Abstract
Background
This work analyzes the effect of social isolation (a mild stressor) on the 24-h variation of pituitary-testicular function in young Wistar rats, assessed by measuring circulating levels of prolactin, FSH, LH and testosterone.
Methods
Animals were either individually caged or kept in groups (4–5 animals per cage) under a 12:12 h light-dark cycle (lights on at 0800 h) for 30 days starting on day 35 of life. Rats were killed at 4-h intervals during a 24-h cycle, beginning at 0900 h.
Results
Isolation brought about a decrease in prolactin, LH and testosterone secretion and an increase of FSH secretion. In isolated rats the 24-h secretory pattern of prolactin and testosterone became modified, i.e., the maximum in prolactin seen in control animals at the beginning of the activity span was no longer detected, whereas the maximum in circulating testosterone taking place at 1700 h in controls was phase-delayed to 2100 h in isolated rats.
Conclusion
Social isolation affects the 24-h variation of pituitary-testicular function in young rats. Secretion of prolactin, LH and testosterone decreases, and secretion of FSH increases, in isolated rats. The maximum in prolactin seen in group-caged rats at the beginning of the activity span is not observed in isolated rats. The maximum in circulating testosterone taking place at the second part of the rest span in controls is phase-delayed to the light-dark transition in isolated rats.
Background
Stemming from the seminal work by Cannon and Selye, stress is defined as "an alteration in the body's hormonal and neuronal secretions caused by the central nervous system in response to a perceived threat" and a stressor is defined as "a change in an organism's internal or external environment which is perceived by the organism as threatening" [1-3]. Within this context, psychosocial stressors like social conflict [4], social isolation [5-7] or overcrowding [8,9] have been identified.
The most profound change that occurs with individual housing is an increase in aggression of males seen in both mice and rats following even relatively brief periods of individual housing [10,11]. Individually housed animals are also hyperresponsive to stressors [12]. For example, in one study it was found that group size per se had limited long-term effects on pathophysiological measures of social stress, although it had a significant influence on many aspects of behavior when rats were first introduced into their groups [13]. Over weeks 1–8, single housed rats continued to spend much more time apparently attempting to escape (sniffing and chewing at the bars and suddenly dashing around their cage) while those housed in groups spent more time sleeping and feeding [13]. This indicates that isolation can be considered as a mild stress for rats.
The objective of the present study was to examine whether social isolation in growing male rats affected 24-h variations of activity of the hypophysial gonadal axis. Indeed, stressors have been shown to modify gonadotropin and testosterone secretion, acute stressors activating and chronic stressors suppressing the activity of the hypophysial gonadal axis [14]. It must be noted that, except for some exceptions [15,16], studies on the subject were performed at single time-points, generally in the morning, a serious drawback in view of the circadian nature of hormone release for most of the hormones considered [17,18]. In this study we measured the daily pattern of plasma prolactin, luteinizing hormone (LH), follicle-stimulating hormone (FSH) and testosterone levels at 6 different time points within a 24-h cycle in growing male rats kept in isolation or group-caged for 30 days.
Methods
Thirty five day-old male Wistar rats were kept under standard conditions of controlled light (12:12 h light/dark schedule; lights on at 0800 h) and temperature (22 ± 2°C). All experiments were conducted in middle spring (May). Rats were either put in individual cages (isolated group) or left in cages of 4–5 animals each. All animals had free access to food and water for the 30 days of the study. The experiments were conducted in accordance with the guidelines of the International Council for Laboratory Animal Science (ICLAS).
Groups of 6–8 rats were killed by decapitation under conditions of minimal stress, at six different time intervals every 4 h throughout a 24-h cycle starting at 0900 h. Blood was collected from the trunk wound in heparinized tubes and was centrifuged at 1500 × g for 15 min. The plasma was collected and stored at -20°C until further analysis.
