Abstract
Two isoforms of inositide-dependent phospholipase C β1 (PI-PLCβ1) are generated by alternative splicing (PLCβ1a and PLCβ1b). Both isoforms are present within the nucleus, but in contrast to PLCβ1a, the vast majority of PLCβ1b is nuclear. In mouse erythroid leukemia cells, PI-PLCβ1 is involved in the regulation of cell division and the balance between cell proliferation and differentiation. It has been demonstrated that nuclear localization is crucial for the enzymatic function of PI-PLCβ1, although the mechanism by which this nuclear import occurs has never been fully characterized. The aim of this study was to characterize both the mechanism of nuclear localization and the molecular function of nuclear PI-PLCβ1 by identifying its interactome in Friend's erythroleukemia isolated nuclei, utilizing a procedure that coupled immuno-affinity purification with tandem mass spectrometry analysis. Using this procedure, 160 proteins were demonstrated to be in association with PI-PLCβ1b, some of which have been previously characterized, such as the splicing factor SRp20 (Srsf3) and Lamin B (Lmnb1). Co-immunoprecipitation analysis of selected proteins confirmed the data obtained via mass spectrometry. Of particular interest was the identification of the nuclear import proteins Kpna2, Kpna4, Kpnb1, Ran, and Rangap1, as well as factors involved in hematological malignancies and several anti-apoptotic proteins. These data give new insight into possible mechanisms of nuclear trafficking and functioning of this critical signaling molecule.
Phosphoinositide-dependent phospholipase C β 1 isoform b (PI-PLCβ1b)1 is one of two existing isoforms of PI-PLCβ1 produced by alternative splicing (1). PI-PLCβ1a (150 kDa) and PI-PLCβ1b (140 kDa) differ only at their carboxyl termini; PI-PLCβ1b is 43 amino acids shorter than PI-PLCβ1a. Both isoforms present with a non-canonical nuclear localization signal comprising a cluster of lysine residues (2). In murine erythroleukemia (MEL) cells, both isoforms are present within the nucleus; however, in contrast to PI-PLCβ1a, PI-PLCβ1b is almost exclusively nuclear.
Nuclear PI-PLCβ1 is known to be involved in specific signal transduction pathways that differ from those occurring in other cellular compartments. The role of PI-PLCβ1 has been extensively studied in the nucleus, and PI-PLCβ1 is now considered a key co-factor for several nuclear processes, including cell growth, proliferation, and differentiation.
In MEL cells, PI-PLCβ1 is involved in regulating G1/S (3) and G2/M (4) cell cycle progression. During G1/S transition, overexpression of PI-PLCβ1 results in up-regulation of the cyclin D3/cdk4 complex. This complex is responsible for the phosphorylation of retinoblastoma protein and the subsequent activation of the E2F-1 transcription factor, forcing cells out of the G1 phase of the cell cycle. In G2/M, the production of inositol-3-phosphate and diacylglycerol (DAG) from the cleavage of phosphatidylinositol-4,5-bisphosphate results in PKCα-dependent phosphorylation of lamin B, leading to nuclear envelope disassembly and cell cycle progression. The regulation of these events falls to the activation of JNK, which translocates to the nucleus and mediates PI-PLCβ1 phosphorylation and activation and DAG production.
PI-PLCβ1 is also implicated in hematopoietic (5–8), skeletal muscle (9, 10), and adipocyte (11) differentiation. In particular, PI-PLCβ1 has different effects depending on the cell type, promoting the differentiation of myoblasts to myotubes (12) and of pre-adipocytes to adipocytes (11) but inhibiting the in vitro erythroid differentiation of both MEL cells and human CD34+ cells (5, 8).
Indeed, nuclear PI-PLCβ1-induced signaling constitutes an autonomous lipid-dependent signaling system independent from its plasma membrane counterpart. The understanding of the nuclear protein network behind PI-PLCβ1 function might give insight as to the downstream target effectors and further clarify its nuclear signaling cascade.
To date, our group has identified few protein targets that physically associate with PI-PLCβ1 in the nucleus, but a systematic analysis of the PI-PLCβ1 protein interactome has not been undertaken previously. By means of immune-affinity purification–mass spectrometry analysis (AP-MS), we identified 160 proteins in complex with PI-PLCβ1b in the nucleus of MEL cells. Among these, we found two interactors already known to associate with PI-PLCβ1, Srsf3 and Lmnb1.
EXPERIMENTAL PROCEDURES
Cells, Antibodies, and Reagents
Murine erythroid leukemia cells (MEL, Friend cells, clone 707) were maintained (105 cells/ml) in RPMI 1640 (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal calf serum (FCS) and 200 mm l-glutamine (Invitrogen, Gaithersburg, MD) at 37 °C. Cells were cultured for 48 h and collected via centrifugation, and nuclei were isolated as previously described (3). The bone-marrow-derived interleukin-3-dependent Ba/F3 cell line was maintained in culture in Fischer's medium supplemented with 10% (v/v) FCS, 2 mm l-glutamine (Invitrogen, Gaithersburg, MD), and 5% (v/v) mIL-3 (conditioned media from X63-Ag-653 cells). The antibodies used are detailed in supplemental Table S1. Protein A/G agarose slurry was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). All other reagents were procured from Sigma-Aldrich.
Cloning and Infection
The full-length cDNA coding for rat PI-PLCβ1b was excised from the expression vector pRc/CMV (Invitrogen, Valencia, CA) and inserted into the retroviral vector pBB-IRES-blast® using BamHI and EcoRI restriction sites. Retroviral infection was carried-out as described by Somervaille and Cleary (13). Briefly, Phi-NxAmpho packaging cells were cultured in DMEM with 10% FCS and transfected with pBB-IRES-blast® or pBB-PI-PLCβ1b-IRES-blast®. After 48 h of incubation at 37 °C, cells were spinoculated with retroviral supernatant in the presence of 5 μg/ml polybrene for 2 h at 1350 × g and 32 °C. Following spinoculation, cells were incubated overnight in the above mentioned medium to allow the expression of blasticidin resistance in transduced cells prior to selection in 4 μg/ml blasticidin for 5 days. Selected cells were given the following nomenclature: MEL/pBBev (vector control) and MEL/PI-PLCβ1b.
Immunoprecipitation for Mass Spectrometry
Isolated nuclei were lysed in radioimmune precipitation assay buffer containing the appropriate phosphatase inhibitor cocktails, calpain I and calpain II inhibitors (Merck Millipore), and benzonase as described elsewhere (14). Glycerol was added to 10% of the lysate volume. Cleared lysates (2 mg) for each control and test sample were then pre-cleared for 1.5 h at 4°C by the addition of 4 μg of nonspecific IgG, and then 20 μl of Protein A/G agarose beads were added for 2 h of incubation. The pre-cleared lysates were centrifuged and transferred to a new microfuge tube. 4 μg of either nonspecific IgG (control) or PI-PLCβ1 specific antibody was added and allowed to complex overnight at 4°C. Fresh Protein A/G agarose beads (20 μl) were added for the last 2 h of incubation. Samples were washed three times in immunoprecipitation wash buffer (10 mm Tris-HCl, pH 7.4, and 1% Nonidet P-40) and then resuspended in 30 μl immunoprecipitation wash buffer containing 10 μl of 3X protein sample buffer. Immunoprecipitates were separated via 4%–15% gradient SDS-PAGE, and the gels were then stained with Coomassie Blue. Each individual lane was fractionated into 2-mm slices for analysis. Additionally, a small fraction of the immunoprecipitate (5 μl) was separated by means of 8% SDS-PAGE, transferred, and immunoblotted with anti-PI-PLCβ1 as a control for PLCβ1b precipitation. Nuclear purity was assessed based on the absence of β-tubulin.
Mass Spectrometry Analysis
Gel slices were washed in 100 mm ammonium bicarbonate (pH 8) and 50% acetonitrile until complete destaining and then digested with sequencing grade trypsin (Promega, Madison, WI) at 37 °C. After overnight incubation, peptides were extracted sequentially three times with 50% acetonitrile and 0.1% formic acid in water. Each extraction involved 5 min of stirring followed by centrifugation and removal of the supernatant. The original supernatant and those obtained from sequential extractions were combined and completely dried down.
Dried peptide fractions were resuspended in 20 μl of 2% (v/v) acetonitrile and 0.1% (v/v) formic acid, and a 1-μl aliquot, corresponding to 1 pmol/μl, was loaded with a 4-μl/min flow rate onto a QTOF G6520 (Agilent Technologies Inc., Santa Clara, CA) equipped with a 1200 series capillary pump, a 1200 series nano pump, and the Chip Cube system. The LC-Chip (Agilent Technologies Inc.) consisted of a Zorbax 300SB-C18 enrichment column (4 mm × 40 nl, 5 μm) and a Zorbax 300SB-C18 analytical column (75 mm × 43 mm, 5 μm).
Elution from the analytical column was performed using a binary solvent mixture composed of 3% (v/v) acetonitrile plus 0.1% (v/v) formic acid (solvent A) and 97% (v/v) acetonitrile plus 0.1% formic acid (solvent B) at a flow rate of 0.4 μl/min with the following gradient: from 4% to 30% B in 17 min, from 30% to 40% B in 3 min, from 40% to 85% B in 3 min, and 85% B for 2 min.
Data were acquired in data-dependent MS/MS mode in which, for each cycle, the three most abundant multiply charged peptides (2+ to 4+) above an absorbance threshold of 200 in the MS scan (m/z full scan acquisition range from 250 to 2450) were selected for MS/MS (m/z tandem mass spectrum acquisition range from 50 to 3200). Each peptide was selected twice and then dynamically excluded for 0.1 min.
