Abstract
Scaffolds derived from naturally occurring polysaccharides have attracted significant interest in bone tissue engineering due to their excellent biocompatibility and hydrophilic nature favorable for cell attachment. In this study, we developed composite chitosan (CH) scaffolds containing anionic carbohydrate, such as chondroitin 4-sulfate (CS) or alginate (AG), with biomimetic apatite layer on their surfaces, and investigate their capacity to deliver progenitor cells (bone marrow stromal cells, BMSC) and model proteins with net-positive (histone) and net-negative charge (bovine serum albumin, BSA). The incorporation of CS or AG in CH scaffolds increased compressive modulus of the scaffolds and enhanced apatite formation. Initial burst release of histone was significantly higher than that of BSA from CH scaffold, while the addition of CS or AG in the scaffolds significantly reduced the initial burst release of histone, indicating strong electrostatic interaction between histone and negatively charged CS or AG. The apatite layer created on scaffold surfaces significantly reduced the initial burst release of both BSA and histone. Furthermore, apatite-coated scaffolds enhanced spreading, proliferation, and osteogenic differentiation of BMSC seeded on the scaffolds compared to non-coated scaffolds as assessed by live/dead and alamarBlue assays, scanning electron microscopy (SEM), alkaline phosphatase (ALP) activity, and Picrosirius red staining. This study suggests that apatite-coated CH/CS composite scaffolds have the potential as a promising osteogenic system for bone tissue engineering applications.
Keywords: Chitosan, Chondroitin Sulfate, Alginate, Biomimetic Apatite, Scaffold, Bone Regeneration
1. Introduction
Bone grafts have been widely used to reconstruct skeletal defects created by tumors, traumas, and bone diseases. However, bone grafts are encumbered by the limited availability of donor sites, donor site morbidity, and complications of increased operating time (Kim, Sun Park, Jeon, Yong Choi & Kim, 2006; Kong, Gao, Cao, Gong, Zhao & Zhang, 2005). Tissue engineering substitutes are alternative strategies using sophisticated biocompatible scaffolds combined with multipotent precursor cells (Thein-Han & Misra, 2009) and appropriate cellular stimulation for treatment of bone defects.
Chitosan (CH) is a naturally derived, cost-effective, and biocompatible polysaccharide used for many pharmaceutical and biomedical applications. Unlike synthetic polymers with hydrophobic surfaces, the high charge density of CH and its structural similarity to glycosaminoglycans, found in extracellular matrix (ECM) of bone and cartilage, are favorable for cell adhesion and proliferation (Li, Ramay, Hauch, Xiao & Zhang, 2005; Manjubala, Ponomarev, Wilke & Jandt, 2008; Sui, Huang, Wang & Bo, 2008). Also, CH is enzymatically degraded in vivo by lysozymes which results in complete absorption without concurrent side reactions or chronic inflammatory responses (Kumar, Muzzarelli, Muzzarelli, Sashiwa & Domb, 2004; Lee, Ha & Park, 1995; Tomihata & Ikada, 1997). This is a considerable advantage over commonly used synthetic resorbable polymers, such as poly(glycolic acid) (PGA) or poly(lactic acid) (PLA), which release acidic degradation by-products that can cause severe inflammation. (Aday & Gumusderelioglu, 2010; Manjubala, Ponomarev, Wilke & Jandt, 2008).
Although CH has been shown to be osteoconductive and enhance bone formation, pure CH scaffolds may not possess the necessary mechanical strength for implantation (Li, Ramay, Hauch, Xiao & Zhang, 2005; Shanmugasundaram, Ravichandran, Reddy, Ramamurty, Pal & Rao, 2001). One of the distinctive properties of CH is its cationic nature and high charge density in solution. This allows the formation of stable ionic complexes with multivalent water-soluble anionic molecules under mild physiological conditions. Although numerous studies have incorporated anionic polymers such as alginate (AG), hyaluronic acid, or chondroitin 4-sulfate (CS) into the CH scaffolds to improve their mechanical properties, and investigated their respective mechanical and biological responses (Li, Ramay, Hauch, Xiao & Zhang, 2005; Manjubala, Ponomarev, Wilke & Jandt, 2008; Murata, Miyamoto & Kawashima, 1996), no single study has reported a comparative assessment of how such additional components influence the cell-scaffold and protein-scaffold interactions, and also apatite formation on the CH scaffolds.
This study investigates protein-scaffold and cell-scaffold interactions with model proteins with net positive charge (histone) and net negative charge proteins (bovine serum albumin, BSA) and bone marrow stromal cells (BMSC) from three substrates: CH, CH/CS, and CH/AG. To enhance bone ingrowth and bonding ability of the scaffolds, we induced a bone mineral-mimicking apatite layer onto the anionic carbohydrate-containing chitosan scaffolds and determined the effects of apatite coating on release kinetics of model proteins, cell spreading, proliferation, and osteogenic differentiation.
2. Materials and Methods
2.1 Materials
CH (Mw 255,000, 75–85% deacetylated), CS sodium salt (Mw 50,000, ≥ 60% type A) from bovine trachea, sodium AG (viscosity ≥2,000 cP, 2% w/v), pentasodium tripolyphosphate (TPP), and BSA-Fluorescein isothiocyanate conjugate (FITC-BSA) were purchased from Sigma-Aldrich (St. Louis, MO) and used as received. Alexa Fluor® 488 Histone H1 conjugate was obtained from Invitrogen (Carlsbad, CA).
2.2 Fabrication of scaffolds
CH scaffolds were prepared as described previously with some modifications (Li, Ramay, Hauch, Xiao & Zhang, 2005; Park, Lee, Lee, Seol, Chung & Lee, 2000; Seol et al., 2004). A solution of CH (3% w/v) was prepared by dissolving CH in 1 N acetic acid. The solution was poured into tissue-culture petri dish, frozen at − 80 °C, and lyophilized in a freeze dryer. For CH/CS composite scaffolds, CH solution (4.5% w/v) in 1 N acetic acid was mixed with CS solution in PBS (3%, 6%, 9% w/v) at a ratio of 2:1 (v/v) to yield CH/CS mixture (3% CH/1% CS, 3% CH/2% CS, 3% CH/3% CS). For CH/AG composite scaffolds, CH solution (6% w/v) in 1 N acetic acid was mixed with AG solution in1 N NaOH (2%, 4%, 6% w/v) at a ratio of 1:1 (v/v) to yield CH/AG mixture (3% CH/1% AG, 3% CH/2% AG, 3% CH/3% AG) and the solution was adjusted to pH 7.4. The mixtures were homogenized with mechanical blender, poured into tissue-culture petri dish, frozen at − 80 °C, and lyophilized in a freeze dryer. The obtained CH and CH/CS scaffolds were neutralized by immersing them into 1 N NaOH for 1 h. CH, CH/CS, and CH/AG scaffolds were crosslinked with 5% (w/v) TPP solution for 30 min. The crosslinked scaffolds were then washed with ddH2O, sterilized by immersing them into 70% ethanol for 30 min, and lyophilized. The dimension of the scaffolds used in following study was 6 mm in diameter and 1 mm in height except the scaffolds used in mechanical testing, those scaffolds were 15 mm in diameter and 15 mm in height.
2.3 Biomimetic apatite coating process
Apatite coating solution was prepared as described previously with some modifications (Chou, Dunn & Wu, 2005; Chou, Huang, Dunn, Miller & Wu, 2005). Briefly, simulated body fluids (SBF) were prepared by sequentially dissolving CaCl2, MgCl2·6H2O, NaHCO3, and K2HPO4·3H2O into ddH2O. The pH of solution was adjusted to pH 6.0 and then Na2SO4, KCl, and NaCl were added. The final pH of solution was adjusted to pH 6.5 (SBF 1). Mg2+ and HCO3− free SBF (SBF 2) was prepared by subsequently dissolving NaCl, CaCl2, and K2HPO4·3H2O and the pH of solution was adjusted to pH 6.4. The obtained scaffolds were subjected to glow discharge argon plasma etching (Harrick Scientific, Ossining, NY). The etched scaffolds were incubated in SBF 1 for 1 day and transferred to SBF 2 for another 2 days at 37 °C. The apatite-coated scaffolds were washed with ddH2O to remove excess ions and lyophilized prior to further studies.