Plasma prolactin, FSH and LH levels were measured by a homologous specific double antibody RIA, using materials kindly supplied by the NIDDK's National Hormone and Pituitary Program. The intra- and interassay coefficients were 6–8%. Sensitivities of the RIAs were 45, 9 and 45 pg/mL for prolactin, FSH and LH using the NIDDK rat prolactin RP-3, rat FSH-RP-2 and rat LH-RP-3, respectively. Results were expressed as ng/mL for prolactin and as pg/mL for FSH and LH [17,18]. Plasma testosterone levels were measured by using a commercial kit (ICN Pharmaceuticals, Inc., Costa Mesa, CA, USA). Sensitivity of the assay was 0.2 ng/mL and the intraassay coefficient of variation was 5%, as previously described [17]; results were expressed as ng testosterone/mL.
Statistical analysis of results was performed by a two-way factorial analysis of variance (ANOVA). Generally, the analysis included assessment of the group effect (i.e. the occurrence of differences in mean values between isolated and control rats), of time-of-day effects (the occurrence of daily changes) and of the interaction between the two factors (manipulation and time, from which inference about differences in timing and amplitude could be obtained). Post-hoc Tukey-Kramer's multiple comparisons tests were then employed to show which time points were significantly different within each experimental group to define existence of peaks. P values lower than 0.05 were considered evidence for statistical significance.
Results
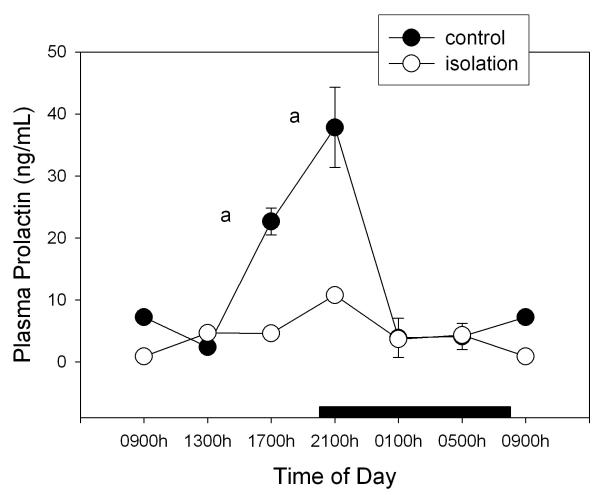
Figure 1 shows the levels of prolactin throughout the day in isolated and control rats. A factorial ANOVA for main effects indicated a significant 74% decrease of circulating prolactin in isolated rats (F1,75= 75.9, p < 0.00001) and the occurrence of significant time of day changes (F5,75= 18.8, p < 0.00001). The maximum seen in control animals at the beginning of the activity span was no longer detected in isolated rats (Fig. 1), as indicated both by a significant interaction between time of day and the experimental procedure in the factorial ANOVA (F5,75= 9.23, p < 0.00001) and by post-hoc Tukey-Kramer's tests (Fig. 1).
Figure 1.
Effect of isolation on 24-h changes of plasma prolactin concentration in young male rats. Groups of 6–8 rats were killed by decapitation at 6 different time intervals throughout a 24 h cycle. Values at 0900 point are repeated on the "second" day. Bar indicates scotophase duration. Shown are the means ± SEM. Letters indicate the existence of significant differences between time points within each group after a Tukey-Kramer's multiple comparisons test, as follows: a p < 0.01 vs. 0900, 1300, 0100 and 0500 h. For further statistical analysis, see text.