For peptide sequence searching, the mass spectra were processed and analyzed using either Mascot v.2.2 (Matrix Science, London, UK) or X!Tandem (integrated within the Trans Proteomic Pipeline, v.4.5 Rapture, rev. 2, Institute for Systems Biology, Seattle, WA) against the mouse-derived UniProtKB–SwissProt database (April 23, 2012; total of 535,698 entries) concatenated with a decoy database constructed by randomizing all of the protein sequences present in the mouse reference database. The search parameters were as follows: one missed cleavage allowed, carbamidomethylation of cysteine as a fixed modification, oxidation of methionines as a variable modification, precursor ion mass tolerance of 10 ppm (Mascot) or 20 ppm (X!Tandem), and fragment ion tolerance of 0.12 Da.
Data Analysis
The Trans Proteomic Pipeline was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95% probability as specified by the Peptide Prophet algorithm (15). Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony (16). Proteins assigned by one identified peptide were accepted only if the peptide was unique and the probability was greater than 99%. Biological replicates were analyzed within iProphet (17), and the probability scores were adjusted according to the number of replicate spectra, the number of sibling ions, the number of sibling modifications, and the number of sibling experiments. The resulting false discovery rate for these experiments was determined to be less than 1%. Gene Ontology classification was performed using the plug-in BiNGO v2.42 within Cytoscape v.2.8.2, assessing over-represented categories with a hypergeometric statistical test and Benjamini & Hochberg False Discovery Rate correction (p < 0.05). As a reference set, the whole mouse repository annotation was used.
Western Blot and Immunoprecipitation Analysis
Nuclear protein lysates were prepared as described elsewhere (3). Briefly, cells were lysed in radioimmune precipitation assay buffer containing protease and phosphatase inhibitor cocktails, calpain I and calpain II inhibitors, and benzonase. The protein concentration was determined via Bradford Protein Assay (Bio-Rad), and 60 μg of nuclear lysate were separated via SDS-PAGE. For validation analysis, 800 μg of nuclear proteins were used, and the immunoprecipitation was carried-out as for mass spectrometry analysis. Gels were transferred onto nitrocellulose membranes and blocked in 5% non-fat dry milk in 1X PBS containing 0.1% Tween-20 (PBST) and then incubated in primary antibody in 1% non-fat dry milk in PBST. Membranes were washed in 1X PBST and incubated for 1 h in the appropriate secondary antibody in 1% non-fat dry milk in PBST. The blots were washed three times with 1X PBST, detected using SuperSignal West Pico Reagent (Pierce, Rockford, IL), and visualized in a ChemiDoc digital image station (Bio-Rad, Hercules, CA). Antibody working dilutions are reported in supplemental Table S1.
RESULTS
AP-MS/MS Identification of Proteins Interacting with PLCβ1b in the Nucleus
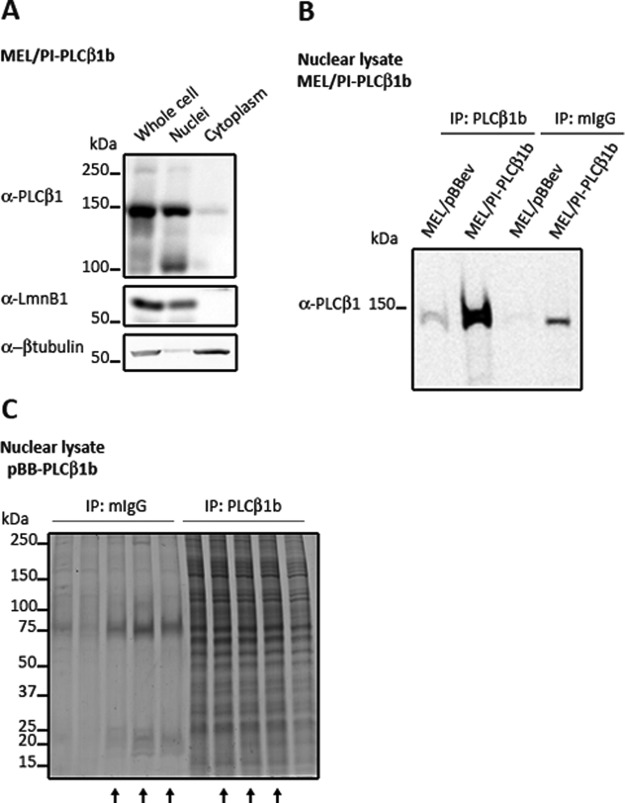
MEL/PI-PLCβ1b cells were obtained by infecting MEL cells with a retroviral vector containing the coding sequence for PI-PLCβ1b and selection in the presence of blasticidin. Obtained clones overexpressed full-length PI-PLCβ1b almost exclusively in the nucleus (Fig. 1A). In order to determine what proteins interact with PI-PLCβ1b in the nucleus, three independent biological experiments were conducted in which nuclei of MEL/PI-PLCβ1b cells were isolated during logarithmic growth. The PI-PLCβ1b complex was affinity purified via immunoprecipitation of MEL/PI-PLCβ1b nuclear lysates. As a control, the normal mouse IgG complex was immunoprecipitated from the same cell line. In order to control for antibody specificity and protein binding, a small fraction of the immunoprecipitates (corresponding to 200 μg of nuclear lysate) was analyzed by means of SDS-PAGE and Western blotting with anti-PI-PLCβ1 (Fig. 1B). The remainder of both the immunoprecipitates (PI-PLCβ1b and mouse IgG control) was separated via SDS-PAGE and Coomassie stained (Fig. 1C). Three lanes from both MEL/pBBev and MEL/PI-PLCβ1b were completely sectioned, and each section was subjected to nano-liquid chromatography electrospray ionization MS/MS to identify the associated proteins. The majority of proteins were identified by at least two distinct peptides; however, proteins identified with a single unique peptide with a probability greater than 99% were also included and are indicated with an asterisk in the tables and text. In total, 160 proteins were discovered to be in complex with PLCβ1b but not with the IgG control (Table I and supplemental Table S2). Two of the proteins identified, Srsf3 (splicing factor SRp20) and Lmnb1, have already been established as PI-PLCβ1 interactants in the nucleus (4, 18).
Fig. 1.
Stable overexpression of PI-PLCβ1b in MEL cells and isolation of PI-PLCβ1b complexes in the nucleus. A, PI-PLCβ1b was cloned into the retroviral vector pBB-IRES-blast® and infected into MEL cells. A clone that stably overexpressed PI-PLCβ1b was selected. 1 × 107 cells were used to isolate nuclei and cytoplasm. 60 μg of cellular, nuclear, and cytoplasmic lysates were separated in 8% SDS-PAGE. Immunoblotted proteins were revealed with anti-PI-PLCβ1 antibody. Lamin B and β-tubulin were used to assess nuclear integrity and purity, respectively. B, specific PI-PLCβ1 antibody was used to isolate potential interactors from the nuclei of MEL cells specifically overexpressing PI-PLCβ1b. A small aliquot of the immunoprecipitate was immunoblotted with anti-PI-PLCβ1 antibody to verify the specificity of the immunoprecipitation. For antibody details, refer to supplemental Table S1. mIgG, mouse IgG. C, immunoprecipitated complex was separated via SDS-PAGE and detected with Coomassie Colloidal Blue. Five independent biological replicates were performed to assess the affinity purification reproducibility. Three lanes from both MEL/pBBev and MEL/PI-PLCβ1b were completely fractionated (indicated by arrows), digested, and subjected to nano-liquid chromatography MS/MS for protein identification.
Table I. List of proteins identified in complex with PI-PLCβ1b in the nucleus of MEL cells.