2.4 Characterization of scaffold morphology and apatite coating
2.4.1 Scanning electron microscopy
The internal morphology of scaffolds was examined by scanning electron microscopy (SEM, Nova Nano SEM 230/FEI, Hillsboro, OR). The cross-sectioned samples were mounted on aluminum stubs and sputter-coated with gold at 20 mA under 70 mTorr for 50 s.
2.4.2 Porosity
The porosity of scaffolds was calculated using a liquid displacement method as described previously (Karageorgiou & Kaplan, 2005; Nazarov, Jin & Kaplan, 2004; Zhang & Ma, 1999b; Zhao et al., 2002). Briefly, scaffolds were submerged to a known volume (V1) of ethanol in graduated cylinder for 5 min. A series of brief evacuation-repressurization cycles were conducted to force ethanol into the pores of scaffolds. The total volume of ethanol and the ethanol-impregnated scaffolds were recorded (V2). The scaffolds were removed from cylinder and the residual ethanol volume was recorded (V3). The porosity (%) was calculated as (V1–V3) / (V2–V3) × 100 wherein V1–V3 is the volume of void within the scaffolds and V2–V3 is the total volume of the scaffolds (Nazarov, Jin & Kaplan, 2004; Zhang & Ma, 1999a).
2.4.3 Apatite-coated area
Total coverage of apatite areas was determined using SEM images. Two of each scaffold was prepared for SEM monographs, and then five different random areas were chosen to take images. A total of 10 SEM images for each scaffold were used to quantify coverage of areas using image analysis system (ImagePro, Rockville, MD). The coverage of apatite coating was expressed in percentage by calculating the ratio of area that covered by apatite to total scaffold area shown on image (Rohanizadeh, Al-Sadeq & Legeros, 2004).
2.4.4 ATR-FTIR
The chemical structure of the scaffolds was analyzed using Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy (ATR-FTIR) before and after apatite coating. The samples were placed in contact with diamond ATR window. FTIR (Avatar 360 Thermo Nicolet spectrometer, Thermo Electron Inc., San Jose, CA) absorbance spectra from 4000 to 400 cm−1 wavenumbers were observed.
2.5 Mechanical Properties
The compressive modulus of scaffolds was measured via indentation experiment with an Instron Electro-Mechanical Testing Machine (Instron, Model 5564, Norwood, MA). The 3 mm diameter flat-ended indenter was used. The specimens were 2 mm thick sections with 15 mm in diameter to ensure an optimal ratio of sample to indenter size as well as flat surface for mechanical test. Three measurements were made per scaffold and three parallel samples were applied. The compressive modulus was calculated as described previously using the poisson’s ratio of 0.3 (Boccaccio, Lamberti, Pappalettere, Carano & Cozzani, 2006; Boccaccio, Lamberti, Pappalettere, Cozzani & Siciliani, 2008; Kanungo, Silva, Van Vliet & Gibson, 2008).
2.6 In vitro release of protein
In vitroprotein release profile was examined using FITC-BSA and Alexa Fluor conjugated histone as model proteins. Briefly, 20 µL of protein solution in phosphate buffered saline (PBS) was dropped onto the scaffolds at a final protein concentration of 1.0 mg/mL. The scaffolds were air dried for 15 mins and further lyophilized in a freeze dryer. The protein-loaded scaffolds were immersed in 1 mL of 10 mM PBS (pH 7.4) at 37 °C. The entire incubating solution was removed and replaced with 1 mL of fresh PBS solution at predetermined time points over 28 days. The amount of released protein in the supernatant was determined by measuring the fluorescence of the sample. The experiment was performed in triplicate, and the amount of protein released was expressed as a percentage of the initial amount of protein loaded.
2.7 Cell seeding and proliferation
Mouse bone marrow stromal cells (BMSC, ATCC, VA) were cultured in Dulbeccos’ modified Eagles’ medium (DMEM, Invitrogen) with low glucose, 10% fetal bovine serum (FBS, Invitrogen), 100 U/mL penicillin, 100 µg/mL streptomycin. Cells were trypsinized upon 70–80% confluence and seeded onto scaffolds at a concentration of 3 × 106 cells/mL. The cell/scaffold constructs were cultured in complete DMEM supplemented with 10 mM β-glycerol phosphate and 50 µg/mL ascorbic acid at 37 °C in 5% CO2 humidified incubators up to 28 days. To observe proliferation of the cells cultured on the scaffolds, the cell/scaffold constructs were washed once with PBS and stained with calcein solution (Invitrogen) at 37 °C for 15 min. Stained samples were observed under a fluorescence microscope (Olympus, Lake Success, NY). Proliferation of cells on the scaffolds was further measured using the alamarBlue assay kit (Invitrogen). The cell/scaffold constructs were washed once with PBS and incubated with sterile alamar blue solution for 3 h at 37 °C. alamarBlue fluorescence was assayed at 535 nm (excitation) and 600 nm (emission). The experiments were performed in triplicate.
2.8 Cell morphology and ECM secretion
The morphology and ECM secretion of the cells cultured on scaffolds were observed using SEM and histological staining at 1, 14, and 28 days. For SEM observation, the cell/scaffold constructs were fixed with 2.5% glutaraldehyde (EM Sciences, Hatfield, PA) and dehydrated using a serial graded ethanol (25, 50, 70, 90, and 100%). After dehydration, the specimens were immersed in hexamethyldisilazane (HMDS, EM Sciences) and air dried (Chou, Dunn & Wu, 2005). The dried specimens were mounted on aluminum stubs and coated with gold as described above. For histological observation, the cultured cell/scaffold constructs were fixed in10% formalin (EM Sciences), embedded in paraffin, and sectioned at 9 µm thickness. The sections were deparaffinized and stained with hematoxylin and eosin (H&E).
2.9 ALP activity and collagen content
After 1, 7, 14, and 28 days of incubation, the cell/scaffold constructs were washed with PBS, minced, and incubated in lysis buffer (0.1% Tween-20). ALP activity was determined colorimetrically using p-nitrophenol phosphate (Sigma) as a substrate and measured at an absorbance of 405 nm. Measurements were performed in triplicate and normalized to total DNA content determined by the PicoGreen Assay (Invitrogen). For collagen content, deparaffinized sections were stained with 0.1% Picrosirius red solution (Polysciences, Inc., Warrington, PA) as described previously (Burns, Rasmussen, Larsen, Schroder & Kassem, 2010; Chai et al., 2012; Junqueira, Bignolas & Brentani, 1979). Stained samples were observed under a microscope. The newly formed collagen appeared as red.
2.10 Statistical analysis
One-way analysis of variance (ANOVA) with Tukey multiple comparison procedure was performed for statistical analysis. Tukey’s post hoc test was performed for comparison and value of p < 0.05 was considered significant.