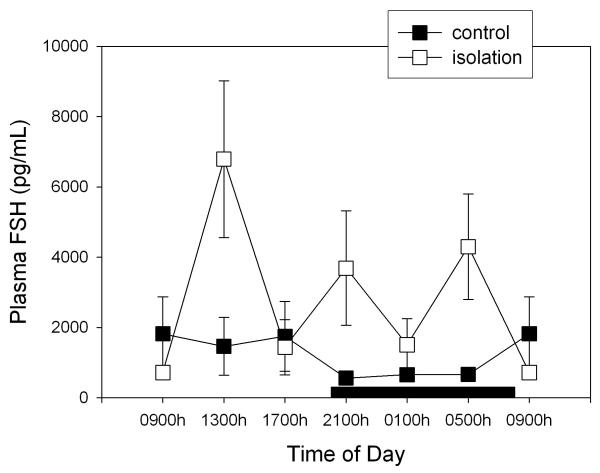
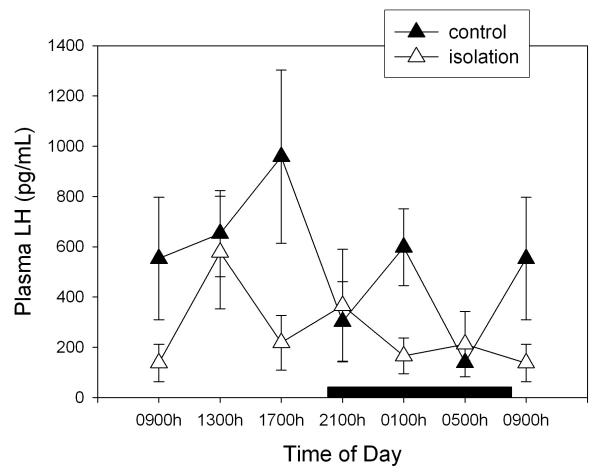
Figure 2 depicts the changes in circulating FSH levels after isolation of rats. Globally, this manoeuvre augmented FSH levels by 64% (F1,79= 8.31, p < 0.005, factorial ANOVA) with absence of significant changes as a function of time of day (F= 1.95, p > 0.9). As shown in Fig. 3, this pattern differed in the case of plasma LH levels, i.e. isolation decreased circulating LH to 51% of controls (F1,78= 4.99, p < 0.03, factorial ANOVA) without any significant effect of time of day (F= 1.41, p > 0.2) (Fig. 3). In comparison with prolactin and testosterone, SEM for FSH and LH values were high. No significant correlation between FSH and concentration values was found, high FSH and LH levels being distributed randomly among animals (results not shown).
Figure 2.
Effect of isolation on 24-h changes of plasma FSH concentration in young male rats. Groups of 6–8 rats were killed by decapitation at 6 different time intervals throughout a 24 h cycle. Values at 0900 point are repeated on the "second" day. Bar indicates scotophase duration. Shown are the means ± SEM. For further statistical analysis, see text.
Figure 3.
Effect of isolation on 24-h changes of plasma LH concentration in young male rats. Groups of 6–8 rats were killed by decapitation at 6 different time intervals throughout a 24 h cycle. Values at 0900 point are repeated on the "second" day. Bar indicates scotophase duration. Shown are the means ± SEM. For further statistical analysis, see text.
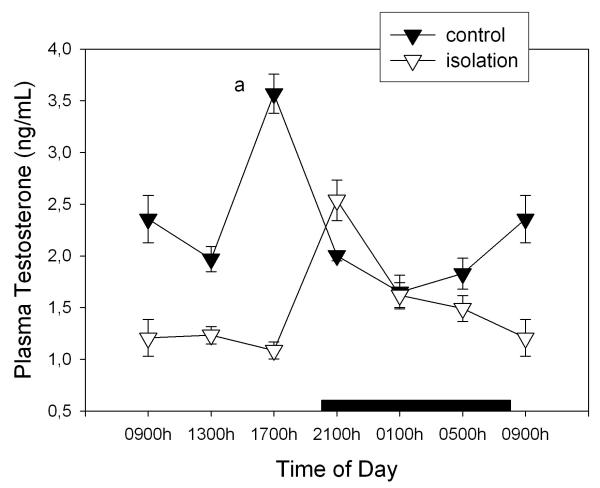
Figure 4 depicts plasma testosterone levels throughout the day in normal and isolated rats. Isolation brought about a 34% decrease of plasma testosterone (factorial ANOVA, F1,77= 58.8, p < 0.00001). Significant effects of time of day (F5,77= 8.71, p < 0.00001) and a significant interaction "time of day × treatment" occurred (F5,77= 21.9, p < 0.00001), i.e., the maximum in circulating testosterone took place at 1700 h in controls and at 2100 h in isolated rats and the decrease of plasma testosterone in isolated rats was seen only during the light phase of daily photoperiod (Fig. 4).