| Acc.nb. | Gene name | Description | Prot prob | Cov % | GO biological process | GO molecular function | GO cellular component |
|---|---|---|---|---|---|---|---|
| Q9Z2N8 | Actl6a | Actin-like protein 6A | 0.9993 | 7.5 | Chromatin remodeling; growth regulation; transcription | ATP binding; chromatin binding | N |
| Q8BK64 | Ahsa1 | Activator of 90-kDa heat shock protein ATPase homolog 1 (*) | 0.9922 | 6.5 | Protein folding; response to stress | ATPase activator | ER; C |
| P05064 | Aldoa | Fructose-bisphosphate aldolase A | 1 | 17.6 | Glycolysis; protein homotetramerization | Aldolase activity | M |
| Q9EST5 | Anp32b | Acidic leucine-rich nuclear phosphoprotein 32 family member B, isoform 2 | 1 | 11.8 | Progression from the G1 to the S phase | Unannotated | N |
| P48036 | Anxa5 | Annexin 5 | 1 | 9.7 | Blood coagulation; apoptosis | Ca2+-dependent phospholipid binding | C |
| P28352 | Apex1 | DNA-(apurinic or apyrimidinic site) lyase | 1 | 9 | DNA demethylation; S phase of mitotic cell cycle; DNA repair; apoptosis | 3′-5′ exonuclease activity; RNA binding | S; N; C |
| P61205 | Arf3 | ADP-ribosylation factor 3 | 0.9969 | 23.3 | Protein transport; small GTPase mediated signal transduction | GTP binding | pN; G |
| P61750 | Arf4 | ADP-ribosylation factor 4 (§) | 0 | See Arf3 | See Arf3 | See Arf3 | |
| Q9D5T0 | Atad1 | ATPase family AAA domain-containing protein 1 (*) | 0.999 | 4.4 | ATP synthesis; transport | ATP binding; ATPase activity | M; Mt |
| Q03265 | Atp5a1 | ATP synthase subunit alpha | 1 | 9.9 | ATP metabolic process; lipid metabolic process | ATP/ADP binding | Mt; M |
| Q9CQQ7 | Atp5f1 | ATP synthase subunit b (*) | 0.999 | 5.9 | ATP synthesis coupled proton transport | ATPase activity | Mt; M |
| Q9DCX2 | Atp5h | ATP synthase subunit d | 1 | 33.5 | ATP synthesis coupled proton transport | TM transporter activity | Mt; M |
| Q64152 | Btf3 | Transcription factor BTF3, isoform 2 | 1 | 40.7 | Regulation of transcription | Unannotated | N |
| P13634 | Ca1 (Car1) | Carbonic anhydrase 1 | 1 | 13.8 | Metabolic process | Dehydratase activity | C |
| P00920 | Ca2 (Car2) | Carbonic anhydrase 2 (*) | 0.999 | 6.2 | Metabolic process; regulation of osteoclast differentiation | Dehydratase activity | C |
| P80314 | Cct2 | T-complex protein 1 subunit beta | 1 | 6.9 | Protein folding | ATP binding | N |
| P80313 | Cct7 | T-complex protein 1 subunit eta | 0.999 | 6.6 | Protein folding | ATP binding | Mt |
| P60766 | Cdc42 | Cell division control protein 42 homolog (*) | 0.9931 | 11.6 | Signaling transduction | GTP binding; GTPase activity | Cs; C |
| P18760 | Cfl1 | Cofilin-1 | 1 | 37.3 | Actin filament organization; protein import into the nucleus | Unannotated | N; C |
| Q6PDQ2 | Chd4 | Chromodomain-helicase-DNA-binding protein 4 | 1 | 2 | Regulation of transcription; chromatin modification | ATP binding | N |
| A2A8L1 | Chd5 | Chromodomain helicase DNA binding protein 5 (§) | 0 | See Cdh4 | See Cdh4 | See Cdh4 | |
| Q6ZQ08 | Cnot1 | CCR4-NOT transcription complex subunit 1, isoform 2 | 1 | 3.1 | Regulation of transcription | Unannotated | C |
| Q6NVF9 | Cpsf6 | Cleavage and polyadenylation specificity factor subunit 6 (*) | 0.9967 | 4.5 | mRNA polyadenylation; mRNA processing | mRNA binding | PS; N |
| Q60737 | Csnk2a1 | Casein kinase II subunit alpha | 1 | 20.7 | Wnt receptor signaling pathway; cell cycle; regulation of transcription; apoptosis | ATP binding; protein Ser/Thr kinase activity | N |
| Q99LI7 | Cstf3 | Cleavage stimulation factor subunit 3 (*) | 0.9949 | 2.2 | mRNA processing | Unannotated | N |
| P16381 | D1Pas1 | Putative ATP-dependent RNA helicase Pl10 (§) | 0 | Cell differentiation | ATP binding | ||
| Q62095 | Ddx3y | ATP-dependent RNA helicase DDX3Y | 1 | 7.9 | Unannotated | ATP binding | N; C |
| O70133 | Dhx9 | ATP-dependent RNA helicase A, isoform 2 | 0.9961 | 2.8 | Cellular response to heat | ATP binding | Nl |
| P54103 | Dnajc2 | DnaJ homolog subfamily C member 2 | 1 | 5.7 | Regulation of transcription; G2 phase of mitotic cell cycle | DNA binding | N; C |
| P10126 | Eef1a | Elongation factor 1-alpha | 0.9998 | 7.8 | Protein biosynthesis | GTP binding | N; C |
| Q6ZWX6 | Eif2s1 | Eukaryotic translation initiation factor 2 subunit 1 | 1 | 27.3 | Protein autophosphorylation; regulation of translation initiation | Ribosome binding | N; C |
| Q9Z1D1 | Eif3g | Eukaryotic translation initiation factor 3 subunit G | 1 | 14.1 | Translational initiation | Nucleotide binding | N; C |
| Q9QZD9 | Eif3i | Eukaryotic translation initiation factor 3 subunit I | 1 | 6.8 | Translational initiation | Initiation factor activity | C |
| Q9DBZ5 | Eif3k | Eukaryotic translation initiation factor 3 subunit K | 1 | 18.8 | Regulation of translational initiation | Ribosome binding | N |
| Q99JX4 | Eif3m | Eukaryotic translation initiation factor 3 subunit M | 1 | 19.8 | Unannotated | Initiation factor activity | C |
| P10630 | Eif4a2 | Eukaryotic initiation factor 4A-II, isoform 2 | 0.9999 | 9.8 | Translational initiation | ATP binding | |
| Q8BGD9 | Eif4b | Eukaryotic translation initiation factor 4 subunit B | 1 | 10.5 | Protein synthesis | Nucleotide binding | |
| P63242 | Eif5a | Eukaryotic translation initiation factor 5A-1 | 1 | 23.4 | Apoptosis; mRNA transport; translation; nucleocytoplasmic transport; cell proliferation | Ribosome binding | N; C |
| Q8BGY2 | Eif5a2 | Eukaryotic translation initiation factor 5A-2 (§) | 0 | Transport; translational elongation; cell proliferation | Ribosome binding | Np | |
| P70372 | Elavl1 | ELAV-like protein 1 | 1 | 9.5 | 3′-UTR-mediated mRNA stabilization; positive regulation of translation | Nucleotide binding | N; C |
| Q8BTM8 | Flna | Filamin-A | 1 | 2.9 | Establishment of protein localization; positive regulation of I-kappaB kinase/NF-kappaB cascade | Signal transducer activity | N; C |
| P35922 | Fmr1 | Fragile X mental retardation protein 1 homolog, isoform ISO10 | 1 | 7.7 | Negative regulation of translational initiation; mRNA transport | RNA binding | N; C |
| Q9CY57 | Fop | Friend of PRMT1 protein, isoform 2 | 1 | 14.7 | Regulation of transcription | RNA binding | N |
| P97855 | G3bp1 | Ras GTPase-activating protein-binding protein 1 | 1 | 8.8 | Wnt receptor signaling pathway; transport | DNA binding | N; C |
| P06745 | Gpi | Glucose-6-phosphate isomerase | 1 | 5.7 | Angiogenesis; glycolysis | Isomerase activity | C |
| P0C0S6 | H2afz | Histone H2A.Z | 1 | 20.3 | Nucleosome assembly | DNA binding | N |
| Q8VDJ3 | Hdlbp | Vigilin | 1 | 7.2 | Transport; lipid metabolic process; lipid transport | RNA binding | N; C |
| Q64525 | Hist2h2bb | Histone H2B type 2-B | 1 | 40.5 | Nucleosome assembly | DNA binding | N |
| P70696 | Hist1h2ba | Histone H2B type 1-A (§) | 0 | Inflammatory response; nucleosome assembly | DNA binding | N | |
| Q64524 | Hist2h2be | Histone H2B type 2-E (§) | 0 | Nucleosome assembly | DNA binding | N | |
| Q8CGP0 | Hist3h2bb | Histone H2B type 3-B (§) | 0 | Nucleosome assembly | DNA binding | N | |
| Q8CGP1 | Hist1h2bk | Histone H2B type 1-K (§) | 0 | Nucleosome assembly | DNA binding | N | |
| P22907 | Hmbs | Porphobilinogen deaminase, isoform 2 | 0.9988 | 11.3 | Heme biosynthesis; porphyrin biosynthesis | Amine binding; coenzyme binding | |
| P63158 | Hmgb1 | High mobility group protein B1 | 1 | 17.7 | Apoptosis; cell proliferation; cell differentation | DNA binding; phosphatidylserine binding | N; C |
| P30681 | Hmgb2 | High mobility group protein B2 | 0.944 | 9 | DNA metabolic process; positive regulation of erythrocyte differentiation; regulation of transcription | DNA binding | N; C |
| O54879 | Hmgb3 | High mobility group protein B3 | 0.999 | 17 | Regulation of cell differentiation | DNA binding | N |
| Q8BG05 | Hnrnpa3 | Heterogeneous nuclear ribonucleoprotein A3 | 1 | 31 | mRNA processing; mRNA splicing | RNA binding | N |
