Abstract
Metabolomics, based on ultraperformance liquid chromatography coupled with electrospray ionization quadrupole mass spectrometry, was used to explore metabolic signatures of tumor growth in mice. Urine samples were collected from control mice and mice injected with squamous cell carcinoma (SCCVII) tumor cells. When tumors reached ∼2 cm, all mice were killed and blood and liver samples collected. The urine metabolites hexanoylglycine, nicotinamide 1-oxide, and 11β,20α-dihydroxy-3-oxopregn-4-en-21-oic acid were elevated in tumor-bearing mice, as was asymmetric dimethylarginine, a biomarker for oxidative stress. Interestingly, SCCVII tumor growth resulted in hepatomegaly, reduced albumin/globulin ratios, and elevated serum triglycerides, suggesting liver dysfunction. Alterations in liver metabolites between SCCVII-tumor-bearing and control mice confirmed the presence of liver injury. Hepatic mRNA analysis indicated that inflammatory cytokines, tumor necrosis factor α, and transforming growth factor β were enhanced in SCCVII-tumor-bearing mice, and the expression of cytochromes P450 was decreased in tumor-bearing mice. Further, genes involved in fatty acid oxidation were decreased, suggesting impaired fatty acid oxidation in SCCVII-tumor-bearing mice. Additionally, activated phospholipid metabolism and a disrupted tricarboxylic acid cycle were observed in SCCVII-tumor-bearing mice. These data suggest that tumor growth imposes a global inflammatory response that results in liver dysfunction and underscore the use of metabolomics to temporally examine these changes and potentially use metabolite changes to monitor tumor treatment response.
The combination of nuclear magnetic resonance and liquid and gas chromatography coupled to mass spectrometry has enabled the global analysis of metabolites in small volumes of biofluids and tissues. Using this technology, the emerging field of metabolomics seeks to elucidate how pathological conditions, genetic modifications, and xenobiotic exposure can lead to alterations in biochemical pathways of an organism as measured via metabolite profile analysis (1–4). The applications of this technology extend beyond those mentioned above (5) and further hold promise for identifying biomarkers that might provide early diagnostic information characterizing a variety of disease processes. Such biomarkers might also be most useful for following the course of therapy and perhaps predicting treatment outcomes.
Cancer is a disease amenable to metabolite interrogation, as many human tumors are difficult to detect at an early stage of development, when they are most vulnerable to treatment. Moreover, many human tumors are unresponsive to a wide variety of therapies, and metabolite profile anomalies specific to cancer might provide targets or pathways for the development of new treatment strategies. A number of different types of human cancers have been subjected to metabolomic analysis (5), and specific metabolites were identified that may provide diagnostic information. Examples include urine sarcosine in prostate cancer (6); urine hydroxyindoleacetate and homovanillate in breast cancer (7); urine hydroxynicotic acid and valine in head and neck cancer (8); and serum methyladenosine, histidine, and inosine in hepatocellular carcinoma (9, 10). Global perturbation of diverse metabolic pathways and the presence of gut microflora metabolites have been reported from urine samples of human colorectal cancer patients (11). Pre-clinical studies using human tumor xenografts have also identified potentially important candidate metabolites for several tumor types (12–15).
In most cancer-related pre-clinical and human metabolomic studies to date, bio-fluids or tissue samples were collected at a single time point. These studies are most informative; however, in order to explore whether cancer-specific metabolites emerge prior to macroscopic tumor development, temporal studies are required. In the present study, rodent squamous cell carcinoma (SCCVII)1 was injected into mice and urine samples were collected periodically prior to and during macroscopic tumor formation. A number of urine metabolites were found to be altered in tumor-bearing mice; however, changes were observed only after a palpable tumor mass developed. Based on metabolite profiles and hepatic mRNA analysis, tumor growth imposed oxidative-stress-mediated inflammation on the host, which resulted in compromised liver function with respect to fatty acid oxidation, tricarboxylic acid (TCA) cycle compromise, and reduced expression of cytochromes P450 (P450).
EXPERIMENTAL PROCEDURES
Chemicals and Reagents
Nicotinamide, nicotinamide 1-oxide, citrate, asymmetric dimethylarginine (ADMA), hexanoylglycine, phosphocholine, and adenosine monophosphate (AMP) were purchased from Sigma (St. Louis, MO). 1-oleoyl-glycero-3-phosphocholine (LPC 18:1) was obtained from Avanti Polar Lipids, Inc. (Alabaster, AL). 11β, 20α-dihydroxy-3-oxopregn-4-en-21-oic acid (DHOPA) was synthesized by Anbilaunch Consulting (San Meteo, CA). All solvents and organic reagents were of the highest grade available.
Animal Studies
Female C3H/Hen mice (National Cancer Institute Animal Production Area, Frederick, MD) were used for this study. Mice were between 7 and 9 weeks of age and weighed between 20 and 30 g at the time of experimentation. SCCVII tumor cells were derived from a spontaneous squamous cell cancer (obtained from Dr. Theodore Phillips, UCSF, San Francisco, CA). For tumor growth studies, 2 × 105 viable SCCVII cells suspended in 100 ml sterile PBS were injected into the subcutaneous space of the right hind leg of C3H mice as previously reported (16). Mice receiving no injection were used as controls. Tumor size and body weight were measured three times per week. 24-h urine was collected from control and SCCVII-injected mice using individual metabolic cages (Jencons, Leighton Buzzard, UK) at day 0, 3, 6, 10, and 13 following the injection of SCCVII cells. Serum, liver, and tumor were harvested when all the mice were sacrificed at day 13. Samples were stored at −80 °C until analysis. Animal studies were performed under a protocol approved by the National Cancer Institute Animal Care and Use Committee and were in compliance with the National Research Council Guide for the Care and Use of Laboratory Animal Resources (1996).
Ultraperformance Liquid Chromatography Coupled with Electrospray Ionization Quadrupole Mass Spectrometry
Urine samples were prepared by adding 20 μl of urine to 180 μl 50% aqueous acetonitrile (50:50 water:acetonitrile). Serum samples were prepared by adding 10 μl of serum to 190 μl 67% aqueous acetonitrile (1:2 water:acetonitrile). Liver samples (100 mg) were minced in 1.0 ml 50% aqueous acetonitrile and shaken for 15 min at room temperature. Samples were vortexed for 5 min and centrifuged at 14,000 rpm for 20 min at 4 °C to remove particulates and precipitate protein. The supernatants were transferred to an autosampler vial for analysis. A 5-μl aliquot of supernatant was chromatographed via ultra-performance liquid chromatography (Waters Corp., Milford, MA) using a 2.1 × 50-mm Waters BEH C18 1.7-μm column and introduced via electrospray into a quadrupole time-of-flight mass spectrometer (UPLC-ESI-QTOFMS). The gradient mobile phase consisted of 0.1% formic acid solution (A) and acetonitrile containing 0.1% formic acid solution (B). The gradient was maintained at 100% A for 0.5 min, increased to 100% B over the next 7.5 min, and returned to 100% A in the last 2 min. Data were collected in positive mode and negative mode, which was operated in full-scan mode from 100 to 1000 m/z. Nitrogen was used as both cone gas (50 l/h) and desolvation gas (600 l/h). The source temperature and desolvation temperature were set at 120 °C and 350 °C, respectively. The capillary voltage and cone voltage were 3000 and 20 V, respectively. Chlorpropamide (5 μm) was added in each sample as the internal standard.