3. Results and discussion
3.1. Scaffold fabrication and characterization
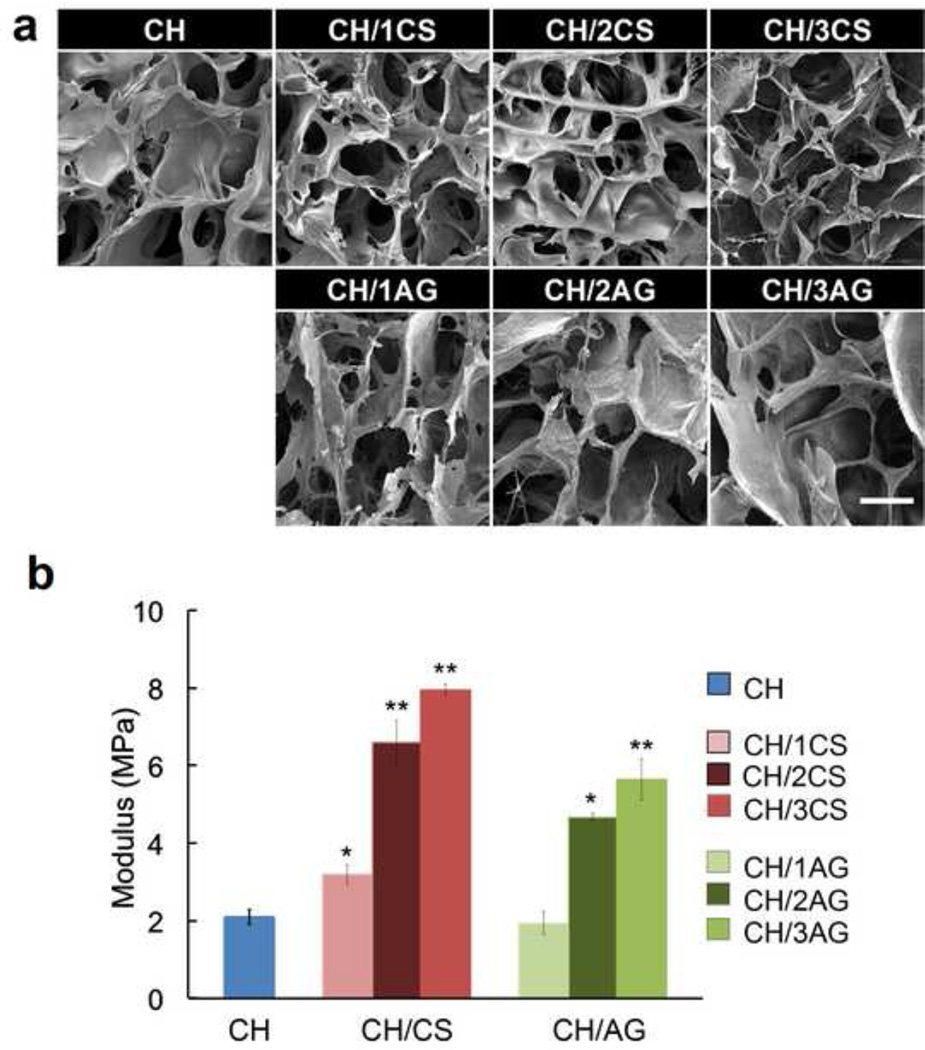
The interior morphology of CH based scaffolds was examined by SEM (Fig. 1a). The cross sectional SEM images of all scaffolds showed highly interconnected porous structures. CH scaffolds (3% w/v) showed a pore size range of 100–150 µm and the addition of CS or AG did not significantly change the pore size of the scaffolds. The porosity of CH scaffolds was approximately 85% and the addition of CS (3% w/v CH/ 3% w/v CS) or AG (3% w/v CH/ 3% w/v AG) significantly increased the porosity of the scaffolds to 93.7% and 95.5%, respectively. This can be attributed to strong ionic interaction between positively charged amino groups in CH and negatively charged carboxyl groups in CS or AG, forming precipitation of polymer solutions and consequently more open network structures. Li et al. reported similar influence on porosity when AG was incorporated in CH scaffolds (Li & Zhang, 2005).
Figure 1.
(a) SEM images of CH (3%), CH/CS (3% CH with 1, 2, or 3% CS, namely CH/1CS, CH/2CS, CH/3CS, respectively), and CH/AG (3% CH with 1, 2, or 3% AG, namely CH/1AG, CH/2AG, CH/3AG, respectively) scaffolds. Scale Bar = 100 µm. (b) Mechanical Properties of CH, CH/CS, and CH/AG scaffolds. Significantly higher compressive modulus was observed in the presence of CS or AG in CH scaffolds (*p<0.05, **p<0.01).
Tissue engineering scaffolds should consider structural properties such as pore size, porosity, and pore interconnectivity to optimize nutrient transport and cell migration to promote new bone formation (Galois & Mainard, 2004; Guillemin, Meunier, Dallant, Christel, Pouliquen & Sedel, 1989; Van Cleynenbreugel, Schrooten, Van Oosterwyck & Vander Sloten, 2006). It is generally considered that an open pore network with minimal pore size of 100 µm (Aday & Gumusderelioglu, 2010; Akman, Tigli, Gumusderelioglu & Nohutcu, 2010; Zeltinger, Landeen, Alexander, Kidd & Sibanda, 2001) and porosity of 65% is optimal (Knabe, Koch, Rack & Stiller, 2008).
It has been demonstrated in previous studies that complex structures created by CS and AG are capable of enhanced mechanical properties (Li, Ramay, Hauch, Xiao & Zhang, 2005). In this study, the mechanical strength of the scaffolds was characterized with various concentrations of CS or AG (Fig. 1b). The compressive modulus of CH (3% w/v) scaffolds significantly increased (p < 0.01) from 2.1 MPa to 3.2 MPa with the addition of CS (1% w/v). The formation of a CH/CS complex by ionic interactions likely contributed to the enhanced mechanical strength of the composite scaffolds. The compressive modulus of CH/CS (3% w/v CH/ 1% w/v CS) scaffolds further increased to 8.0 MPa as the CS concentration increased to 3%, indicating stronger ionic interactions between CH and CS. The CH/AG scaffolds showed a greater compressive modulus than pure CH scaffolds showed when the AG concentration was equal to or higher than 2% (w/v). CH (3% w/v) scaffolds containing 3% of CS or AG were used in further studies because higher concentrations over 3% were too viscous for handling and uniform mixing.