Figure 4.
Effect of isolation on 24-h changes of plasma testosterone concentration in young male rats. Groups of 6–8 rats were killed by decapitation at 6 different time intervals throughout a 24 h cycle. Values at 0900 point are repeated on the "second" day. Bar indicates scotophase duration. Shown are the means ± SEM. Letters indicate the existence of significant differences between time points within each group after a Tukey-Kramer's multiple comparisons test, as follows: a p < 0.01 vs. all time points. For further statistical analysis, see text.
Discussion
Our results indicate that social isolation of young male rats for 30 days brings about changes in the 24-h variation of pituitary-testicular function. Overall, the secretion of prolactin, LH and testosterone decreased whereas that of FSH augmented in isolated rats. The maximum in prolactin seen in group-caged rats at the beginning of the activity span was not observed in isolated rats. In addition, the maximum in circulating testosterone taking place at the second part of rest span in controls was phase-delayed to light-dark transition in isolated rats. A decrease of plasma testosterone in isolated rats was seen only during the light phase of daily photoperiod.
Solitary housing of usually social animals such as rats and mice causes complex neurobiological changes. Socially isolated animals exhibit a decrease in the electrical activity of neurons within the hypothalamus and have lower basal plasma corticosterone levels than do animals raised in social conditions [10]. Although this could be interpreted as indicating less psychosocial stress in isolation, individual housing of animals is associated with an increase in aggression of males [10,19], hyperresponsiveness to several stressors [12] and an increase in time spent attempting to escape and decrease in time spent sleeping and feeding [13]. Decreases in plasma levels of prolactin were found in subordinate hamsters after exposure to social conflict [20] and in isolated male hamsters as compared to hamsters with a family [21]. Therefore, the decrease levels of plasma prolactin herein described after a 1-month isolation of growing male rats agrees with the reported modifications of prolactin seen in isolated hamsters [21].
The role of prolactin in males is yet far to be understood. Prolactin is a versatile compound that has a dual function – as a circulating hormone and as a cytokine. The prolactin receptor is a member of the cytokine receptor superfamily, linked to activation of signaling pathways that promote cell growth and survival. Through these mechanisms prolactin regulates diverse physiological functions via its effects on cellular processes such as proliferation, differentiation, and cell survival [22,23]. In addition, prolactin may represent a peripheral regulatory factor for reproductive function in males, and/or a feedback mechanism that signals CNS centers controlling sexual arousal and behavior. For example, studies on sexual hormonal response in males demonstrated that plasma prolactin concentrations are substantially increased for over 1 h following orgasm in men but unchanged following sexual arousal without orgasm [24]. Evidence exists for a brain prolactin receptor-mediated anxiolytic action and for inhibitory actions on the reactivity of the hypothalamic-pituitary-adrenal axis and the neurohypophysial oxytocin system [25]. Known relationships also exist between prolactin and the expression of mammalian paternal behavior [26]. Hyperprolactinemia in males induces hypogonadism by inhibiting gonadotropin-releasing hormone pulsatile secretion and, consequently, FSH, LH and testosterone release. This leads to spermatogenic arrest, impaired motility, and sperm quality and results in morphologic alterations of the testes similar to those observed in prepubertal testes [27].
The present results on decreased prolactin levels in isolated male rats disagree with the previously reported increase in prolactin levels in a similar psychosocial stress paradigm [16]. Some conditions of the experiments, like the age of rats, i.e., growing rats in this study vs. adult rats in the study by Gambardella and colleagues, could explain this discrepancy.
The changes in gonadotropin secretion found in isolated rats include a decrease of plasma LH and an increase of plasma FSH levels. The reduction in circulating LH was accompanied by a concomitant reduction of testosterone. Since FSH levels augmented in isolated rats, the possible decrease of testicular inhibin should be considered [28]. Further studies are needed to document this point.