Data Processing and Multivariate Data Analysis
Centroided and integrated chromatographic data were processed using MarkerLynx software (Waters) to generate a data matrix consisting of peak areas corresponding to a unique m/z and retention time without normalization. The multivariate data matrix was exported into SIMCA-p + 12.0 (Umetrics, Kinnelon, NJ) for principal component analysis (PCA) and partial least-squares discriminant analysis (PLS-DA). The ions with a correlation of 0.8 or higher to the model contributed significantly to the separation between wild-type and SCCVII-tumor-bearing mice, and their identity was further investigated through searches of metabolomics databases and comparison with authentic compounds.
Quantification of Metabolites in Urine, Serum, and Liver Homogenate
Quantitation of the endogenous metabolites was performed using an ACQUITY UPLC system coupled to a Xevo triple quadrupole tandem mass spectrometer (Waters Corp.). Chromatography was as described for UPLC-ESI-QTOFMS analysis. Calibration curves (0.2 to 25 μm) were generated for each metabolite. The following multiple reaction monitoring transitions were monitored: nicotinamide 1-oxide (139 → 106; ESI+), ADMA (203 → 70; ESI+), DHOPA (363 → 121; ESI+), citrate (193 → 129; ESI+), hexanoylglycine (174 → 76; ESI+), LPC 18:1 (522 → 104; ESI+), LPC 16:1 (494 → 104; ESI+), creatinine (114 → 86; ESI+), and chlorpropamide (277 → 111; ESI+). Chlorpropamide (0.5 μm) was used as the internal standard. All values are expressed as μmol/mmol creatinine.
Gene Expression Analysis
Total RNA was prepared from frozen liver using TRIzol reagent (Invitrogen, Carlsbad, CA). Complementary DNA was synthesized from 1 μg RNA using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). Quantitative real-time PCR (qPCR) was carried out using SYBR green PCR master mix in an ABI Prism 7900HT sequence detection system (Applied Biosystems, Foster City, CA). qPCR primer sequences are shown in supplemental Table S1. Messenger RNA levels were normalized to those of β-actin mRNA and expressed as the fold change relative to those of wild-type mice.
Serum Chemistry
Serum alkaline phosphatase, albumin, globulin, total protein, and other physiological serum parameters were measured using the VetScan VS2 Comprehensive Diagnostic Profile (Abaxis, Inc., Union City, CA).
Data Analysis
Statistical analysis was performed using GraphPad Prism software (San Diego, CA). Two-tailed Student's t test was used to compare the levels of metabolites and changes in gene expression between wild-type mice and SCCVII-tumor-bearing mice. Experimental values are presented as the mean ± S.E. p values of less than 0.05 are considered significant.
RESULTS
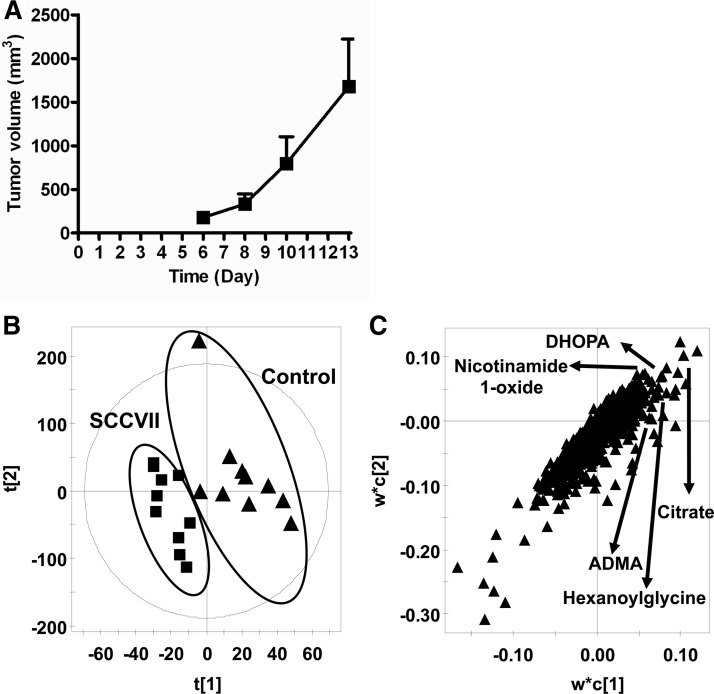
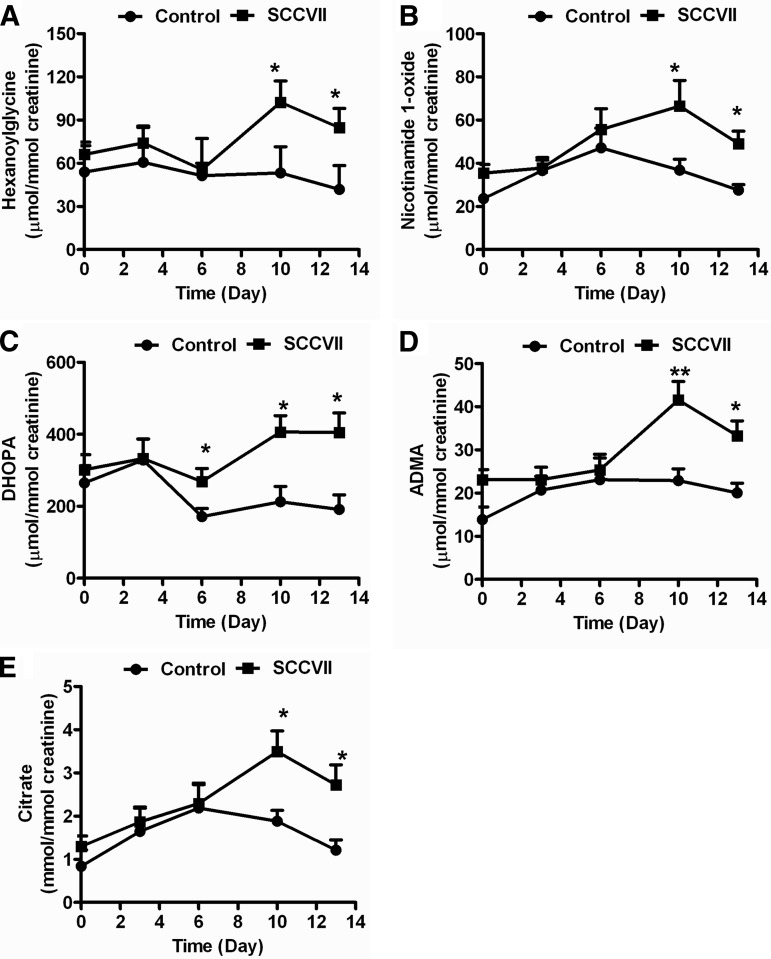
SCCVII tumor growth was rapid, with measurable tumor formation by day 6 (Fig. 1A). On day 13, tumor volumes approached 2000 mm3, at which time both control and SCCVII-tumor-bearing mice were killed. Urine samples were collected on days 0, 3, 6, 10, and 13 and analyzed using UPLC-ESI-QTOFMS operating in positive and negative ionization mode. After the chromatographic data were processed using MarkerLynx software, a data table was generated consisting of peak areas corresponding to unique m/z and retention times without normalization. The peak areas of all ions were normalized according to the peak area of the internal standard, chlorpropamide, before PCA and PLS-DA analysis. Globally, the urine metabolite profiles of control and SCCVII-tumor-bearing mice did not differ significantly up to day 6, as shown in supplemental Fig. S1C. Thus, no tumor-specific metabolites were discernible prior to palpable tumor mass formation (day 6). However, the PCA and PLS-DA models distinguished SCCVII-tumor-bearing mice from control mice at day 13 (Fig. 1B and supplemental Fig. S1E). The ions that contributed to the separation of SCCVII-tumor-bearing and control mice were those that deviated from the ion cloud in the loading scatter plot (Fig. 1C). The candidate biomarkers were identified by searching the Madison-Qingdao Metabolomics Consortium Database and METLIN databases. The identity of ions was further confirmed through the comparison of retention times and mass fragmentation patterns to authentic standards (supplemental Figs. S2A–S2E). The five top ions in urine, m/z 174.11123+, 215.0187+, 139.0513+, 363.2168+, and 203.1502+, were identified as hexanoylglycine, nicotinamide 1-oxide, DHOPA, ADMA, and