3.2. Biomimetic Apatite Coating
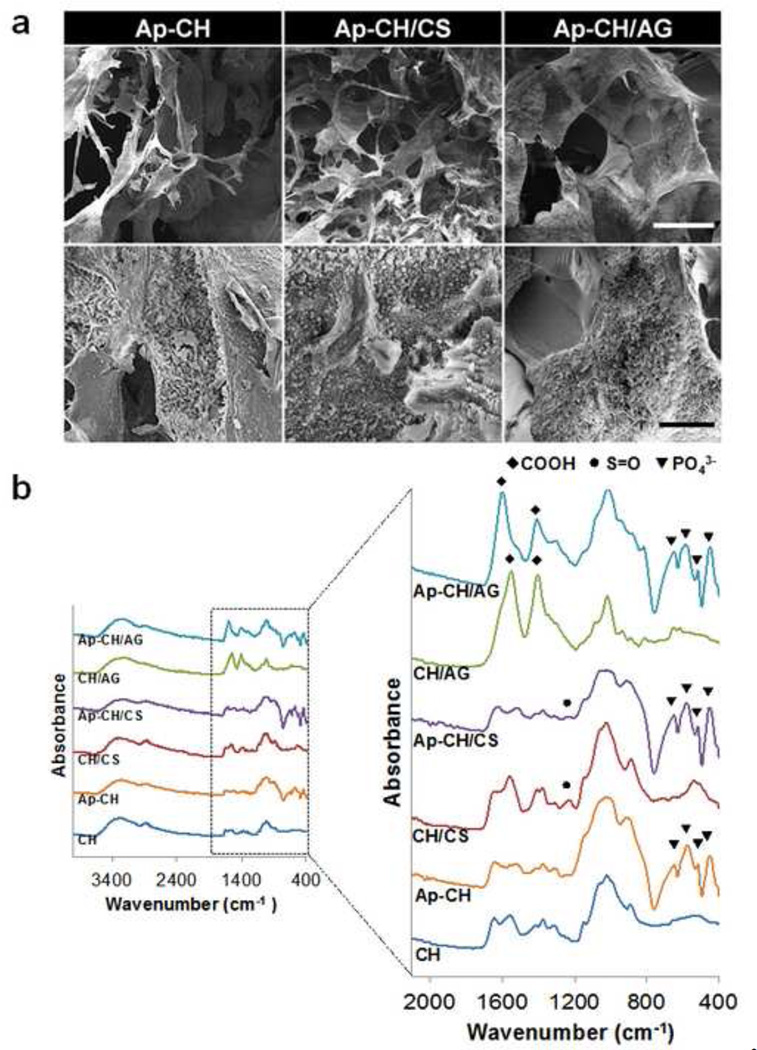
Although CH scaffolds provide a substrate for cell attachment and physical support for bone repair, they do not truly induce bone regeneration. Many studies have suggested calcium phosphate can promote cell proliferation and osteogenic differentiation. (Shiqian, Dongjie, Fei, Tian, Feng & Jiawei, 2013; Zo, Singh, Kumar, Cho, Oh & Han, 2012) We previously reported biomimetic processing strategies to confer bone mineral-mimicking apatite microenvironments onto various biomaterial surfaces such as Poly Lactic-co-Glycolic-Acid (PLGA) and tricalcium phosphate (TCP) (Chou, Dunn & Wu, 2005; Chou, Huang, Dunn, Miller & Wu, 2005; Lee et al., 2009; Yamashita et al., 1996). In addition to enhancing overall osteoinductivity of scaffolds, apatite coating also allowed bioactive molecules to be delivered in a sustained manner. Apatite coating of CH scaffolds was achieved by incubating scaffolds in simulated body fluids as described in our previous studies. The apatite coating created on scaffolds exhibited plate-like morphology (Fig. 2a). The addition of CS or AG into CH scaffolds accelerated apatite deposition and increased the percentage of areas covered with apatite. In comparison to pure CH scaffolds, apatite covered areas increased from approximately 50% to 90% and 60% for CH/CS and CH/AG, respectively. The enhanced apatite formation may be due to negatively charged CS (pKa of carboxyl group = 3.5 ~ 4 and pKa of sulfate group = 2 ~ 2.5) and AG (pKa of carboxyl group = 3.3) incorporated in CH scaffolds that attract Ca2+ ions, accelerating nucleation of apatite on CH/CS and CH/AG surfaces. Yamashita et al. reported that positively charged TCP surface first absorbed chloride ions in SBF solution, which was unfavorable for crystal growth of hydroxyapatite, while negatively charged TCP surface first absorbed Ca2+ ions, which accelerated the apatite crystal growth (Yamashita et al., 1996). Zhu et al. demonstrated that the electrostatic accumulation of Ca2+ ions in the surrounding region can induce apatite nucleation on the material surface. Therefore the apatite nucleation of negatively charged hydroxyl self-assembled monolayers (OH-SAM) was greater than that of positively charged amino SAM (NH2-SAM) (Zhu, Masuda & Koumoto, 2004). More uniform apatite coating was observed on CH/CS scaffolds compared to CH/AG scaffolds. It is possible that CS was better dispersed in a CH solution, inducing more homogeneous distribution of CS in CH scaffolds.
Figure 2.
(a) SEM images of apatite-coated CH (3%), CH/CS (3% CH with 3% CS), and CH/AG (3% CH with 3% AG) scaffolds: upper panel: low magnification. Scale Bar = 100 µm; lower panel: high magnification. Scale Bar = 30 µm (b) ATR-FTIR Spectra of CH (3%), CH/CS (3% CH with 3% CS), and CH/AG (3% CH with 3% AG) scaffolds before (CH, CH/CS, CH/AG) and after (Ap-CH, Ap-CH/CS, Ap-CH/AG) apatite coating. A plate-like apatite layer was formed on the surface of scaffolds after apatite coating.
The incorporation of CS or AG in CH scaffolds and formation of apatite layer on the scaffolds was confirmed by ATR-FTIR analysis (Fig. 2b). CH scaffolds showed their characteristic peaks at 1627 cm−1 (amide I, C=O), 1535 cm−1 (amide II, N-H), 1350 cm−1 (C-N), 1141 cm−1, 1064 cm−1, and 1010 cm−1 (C-O-C). The absorption peak of sulfate group (S=O) was appeared at 1218 cm−1 after CS was added to CH scaffolds, indicating incorporation of CS into CH scaffolds. The newly formed carboxylic groups (COO−) were observed at 1542 cm−1 and 1396 cm−1 in CH/AG scaffolds, suggesting incorporation of AG into CH scaffolds. After incubating in SBF, ATR-FTIR spectra exhibited the unique peaks of phosphate groups (PO43−) at 640 cm−1, 570 cm−1, 520 cm−1, and 450 cm−1 in all three scaffolds (CH, CH/CS, CH/AG), indicating apatite formation on the scaffolds (Chou, Dunn & Wu, 2005; Chou, Huang, Dunn, Miller & Wu, 2005).
3.3. In vitro Protein release
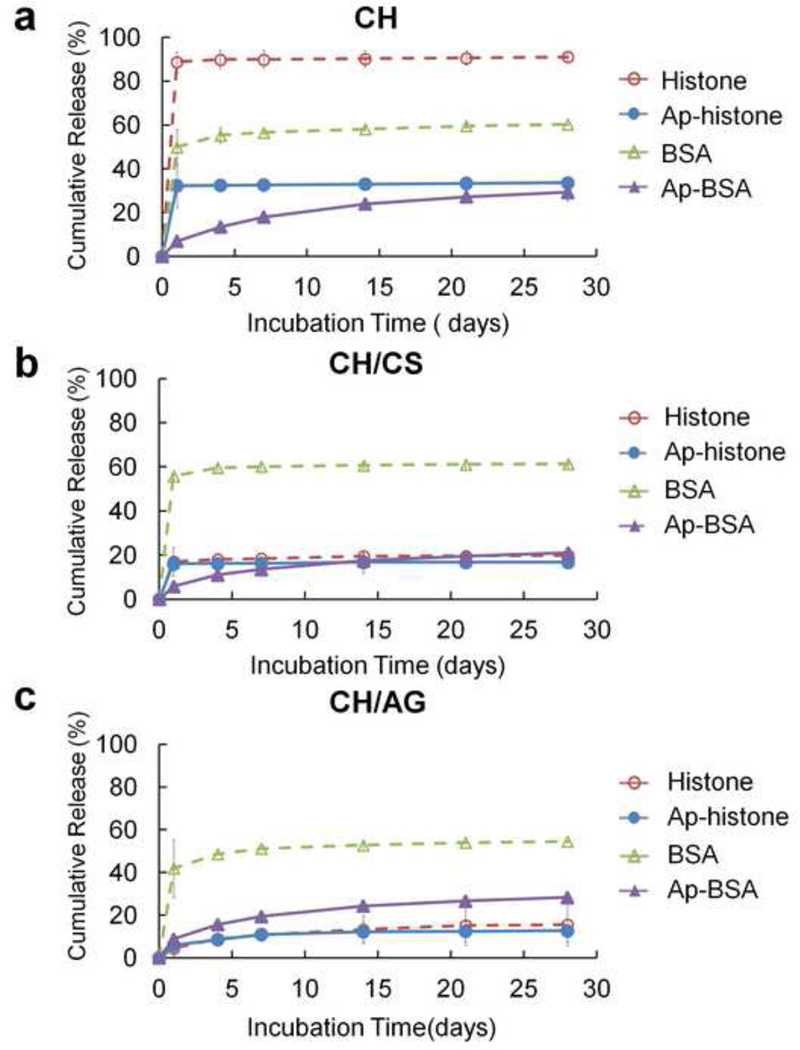
To investigate the protein delivery capacity of scaffolds, we examined release kinetics of proteins from scaffolds using two oppositely charged proteins, BSA and histone, by incubating them in PBS (Fig. 3). Approximately 90% of the initially loaded histone was released from CH scaffolds during the first day, followed by a plateau release up to 28 days. In contrast, the burst release of BSA was significantly reduced to ~50%, indicating that negatively charged BSA (pI = 4.9) has stronger electrostatic interaction with CH. The addition of CS or AG significantly reduced the burst release of histone to 17% and 5% on CH/CS and CH/AG scaffolds, respectively. This could be explained by a shielding of the positive charge on CH scaffolds, by the incorporated anionic CS or AG, which suppresses electrostatic repulsion of cationic histone on the scaffolds. The influence of electrostatic interaction on protein release has been demonstrated in other studies. Release of cationic drugs (chlorpheniramine maleate) was slower from an anionic AG gels compared with that of anionic drugs (sodium salicylate) (Davis, Hardy, Taylor, Stockwell, Whalley & Wilson, 1984). Other studies reported slower release of bFGF (pI = 8.6) from negatively charged gelatin than that from positively charged gelatin (Ikada & Tabata, 1998).