| P49312 | Hnrnpa1 | Heterogeneous nuclear ribonucleoprotein A1 (§) | 1 | 27.2 | Alternative nuclear mRNA splicing; transport | RNA binding | N; C |
| Q99020 | Hnrnpab | Heterogeneous nuclear ribonucleoprotein A/B | 0.9999 | 12.7 | Transcription | DNA/RNA binding | N; C |
| Q9Z204 | Hnrnpc | Heterogeneous nuclear ribonucleoproteins C1/C2, isoform C1 | 1 | 13.7 | RNA splicing; mRNA processing | mRNA binding | N |
| Heterogeneous nuclear ribonucleoproteins C1/C2, isoform 3 (§) | 0 | RNA splicing; mRNA processing | mRNA binding | N | |||
| P61979 | Hnrnpk | Heterogeneous nuclear ribonucleoprotein K, isoform 2 | 1 | 22.1 | RNA splicing; regulation of transcription | RNA binding | N; C |
| Q3TEA8 | Hp1bp3 | Heterochromatin protein 1-binding protein 3, isoform 2 | 0.9996 | 7.5 | Nucleosome assembly | DNA binding | N |
| P38647 | Hspa9 | Stress-70 protein | 0.9976 | 8.2 | Protein folding; response to stress; protein export from nucleus | ATP binding | Mt; Nl |
| P52293 | Kpna2 | Importin subunit alpha-2 | 0.9994 | 7.5 | Protein import into nucleus; NLS-bearing substrate import into nucleus | Protein transporter activity | N; C |
| O35343 | Kpna4 | Importin subunit alpha-4 (*) | 0.9917 | 3.3 | Protein import to the nucleus | Protein transporter activity | N; C |
| P70168 | Kpnb1 | Importin subunit beta-1 | 1 | 12.4 | Protein import to the nucleus | Protein transporter activity | N; C |
| P14733 | Lmnb1 | Lamin-B1 | 1 | 11.6 | G2/M-specific positive regulation of cyclin-dependent protein kinase activity; positive regulation of JNK cascade | Structural molecule activity | N |
| Q922Q8 | Lrrc59 | Leucine-rich repeat-containing protein 59 | 1 | 35.5 | Unannotated | Unannotated | M; ER |
| Q08288 | Lyar | Cell-growth-regulating nucleolar protein (*) | 0.999 | 10.7 | Unannotated | Metal ion binding | Nl |
| Q9Z2D8 | Mbd3 | Methyl-CpG-binding domain protein 3, isoform 2 | 1 | 17.8 | Regulation of transcription; methylation-dependent chromatin silencing | DNA binding; chromatin binding | N; C |
| Q61881 | Mcm7 | DNA replication licensing factor MCM7 | 1 | 12.5 | Cell proliferation; DNA replication; regulation of phosphorylation; cell cycle; response to DNA damage stimulus | ATP binding | N; C |
| P08249 | Mdh2 | Malate dehydrogenase | 1 | 16 | Metabolic process | Dehydrogenase activity | Mt; N |
| P70670 | Naca | Nascent polypeptide-associated complex subunit alpha | 0.9919 | 25.6 | Protein transport; regulation of transcription | DNA binding | N; C |
| Q9DC69 | Ndufa9 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 9 | 1 | 8.5 | Electron transport chain | Nucleotide binding | Mt |
| Q9DCT2 | Nduhs3 | NADH dehydrogenase [ubiquinone] iron-sulfur protein 3 | 1 | 9.9 | Electron transport chain; induction of apoptosis | Dehydrogenase activity | Mt |
| Q9D6J6 | Ndufv2 | NADH dehydrogenase [ubiquinone] flavoprotein 2, isoform 2 (*) | 0.9895 | 6.6 | Cardiac muscle tissue development; mitocondrial electron transport | Metal ion binding | Mt |
| P15532 | Nme1 | Nucleoside diphosphate kinase A | 0.9995 | 17.8 | Negative regulation of myeloid leukocyte differentiation; positive regulation of DNA binding | ATP binding; nucleoside diphosphate kinase activity | Mt; N; C |
| Q61937 | Npm1 | Nucleophosmin 1 | 0.9962 | 22.2 | Regulation of cell cycle; nucleocytoplasmic transport; apoptosis | Ribosomal small and large subunit binding; DNA binding | S; N; C |
| Q9CPP0 | Npm3 | Nucleoplasmin-3 | 0.9999 | 13.1 | rRNA processing; rRNA transcription | Nucleic acid binding | Nl |
| E9Q7G0 | Numa1 | Uncharacterized protein | 0.9996 | 1.8 | Establishment of mitotic spindle orientation (predicted) | Unannotated | N; C |
| Q99JX7 | Nxf1 | Nuclear RNA export factor 1 | 1 | 4.9 | mRNA export from nucleus | Nucleocytoplasmic transporter activity | S; N; C |
| Q9CZ30 | Ola1 | Obg-like ATPase 1, isoform 2 | 0.9995 | 9.6 | ATP catabolic process | ATP binding; GTP binding | C |
| P60335 | Pcbp1 | Poly(rC)-binding protein 1 | 1 | 17.4 | mRNA processing | RNA binding | N |
| Q61990 | Pcbp2 | Poly(rC)-binding protein 2, isoform 2 | 1 | 16 | Innate immune response | DNA/RNA binding | N; C |
| Q3UHX2 | Pdap1 | 28 kDa heat- and acid-stable phosphoprotein | 1 | 13.3 | Unannotated | Unannotated | |
| Q9DBD5 | Pelp1 | Proline-, glutamic acid-, and leucine-rich protein 1 (*) | 0.999 | 1.2 | Transcription | Unannotated | C |
| Q9DBJ1 | Pgam1 | Phosphoglycerate mutase 1 | 1 | 18.9 | Metabolic process; regulation of glycolysis | Biphosphoglycerate mutase and phosphatase activity | C |
| O70250 | Pgam2 | Phosphoglycerate mutase 2 (§) | 0 | Metabolic process; spermatogenesis; striated muscle contraction | Biphosphoglycerate mutase and phosphatase activity | N; C | |
| O35129 | Phb2 | Prohibitin-2 | 1 | 39.5 | Regulation of transcription | Unannotated | Mt; N |
| P17742 | Ppia | Peptidyl-prolyl cis-trans isomerase A | 1 | 26.8 | Protein folding | Peptide binding | N; C |
| Q9CR16 | Ppid | Peptidyl-prolyl cis-trans isomerase D | 0.9948 | 3.2 | Protein folding | Peptide binding | C |
| P62137 | Ppp1ca | Serine/threonine-protein phosphatase PP1-alpha catalytic subunit | 0.9999 | 9.6 | Cell division; glycogen metabolic process; regulation of translation | Protein serine/threonine phosphatase activity | Nl; C |
| P35700 | Prdx1 | Peroxiredoxin-1 | 1 | 22.4 | Cell proliferation; erythrocyte homeostasis; regulation of NF-kB import to the nucleus | Thioredoxin peroxidase activity | Mt; N |
| O08807 | Prdx4 | Peroxiredoxin-4 (§) | 0 | Reactive oxygen species metabolic process | Peroxidase activity | Mt | |
| Q61171 | Prdx2 | Peroxiredoxin-2 | 1 | 27.9 | Activation of MAPK activity; anti-apoptosis; negative regulation of NF-kB | Thioredoxin peroxidase activity | Mt |
| O08709 | Prdx6 | Peroxiredoxin-6 (*) | 0.9938 | 7 | Response to reactive oxygen species; phospholipid catabolic process | Glutathione peroxidase activity | Mt; C |
| P70388 | Rad50 | DNA repair protein RAD50, isoform 2 | 1 | 5.8 | Meiosis; cell cycle; DNA repair | ATP binding; nuclease activity | N |
| DNA repair protein RAD50, isoform 4 (§) | 0 | ||||||
| P62827 | Ran | GTP-binding nuclear protein Ran | 1 | 14.8 | Protein import into nucleus; mitosis; cell cycle; signal transduction | GTP binding; GTPase activity | N; C |
| P46061 | Rangap1 | Ran GTPase-activating protein 1 (*) | 0.9923 | 3 | Signal transduction | Ran GTPase activator activity | N; C |
| Q91VM5 | Rbmxl1 (Rbmxrt) | Heterogeneous nuclear ribonucleoprotein G-like 1 | 0.9995 | 34 | RNA splicing | Chromatin binding; mRNA binding | N |
| P47955 | Rplp1 | 60S acidic ribosomal protein P1 | 0.9998 | 19.3 | Translational elongation | Structural constituent of ribosome | R |
| Q6ZWV3 | Rpl10 | 60S ribosomal protein L10 | 1 | 26.2 | Translation | Structural constituent of ribosome | R |
| Q9CR57 | Rpl14 | 60S ribosomal protein L14 | 0.9999 | 8.8 | rRNA processing; translation | Structural constituent of ribosome | R |
| Q9CZM2 | Rpl15 | 60S ribosomal protein L15 | 1 | 21.6 | Translation | Structural constituent of ribosome | R |
| Q9CPR4 | Rpl17 | 60S ribosomal protein L17 | 1 | 27.2 | Translation | Structural constituent of ribosome | R |
| P62717 | Rpl18a | 60S ribosomal protein L18a | 0.9986 | 10.8 | Translation | Structural constituent of ribosome | R |
| P61358 | Rpl27 | 60S ribosomal protein L27 | 1 | 36 | Translation | Structural constituent of ribosome | R |
| P47915 | Rpl29 | 60S ribosomal protein L29 | 0.9767 | 14.9 | Translation | Structural constituent of ribosome | R |
| P27659 | Rpl3 | 60S ribosomal protein L3 | 1 | 11.4 | Translation | Structural constituent of ribosome | R |
| P62889 | Rpl30 | 60S ribosomal protein L30 | 1 | 10.4 | Translation | Structural constituent of ribosome | R |
| P62900 | Rpl31 | 60S ribosomal protein L31 | 1 | 48.8 | Translation | Structural constituent of ribosome | R |