citrate, respectively. The levels of these metabolities were analyzed at the various time points (Fig. 2 and Table I). Hexanoylglycine was increased 1.9- and 2.1-fold in SCCVII mice at days 10 and 13 relative to control mice (p < 0.05) (Fig. 2A). Nicotinamide 1-oxide concentrations were increased from 27.5 μmol/mmol creatinine in control mice to 49.1 μmol/mmol creatinine in SCCVII-tumor-bearing mice at day 13 (p < 0.05) (Fig. 2B), and DHOPA was increased from 191 μmol/mmol creatinine in control mice to 405 μmol/mmol creatinine in SCCVII-tumor-bearing mice (p < 0.05) (Fig. 2C). ADMA, a metabolite involved in oxidative stress, had an average concentration of 33.2 μmol/mmol creatinine in SCCVII-tumor-bearing mice at day 13, compared with 20.0 μmol/mmol creatinine in control mice (Fig. 2D). Citrate was increased in SCCVII-tumor-bearing mice from day 6 to day 13, reaching levels of 2.7 mmol/mmol creatinine at day 13, compared with 1.2 mmol/mmol creatinine in control mice (p < 0.05) (Fig. 2E). The five metabolites shown in Fig. 2—hexanoylglycine, DHOPA, nicotinamide 1-oxide, ADMA, and citrate—were increased in SCCVII-tumor-bearing mouse urine and are related to fatty acid oxidation, the TCA cycle, and oxidative stress, suggesting possible liver dysfunction.
Fig. 1.
SCCVII tumor growth and metabolomics analysis of urinary metabolites. A, growth curve of SCCVII tumors from day 6 to day 13 (n = 10 mice/group). B, PLS-DA model of urinary metabolites between SCCVII (n = 10, solid squares) and control (n = 10, solid triangles). C, loadings scatter plot of PLS-DA.
Fig. 2.
Quantitation of urinary hexanoylglycine, nicotinamide 1-oxide, DHOPA, ADMA, and citrate. Urinary levels of hexanoylglycine (A), nicotinamide 1-oxide (B), DHOPA (C), ADMA (D), and citrate (E) in SCCVII-tumor-bearing and control mice at various times following tumor cell injection. The levels of metabolites were normalized according to the concentration of urinary creatinine. Statistical analysis was performed using two-tailed Student's t test (n = 10 in each group). *, p < 0.05; **, p < 0.01.
Table I. Summary of ions altered in SCCVII mice relative to control mice.
| Number | Retention time (min) | Mass (m/z) | Empirical formula | Mass error (ppm) | Identity | Up/down | Fold change |
|---|---|---|---|---|---|---|---|
| Liver | |||||||
| 1 | 0.32 | 123.0555 | C6H6N2O [H+] | −2.4 | Nicotinamide | ↓ | 1.2 |
| 2 | 0.28 | 184.0727 | C5H14NO4P [H+] | −6.5 | Phosphocholine | ↓ | 1.6 |
| 3 | 0.29 | 348.0684 | C10H14N5O7P [H+] | −7.2 | AMP | ↓ | 1.2 |
| Urine | |||||||
| 4 | 0.34 | 139.0513 | C6H6N2O2 [H+] | 3.6 | Nicotinamide 1-oxide | ↑ | 1.8 |
| 5 | 5.22 | 363.2168 | C21H30O5 [H+] | −0.8 | DHOPA | ↑ | 2.1 |
| 6 | 0.38 | 215.0187 | C6H8O7 [Na+] | 8.8 | Citrate | ↑ | 2.2 |
| 7 | 3.01 | 174.1123 | C8H15NO3 [H+] | −0.4 | Hexanoylglycine | ↑ | 2.1 |
| 8 | 0.31 | 203.1502 | C8H18N4O2 [H+] | −2.9 | ADMA | ↑ | 1.7 |
| Serum | |||||||
| 9 | 4.90 | 522.3553 | C26H52NO7P [H+] | −1.3 | LPC 18:1 | ↓ | 1.4 |
| 10 | 4.37 | 494.3244 | C24H48NO7P[H+] | −0.6 | LPC 16:1 | ↓ | 1.4 |
↑, up-regulation; ↓, down-regulation.
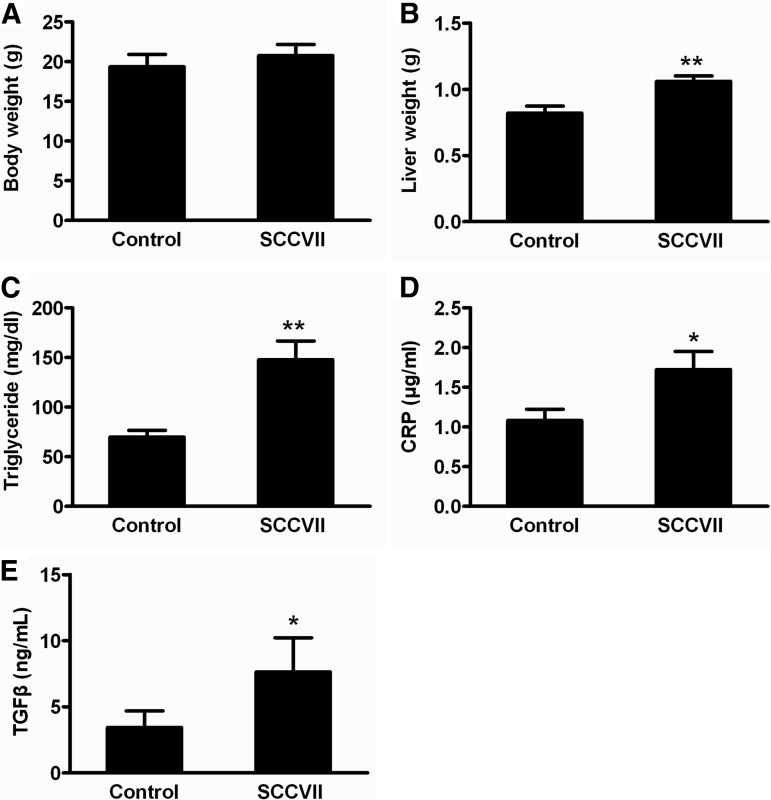
Although overall body weights were similar for both groups, liver weights were significantly elevated by ∼20% at day 13 in tumor-bearing mice relative to controls (Figs. 3A and 3B). Further, clinical serum chemistry was assessed for the important parameters of liver function, including alkaline phosphatase, total protein, albumin, and albumin/globulin ratio (Table II). Serum alkaline phosphatase levels were decreased by 67% (p < 0.01) in SCCVII-tumor-bearing mice, which was indicative of protein deficiency and consistent with the decrease in the total protein (18%) and albumin (33%) in serum from SCCVII-tumor-bearing mice. The albumin/globulin ratio, which directly reflects liver function, was significantly diminished by ∼50% in SCCVII-tumor-bearing mice relative to control mice (p < 0.01). In addition, total serum triglycerides were increased in SCCVII-tumor-bearing mice at day 13, reaching levels of 148 mg/dl, compared with 69.8 mg/dl in control mice (p < 0.05) (Fig. 3C). Serum C-reactive protein levels and transforming growth factor β (TGFβ), which is elevated in response to inflammation, were significantly enhanced by SCCVII tumor growth (Figs. 3D and 3E). Collectively, the elevated liver weights, triglyceride, C-reactive protein, and TGFβ levels, along with compromised liver function markers, suggested significant liver injury during tumor growth.
