatically increased the apoptotic fraction to 14.7 ± 2.21 times the normal (Fig. 5B). These findings suggest that Stattic is a potential radiosensitizer of HNSCC and STAT3 inhibition may represent a valuable addition to current treatment strategies.
Figure 5. Stattic treatment induced apoptosis in UM-SCC-17B tumors.
(A) Representative sections of UM-SCC-17B tumors, stained with DAPI (blue) and TUNEL (green), confirm the increase in apoptosis with Stattic and combination treatment; XRT = radiotherapy. (B) To determine the apoptotic fraction, doubly stained cells (blue and green) were counted and divided by DAPI stained cells (blue) at 200× magnification. Stattic or radiation treatment alone induced apoptosis as compared to the controls, but the combined treatment was the most effective; XRT = radiotherapy; *, p < 0.05; **, p < 0.005; ***, p < 0.005.
Discussion
We present the effective inhibition of STAT3 activation by the small molecule inhibitor, Stattic, which results in decreased STAT3-mediated HIF-1 expression and subsequent radiosensitization of HNSCC cells. This effect was more pronounced in hypoxia, but Stattic was able to reduce colony formation in normoxia as well. Despite evidence of Stattic activity on STAT3 and HIF-1, UM-SCC-22B cells did not exhibit the radiosensitization observed in other HNSCC cells. These findings suggest that alternate mechanisms of radioresistance are present in HNSCC, which are currently being investigated.
Inhibiting the STAT3 signaling pathway may represent an effective strategy in the treatment of HNSCC and here we present the first evidence of Stattic activity in vivo. Stattic pre-treatment sensitized orthotopic xenograft HNSCC tumors to radiation and resulted in significantly reduced tumor growth, although this effect was not as dramatic as anticipated by the in vitro studies. Given this finding, we examined the potential effects of Stattic and XRT on tumor cell apoptosis. TUNEL assay results revealed that Stattic combined with radiotherapy dramatically increased the apoptotic fraction as compared to Stattic and radiation therapy alone. Fractionated radiation treatment (2 Gy × 3 days) may not have been sufficient to effectively demonstrate the radiosensitizing activity of Stattic in vivo. In addition, drug dosing, choice of vehicle, route and schedule of delivery need to be further optimized in future experiments to assess the value of Stattic or other STAT3 inhibitors as a complement to radiation therapy.
It has been previously reported that pSTAT3 not only binds to HIF-1 directly but also suppresses the HIF-1 degradation machinery (Prolyl hydroxylase domain proteins/von Hippel-Lindau protein) while promoting its synthesis (PI3K/Akt/mTOR signaling pathways) in human renal cell carcinoma.12 STAT3 has also been shown to enhance HIF-1 mediated expression of VEGF.13 Our results provide additional evidence for the involvement of STAT3 in the regulation of HIF-1 by demonstrating the sensitization of HNSCC cells to radiotherapy through the inhibition of both transcription factors by Stattic. A similar therapeutic strategy has been reported in prostate cancer, where combining the STAT3 inhibitor, T40214, with the HIF-1 inhibitor, JG244, circumvented hypoxia-induced drug resistance.19 Additionally, work with STAT3 targeted shRNA demonstrated enhanced radiosensitivity in the human squamous cell carcinoma cell line A431.20 By targeting multiple oncogenic signaling pathways within cells, Stattic may be able to sensitize tumors to radiotherapy and perhaps chemotherapy.
In addition to Stattic, several other small molecule inhibitors of STAT3 have been described in the literature and continuing efforts to develop more potent STAT3 inhibitors are underway23,24. In particular, STA-21 and S3I-201 selectively target the DNA-binding domain of STAT3 and effectively suppresses its activity in rhabdomyosarcoma, osteosarcoma and breast cancer.14,21,22 This new generation of small molecule inhibitors is based on virtual screening of the crystalline structure of STAT3 and has offered promising results. Given the role of STAT3 in modulating tumor viability and radiosensitivity, the development of an efficient STAT3 inhibitor is critical in the development of novel treatment regimens for solid tumors.
Our findings emphasize the importance of STAT3 and HIF-1 in tumor viability and resistance to radiotherapy. Having demonstrated a valuable therapeutic strategy involving STAT3 inhibition in HNSCC, this work should provide impetus for the clinical evaluation of biological modifiers that may enhance radiation treatment and potentially reduce undesirable side effects associated with currently available treatment strategies.
Acknowledgments
Role of Funding Sources This work was supported by a NIH Mentored Career Development Award (K08 DE018061; SYL), SPORE Career Development Award (SYL), a NIH SPORE Collaborative Developmental Research Project Grant (SYL), the Uehara Memorial Foundation Postdoctoral Fellowship (MA), and the Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad (MA). This research was supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant CA016672.
None of the study sponsors had any role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement None declared.