| P62911 | Rpl32 | 60S ribosomal protein L32 | 1 | 17.8 | Translation | Structural constituent of ribosome | R |
| Q9D8E6 | Rpl4 | 60S ribosomal protein L4 | 1 | 15.3 | Translation | Structural constituent of ribosome | Nl; C |
| P51410 | Rpl9 | 60S ribosomal protein L9 | 1 | 52.1 | Translation | Structural constituent of ribosome | R |
| P99027 | Rplp2 | 60S acidic ribosomal protein P2 | 1 | 38.3 | Translational elongation | Structural constituent of ribosome | R |
| P63325 | Rps10 | 40S ribosomal protein S10 | 1 | 26.7 | Ribosomal small subunit assembly | Structural constituent of ribosome | Nl; C |
| P62843 | Rps15 | 40S ribosomal protein S15 | 1 | 46.8 | rRNA processing; ribosomal small subunit biogenesis; translation | RNA binding; structural constituent of ribosome | C |
| P62245 | Rps15a | 40S ribosomal protein S15a | 0.9999 | 32.3 | Positive regulation of cell cycle and cell proliferation; translation | Structural constituent of ribosome | Mt; C |
| P63276 | Rps17 | 40S ribosomal protein S17 | 0.9999 | 23.7 | Ribosomal small subunit assembly; translational elongation | Structural constituent of ribosome | C |
| P60867 | Rps20 | 40S ribosomal protein S20 | 1 | 36.1 | Translation | Structural constituent of ribosome | R |
| P62267 | Rps23 | 40S ribosomal protein S23 | 1 | 23.8 | Translation | Structural constituent of ribosome | C |
| P62849 | Rps24 | 40S ribosomal protein S24, isoform 2 | 1 | 37.7 | Erythrocyte homeostasis; translational elongation | Nucleotide binding; structural constituent of ribosome | Nl; C |
| P07742 | Rrm1 | Ribonucleoside-diphosphate reductase large subunit | 1 | 5.7 | DNA replication | ATP binding | N |
| Q9CY58 | Serbp1 | Plasminogen activator inhibitor 1 RNA-binding protein, isoform 2 | 1 | 15 | Regulation of anti-apoptosis | mRNA 3′-UTR binding | N |
| Q9EQU5 | Set | Isoform 2 of Protein SET | 0.9902 | 7.5 | Negative regulation of transcription; some assembly | DNA binding | N; C |
| Q99NB9 | Sf3b1 | Splicing factor 3B subunit 1 | 1 | 4.5 | RNA splicing; mRNA processing | Unannotated | N |
| Q8VEM8 | Slc25a3 | Phosphate carrier protein | 0.9913 | 6.2 | Transport | Symporter activity | Mt; C |
| P51881 | Slc25a5 | ADP/ATP translocase 2 | 1 | 34.9 | Transmembrane transport; chromosome segregation | Transporter activity | M; Mt |
| Q3V132 | Slc25a31 | ADP/ATP translocase 4 (§) | 0 | Transmembrane transport | Transporter activity | M; Mt | |
| Q3TKT4 | Smarca4 | Transcription activator BRG1, isoform 2 | 1 | 3.5 | DNA methylation; regulation of transcription, chromatin modification and remodeling | ATP binding; chromatin binding | N |
| Q9CU62 | Smc1a | Structural maintenance of chromosomes protein 1A | 1 | 3.5 | DNA repair; cell cycle | ATP binding; chromatin binding | N |
| P62315 | Snrpd1 | Small nuclear ribonucleoprotein Sm D1 | 0.9999 | 27.7 | RNA splicing; mRNA processing | Unannotated | N; C |
| P62317 | Snrpd2 | Small nuclear ribonucleoprotein Sm D2 | 1 | 23.7 | RNA splicing; mRNA processing | Unannotated | N; C |
| P62320 | Snrpd3 | Small nuclear ribonucleoprotein Sm D3 | 1 | 15.1 | RNA splicing; mRNA processing | Histone pre-mRNA DCP binding | N; C |
| P16546 | Sptan1 (Spna1) | Spectrin alpha chain, brain, isoform 2 | 1 | 1.6 | Calmodulin binding; syntaxin binding; spectrin binding; actin filament capping | Calcium ion binding | C |
| Q8BMA6 | Srp68 | Signal recognition particle 68 kDa protein | 1 | 7.2 | Response to drug | RNA binding | Nl |
| Q62093 | Srsf2 | Serine/arginine-rich splicing factor 2 | 0.9995 | 7.2 | RNA splicing; mRNA processing | RNA binding; nucleotide binding | S; N |
| P84104 | Srsf3 | Serine/arginine-rich splicing factor 3, isoform short | 0.9526 | 11.3 | RNA splicing; insulin receptor signaling pathway; mRNA processing | RNA binding; nucleotide binding | S; N |
| P32067 | Ssb | Lupus La protein homolog (*) | 0.9937 | 7.6 | RNA processing | RNA binding; nucleotide binding | N |
| Q62186 | Ssr4 | Translocon-associated protein subunit delta | 1 | 17.4 | Unannotated | Unannotated | |
| Q99JB2 | Stoml2 | Stomatin-like protein 2 | 1 | 18.7 | Unannotated | Unannotated | |
| P61957 | Sumo2 | Small ubiquitin-related modifier 2 | 0.9858 | 16.9 | Cellular protein localization; protein sumoylation | SUMO ligase activity | N |
| P11031 | Sub1 | Activated RNA polymerase II transcriptional coactivator p15 | 1 | 23.6 | Regulation of transcription | Single-stranded DNA binding | Nl |
| Q7TMK9 | Syncrip | Heterogeneous nuclear ribonucleoprotein Q, isoform 2 | 1 | 8.5 | RNA splicing; mRNA processing | Nucleotide binding | N |
| Q921F2 | Tardbp | TAR DNA-binding protein 43 | 1 | 7.1 | mRNA stabilization, splicing and processing; transcription | Double-stranded DNA binding | N |
| P26039 | Tln1 | Talin-1 | 1 | 1.4 | Cell adhesion | Structural constituent of cytoskeleton | C |
| Q61029 | Tmpo | Lamina-associated polypeptide 2 | 1 | 13.4 | Regulation of transcription | DNA binding | N |
| Q9CPQ3 | Tomm22 | Import receptor subunit TOM22 homolog | 1 | 16.2 | Protein import | Transmembrane transporter activity | Mt |
| P17751 | Tpi1 | Triosephosphate isomerase | 0.9999 | 16.5 | Metabolic process | Triose-phosphate esomerase activity | N; C |
| P21107 | Tpm3 | Tropomyosin alpha-3 chain | 0.9939 | 8.1 | Unannotated | Unannotated | C |
| Q7M739 | Tpr | Nuclear pore complex-associated intranuclear coiled-coil protein TPR | 1 | 3.3 | Mitotic cell-cycle spindle assembly checkpoint; protein import into nucleus | ATP binding; serine-tRNA ligase activity | N; C |
| P63028 | Tpt1 | Translationally controlled tumor protein (*) | 0.9947 | 8.1 | Anti-apoptosis; stem cell maintenance | Calcium ion binding | N; C |
| Q6NV83 | U2surp (Sr140) | U2 snRNP-associated SURP motif-containing protein, isoform 2 | 1 | 6.6 | RNA processing | RNA binding; nucleotide binding | N |
| Q9CZ13 | Uqcrc1 | Cytochrome b-c1 complex subunit 1 | 1 | 11.5 | Transport; proteolysis | Metal ion binding | Mt |
| Q60930 | Vdac2 | Voltage-dependent anion-selective channel protein 2 | 0.9988 | 8.1 | Negative regulation of intrinsic apoptotic signaling pathway | Nucleotide binding; porin activity | Pc; Mt |
| Q60931 | Vdac3 | Voltage-dependent anion-selective channel protein 3 | 0.9992 | 10.2 | Synaptic transmission, learning | Nucleotide binding; porin activity | Pc; Mt |
| Q9EQH3 | Vps35 | Vacuolar protein sorting-associated protein 35 | 1 | 5.9 | Protein transport; vacuolar protein processing | Unannotated | C |
| P62960 | Ybx1 | Nuclease-sensitive element-binding protein 1 (*) | 0.9958 | 6 | Regulation of transcription; regulation of cell proliferation; RNA splicing and processing | RNA binding, single-stranded DNA binding | N; C |
Notes: Proteins identified in the nuclear PI-PLCβ1b complex. Proteins are listed in alphabetical order according to the gene name. Identified proteins are listed according to the search engine used for the analysis (proteins identified in both Mascot and X!Tandem, or proteins provided by either Mascot or X!Tandem). For proteins identified with multiple search engines (Mascot and X!Tandem), the best score is reported. The first three columns report the accession number (Acc.nb.), gene name, and description according to the UniProtKB-SwissProt database (April 2012). The next two columns, the protein probability (“Prot prob”) and the percentage of coverage (“Cov %”), report data provided by iProphet analysis. The last three columns, “GO biological process,” “GO molecular function,” and “GO cellular component,” are as annotated in the GO database and present data retrieved from the UniProtKB-SwissProt database.
(*) Indicates proteins identified with one unique single peptide; the protein probability refers to the score of the unique peptide.
(§) Indicates proteins that have been grouped together according to the Peptide Prophet algorithm, mainly because they are isoforms or homologues and thus share the peptides identified.