Fig. 3.
Body weights, liver weights, and serum parameters after SCCVII tumor xenograft. A, B, body weight and liver weight of control and SCCVII-tumor-bearing mice at day 13. C–E, serum triglyceride, C-reactive protein, and TGFβ levels in control and SCCVII-tumor-bearing mice at day 13. Statistical analysis was performed using two-tailed Student's t test (n = 10 in each group). *, p < 0.05; **, p < 0.01.
Table II. Clinical serum chemistry of SCCVII.
| Control | SCCVII | |
|---|---|---|
| ALB (g/dl) | 3.7 ± 0.1 | 2.5 ± 0.3** |
| ALT (U/l) | 53.2 ± 16.9 | 147 ± 90.3 |
| ALP (U/l) | 116 ± 9.5 | 46.4 ± 5.7** |
| Glucose (mg/dl) | 208 ± 26.9 | 230 ± 21.2 |
| Bilirubin (mg/dl) | 0.4 ± 0 | 0.4 ± 0 |
| Amylase (U/l) | 880 ± 55.8 | 698 ± 48.5** |
| BUN (mg/dl) | 12.8 ± 4.1 | 10.8 ± 3.0 |
| Calcium (mg/dl) | 10.8 ± 0.3 | 11.7 ± 0.6* |
| Phosphate (mg/dl) | 11.2 ± 0.7 | 10.0 ± 1.3 |
| Potassium (mmol/l) | 6.5 ± 0.5 | 7.6 ± 0.6* |
| Total protein (g/dl) | 5.2 ± 0.08 | 4.4 ± 0.3** |
| ALB/GLOB | 2.2 ± 0.1 | 1.2 ± 0.2** |
Comparison of diagnostic biomarkers between SCCVII and control mice (n = 10).
*, p < 0.05;
**, p < 0.01.
ALB, albumin; ALT, alanine aminotransferase; ALP, alkaline phosphatase; BUN, blood urea nitrogen; ALB/GLOB, albumin/globulin.
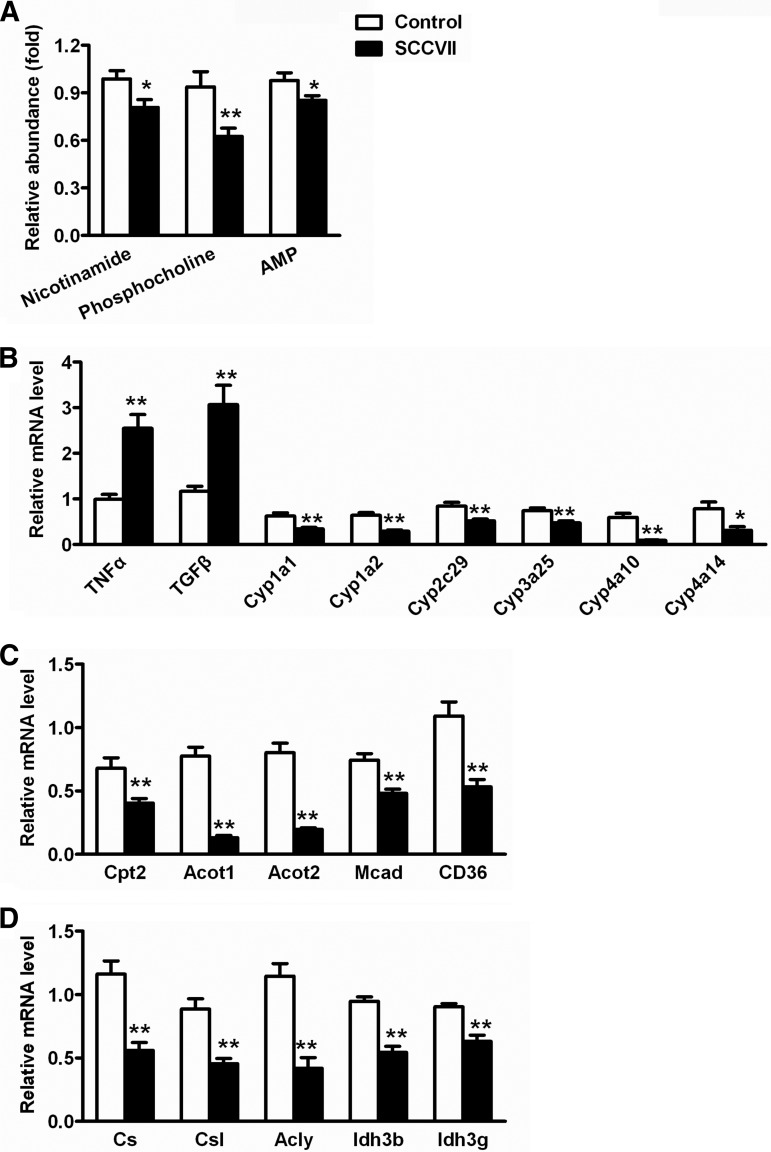
On day 13, the PLS-DA model distinguished the liver homogenates of SCCVII-tumor-bearing mice from those of control mice (supplemental Fig. S3A). Three ions, m/z 123.0555+, 184.0727+, and 348.0684+, that were decreased in SCCVII-tumor-bearing mice were identified as nicotinamide, phosphocholine, and AMP (supplemental Figs. S3B–S3E). Nicotinamide, phosphocholine, and AMP, which directly reflect inflammation in the body, were decreased by 21%, 40%, and 17%, respectively, in SCCVII-tumor-bearing mice liver at day 13 relative to control mice (Fig. 4A). Gene expression analysis indicated that mRNAs encoding tumor necrosis factor α (TNFα) and TGFβ, two typical pro-inflammatory cytokines, were activated 1.5- and 2.0-fold in SCCVII-tumor-bearing mouse liver, respectively (Fig. 4B). Additionally, mRNAs encoding several P450s involved in the metabolism of xenobiotic and endogenous compounds were significantly reduced in SCCVII-tumor-bearing mice relative to control mice, including Cyp1a1, Cyp1a2, Cyp3a25, Cyp2c29, Cyp4a10, and Cyp4a14 mRNAs (Fig. 4B).
Fig. 4.