References
- 1.Choong N, Vokes E. Expanding role of the medical oncologist in the management of head and neck cancer. CA Cancer J Clin. 2008;58(1):32–53. doi: 10.3322/CA.2007.0004. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira MB, De Souza JA, Cohen EE. Role of molecular markers in the management of head and neck cancers. Curr Opin Oncol. 2011;23(3):259–264. doi: 10.1097/CCO.0b013e328344f53a. [DOI] [PubMed] [Google Scholar]
- 3.Lai SY, Johnson FM. Defining the role of the JAK-STAT pathway in head and neck and thoracic malignancies: implications for future therapeutic approaches. Drug Resist Updat. 2010;13(3):67–78. doi: 10.1016/j.drup.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal BB, Kunnumakkara AB, Harikumar KB, et al. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunning PT, Glenn MP, Siddiquee KA, et al. Targeting protein-protein interactions: suppression of Stat3 dimerization with rationally designed small-molecule, nonpeptidic SH2 domain binders. Chembiochem. 2008;9(17):2800–2803. doi: 10.1002/cbic.200800291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darnell JE., Jr Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2(10):740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- 7.Yue P, Turkson J. Targeting STAT3 in cancer: how successful are we? Expert Opin Investig Drugs. 2009;18(1):45–56. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jing N, Zhu Q, Yuan P, Li Y, Mao L, Tweardy DJ. Targeting signal transducer and activator of transcription 3 with G-quartet oligonucleotides: a potential novel therapy for head and neck cancer. Mol Cancer Ther. 2006;5(2):279–286. doi: 10.1158/1535-7163.MCT-05-0302. [DOI] [PubMed] [Google Scholar]
- 9.Leeman RJ, Lui VW, Grandis JR. STAT3 as a therapeutic target in head and neck cancer. Expert Opin Biol Ther. 2006;6(3):231–241. doi: 10.1517/14712598.6.3.231. [DOI] [PubMed] [Google Scholar]
- 10.Karar J, Maity A. Modulating the tumor microenvironment to increase radiation responsiveness. Cancer Biol Ther. 2009;8(21):1994–2001. doi: 10.4161/cbt.8.21.9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummins EP, Taylor CT. Hypoxia-responsive transcription factors. Pflugers Arch. 2005;450(6):363–371. doi: 10.1007/s00424-005-1413-7. [DOI] [PubMed] [Google Scholar]
- 12.Jung JE, Lee HG, Cho IH, et al. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 2005;19(10):1296–1298. doi: 10.1096/fj.04-3099fje. [DOI] [PubMed] [Google Scholar]
- 13.Xu Q, Briggs J, Park S, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24(36):5552–5560. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 14.Song H, Wang R, Wang S, Lin J. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc Natl Acad Sci U S A. 2005;102(13):4700–4705. doi: 10.1073/pnas.0409894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker S, Groner B, Muller CW. Three-dimensional structure of the Stat3beta homodimer bound to DNA. Nature. 1998;394(6689):145–151. doi: 10.1038/28101. [DOI] [PubMed] [Google Scholar]
- 16.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13(11):1235–1242. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Leong PL, Andrews GA, Johnson DE, et al. Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc Natl Acad Sci U S A. 2003;100(7):4138–4143. doi: 10.1073/pnas.0534764100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gule MK, Chen Y, Sano D, et al. Targeted therapy of VEGFR2 and EGFR significantly inhibits growth of anaplastic thyroid cancer in an orthotopic murine model. Clin Cancer Res. 2011;17(8):2281–2291. doi: 10.1158/1078-0432.CCR-10-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy KR, Guan Y, Qin G, Zhou Z, Jing N. Combined treatment targeting HIF-1alpha and Stat3 is a potent strategy for prostate cancer therapy. Prostate. doi: 10.1002/pros.21397. [DOI] [PubMed] [Google Scholar]
- 20.Bonner JA, Trummell HQ, Willey CD, Plants BA, Raisch KP. Inhibition of STAT-3 results in radiosensitization of human squamous cell carcinoma. Radiother Oncol. 2009;92(3):339–344. doi: 10.1016/j.radonc.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CL, Loy A, Cen L, et al. Signal transducer and activator of transcription 3 is involved in cell growth and survival of human rhabdomyosarcoma and osteosarcoma cells. BMC Cancer. 2007;7:111. doi: 10.1186/1471-2407-7-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siddiquee K, Zhang S, Guida WC, et al. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci U S A. 2007;104(18):7391–7396. doi: 10.1073/pnas.0609757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X, Kasembeli MM, Jiang X, Tweardy BJ, Tweardy DJ. Chemical probes that competitively and selectively inhibit Stat3 activation. PLoS One. 2009;4(3):e4783. doi: 10.1371/journal.pone.0004783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redell MS, Ruiz MJ, Alonzo TA, Gerbing RB, Tweardy DJ. Stat3 signaling in acute myeloid leukemia: ligand-dependent and -independent activation and induction of apoptosis by a novel small-molecule Stat3 inhibitor. Blood. 117(21):5701–5709. doi: 10.1182/blood-2010-04-280123. [DOI] [PMC free article] [PubMed] [Google Scholar]