Figure 3.
In vitro release of proteins (BSA and Histone) from (a) CH, (b) CH/CS, and (c) CH/AG scaffolds before (BSA, Histone) and after (Ap-BSA, Ap-Histone) apatite coating (n = 3, mean ± SD).
The release kinetics of proteins from apatite-coated and uncoated scaffolds was investigated to determine if apatite coating could reduce initial burst release (Fig. 3). The initial burst release of histone and BSA from CH scaffolds, 88.9% and 49.9%, respectively, was significantly reduced after apatite coating (32.3% for histone and 7.0% for BSA). In addition, the burst release of BSA from CH/CS (55.8%) and CH/AG (41.9%) scaffolds significantly decreased to 5.9% and 8.9% from apatite coated CH/CS and CH/AG scaffolds, respectively. The observed sustained burst release of proteins from apatite-coated scaffolds can be attributed to high protein binding affinity for apatite and also the high surface area of their plate-like morphology providing more non-specific absorption sites for proteins.
This further confirms our previous report that biomimetic apatite coating can provide more sustained release of osteogenic proteins with greatly reduced burst release from CH/AG microparticles (Hu, Hou, Park & Lee, 2012; Lee et al., 2009). We also demonstrated that large dose of osteogenic proteins was successfully delivered over prolonged periods from apatite coated β-TCP particles (Hu, Hou, Park & Lee, 2012).
3.4. Cell Proliferation
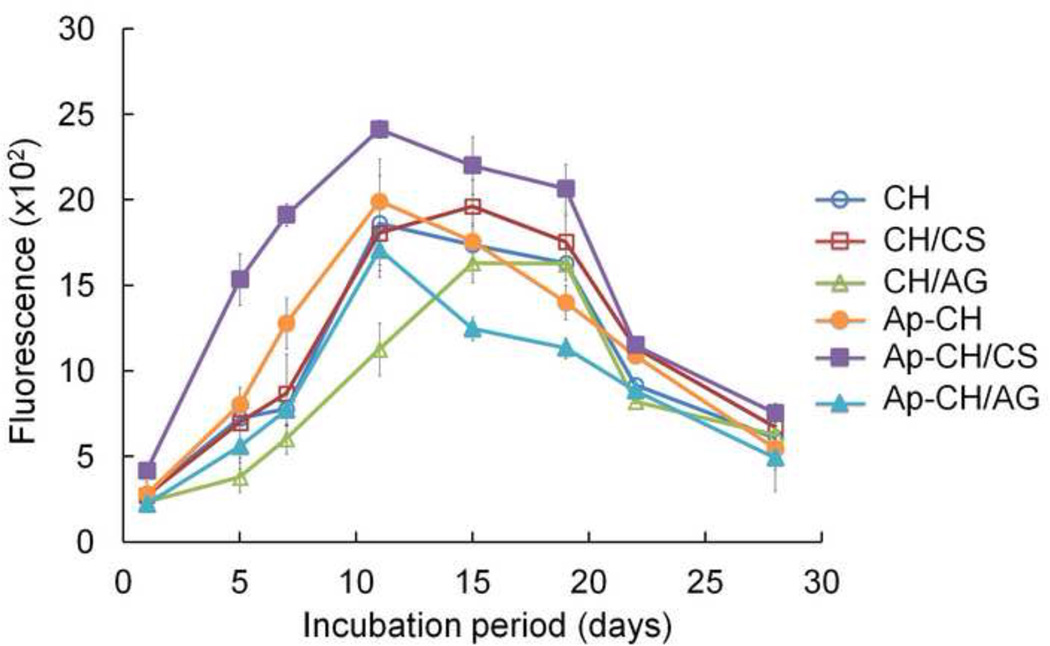
Viability and proliferation of BMSCs seeded on scaffolds were observed to investigate the feasibility of these scaffolds to support cell growth and osteoconduction (Fig. 4). BMSCs showed good viability of > 95% (data not shown) in all scaffolds over the 28 days culture period (Fig. 4a). Proliferation of seeded BMSCs on scaffolds was studied by use of alamarBlue assay (Fig. 4b). AlamarBlue fluorescence increased over culture time in all scaffolds, indicating proliferation of BMSCs. BMSCs grew most rapidly on apatite-coated CH/CS scaffolds, correlating well with Live/Dead staining observed in Fig. 4a, indicating that uniform apatite coating enhanced cell proliferation rate. AlamarBlue fluorescence decreased 14 days after culture in all scaffolds. Given that the level of cell viability appeared to remain very high (over 90%) over the 28 day period, as observed by use of Live/Dead staining and that alamarBlue assay detected changes in metabolic rates of cells is not directly related to cell viability and proliferation (Erikstein et al., 2010), the observed decrease in fluorescence signals does not necessarily signify a decrease in proliferation. It could be due to the increase of aged cells that decreased their metabolic activity as the cells grew tightly each other and reached 100% confluence. It is also possible that the aggregation of cells into clusters and secretion of ECM limited the supply of oxygen and nutrients to cells and subsequently reduced metabolic activity (Goegan, Johnson & Vincent, 1995).
Figure 4.
(a) Live-dead fluorescent staining of BMSCs seeded on non-coated (CH, CH/CS, CH/AG) and apatite-coated (Ap-CH, Ap-CH/CS, Ap-CH/AG) scaffolds after 1, 14, and 28 days in culture. Scale bar = 200 µm. (b) AlamarBlue assay showing proliferation of BMSCs seeded on non-coated (CH, CH/CS, CH/AG) and apatite-coated (Ap-CH, Ap-CH/CS, Ap-CH/AG) scaffolds.
3.5. Cellular Morphology and Extracellular Matrix secretion
The BMSCs morphology and extracellular matrix (ECM) secretion on scaffolds during culture period were investigated by SEM (Fig. 5). Morphological change was observed by Live/Dead staining. Most cells were spherical and existed as single cells at day 1 in all scaffolds. On non-apatite coated scaffolds, cells aggregated to form cell clusters after 14 days of culture, and these clusters increased in both number and size by day 28. In contrast, the loaded cells of apatite coated cells demonstrated varying degrees of cellular spreading. Cells on the apatite-coated CH/CS scaffolds demonstrated complete spreading by day 14. By day 28, the spread cells and secreted ECM covered the entire porous structure of the apatite-coated CH/CS scaffold. Cells loaded on apatite-coated CH and apatite-coated CH/AG scaffolds demonstrated partial spreading at day 14. By day 28, though there was a marked increase in the number of spread cells, the majority of these cells was spherical and remained as clusters. The lack of morphological change in CH scaffolds may be attributed to the decreased amount of apatite coating in comparison to CH/CS scaffolds. The lack of morphological change in CH/AG is likely due to a low level of apatite coating uniformity as observed in section 3.2. Furthermore, AG has been reported to inhibit spreading of cells and keep spherical morphology. Cells formed clusters and remained in their isolated forms on CH/AG scaffolds (Li & Zhang, 2005). Other studies reported that rat osteosarcoma UMR106 cells cultured on AG/hydroxyapatite composite scaffolds exhibited spherical-shaped aggregates and formed clusters over time (Lin & Yeh, 2004).