Hepatic levels of metabolites and gene expression in SCCVII mice. A, liver levels of nicotinamide, phosphocholine, and AMP. B, hepatic mRNA levels of genes associated with inflammation and P450s. The mRNA levels were normalized to those of β-actin mRNA and subsequently normalized to those of control mice. C, hepatic mRNA levels of genes associated with fatty acid oxidation. D, hepatic mRNAs from genes associated with the TCA cycle. The mRNA levels were normalized to those of β-actin mRNA and subsequently normalized to those of control mice. Statistical analysis was performed using two-tailed Student's t test (n = 10 in each group). *, p < 0.05; **, p < 0.01.
These results suggest that liver function was abnormal in SCCVII-tumor-bearing mice. The expression levels of several important P450s were decreased in the liver following injection of the SCCVII cells, including CYP4A10 and CYP4A14 (Fig. 4B). Both enzymes can carry out fatty acid ω-oxidation. The suppression of CYP4A10 and CYP4A14 in liver, as well as the increased triglycerides in serum, suggested that fatty acid oxidation was indeed disrupted in SCCVII-tumor-bearing mice. To further determine whether fatty acid oxidation was affected in SCCVII-tumor-bearing mice, the hepatic expression level of the genes involved in fatty acid oxidation was measured using qPCR. The results indicated that carnitine palmitoyltransferase II deficiency (Cpt2), acyl-CoA thioesterase 1 (Acot1), acyl-CoA thioesterase 2 (Acot2), medium-chain acyl-CoA dehydrogenase (Mcad), and cluster of differentiation 36 (Cd36) mRNAs were also reduced in SCCVII-tumor-bearing mouse liver (Fig. 4C). Additionally, urinary metabolomics revealed that three metabolites related to fatty acid oxidation—hexanoylglycine, DHOPA, and nicotinamide 1-oxide—were increased in SCCVII-tumor-bearing mice (Figs. 2A–2C). The gene expression and metabolite change demonstrated that the impaired fatty acid oxidation resulted from SCCVII tumor cell growth.
Urinary citrate levels were increased 1.9- and 2.2-fold in SCCVII-tumor-bearing mice at days 10 and 13, suggesting influence on the TCA cycle by SCCVII tumor growth. The fatty acid oxidation products produced by MCAD, citrate synthase, citrate synthase like, and ATP citrate lyase, can enter the TCA cycle via the oxidized metabolite, acetyl coenzyme A (acetyl-CoA). The expression level of MCAD, which breaks down medium chain fatty acids into acetyl-CoA, was significantly diminished in SCCVII-tumor-bearing mouse liver (Fig. 4C). Citrate synthase, citrate synthase like, and ATP citrate lyase, which catalyze the generation of citrate and CoASH from acetyl-CoA, were also reduced in SCCVII-tumor-bearing mice (Fig. 4D). Additionally, ACOT1 and ACOT2, which regulate the hydrolysis of acyl-CoA to the coenzyme A (CoASH), were dramatically decreased in SCCVII-tumor-bearing mice relative to control mice (Fig. 4C). Further, other enzymes involved in the TCA cycle, isocitrate dehydrogenase 3b (IDH3b) and isocitrate dehydrogenase 3g (IDH3g), were also significantly decreased in SCCVII-tumor-bearing mice relative to control mice (Fig. 4D). Collectively, the results of urinary metabolomics and gene expression provide compelling evidence that the liver TCA cycle was disrupted in SCCVII-tumor-bearing mice.
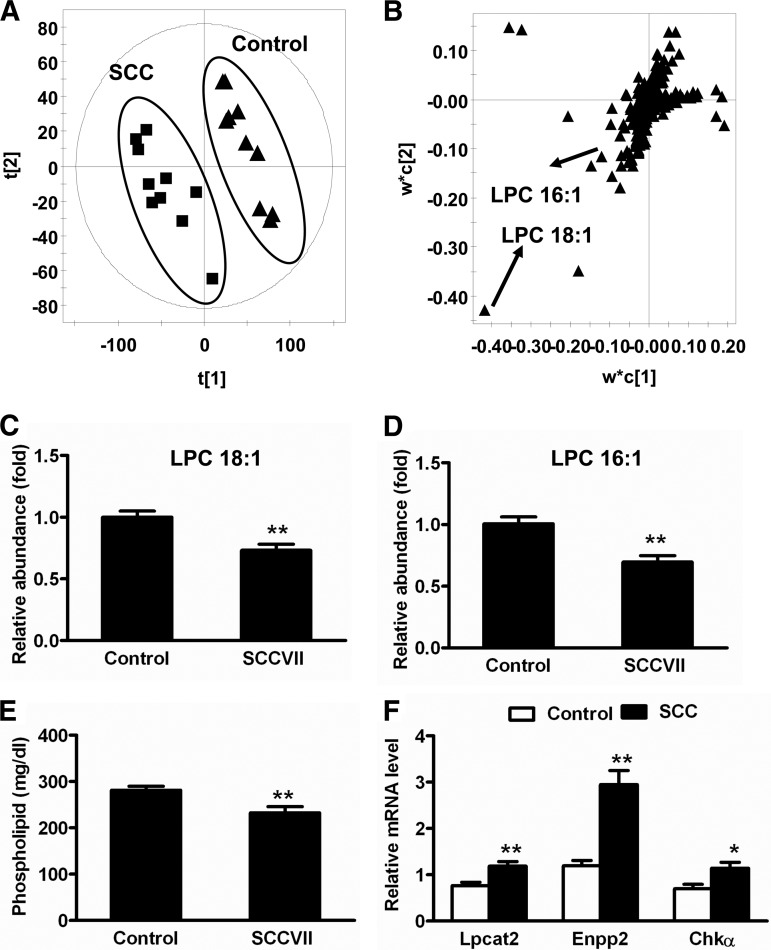
Serum samples collected at day 13 were analyzed using UPLC-ESI-QTOFMS operating in positive and negative ionization mode. The raw data exported from MassLynx were subjected to PLS-DA modeling. The separation between control and SCCVII-tumor-bearing mice was clear in the score scatter plot (Fig. 5A). Those ions that contributed to the separation of SCCVII-tumor-bearing and control groups deviated from the ion cloud in the loading scatter plot (Fig. 5B). In serum, the top decreased ions were m/z 496.3244+ and 522.3553+, which were unsaturated lysophosphatidylcholines, LPC 16:1 and LPC 18:1 (supplemental Figs. 4A and 4B). Serum LPC 16:1 was decreased by 31% in SCCVII tumor mice relative to control mice (p < 0.01), and LPC 18:1 was decreased by 27% in SCCVII-tumor-bearing mice (p < 0.01) (Figs. 5C and 5D). No significant changes in saturated LPC were found in the serum of SCCVII-tumor-bearing mice (data not shown). The total phospholipid level was decreased by 18% in SCCVII mouse serum relative to control mice (Fig. 5E). Hepatic gene expression data showed that mRNAs encoding several enzymes involved in LPC metabolism and synthesis were induced in SCCVII-tumor-bearing mouse liver, including lysophosphatidylcholine acyltransferase 2 (LPCAT2), ectonucleotide pyrophosphatase/phosphodiesterase 2 (ENPP2), and choline kinase α (CHKα) (Fig. 5F). Collectively, these data suggest that SCCVII tumor growth directly activated liver phospholipid metabolism.
Fig. 5.