Temporal organization is an important feature of biological systems and its main function is to facilitate adaptation of the organism to the environment [29]. The daily variation of biological variables arises from an internal time-keeping system, and the major action of the environment is to synchronize this internal clock to a period of exactly 24 h. The light-dark cycle, food, ambient temperature, scents and social cues have been identified as environmental synchronizers or "Zeitgebers" in rats [29,30]. Stress is also capable of perturbing temporal organization by affecting the shape and amplitude of a rhythm or by modifying the intrinsic oscillatory mechanism itself. In particular, social stress in rodents has been found to cause disruptions of the body temperature, heart rate and locomotor activity rhythms [31-33].
Further experiments are needed to assess whether the changes in amplitude as well in timing of 24-h rhythms of prolactin and testosterone secretion seen in socially isolated rats can be attributed to an effect on the endogenous clock that modulates the circadian variation of pituitary testicular hormones or to a masking effect on some output(s) of the clock. Likewise, to what extent the differences between group- and single-housed rats in the plasma levels of the various hormones can affect reproduction or the immune response should be further explored. Our results concerning FSH plasma levels indicate that the increase in secretion induced by isolation is several fold larger than the daily variation in group-housed animals (Fig. 2), which suggests that measurements of FSH secretion in isolated animals may misrepresent natural pituitary function in this species.
Conclusions
Secretion of prolactin, LH and testosterone decreases, and secretion of FSH increases, in isolated rats. The maximum in prolactin seen in group-caged rats at the beginning of the activity span is not observed in isolated rats. The maximum in circulating testosterone taking place at the second part of the rest span in controls is phase-delayed to the light-dark transition in isolated rats.
Competing interests
None declared.
Authors' contributions
AIE conceived of the study and supervised its technical implementation. FC and VJ took care of the experimental animals and carried out the immunoassays. CFRY performed the statistical analysis. DPC conceived of the study and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This work was supported by grants from DGES, Spain, Fundación Rodríguez-Pascual, Spain, Agencia Nacional de Promoción Científica y Tecnológica, Argentina (PICT 6153), Fundación Bunge y Born, Argentina and Fundación Antorchas, Argentina. The gift of the reagents to measure prolactin, LH and FSH levels by the NIDDK's National Hormone and Pituitary Program and Dr. A. Parlow (Harbor UCLA Medical Center, Torrance CA) is gratefully acknowledged.
Contributor Information
Ana I Esquifino, Email: pelayos@med.ucm.es.
Fernando Chacón, Email: pelayos@med.ucm.es.
Vanessa Jimenez, Email: pelayos@med.ucm.es.
Carlos F Reyes Toso, Email: creyestoso@intramed.net.ar.
Daniel P Cardinali, Email: cardinal@mail.retina.ar.