Figure 5.
SEM images of BMSCs cultured on non-coated (CH, CH/CS, CH/AG) and apatite-coated (Ap-CH, Ap-CH/CS, Ap-CH/AG) scaffolds for 28 days. Scale Bar = 100 µm. Solid arrows indicate cell clusters and open arrows indicate spread cells with secreted extracellular matrix (ECM).
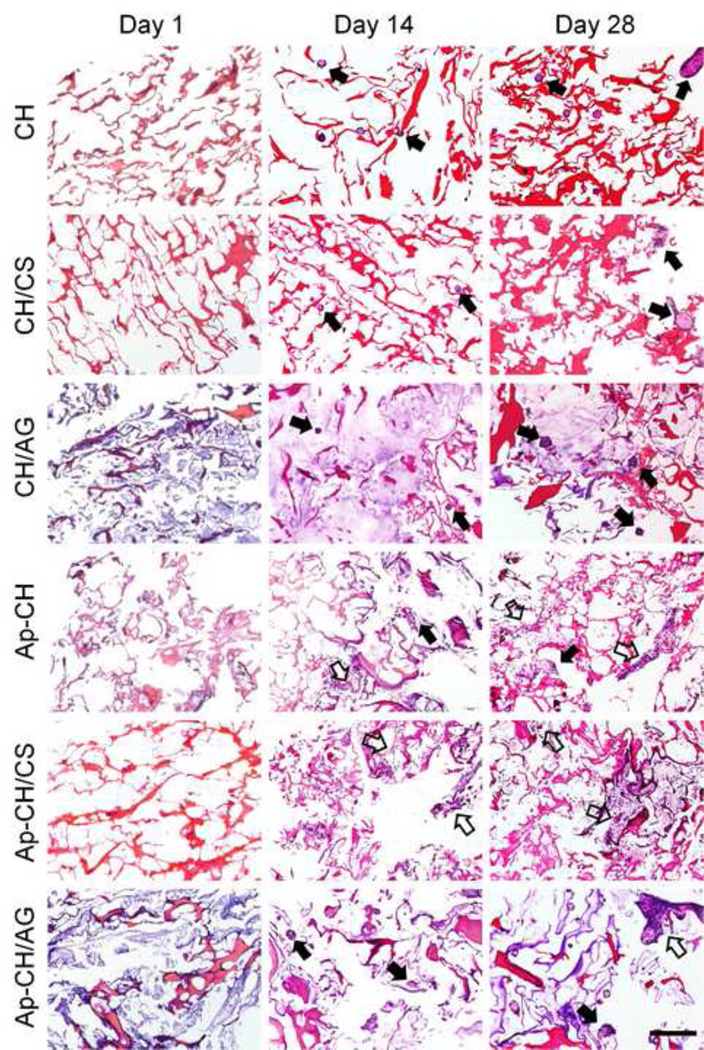
The morphology and distribution of cells cultured on scaffolds were further examined by H&E staining (Fig. 6). At day 1, most of seeded cells were attached as individual cells on all scaffolds and were homogeneously distributed throughout the scaffolds. Cell clusters formed over the culture period on non-apatite coated scaffolds and more clusters were observed at day 28. In contrast, cells spread on apatite-coated scaffolds and significant spreading was observed on apatite-coated CH/CS scaffolds, which is in good agreement with those observed in Live/Dead staining (Fig. 4a) and SEM (Fig. 5).
Figure 6.
Hematoxylin-eosin staining of BMSCs cultured on non-coated (CH, CH/CS, CH/AG) and apatite-coated (Ap-CH, Ap-CH/CS, Ap-CH/AG) scaffolds for 1, 14, and 28 days. Scale Bar = 200 µm. Solid arrows indicate cell clusters and open arrows indicate spread cells.
3.6. Cell Differentiation
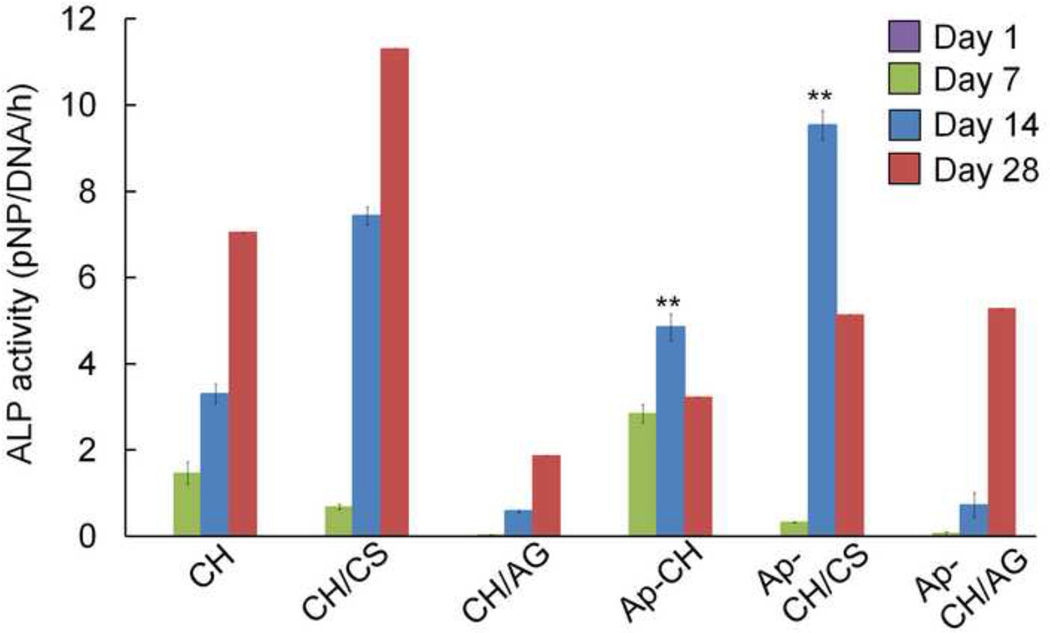
Osteogenic differentiation of BMSCs cultured on scaffolds was determined by quantifying ALP activity (Fig. 7) and collagen deposition (Fig. 8). ALP activity of cells seeded on scaffolds increased over the 28 days culture period. Although the cells cultured on apatite coated scaffolds exhibited significantly higher ALP activity compared with non-apatite coated scaffolds by day 14, ALP expression appears to be suppressed at day 28. This is expected since ALP is generally considered as a marker that is expressed in early osteogenic development. The ALP expression was found to peak at the beginning of osteoblastic differentiation and started to decline as differentiation progresses (Mygind et al., 2007; Quarles, Yohay, Lever, Caton & Wenstrup, 1992; Whited, Whitney, Hofmann, Xu & Rylander, 2011). A previous study using MC3T3 preosteoblasts showed that apatite environments decreased the expression of ALP but increased late osteogenic markers such as osteocalcin (Chou, Dunn & Wu, 2005).
Figure 7.
Alkaline Ph osphatase (ALP) activity of BMSCs cultured on non-coated (CH, CH/CS, CH/AG) and apatite-coated (Ap-CH, Ap-CH/CS, Ap-CH/AG) scaffolds for 28 days. On day 14, Ap-CH and Ap-CH/CS scaffolds showed significantly higher (**p<0.01) ALP activity compared to its corresponding non-coating scaffolds.
Figure 8.
Picrosirius Red staining of BMSCs cultured on (a) non-coated (CH, CH/CS, CH/AG) and (b) apatite coated (Ap-CH, Ap-CH/CS, Ap-CH/AG) scaffolds for 1 and 28 days. Scale Bar = 100 µm. Collagen deposition was observed in cell clusters (solid arrows) and spread cells (open arrows).