Activation of phospholipid metabolism during SCCVII tumor growth. A, PLS-DA model of serum metabolites in SCCVII (n = 10, solid squares) and control (n = 10, solid triangles). B, loadings scatter plot of PLS-DA. C, serum levels of LPC 18:1 in SCCVII and control mice. D, serum levels of LPC 16:1 in SCCVII-tumor-bearing and control mice. The levels of LPC were normalized to those of control mice and were expressed as the relative abundance. E, serum phospholipid in SCCVII-tumor-bearing and control mice. F, hepatic mRNA levels of genes associated with LPC metabolism and synthesis. The mRNA levels were normalized to those of β-actin mRNA and subsequently normalized to those of control mice. Statistical analysis was performed using two-tailed Student's t test (n = 10 in each group). *, p < 0.05; **, p < 0.01.
DISCUSSION
UPLC-ESI-QTOFMS-based metabolomics was used to determine whether dynamic metabolic signatures emerge during SCCVII tumor growth in vivo. PCA analysis of urine metabolites 3 days after cell injection showed no significant differences between control and SCCVII-tumor-bearing mice, indicating that no tumor-specific biomarkers emerged prior to a palpable tumor mass in this model at the level of sensitivity of our instrumentation. Six days after tumor cell injection, when the tumor was clearly established, there were no differences between the metabolite profiles of control and SCCVII-tumor-bearing mice, with the exception of DHOPA, which was significantly elevated in tumor-bearing mice. By days 10 and 13, however, numerous differences were evident between the two groups. The ions representing potential biomarkers in the loadings plot that give rise to the separation of the SCCVII and control groups are those that deviate from the central ion cloud. The change of ions was based on the model fit and abundance. The identities of these potential biomarkers were determined by searching metabolomics databases, and their structures were verified through comparison of retention times and their fragmentation patterns with those from authentic compounds. From these data, combined with the growth curve of SCCVII tumor, five significant biomarkers (hexanoylglycine, nicotinamide 1-oxide, DHOPA, ADMA, and citrate) were identified, quantified, and determined to be associated with SCCVII tumor growth from days 3, 6, 10, and 13 of tumor progression. Notably, hexanoylglycine, DHOPA, and nicotinamide 1-oxide were clearly increased in the SCCVII-tumor-bearing mice. These metabolites are correlated with activation of peroxisome proliferator-activated receptor α, a nuclear hormone receptor regulating peroxisomal and mitochondrial fatty acid oxidation (17). During the experiment, the body weight and food intake did not change in SCCVII-tumor-bearing mice, indicating that alteration of these metabolites directly resulted from the SCCVII tumor growth. Further, hexanoylglycine can act as a diagnostic biomarker for MCAD deficiency, a disorder associated with compromised fatty acid metabolism (18). ADMA was also found to be elevated in the urine of SCCVII mice relative to that of the control mice. ADMA, an inhibitor of nitric oxide synthase, is an endogenous modulator of endothelial function and oxidative stress, and increased levels of this metabolite were reported in some metabolic disorders (19). In hypertensive patients, elevated levels of ADMA and oxidative stress contribute to microvascular endothelial dysfunction and elevated blood pressure (20). The time course of ADMA levels and oxidative stress in hypertension indicated that the elevated ADMA observed could in part be secondary to the early development of oxidative stress (21). Further, ADMA was identified as a biomarker for oxidative stress in caveolin-1 knockout mice (22). Tissue and serum metabolites from renal cancer xenografts show extremely high levels of cysteine-glutathione disulfide, a signature of oxidative stress (12). In further support of tumor-mediated oxidative stress are studies showing expression of the DNA damage marker H2AX in tissues distant from tumor sites (23). Lastly, citrate was found to be elevated in SCCVII-tumor-bearing mouse urine. Increased urinary citrate is correlated with gastric cancer tumor growth in xenografts (24) and in human head and neck squamous cell carcinoma (25). Increased TCA cycle metabolites can originate from enhanced aerobic glycolysis in SCCVII tumors, as revealed by previous studies (26, 27). Such was the case for tissue taken from renal cancer xenografts, which exhibited a 21-fold increase in citrate levels (12). The expression levels of Idh3b and Idh3g were decreased in SCCVII-tumor-bearing mice; however, the expression of Idh1 and Idh2 did not change. IDH3 is the main form of IDH functioning in the TCA cycle under physiological conditions (28, 29). In addition, three enzymes responsible for the synthesis of citrate—citrate synthase, citrate synthase like, and ATP citrate lyase—also were decreased in SCCVII-tumor-bearing mice. Further, the significant decrease in Acot1 and Acot2 expression potentially leading to the inhibition of fatty acid oxidation in SCCVII-tumor-bearing mice could affect the generation of acyl-CoA. Alteration of acyl-CoA levels can cause disruption of the TCA cycle. The changes in urine metabolites; alteration of TCA cycle metabolites; and the presence of oxidative stress markers, liver hepatomegaly, and alterations in serum liver function markers suggested that liver metabolism was compromised, perhaps by the presence of tumor growth.
Indeed, several liver metabolites (nicotinamide, phosphocholine, and AMP) were reduced in SCCVII-tumor-bearing mouse liver relative to control mice. Decreases in these metabolites have been associated with the injury of ionizing radiation, which can cause inflammation resulting in the release of proinflammatory cytokines (30, 31). Consistent with this finding, the expression of two key inflammatory cytokines, TNFα and TGFβ, and C-reactive protein was significantly enhanced in the liver of SCCVII-tumor-bearing mice. Further, the expression of several hepatic P450s was suppressed in SCCVII-tumor-bearing mice, in keeping with the observation that inflammatory stimuli can suppress the activity and expression levels of P450 in the liver (32). It was also established that tumor growth can compromise P450 activity (33, 34). A major implication of these findings centers on the ability of the host to detoxify xenobiotics and, in particular, cytotoxic chemotherapy drugs in the setting of cancer treatment (33). Reduced activities of P450s would affect the maximum tolerated doses of the chemotherapy used to treat the tumor. If the host were compromised with respect to drug detoxification, lower non-curative doses of chemotherapy would by necessity be used. It is interesting to speculate that if tumor-mediated induction of inflammation leading to reduced P450 activity could be prevented, higher, more potentially curative doses of chemotherapy could be administered.
Total triglyceride levels were significantly increased in serum taken from SCCVII-tumor-bearing mice, suggesting the inhibition of fatty acid oxidation during SCCVII tumor growth. The elevated levels of serum triglycerides are consistent with the reduction in expression levels of hepatic genes encoding the enzymes CPT2, ACOT1, ACOT2, MCAD, and the receptor CD36. Levels of urinary DHOPA regulated by 21-hydroxysteroid dehydrogenase can be elevated in other pathological conditions in which fatty acid oxidation is inhibited (3, 35). In addition, liver inflammation might have resulted in the activation of phospholipid metabolism. Indeed, two serum lysophosphatidylcholines, LPC 16:1 and LPC 18:1, were significantly decreased in SCCVII-tumor-bearing mice relative to control mice. LPCs, which are derived from phosphatidylcholine via hydrolysis, are the major phospholipid species in serum. LPCs can regulate different biological processes, including inflammation, tumor cell invasiveness, and cell proliferation (36). It is possible that the reduction in serum LPCs in SCCVII-tumor-bearing mice is a result of tumor utilization for energy and bio-membrane synthesis. It was reported that TGFβ can regulate phospholipid metabolism through the induction of lysophosphatidylcholine acyltransferase 2 (37). Therefore, hepatic TGFβ activation in SCCVII-tumor-bearing mice also might have contributed to the decrease in serum LPCs.