In Vitro Binding of Identified Proteins with PI-PLCβ1b
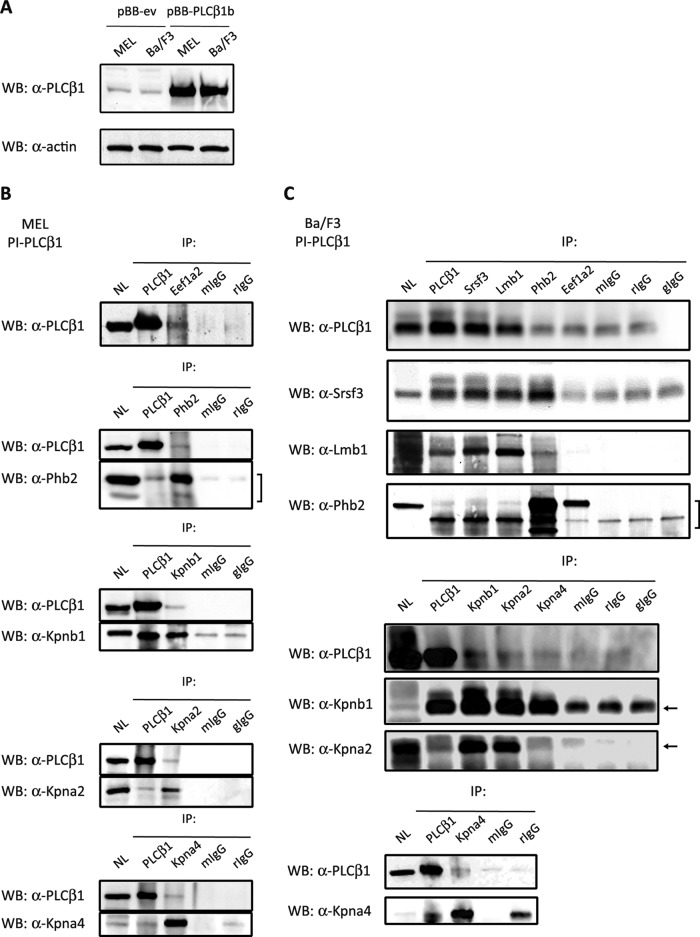
Data obtained from mass spectrometry analysis were validated in co-immunoprecipitation experiments performed with the following proteins of interest from isolated nuclei of MEL and Ba/F3 cells: Eef1a2, Kpna2, Kpna4, Kpnb1, Lmnb1 (Ba/F3 only), Phb2, and Srsf3 (Ba/F3 only) (Fig. 2). MEL and Ba/F3 cells demonstrated equal expression of PI-PLCβ1b both when the wild-type cells were compared and when cells stably overexpressing PI-PLCβ1b were compared (Fig. 2A). Co-immunoprecipitation is the most common method used to test whether two proteins of interest are associated; in this method, target-protein-specific antibodies are used to indirectly capture both proteins considered to be bound together. The crossed pull-down acted as a double-check on the validity of the results obtained in the mass spectrometry identifications, as PI-PLCβ1 immunoprecipitation results in the co-immunoprecipitation of protein B and targeted immunoprecipitation of protein B co-immunoprecipitates PI-PLCβ1. In MEL cells, Eef1a2, which we previously reported to be phosphorylated by PKCβI (conventional protein kinase C, DAG-dependent) in the nuclear compartment (19), was verified by means of crossed co-immunoprecipitation as associated specifically with PI-PLCβ1b but not the IgG control (Fig. 2B). Similarly, PI-PLCβ1b was shown to interact with Phb2, a protein that shuttles between the nucleus and mitochondria, and with importins Kpna2, Kpna4, and Kpnb1, which are required for the nucleo-cytoplasmic transport of a certain set of proteins. Similar to MEL/PI-PLCβ1b cells, PI-PLCβ1b was also found in complex with Srsf3, Lmnb1, Phb2, Kpnb1, and Kpna2 in nuclei of the murine pro-B lymphoid Ba/F3 cells overexpressing PI-PLCβ1b (Fig. 2C). The interaction of PI-PLCβ1b with Eef1a2 and Kpna4 was not as evident in Ba/F3 as in MEL cells. Unfortunately, because of the antibody background it was not possible to detect bands in Western blots for Eef1a2 following immunoprecipitation with PI-PLCβ1. These data provided further support that proteins identified via AP-MS were specifically complexed with nuclear PI-PLCβ1b.
Fig. 2.
In vitro validation of mass spectrometry identifications. MEL and Ba/F3 cells were stably infected with the retroviral pBB-IRES-blast® vector. Clones were selected with 4 μg/ml blasticidin for 5 days. PI-PLCβ1 expression was evaluated by means of Western blot analysis in both MEL and Ba/F3 cells, wild-type (pBB-ev) and overexpressing PI-PLCβ1b (pBB-PLCβ1b) (A). The association of PI-PLCβ1b with Eef1a2, Kpna2, Kpna4, Kpnb1, Lmnb1, Phb2, and Srsf3 was verified in MEL nuclei (B), Ba/F3 nuclei (C), or both by immunoprecipitating 800 μg of the respective nuclear lysate as described in “Experimental Procedures.” Total nuclear lysate (60 μg) and the immunoprecipitates were separated via 4%–15% gradient SDS-PAGE and immunoblotted with α-PI-PLCβ1 or a specific antibody directed against Eef1a2, Kpna2, Kpna4, Kpnb1, Lmnb1, Phb2, or Srsf3. Detailed information about antibodies is listed in supplemental Table S1. mIgG, mouse IgG; rIgG, rabbit IgG; gIgG, goat IgG.
Biological Classification of Proteins Identified in Complex with Nuclear PI-PLCβ1b
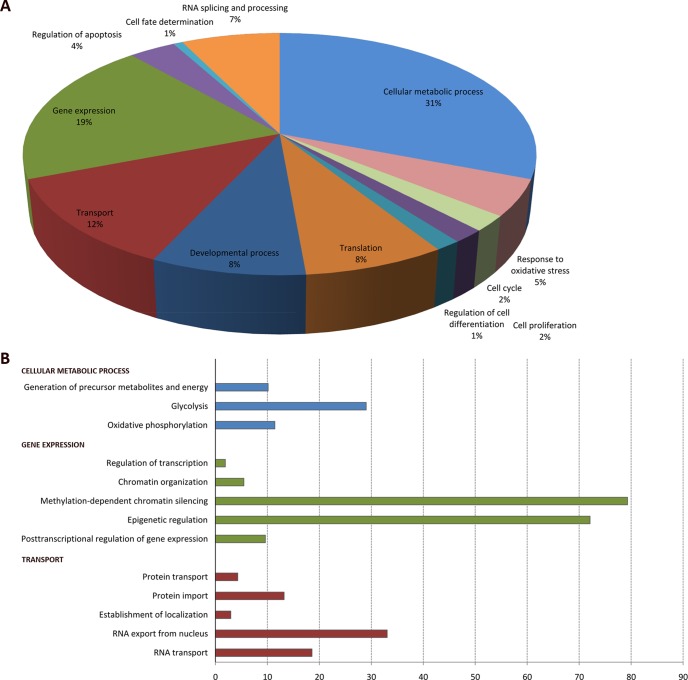
Proteins identified in complex with nuclear PI-PLCβ1b were analyzed in Gene Ontology (GO) and classified according to their biological role(s). The over-representation of each GO term was determined using the plug-in BiNGO and Cytoscape, utilizing the whole mouse repository annotation as a reference dataset. GO categories that were significantly over-represented, with p values < 0.05, are shown in Fig. 3. PI-PLCβ1b was associated with proteins involved in cellular metabolic processes (31%), gene expression (19%), transport (12%), developmental processes (8%), translation (8%), RNA splicing and processing (7%), response to oxidative stress (5%), and regulation of apoptosis (4%). A minor percentage of proteins were related to the cell cycle (2%), cell proliferation (2%), regulation of (myeloid) differentiation (1%), and cell fate determination (1%) (Fig. 3A). Sub-dividing the gene expression categories revealed that PI-PLCβ1b specifically associated with chromatin organization proteins, chromatin silencing by methylation, and epigenetic regulation (Fig. 3B). Within the GO term “transport,” proteins involved in RNA and protein transport, as well as the establishment of sub-cellular localization, were found in complex with PI-PLCβ1b. Among the proteins involved in cellular metabolic processes were proteins involved in glycolysis and oxidative phosphorylation.
Fig. 3.
Gene Ontology of nuclear PI-PLCβ1b complex biological processes. A, biological processes involving proteins identified in complex with PI-PLCβ1b. The percentage refers to the frequency of the process in the dataset, not in the whole mouse repository annotation. The frequency represents the percentage of protein entries (by gene name) in a particular GO category relative to the respective total number of entries. B, sub-division of cellular metabolic processes (blue bars), gene expression (green bars), and transport (red bars). The overrepresentation of each sub-category was calculated as the ratio among the dataset frequency and the mouse reference annotation frequency.
PI-PLCβ1b Interactome Network
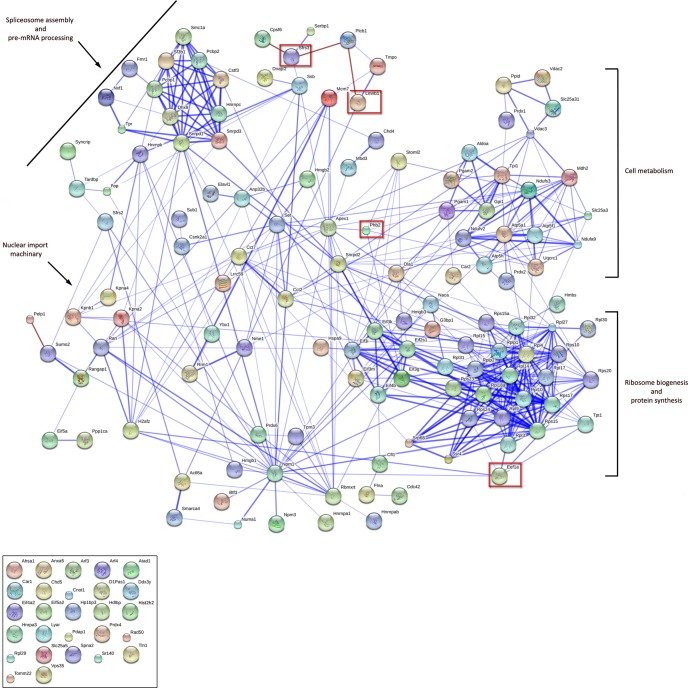
Network analysis was conducted with the open-source software String 9.0, which was used to build functional protein association networks based on data present in the database, including physical and functional associations of proteins derived from genomic, high-throughput experiments, co-expression, and literature knowledge (5,214,234 proteins from 1133 organisms; January 2013) (20). The generation of a virtual protein network for PI-PLCβ1b interactants highlighted that PI-PLCβ1b predominantly associated with protein complexes involved in protein/RNA transport, transcriptional and translational regulation (epigenetic control), splicing, response to oxidative stress, and metabolic processes (glycolysis and heme biosynthesis; Fig. 4).
Fig. 4.
Network analysis of nuclear PI-PLCβ1b complex protein–protein interactions. Analysis was performed using STRING 9.0, setting neighborhood, gene fusion, co-occurrence, co-expression, experiments, databases, and text-mining as prediction methods, with a medium confidence threshold (0.04). Protein–protein connections are presented in the confidence mode (heavier connecting lines indicate more evidence/high score for the interaction). In the box are listed proteins for which no connections were found above the score threshold. Red lines represent manually added connections based on literature evidence (4, 18, 21). PI-PLCβ1 binding partners Srsf3, Lmnb1, Phb2, and Eef1a are highlighted in red.