The collagen expression is a predictive marker for new bone formation and the extent of collagen deposition was confirmed by picrosirius red staining (Chai et al., 2012; De Bari et al., 2008). Collagen deposition by BMSCs cultured on non-apatite coated scaffolds (Fig. 8a) was observed only in cell clusters. Cells cultured on apatite-coated CH or CH/CS scaffolds (Fig. 8b), however, showed strong collagen deposition by day 28. Cells cultured on apatite-coated CH/CS scaffolds deposited collagen throughout the entireties of the construct by day 28.
4. Conclusion
In this study, we investigated the ability of CH scaffolds to deliver model proteins and support cell growth for bone regeneration. The incorporation of CS or AG in CH scaffolds significantly increased the mechanical strength of scaffolds and enhanced the formation of an apatite layer on scaffolds. The apatite coating created on scaffolds provided a sustained release of loaded proteins with reduced initial burst. Furthermore, the apatite coating enhanced spreading, proliferation, and osteogenic differentiation of BMSCs. Among experimental groups, CH/CS composite scaffolds were the most promising. These results indicate that the addition of CS to CH scaffolds and the modification of their surfaces with apatite might be a useful system for bone tissue engineering.
Highlights.
Incorporation of CS or AG in CH scaffold increases the mechanical strength.
Incorporation of CS or AG in CH scaffold enhanced apatite formation on the surface.
Apatite coating provides a sustained protein release with reduced initial burst.
Apatite coating enhances spreading, proliferation, and osteogenesis of BMSCs.
The apatite-coated CH/CS scaffold has a potential as a promising osteogenic system.
Acknowledgements
This work was supported by the National Institutes of Health grants R01 AR060213 and R21 DE021819, the International Association for Dental Research, and the Academy of Osseointegration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aday S, Gumusderelioglu M. Bone-Like Apatite-Coated Chitosan Scaffolds: Characterization and Osteoblastic Activity. Polymer Composites. 2010;31(8):1418–1426. [Google Scholar]
- Akman AC, Tigli RS, Gumusderelioglu M, Nohutcu RM. bFGF-loaded HA-chitosan: a promising scaffold for periodontal tissue engineering. J Biomed Mater Res A. 2010;92(3):953–962. doi: 10.1002/jbm.a.32428. [DOI] [PubMed] [Google Scholar]
- Boccaccio A, Lamberti L, Pappalettere C, Carano A, Cozzani M. Mechanical behavior of an osteotomized mandible with distraction orthodontic devices. J Biomech. 2006;39(15):2907–2918. doi: 10.1016/j.jbiomech.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Boccaccio A, Lamberti L, Pappalettere C, Cozzani M, Siciliani G. Comparison of different orthodontic devices for mandibular symphyseal distraction osteogenesis: a finite element study. Am J Orthod Dentofacial Orthop. 2008;134(2):260–269. doi: 10.1016/j.ajodo.2006.09.066. [DOI] [PubMed] [Google Scholar]
- Burns JS, Rasmussen PL, Larsen KH, Schroder HD, Kassem M. Parameters in three-dimensional osteospheroids of telomerized human mesenchymal (stromal) stem cells grown on osteoconductive scaffolds that predict in vivo bone-forming potential. Tissue Eng Part A. 2010;16(7):2331–2342. doi: 10.1089/ten.TEA.2009.0735. [DOI] [PubMed] [Google Scholar]
- Chai YC, Roberts SJ, Desmet E, Kerckhofs G, van Gastel N, Geris L, Carmeliet G, Schrooten J, Luyten FP. Mechanisms of ectopic bone formation by human osteoprogenitor cells on CaP biomaterial carriers. Biomaterials. 2012;33(11):3127–3142. doi: 10.1016/j.biomaterials.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Chou YF, Chiou WA, Xu Y, Dunn JC, Wu BM. The effect of pH on the structural evolution of accelerated biomimetic apatite. Biomaterials. 2004;25(22):5323–5331. doi: 10.1016/j.biomaterials.2003.12.037. [DOI] [PubMed] [Google Scholar]
- Chou YF, Dunn JC, Wu BM. In vitro response of MC3T3-E1 pre-osteoblasts within three-dimensional apatite-coated PLGA scaffolds. J Biomed Mater Res B Appl Biomater. 2005;75(1):81–90. doi: 10.1002/jbm.b.30261. [DOI] [PubMed] [Google Scholar]
- Chou YF, Huang W, Dunn JC, Miller TA, Wu BM. The effect of biomimetic apatite structure on osteoblast viability, proliferation, and gene expression. Biomaterials. 2005;26(3):285–295. doi: 10.1016/j.biomaterials.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Davis SS, Hardy JG, Taylor MJ, Stockwell A, Whalley DR, Wilson CG. The in-vivo evaluation of an osmotic device (Osmet) using gamma scintigraphy. J Pharm Pharmacol. 1984;36(11):740–742. doi: 10.1111/j.2042-7158.1984.tb04862.x. [DOI] [PubMed] [Google Scholar]
- De Bari C, Dell'Accio F, Karystinou A, Guillot PV, Fisk NM, Jones EA, McGonagle D, Khan IM, Archer CW, Mitsiadis TA, Donaldson AN, Luyten FP, Pitzalis C. A biomarker-based mathematical model to predict bone-forming potency of human synovial and periosteal mesenchymal stem cells. Arthritis Rheum. 2008;58(1):240–250. doi: 10.1002/art.23143. [DOI] [PubMed] [Google Scholar]
- Erikstein BS, Hagland HR, Nikolaisen J, Kulawiec M, Singh KK, Gjertsen BT, Tronstad KJ. Cellular stress induced by resazurin leads to autophagy and cell death via production of reactive oxygen species and mitochondrial impairment. Journal of Cellular Biochemistry. 2010;111(3):574–584. doi: 10.1002/jcb.22741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galois L, Mainard D. Bone ingrowth into two porous ceramics with different pore sizes: an experimental study. Acta Orthop Belg. 2004;70(6):598–603. [PubMed] [Google Scholar]
- Goegan P, Johnson G, Vincent R. Effects of serum protein and colloid on the alamarBlue assay in cell cultures. Toxicol In Vitro. 1995;9(3):257–266. doi: 10.1016/0887-2333(95)00004-r. [DOI] [PubMed] [Google Scholar]
- Guillemin G, Meunier A, Dallant P, Christel P, Pouliquen JC, Sedel L. Comparison of coral resorption and bone apposition with two natural corals of different porosities. J Biomed Mater Res. 1989;23(7):765–779. doi: 10.1002/jbm.820230708. [DOI] [PubMed] [Google Scholar]
- Hu J, Hou Y, Park H, Lee M. Beta-tricalcium phosphate particles as a controlled release carrier of osteogenic proteins for bone tissue engineering. J Biomed Mater Res A. 2012;100(7):1680–1686. doi: 10.1002/jbm.a.34115. [DOI] [PubMed] [Google Scholar]
- Ikada Y, Tabata Y. Protein release from gelatin matrices. Adv Drug Deliv Rev. 1998;31(3):287–301. doi: 10.1016/s0169-409x(97)00125-7. [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11(4):447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Kanungo BP, Silva E, Van Vliet K, Gibson LJ. Characterization of mineralized collagen-glycosaminoglycan scaffolds for bone regeneration. Acta Biomater. 2008;4(3):490–503. doi: 10.1016/j.actbio.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kim SS, Sun Park M, Jeon O, Yong Choi C, Kim BS. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(8):1399–1409. doi: 10.1016/j.biomaterials.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Knabe C, Koch C, Rack A, Stiller M. Effect of beta-tricalcium phosphate particles with varying porosity on osteogenesis after sinus floor augmentation in humans. Biomaterials. 2008;29(14):2249–2258. doi: 10.1016/j.biomaterials.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Kong L, Gao Y, Cao W, Gong Y, Zhao N, Zhang X. Preparation and characterization of nano-hydroxyapatite/chitosan composite scaffolds. J Biomed Mater Res A. 2005;75(2):275–282. doi: 10.1002/jbm.a.30414. [DOI] [PubMed] [Google Scholar]
- Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104(12):6017–6084. doi: 10.1021/cr030441b. [DOI] [PubMed] [Google Scholar]
- Lee KY, Ha WS, Park WH. Blood compatibility and biodegradability of partially N-acylated chitosan derivatives. Biomaterials. 1995;16(16):1211–1216. doi: 10.1016/0142-9612(95)98126-y. [DOI] [PubMed] [Google Scholar]
- Lee M, Li W, Siu RK, Whang J, Zhang X, Soo C, Ting K, Wu BM. Biomimetic apatite-coated alginate/chitosan microparticles as osteogenic protein carriers. Biomaterials. 2009;30(30):6094–6101. doi: 10.1016/j.biomaterials.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Ramay HR, Hauch KD, Xiao D, Zhang M. Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials. 2005;26(18):3919–3928. doi: 10.1016/j.biomaterials.2004.09.062. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang M. Chitosan-alginate as scaffolding material for cartilage tissue engineering. J Biomed Mater Res A. 2005;75(2):485–493. doi: 10.1002/jbm.a.30449. [DOI] [PubMed] [Google Scholar]
- Lin HR, Yeh YJ. Porous alginate/hydroxyapatite composite scaffolds for bone tissue engineering: preparation, characterization, and in vitro studies. J Biomed Mater Res B Appl Biomater. 2004;71(1):52–65. doi: 10.1002/jbm.b.30065. [DOI] [PubMed] [Google Scholar]
- Manjubala I, Ponomarev I, Wilke I, Jandt KD. Growth of osteoblast-like cells on biomimetic apatite-coated chitosan scaffolds. J Biomed Mater Res B Appl Biomater. 2008;84(1):7–16. doi: 10.1002/jbm.b.30838. [DOI] [PubMed] [Google Scholar]
- Murata Y, Miyamoto E, Kawashima S. Additive effect of chondroitin sulfate and chitosan on drug release from calcium-induced alginate gel beads. Journal of Controlled Release. 1996;38(2–3):101–108. [Google Scholar]
- Mygind T, Stiehler M, Baatrup A, Li H, Zou X, Flyvbjerg A, Kassem M, Bunger C. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials. 2007;28(6):1036–1047. doi: 10.1016/j.biomaterials.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Nazarov R, Jin HJ, Kaplan DL. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules. 2004;5(3):718–726. doi: 10.1021/bm034327e. [DOI] [PubMed] [Google Scholar]
- Park YJ, Lee YM, Lee JY, Seol YJ, Chung CP, Lee SJ. Controlled release of platelet-derived growth factor-BB from chondroitin sulfate-chitosan sponge for guided bone regeneration. J Control Release. 2000;67(2–3):385–394. doi: 10.1016/s0168-3659(00)00232-7. [DOI] [PubMed] [Google Scholar]
- Quarles LD, Yohay DA, Lever LW, Caton R, Wenstrup RJ. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. Journal of Bone and Mineral Research. 1992;7(6):683–692. doi: 10.1002/jbmr.5650070613. [DOI] [PubMed] [Google Scholar]
- Rohanizadeh R, Al-Sadeq M, Legeros RZ. Preparation of different forms of titanium oxide on titanium surface: effects on apatite deposition. J Biomed Mater Res A. 2004;71(2):343–352. doi: 10.1002/jbm.a.30171. [DOI] [PubMed] [Google Scholar]
- Seol YJ, Lee JY, Park YJ, Lee YM, Young K, Rhyu IC, Lee SJ, Han SB, Chung CP. Chitosan sponges as tissue engineering scaffolds for bone formation. Biotechnol Lett. 2004;26(13):1037–1041. doi: 10.1023/B:BILE.0000032962.79531.fd. [DOI] [PubMed] [Google Scholar]
- Shanmugasundaram N, Ravichandran P, Reddy PN, Ramamurty N, Pal S, Rao KP. Collagen-chitosan polymeric scaffolds for the in vitro culture of human epidermoid carcinoma cells. Biomaterials. 2001;22(14):1943–1951. doi: 10.1016/s0142-9612(00)00220-9. [DOI] [PubMed] [Google Scholar]
- Shiquian S, Dongjie F, Fei X, Tian L, Feng H, Jiawei W. The design and features of apatite-coated chitosan microspheres as injectable scaffold for bone tissue engineering. Biomed Mater. 2013;8(2):025007. doi: 10.1088/1748-6041/8/2/025007. [DOI] [PubMed] [Google Scholar]
- Sui W, Huang L, Wang J, Bo Q. Preparation and properties of chitosan chondroitin sulfate complex microcapsules. Colloids Surf B Biointerfaces. 2008;65(1):69–73. doi: 10.1016/j.colsurfb.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Tan W, Krishnaraj R, Desai TA. Evaluation of nanostructured composite collagen--chitosan matrices for tissue engineering. Tissue Eng. 2001;7(2):203–210. doi: 10.1089/107632701300062831. [DOI] [PubMed] [Google Scholar]
- Thein-Han WW, Misra RD. Biomimetic chitosan-nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater. 2009;5(4):1182–1197. doi: 10.1016/j.actbio.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Tomihata K, Ikada Y. In vitro and in vivo degradation of films of chitin and its deacetylated derivatives. Biomaterials. 1997;18(7):567–575. doi: 10.1016/s0142-9612(96)00167-6. [DOI] [PubMed] [Google Scholar]
- Van Cleynenbreugel T, Schrooten J, Van Oosterwyck H, Vander Sloten J. Micro-CT-based screening of biomechanical and structural properties of bone tissue engineering scaffolds. Med Biol Eng Comput. 2006;44(7):517–525. doi: 10.1007/s11517-006-0071-z. [DOI] [PubMed] [Google Scholar]
- Whited BM, Whitney JR, Hofmann MC, Xu Y, Rylander MN. Pre-osteoblast infiltration and differentiation in highly porous apatite-coated PLLA electrospun scaffolds. Biomaterials. 2011;32(9):2294–2304. doi: 10.1016/j.biomaterials.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Oikawa N, Umegaki T. Acceleration and deceleration of bone-like crystal growth on ceramic hydroxyapatite by electric poling. Chem Mater. 1996;8(12):2697–2700. [Google Scholar]
- Zeltinger J, Landeen LK, Alexander HG, Kidd ID, Sibanda B. Development and characterization of tissue-engineered aortic valves. Tissue Eng. 2001;7(1):9–22. doi: 10.1089/107632701300003250. [DOI] [PubMed] [Google Scholar]
- Zhang R, Ma PX. Poly(alpha-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering I Preparation and morphology. J Biomed Mater Res. 1999a;44(4):446–455. doi: 10.1002/(sici)1097-4636(19990315)44:4<446::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Zhang R, Ma PX. Porous poly(L-lactic acid)/apatite composites created by biomimetic process. J Biomed Mater Res. 1999b;45(4):285–293. doi: 10.1002/(sici)1097-4636(19990615)45:4<285::aid-jbm2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Zhao F, Yin Y, Lu WW, Leong JC, Zhang W, Zhang J, Zhang M, Yao K. Preparation and histological evaluation of biomimetic three-dimensional hydroxyapatite/chitosan-gelatin network composite scaffolds. Biomaterials. 2002;23(15):3227–3234. doi: 10.1016/s0142-9612(02)00077-7. [DOI] [PubMed] [Google Scholar]
- Zhu P, Masuda Y, Koumoto K. The effect of surface charge on hydroxyapatite nucleation. Biomaterials. 2004;25(17):3915–3921. doi: 10.1016/j.biomaterials.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Zo SM, Singh D, Kumar A, Cho YW, Oh TH, Han SH. Chitosan-hydroxyapatite macroporous matirx for bone tissue engineering. Curr Sci. 2012;103(12):1438–1446. [Google Scholar]