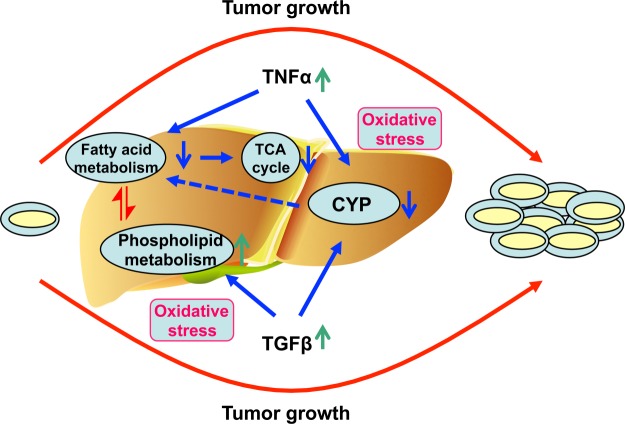
In conclusion, metabolomics was used to profile metabolic changes that occur during SCCVII tumor growth in mice. Alterations in urine metabolites, blood markers, and liver hepatomegaly in tumor-bearing mice prompted closer interrogation of liver function, revealing that SCCVII tumor growth resulted in liver dysfunction (Fig. 6). These findings support the view that tumor growth exerts a systemic global response that may be easily monitored via metabolomics. Further, relevant metabolite biomarkers might be used to monitor tumor treatment response.
Fig. 6.
Proposed mechanism of liver dysfunction during SCCVII tumor growth. During SCCVII tumor growth, oxidative stress can be increased in the body, which can activate the inflammatory cytokines TNFα and TGFβ. The enhanced TNFα suppresses fatty acid oxidation and CYP450s. The suppression of fatty acid oxidation contributes to the disruption of the TCA cycle. The enhancement of TGFβ activates phospholipid metabolism and can also suppress P450s. The disruption of all these metabolic pathways contributes to liver dysfunction. ↑, up-regulation; ↓, down-regulation.
Supplementary Material
Footnotes
* This work was supported in part by the Intramural Research Program of the Center for Cancer Research, NCI, National Institutes of Health.
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- acetyl-CoA
- acetyl coenzyme A
- Acot
- acyl-CoA thioesterase
- ADMA
- asymmetric dimethylarginine
- AMP
- adenosine monophosphate
- DHOPA
- 11β, 20α-dihydroxy-3-oxopregn-4-en-21-oic acid
- IDH
- isocitrate dehydrogenase
- LPC
- lysophosphatidylcholine
- MCAD
- medium-chain acyl-CoA dehydrogenase
- P450
- cytochromes P450
- PCA
- principal component analysis
- PLS-DA
- partial least-squares discriminant analysis
- SCCVII
- squamous cell carcinoma
- TCA
- tricarboxylic acid
- TGFβ
- transforming growth factor β
- TNFα
- tumor necrosis factor α
- UPLC-ESI-QTOFMS
- ultra-performance liquid chromatography coupled with electrospray ionization quadrupole mass spectrometry.
REFERENCES
- 1. Chen C., Krausz K. W., Shah Y. M., Idle J. R., Gonzalez F. J. (2009) Serum metabolomics reveals irreversible inhibition of fatty acid beta-oxidation through the suppression of PPARalpha activation as a contributing mechanism of acetaminophen-induced hepatotoxicity. Chem. Res. Toxicol. 22, 699–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li F., Patterson A. D., Krausz K. W., Dick B., Frey F. J., Gonzalez F. J., Idle J. R. (2012) Metabolomics reveals the metabolic map of procainamide in humans and mice. Biochem. Pharmacol. 83, 1435–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li F., Patterson A. D., Krausz K. W., Tanaka N., Gonzalez F. J. (2012) Metabolomics reveals an essential role for peroxisome proliferator-activated receptor alpha in bile acid homeostasis. J. Lipid Res. 53, 1625–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patterson A. D., Bonzo J. A., Li F., Krausz K. W., Eichler G. S., Aslam S., Tigno X., Weinstein J. N., Hansen B. C., Idle J. R., Gonzalez F. J. (2011) Metabolomics reveals attenuation of the SLC6A20 kidney transporter in nonhuman primate and mouse models of type 2 diabetes mellitus. J. Biol. Chem. 286, 19511–19522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Monteiro M. S., Carvalho M., Bastos M. D., de Pinho P. G. (2013) Metabolomics analysis for biomarker discovery: advances and challenges. Curr. Med. Chem. 19, 5601–5606 [DOI] [PubMed] [Google Scholar]
- 6. Sreekumar A., Poisson L. M., Rajendiran T. M., Khan A. P., Cao Q., Yu J., Laxman B., Mehra R., Lonigro R. J., Li Y., Nyati M. K., Ahsan A., Kalyana-Sundaram S., Han B., Cao X., Byun J., Omenn G. S., Ghosh D., Pennathur S., Alexander D. C., Berger A., Shuster J. R., Wei J. T., Varambally S., Beecher C., Chinnaiyan A. M. (2009) Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 457, 910–914 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 7. Nam H., Chung B. C., Kim Y., Lee K., Lee D. (2009) Combining tissue transcriptomics and urine metabolomics for breast cancer biomarker identification. Bioinformatics 25, 3151–3157 [DOI] [PubMed] [Google Scholar]
- 8. Xie G. X., Chen T. L., Qiu Y. P., Shi P., Zheng X. J., Su M. M., Zhao A. H., Zhou Z. T., Jia W. (2012) Urine metabolite profiling offers potential early diagnosis of oral cancer. Metabolomics 8, 220–231 [Google Scholar]
- 9. Chen F., Xue J., Zhou L., Wu S., Chen Z. (2011) Identification of serum biomarkers of hepatocarcinoma through liquid chromatography/mass spectrometry-based metabonomic method. Anal. Bioanal. Chem. 401, 1899–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen T., Xie G., Wang X., Fan J., Qiu Y., Zheng X., Qi X., Cao Y., Su M., Xu L. X., Yen Y., Liu P., Jia W. (2011) Serum and urine metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma. Mol. Cell. Proteomics 10, M110.004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng Y., Xie G., Chen T., Qiu Y., Zou X., Zheng M., Tan B., Feng B., Dong T., He P., Zhao L., Zhao A., Xu L. X., Zhang Y., Jia W. (2012) Distinct urinary metabolic profile of human colorectal cancer. J. Proteome Res. 11, 1354–1363 [DOI] [PubMed] [Google Scholar]
- 12. Ganti S., Taylor S. L., Abu Aboud O., Yang J., Evans C., Osier M. V., Alexander D. C., Kim K., Weiss R. H. (2012) Kidney tumor biomarkers revealed by simultaneous multiple matrix metabolomics analysis. Cancer Res. 72, 3471–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li S., Liu H., Jin Y., Lin S., Cai Z., Jiang Y. (2011) Metabolomics study of alcohol-induced liver injury and hepatocellular carcinoma xenografts in mice. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 879, 2369–2375 [DOI] [PubMed] [Google Scholar]
- 14. Moroz J., Turner J., Slupsky C., Fallone G., Syme A. (2011) Tumour xenograft detection through quantitative analysis of the metabolic profile of urine in mice. Phys. Med. Biol. 56, 535–556 [DOI] [PubMed] [Google Scholar]
- 15. Wang X., Yan S. K., Dai W. X., Liu X. R., Zhang W. D., Wang J. J. (2010) A metabonomic approach to chemosensitivity prediction of cisplatin plus 5-fluorouracil in a human xenograft model of gastric cancer. Int. J. Cancer 127, 2841–2850 [DOI] [PubMed] [Google Scholar]