References
- Mason JW. A historical view of the stress field. J Hum Stress. 1975;1:6–12. doi: 10.1080/0097840X.1975.9940399. [DOI] [PubMed] [Google Scholar]
- Mason JW. A historical view of the stress field. J Hum Stress. 1975;1:22–36. doi: 10.1080/0097840X.1975.9940405. [DOI] [PubMed] [Google Scholar]
- Selye H. Confusion and controversy in the stress field. J Hum Stress. 1975;1:37–44. doi: 10.1080/0097840X.1975.9940406. [DOI] [PubMed] [Google Scholar]
- Sachser N. Short-term responses of plasma norepinephrine, epinephrine, glucocorticoid and testosterone titers to social and non-social stressors in male guinea pigs of different social status. Physiol Behav. 1987;39:11–20. doi: 10.1016/0031-9384(87)90338-6. [DOI] [PubMed] [Google Scholar]
- Gentsch C, Lichtsteiner M, Kraeuchi K, Feer H. Different reaction patterns in individually and socially reared rats during exposures to novel environments. Behav Brain Res. 1982;4:45–54. doi: 10.1016/0166-4328(82)90163-2. [DOI] [PubMed] [Google Scholar]
- Holson RR, Scallet AC, Ali SF, Turner BB. isolation stress revisited: Isolation-rearing effects depend on animal care methods. Physiol Behav. 1991;49:1107–1118. doi: 10.1016/0031-9384(91)90338-O. [DOI] [PubMed] [Google Scholar]
- Castro WLR, Matt KS. Neuroendocrine correlates of separation stress in the Siberian dwarf hamster (Phodopus sungorus) Physiol Behav. 1997;61:477–484. doi: 10.1016/S0031-9384(96)00456-8. [DOI] [PubMed] [Google Scholar]
- Rao AM, Purushotham K. Adreno-gonadal responses to crowding stress in the Indian field mouse, Mus booduga. Indian J Comp Physiol. 1984;2:4–7. [Google Scholar]
- Viveros MP, Hernandez R, Martinez I, Gonzalez P. Effects of social isolation and crowding upon adrenocortical reactivity and behavior in the rat. Rev Esp Fisiol. 1988;44:315–321. [PubMed] [Google Scholar]
- Brain PF. What does individual housing mean to a mouse? Minireview. Life Sci. 1975;16:187–200. doi: 10.1016/0024-3205(75)90017-X. [DOI] [PubMed] [Google Scholar]
- Baurmel I, de Feo JJ, Lal H. Alterations in brain sensitivity and barbiturate metabolism unrelated to aggression in socially deprived mice. Psychopharmacology. 1978;18:320–324. doi: 10.1007/BF00412678. [DOI] [PubMed] [Google Scholar]
- Giralt M, Armario A. Individual housing does not influence the adaptation of the pituitary adrenal axis and other physiological variables to chronic stress in adult male rats. Physiol Behav. 1989;45:477–481. doi: 10.1016/0031-9384(89)90061-9. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Barnard J, Tolladay U, Nevison CM, West CD. Housing and welfare in laboratory rats: effects of cage stocking density and behavioural predictors of welfare. Animal Behav. 1999;58:563–586. doi: 10.1006/anbe.1999.1165. [DOI] [PubMed] [Google Scholar]
- Rivier C, Rivest S. Effect of stress on the activity of the hypothalamic pituitary gonadal axis: Peripheral and central mechanisms. Biol Reprod. 1991;45:523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- Dunn JD, Arimura A, Scheving LE. Effect of stress on circadian periodicity in serum LH and prolactin concentration. Endocrinology. 1972;90:29–33. doi: 10.1210/endo-90-1-29. [DOI] [PubMed] [Google Scholar]
- Gambardella P, Greco AM, Sticchi R, Bellotti R, Di Renzo G. Individual housing modulates daily rhythms of hypothalamic catecholaminergic system and circulating hormones in adult male rats. Chronobiol Int. 1994;11:213–221. doi: 10.3109/07420529409067790. [DOI] [PubMed] [Google Scholar]
- Garcia Bonacho M, Esquifino AI, Castrillon P, Reyes Toso C, Cardinali DP. Age-dependent effect of Freund's adjuvant on 24-hour rhythms in plasma prolactin, growth hormone, thyrotropin, insulin, follicle-stimulating hormone, luteinizing hormone and testosterone in rats. Life Sciences. 2000;66:1969–1977. doi: 10.1016/S0024-3205(00)00522-1. [DOI] [PubMed] [Google Scholar]