Trafficking and Transport
Several proteins involved in nucleocytoplasmic transport, specifically in nuclear protein import, such as Kpna2, Kpna4 (*), Kpnb1, Ran, and Rangap1 (*), were found in complex with PI-PLCβ1b. Also found were the co-chaperone Ahsa1 (*); the GTP binding proteins Arf3 and Arf4; Hnrnpa3, involved in protein trafficking; and Fmr1, Hnrnpa1, and Nxf1, involved in RNA transport.
Spliceosome Assembly and Pre-mRNA Processing
Multiple proteins of the spliceosome C complex were associated with PI-PLCβ1b, including Hnrnpa1, Hnrnpc, Hnrnpk, Syncrip, Sf3b1, Snrpd1, Snrpd2, Snrpd3, and Srsf2. Also interacting with PI-PLCβ1b were Cstf3, Cpsf6 (mRNA processing proteins), Snrpd2 (pre-mRNA processing and polyadenylation), Sr140 (spliceosome-associated protein), Serbp1 (mRNA stability), Rbmx (RNA-binding protein) and Hdlbp (mRNA stabilization). In addition to these RNA splicing proteins, mRNP complex proteins (Dhx9, Syncrip, Elavl1, Ybx1, and Pcbp2) were also in complex with PI-PLCβ1b.
Chromatin Remodeling and Gene Expression
In PI-PLCβ1b clusters, the chromatin remodeling proteins Actl6a, Smarca4 (SWI/SNF chromatin remodeling and histone acetyltransferase complex proteins), Chd4 (a central component of the nucleosome remodeling and histone deacetylase repressor complex, NuRD), and Dnajc2 (recruited at histone H2A sites; promotes the activation of polycomb genes) were identified. Proteins associated with chromatin (Hmgb1), a component of heterochromatin (Hp1bp3) and histones (H2A and H2B types), were also found. In addition, proteins involved in the regulation of transcription were identified in complex with PI-PLCβ1b, including Btf3 (a general transcription factor required for transcription initiation), Cnot1 (a transcriptional repressor belonging to the CCR4-NOT complex), Dhx9 (transcriptional activator), Hnrnpab (transcriptional repressor), Hspa9 (a heat shock protein that modulates demethylation and chromatin remodeling), Mbd3 (a transcriptional repressor and component of the NuRD complex), Pelp1 (*) (a co-activator of estrogen receptor-mediated transcription), Phb2 (a mediator of transcriptional repression through recruitment of histone deacetylases), and Sub1 (a general transcription co-activator). Among the proteins cited above, Chd4, Actl6a, Smarca4, Dnajc2, and Mbd3 play a role in regulating chromatin remodeling and DNA methylation, thereby acting as epigenetic regulators (21).
Apoptosis
Several pro- and anti-apoptotic proteins were discovered to be in complex with PI-PLCβ1b. Components of the SET complex (Set, Apex1, Hmgb2, and Nme1), involved in cytotoxic T-lymphocyte-induced apoptosis, Anp32b (a caspase 3 inhibitor), annexin 5 (Anxa5), Eif5a (a regulator of p53-dependent apoptosis, in complex with syntenin), and Tpt1 (*) (an anti-apoptotic protein) were all identified via mass spectrometry.
Ribosome Biogenesis and Protein Synthesis
Large (60S) ribosomal subunit constituent proteins (Rpl10, Rpl14, Rpl15, Rpl20, Rpl27, Rpl29, Rpl3, Rpl30, Rpl31, Rpl17, Rpl18a, Rpl32, Rpl4, and Rpl9) and small (40S) ribosomal subunit proteins (Rps10, Rps15, Rps15a, Rps17, Rps23, and Rps24) were found to be in complex with PI-PLCβ1b. Interestingly, several proteins involved in translation initiation (Eif2a, members of the Eif3 complex, Eif4a2, and Eif4b) and elongation (Rplp1, Rplp2, and Eef1a1/2) were found to be associated with PI-PLCβ1b, as was a putative chaperone involved in ribosome biogenesis, Npm3. Other proteins involved in sub-cellular targeting (Nacam and Srp68), protein folding (Pcpb1, Ppia, Ppid, Ssb (*), Cct2, and Cct7), and post-translational modification (Sumo2) were also bound to PI-PLCβ1b.
Miscellaneous
In addition to the previously mentioned proteins involved in specific processes, we found several multifunctional proteins. Among these were Apex1, which plays a central role in the oxidative stress response, DNA repair, epigenetic regulation of gene expression by DNA methylation, and regulation of transcription factors such as Fos/Jun; Npm1, which is involved in ribosome biogenesis, histone assembly, cell proliferation, and the regulation of p53; and Csnk2a1, a regulator of cell cycle progression, apoptosis, and transcription. Csnk2a1 is a Ser/Thr kinase with many known substrates, several of which were found to be in complex with PI-PLCβ1b: Anp32b (G1-S progression of the cell cycle), Ssb (pre-mRNA folding and maturation), Sptan1 (secretion), Hnrpa1, Hnrpc, Fmr1, Eif5, Gpi, and Rangap1 (22). Moreover, several glycolytic enzymes were associated with PI-PLCβ1b, including Aldoa, Pgam1, Pgam2, Tpi, and Gpi1. In addition, the oxidative-phosphorylation-associated proteins Ndufv2, Ndufa9 (*), Ndufs3, Uqcrc1, Atad1 (*), Atp5a1, Atp5f1 (*), Atp5h, and Mdh2, as well as proteins that function during reactive oxygen species metabolic processes (Prdx1, Prdx2, Prdx6 (*), Prdx4, and Ola1), were identified. Some of these enzymes are known to be present within the nucleus (Table I) and to be involved in diseases such as cancer and leukemia (23–28). Finally, Hmbs, responsible for heme and porphyrin biosynthesis, was identified in complex with PI-PLCβ1b.
DISCUSSION
AP-MS has emerged as a powerful tool for studying the protein interaction network of individual proteins of interest, coupling the specificity of the pray:bait protein isolation procedure and the sensitivity of high-throughput mass spectrometry analysis. In comparison to other systems (i.e. yeast-2-hybrid and in vitro tagged bait systems), the major advantage of AP-MS is the isolation of multiprotein complexes in their endogenous forms, thereby preserving the native conditions and post-translational modifications. Often, though, unfiltered datasets may present with a large number of false-positive protein interactions, which basically derive from incorrect protein identifications and antibody background (29–32). To avoid such false-positive identifications, we performed multiple-step AP experiments from three independent biological replicates using an antibody specific to PI-PLCβ1 and the corresponding normal mouse immunoglobulin, allowing for the identifications of 160 proteins in complex with PI-PLCβ1, which included direct interactors as well as proteins with varying degrees of interconnectivity.
Nuclear localized PI-PLCβ1 has been associated with multiple cellular processes, including proliferation, survival, differentiation, and metabolism (33). In particular, nuclear PI-PLCβ1 seems to play a critical role in the self-renewal and differentiation of leukemic cells both by regulating the cell cycle during the G1-S and G2-M phases and by promoting cell differentiation in an expression-dependent manner. Based on the evidence that PI-PLCβ1 nuclear localization is critical to its function, our group is attempting to characterize the mechanism(s) by which the PI-PLCβ1 signaling network is exerted. We previously reported that in MEL cells, cyclin D3 (3), the transcription factor p45/NF-E2 (enhancer binding protein for the β-globin gene) (6), the antigen CD24 (involved in differentiation and hematopoiesis) (7), the splicing factor Sfrs3 (Srp20) (18), and lamin B1 (4) are affected by nuclear PI-PLCβ1, among which Srp20 and lamin B1 were found to be associated with PI-PLCβ1 in co-immunoprecipitation experiments. In recent years, the involvement of PI-PLCβ1 in the development of cancer has been proposed, as studies on myelodysplastic syndrome (MDS) and acute myeloid leukemia have demonstrated that epigenetic and genetic modifications of the PI-PLCβ1 locus occur in patients during MDS progression to acute myeloid leukemia (34). All together, these data make the need to understand the effectors and interactors of PI-PLCβ1 even more urgent, in order to precisely target the mechanisms implicated in these diseases. This study reports the identification of 160 proteins in complex with nuclear PI-PLCβ1, many of which are associated not only with established mechanisms (e.g. regulation of the cell cycle and differentiation), but also with hypothesized roles for PI-PLCβ1 (e.g. apoptosis and RNA splicing), while in addition providing some completely new insights into PI-PLCβ1's function in the nucleus (e.g. nuclear transport mechanism).