- 16. Cotrim A. P., Hyodo F., Matsumoto K., Sowers A. L., Cook J. A., Baum B. J., Krishna M. C., Mitchell J. B. (2007) Differential radiation protection of salivary glands versus tumor by Tempol with accompanying tissue assessment of Tempol by magnetic resonance imaging. Clin. Cancer Res. 13, 4928–4933 [DOI] [PubMed] [Google Scholar]
- 17. Zhen Y., Krausz K. W., Chen C., Idle J. R., Gonzalez F. J. (2007) Metabolomic and genetic analysis of biomarkers for peroxisome proliferator-activated receptor alpha expression and activation. Mol. Endocrinol. 21, 2136–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Downing M., Manning N. J., Dalton R. N., Krywawych S., Oerton J. (2008) Detection of urinary hexanoylglycine in the diagnosis of MCAD deficiency from newborn screening. J. Inherit. Metab. Dis. 31, 550. [DOI] [PubMed] [Google Scholar]
- 19. Boger R. H. (2005) Asymmetric dimethylarginine (ADMA) and cardiovascular disease: insights from prospective clinical trials. Vasc. Med. 10 Suppl 1, S19-S25 [DOI] [PubMed] [Google Scholar]
- 20. Wang D., Strandgaard S., Iversen J., Wilcox C. S. (2009) Asymmetric dimethylarginine, oxidative stress, and vascular nitric oxide synthase in essential hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R195–R200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Korandji C., Zeller M., Guilland J. C., Collin B., Lauzier B., Sicard P., Duvillard L., Goirand F., Moreau D., Cottin Y., Rochette L., Vergely C. (2011) Time course of asymmetric dimethylarginine (ADMA) and oxidative stress in fructose-hypertensive rats: a model related to metabolic syndrome. Atherosclerosis 214, 310–315 [DOI] [PubMed] [Google Scholar]
- 22. Pavlides S., Tsirigos A., Migneco G., Whitaker-Menezes D., Chiavarina B., Flomenberg N., Frank P. G., Casimiro M. C., Wang C., Pestell R. G., Martinez-Outschoorn U. E., Howell A., Sotgia F., Lisanti M. P. (2010) The autophagic tumor stroma model of cancer: role of oxidative stress and ketone production in fueling tumor cell metabolism. Cell Cycle 9, 3485–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Redon C. E., Dickey J. S., Nakamura A. J., Kareva I. G., Naf D., Nowsheen S., Kryston T. B., Bonner W. M., Georgakilas A. G., Sedelnikova O. A. (2010) Tumors induce complex DNA damage in distant proliferative tissues in vivo. Proc. Natl. Acad. Sci. U.S.A. 107, 17992–17997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim K. B., Yang J. Y., Kwack S. J., Park K. L., Kim H. S., Ryu do H., Kim Y. J., Hwang G. S., Lee B. M. (2010) Toxicometabolomics of urinary biomarkers for human gastric cancer in a mouse model. J. Toxicol. Environ. Health A 73, 1420–1430 [DOI] [PubMed] [Google Scholar]
- 25. Somashekar B. S., Kamarajan P., Danciu T., Kapila Y. L., Chinnaiyan A. M., Rajendiran T. M., Ramamoorthy A. (2011) Magic angle spinning NMR-based metabolic profiling of head and neck squamous cell carcinoma tissues. J. Proteome Res. 10, 5232–5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Furusawa M., Onomichi M., Morishita S., Hamatake S., Takahashi M., Ito Y. H. (1997) Carbon-13 magnetic resonance spectroscopy of glucose metabolism in SCC-VII tumors. Radiat. Med. 15, 149–153 [PubMed] [Google Scholar]
- 27. Matsumoto S., Saito K., Yasui H., Morris H. D., Munasinghe J. P., Lizak M., Merkle H., Ardenkjaer-Larsen J. H., Choudhuri R., Devasahayam N., Subramanian S., Koretsky A. P., Mitchell J. B., Krishna M. C. (2013) EPR oxygen imaging and hyperpolarized (13) C MRI of pyruvate metabolism as noninvasive biomarkers of tumor treatment response to a glycolysis inhibitor 3-bromopyruvate. Magn. Reson. Med. 69, 1443–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hartong D. T., Dange M., Mcgee T. L., Berson E. L., Dryja T. P., Colman R. F. (2008) Insights from retinitis pigmentosa into the roles of isocitrate dehydrogenases in the Krebs cycle. Nat. Genet. 40, 1230–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prensner J. R., Chinnaiyan A. M. (2011) Metabolism unhinged: IDH mutations in cancer. Nat. Med. 17, 291–293 [DOI] [PubMed] [Google Scholar]
- 30. Patterson A. D., Li H., Eichler G. S., Krausz K. W., Weinstein J. N., Fornace A. J., Jr., Gonzalez F. J., Idle J. R. (2008) UPLC-ESI-TOFMS-based metabolomics and gene expression dynamics inspector self-organizing metabolomic maps as tools for understanding the cellular response to ionizing radiation. Anal. Chem. 80, 665–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lorimore S. A., Coates P. J., Scobie G. E., Milne G., Wright E. G. (2001) Inflammatory-type responses after exposure to ionizing radiation in vivo: a mechanism for radiation-induced bystander effects? Oncogene 20, 7085–7095 [DOI] [PubMed] [Google Scholar]
- 32. Morgan E. T. (1997) Regulation of cytochromes P450 during inflammation and infection. Drug Metab. Rev. 29, 1129–1188 [DOI] [PubMed] [Google Scholar]
- 33. Robertson G. R., Liddle C., Clarke S. J. (2008) Inflammation and altered drug clearance in cancer: transcriptional repression of a human CYP3A4 transgene in tumor-bearing mice. Clin. Pharmacol. Ther. 83, 894–897 [DOI] [PubMed] [Google Scholar]
- 34. Sharma R., Kacevska M., London R., Clarke S. J., Liddle C., Robertson G. (2008) Downregulation of drug transport and metabolism in mice bearing extra-hepatic malignancies. Br. J. Cancer 98, 91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang Y., Li F., Patterson A. D., Wang Y., Krausz K. W., Neale G., Thomas S., Nachagari D., Vogel P., Vore M., Gonzalez F. J., Schuetz J. D. (2012) Abcb11 deficiency induces cholestasis coupled to impaired beta-fatty acid oxidation in mice. J. Biol. Chem. 287, 24784–24794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moolenaar W. H. (1999) Bioactive lysophospholipids and their G protein-coupled receptors. Exp. Cell Res. 253, 230–238 [DOI] [PubMed] [Google Scholar]
- 37. Matsubara T., Tanaka N., Patterson A. D., Cho J. Y., Krausz K. W., Gonzalez F. J. (2011) Lithocholic acid disrupts phospholipid and sphingolipid homeostasis leading to cholestasis in mice. Hepatology 53, 1282–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.