- Castrillon P, Cardinali DP, Pazo D, Cutrera RA, Esquifino AI. Effect of superior cervical ganglionectomy on 24-h variations in hormone secretion from anterior hypophysis and in hypothalamic monoamine turnover, during the preclinical phase of Freund's adjuvant arthritis in rats. Journal of Neuroendocrinology. 2001;13:288–295. doi: 10.1046/j.1365-2826.2001.00627.x. [DOI] [PubMed] [Google Scholar]
- Sayegh JF, Kobor G, Lajtha A, Vadasz C. Effects of social isolation and the time of day on testosterone levels in plasma of C57BL/6By and Balb/cBy mice. Steroids. 1990;55:79–82. doi: 10.1016/0039-128X(90)90029-B. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Moore TO, Mougey EH, Meyerhoff JL. Hormonal responses to fighting in hamsters: Separation of physical and psychological causes. Physiol Behav. 1992;51:1083–1086. doi: 10.1016/0031-9384(92)90097-L. [DOI] [PubMed] [Google Scholar]
- Matt KS, Soares MJ, Talamantes F, Bartke A. Effects of handling and ether anesthesia on serum prolactin levels in the golden hamster. Proc Soc Exp Biol Med. 1983;173:463–466. doi: 10.3181/00379727-173-4-rc1. [DOI] [PubMed] [Google Scholar]
- Vera-Lastra O, Jara LJ, Espinoza LR. Prolactin and autoimmunity. Autoimmun Rev. 2002;1:360–364. doi: 10.1016/S1568-9972(02)00081-2. [DOI] [PubMed] [Google Scholar]
- Yu-Lee LY. Prolactin modulation of immune and inflammatory responses. Recent Prog Horm Res. 2002;57:435–455. doi: 10.1210/rp.57.1.435. [DOI] [PubMed] [Google Scholar]
- Kruger TH, Haake P, Hartmann U, Schedlowski M, Exton MS. Orgasm-induced prolactin secretion: feedback control of sexual drive? Neurosci Biobehav Rev. 2002;26:31–44. doi: 10.1016/S0149-7634(01)00036-7. [DOI] [PubMed] [Google Scholar]
- Torner L, Neumann ID. The brain prolactin system: involvement in stress response adaptations in lactation. Stress. 2002;5:249–257. doi: 10.1080/1025389021000048638. [DOI] [PubMed] [Google Scholar]
- Wynne-Edwards KE. Hormonal changes in mammalian fathers. Horm Behav. 2001;40:139–145. doi: 10.1006/hbeh.2001.1699. [DOI] [PubMed] [Google Scholar]
- De Rosa M, Zarrilli S, Di Sarno A, Milano N, Gaccione M, Boggia B, Lombardi G, Colao A. Hyperprolactinemia in men: clinical and biochemical features and response to treatment. Endocrine. 2003;20:75–82. doi: 10.1385/ENDO:20:1-2:75. [DOI] [PubMed] [Google Scholar]
- de Kretser DM, Loveland KL, Meehan T, O'Bryan MK, Phillips DJ, Wreford NG. Inhibins, activins and follistatin: actions on the testis. Mol Cell Endocrinol. 2001;180:87–92. doi: 10.1016/S0303-7207(01)00502-0. [DOI] [PubMed] [Google Scholar]
- Moore-Ede MC. Physiology of the circadian system: Predictive versus reactive homeostasis. Am J Physiol. 1986;250:R737–R752. doi: 10.1152/ajpregu.1986.250.5.R737. [DOI] [PubMed] [Google Scholar]
- Hastings MH. Circadian clocks: self-assembling oscillators? Curr Biol. 2003;13:R681–R682. doi: 10.1016/S0960-9822(03)00608-0. [DOI] [PubMed] [Google Scholar]
- Greco AM, Gambardella P, Sticchi R, D'Aponte D, Di Renzo G, de Franciscis P. Effects of individual housing on circadian rhythms of adult rats. Physiol Behav. 1989;45:363–366. doi: 10.1016/0031-9384(89)90141-8. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Pozzato C, Meerlo P, Costoli T, Manghi M, Stilli D, Olivetti G, Musso E. Intermittent exposure to social defeat and open-field test in rats: acute and long-term effects on ECG, body temperature and physical activity. Stress. 2002;5:23–35. doi: 10.1080/102538902900012387. [DOI] [PubMed] [Google Scholar]
- Spani D, Arras M, Konig B, Rulicke T. Higher heart rate of laboratory mice housed individually vs in pairs. Lab Anim. 2003;37:54–62. doi: 10.1258/002367703762226692. [DOI] [PubMed] [Google Scholar]