PI-PLCβ1 was the first phospholipase C isoform to be described that was recruited to the nucleus following mitogenic or differentiating stimuli. It has been demonstrated that PI-PLCβ1 contains a putative non-canonical nuclear localization signal in the carboxy-terminal region (35); nevertheless, the mechanism by which translocation occurs has never been further explored. One of the more interesting findings that have emerged from this study is the association of PI-PLCβ1b with the classical import proteins Kpna2, Kpna4, Kpnab1, Ran, and Rangap1. The classical import mechanism purports that a cargo protein binds to the adapter protein importin α (Kpna) via its nuclear localization signal sequence, and this promotes the association with importin β (Kpnb1), forming a ternary complex. The complex then translocates to the nucleus through the binding of Kpnb1 with the pore complex proteins in a Ran-dependent process that promotes dissociation of the complex at the nucleoplasmic side (36). However, non-conventional nuclear import mechanisms have also been established (37); for example, Yagisawa reported that PI-PLCδ1, although bearing a non-canonical nuclear localization signal, is still imported into the nucleus following an increase in the levels of intracellular Ca2+ via a mechanism that is only importin b1–dependent (38). Thus, the findings presented here suggest that, in contrast to PI-PLCδ1, the nuclear translocation of PI-PLCβ1 is most likely mediated via the classical mechanism, in which both alpha and beta importins are required for nuclear import. Unlike other PI-PLC isozymes, PI-PLCβs possess a long C-terminal sequence of about 400 amino acids, containing an unusually high proportion of lysine and arginine residues, downstream of their catalytic domains (35). The two isoforms of PI-PI-PLCβ1, 1a and 1b, show 94% homology and differ only at their C-terminal ends for a sequence of 75 amino acids in the 1a isoform and 32 amino acids in the 1b isoform. Both PI-PLCβ1a and PI-PLCβ1b present with a bipartite consensus sequence for nuclear localization (K1055, K1056 separated from K1069, K1071 by a linker of 12 amino acids), but a cluster of three lysines (K1056, K1063, and K1070) was demonstrated to be essential for nuclear translocation. It was therefore suggested these three lysines were critical sites for establishing interactions that retain PI-PLCβ1 within the nucleus (2). In a recently published paper, Scarlata and colleagues report that PKC phosphorylation on S887 (in the C-terminal region of both PI-PLCβ 1a and 1b) also regulates the subcellular distribution of PI-PLCβ1, as the lack of phosphorylation keeps the enzyme located within the nucleus (39). The sequence of 75 amino acids exclusive to PI-PLCβ1a also contains a putative nuclear export sequence, as predicted by NetNES 1.1, which likely explains the predominant presence of PI-PLCβ1a in the cytoplasm. Together these findings suggest that PI-PLCβ1b (and probably PI-PLCβ1a) enters the nucleus complexed with the alpha/beta importin system, and then PI-PLCβ1b is retained inside the nucleus through binding to negatively charged components (2), whereas PI-PLCβ1a can be exported to the cytoplasm via its nuclear export sequence, thus providing an explanation for the different sub-cellular localizations of the two isoforms of PI-PLCβ1.
Several proteins identified as nuclear PI-PLCβ1b interactors are associated with leukemic malignancies, as either prognostic markers or potential targets for therapeutic intervention. Of particular interest was the identification of two splicing factors whose genes are often mutated in MDS, Srsf2 and Sf3b1 (40, 41). Srsf2 is associated with a negative prognostic impact, as patients bearing mutations in Srsf2 have significantly inferior overall survival and a more rapid and frequent progression to acute myeloid leukemia (42, 43). Depending on the disease classification and how the statistical analysis was performed and applied, Sf3b1, important for anchoring the spliceosome to precursor mRNA, has been proposed to serve either as a favorable marker in MDS or as an independent prognostic factor for progression (44, 45). These findings suggest that imbalances in the spliceosome machinery can have a significant role in promoting leukemogenesis. Interestingly, other proteins associated with PI-PLCβ1b serve in mRNA processing as part of the spliceosome C complex (see “Results”) or as independent splicing factors, such as Srsf2, Tarbp, and Srsf3/SRp20. In mammalian cells, constituents of the pre-mRNA splicing machinery are associated with a specific nuclear sub-compartment, called speckles (46). Noteworthy, PI-PLCβ1 is also localized to nuclear speckles with its binding partner Srp20 (18) and additional proteins implicated in inositide-dependent signal transduction, including PIP kinases, PI(4,5)P2, DGKθ, PLCδ4, PI3K C2α, and phosphatases PTEN and SHIP2, as reviewed in Ref. 47. As for the current concepts concerning factors promoting hematological malignancies, in which gene mutations, deregulated gene expression, and epigenetic changes are seen as key steps in disease pathogenesis, PI-PLCβ1 was found to be involved in each of these processes (48). The identification of proteins implicated in the epigenetic regulation of gene expression through DNA methylation as PI-PLCβ1 binders was particularly relevant. The helicase DNA-binding protein Chd4 and the methyl-CpG-binding domain protein Mbd3 were identified in complex with nuclear PI-PLCβ1b. These two proteins are components of the nucleosome remodeling and deacetylase complex, which functions as a determinant epigenetic regulator, associating with methylated DNA, in order to bring about the deacetylation and demethylation of histones (49). Deregulation of the nucleosome remodeling and deacetylase complex by oncogenes can be a cause for aberrant cell proliferation in leukemogenesis that should be further exploited for therapies.
The overexpression of PI-PLCβ1 acts as a negative regulator of erythroid-induced differentiation in both murine and human erythroleukemia cells (5). Recently, our group reported that low-risk MDS patients refractory to erythropoietin therapy presented with increased PI-PLCβ1b expression at the end of the treatment (8). In the present study, mass spectrometry analysis identified proteins involved in red cell metabolism and in the regulation of erythropoietin mRNA. Among these proteins were the poly(rC)-binding proteins Pcbp1 and Pcbp2, which are reportedly associated with the erythropoietin mRNA 3′-UTR, in a region required for messenger RNA stability (50). Depletion of Pcbp1 and Pcbp2 in human erythroleukemia cells (K562) was shown to decrease cell proliferation, leading to G1 arrest via the induction of p21WAF (51).
In 2000, a study by Lee et al. (52) demonstrated that PI-PLCβ1 exerts a protective effect against oxidative-stress-induced cell death, although the mechanism was not elucidated. In accordance with these previous findings, mass spectrometry identified several proteins involved in apoptosis that were associated with nuclear PI-PLCβ1b, some specifically exerting a negative effect against apoptosis. One of these proteins, Anp32b, is an anti-apoptotic protein that acts as a decoy caspase 3 substrate, inhibiting the pro-apoptotic function of caspase 3 (53). Another protein in complex with PI-PLCβ1b was Apex 1 (Ape/Ref1), a multifunctional protein that stimulates the DNA binding activity of numerous transcription factors involved in cancer promotion and progression, such as the Fos/Jun Ap1 complex, NFkB, p53, and CREB. In addition, Apex 1 is also a DNA-damage response protein. The lyase activity of Apex 1 can repair apurinic/apyrimidinic sites of DNA following damage by reactive oxygen species. Apex1 also possesses redox activity that can control transcription factors such as Jun and Fos, and this activity is enhanced following Apex1 phosphorylation by PKC and CK2, both of which can be activated through PI-PLCβ1-mediated DAG production (54). In the nucleus, DAG production leads to the activation of cPKCs and a specific isoform of CK2, Csnk2a1, which was identified in complex with PI-PLCβ1b. Moreover, our group previously determined that PI-PLCβ1 can affect cyclin D3 promoter activity during differentiation through activation of the c-jun/AP1 complex (9). As PI-PLCβ1 does not directly bind to cyclin D3 but largely affects its expression and regulation, the identification of Apex1 and Cnsk2a1 opens a new field of investigation. Npm1, another multifunctional protein identified in complex with PI-PLCβ1, exerts control over Apex1 endonuclease activity, which has been implicated in the DNA repair of peroxide-damaged cells (55). Finally, Tpt1 and Eef1a2 were recently identified as anti-apoptotic proteins. Tpt1 controls the stability of p53, and Eef1a2 inhibits apoptosis and promotes the G1-S progression of the cell cycle in human multiple myeloma (56, 57). The fact that PI-PLCβ1 bound with Eef1a2, together with its previous identification as a substrate of PKCβI (19), suggests that it can act as an intermediate effector for nuclear inositide signaling elicited by PI-PLCβ1, not only during insulin-induced differentiation, but also in the regulation of the cell cycle progression (3) and apoptosis. These data give significant insight into the molecular environment that surrounds PI-PLCβ1 and provide evidence of the interaction of nuclear PI-PLCβ1b with a number of proteins involved in nuclear import, differentiation, mRNA processing, and apoptosis, therefore hinting at multiple novel targets for therapeutic intervention in hematological malignancies.
Supplementary Material
Acknowledgments
We thank the Cassa di Risparmio di Modena (CARISMO) for purchasing the Chip Cube-QTOF Instrumentation (Agilent). We also thank Dr. Diego Pinetti for technical assistance.
Footnotes
* This work was supported by the Italian MIUR-FIRB HumanProteomeNet, Italian MIUR-PRIN, and CARISBO Foundation (to L.C.).
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- AP-MS
- affinity purification–mass spectrometry
- DAG
- diacylglycerol
- GO
- gene ontology
- MDS
- myelodysplastic syndrome
- MEL
- murine erythroleukemia
- PI-PLCβ1
- phosphoinositide-dependent phospholipase C beta 1.
REFERENCES
- 1. Bahk Y. Y., Lee Y. H., Lee T. G., Seo J., Ryu S. H., Suh P. G. (1994) Two forms of phospholipase C-beta 1 generated by alternative splicing. J. Biol. Chem. 269, 8240–8245 [PubMed] [Google Scholar]
- 2. Kim C. G., Park D., Rhee S. G. (1996) The role of carboxyl-terminal basic amino acids in Gqalpha-dependent activation, particulate association, and nuclear localization of phospholipase C-beta1. J. Biol. Chem. 271, 21187–21192 [DOI] [PubMed] [Google Scholar]
- 3. Faenza I., Matteucci A., Manzoli L., Billi A. M., Aluigi M., Peruzzi D., Vitale M., Castorina S., Suh P. G., Cocco L. (2000) A role for nuclear phospholipase Cbeta 1 in cell cycle control. J. Biol. Chem. 275, 30520–30524 [DOI] [PubMed] [Google Scholar]
- 4. Fiume R., Ramazzotti G., Teti G., Chiarini F., Faenza I., Mazzotti G., Billi A. M., Cocco L. (2009) Involvement of nuclear PLCbeta1 in lamin B1 phosphorylation and G2/M cell cycle progression. FASEB J. 23, 957–966 [DOI] [PubMed] [Google Scholar]
- 5. Matteucci A., Faenza I., Gilmour R. S., Manzoli L., Billi A. M., Peruzzi D., Bavelloni A., Rhee S. G., Cocco L. (1998) Nuclear but not cytoplasmic phospholipase C beta 1 inhibits differentiation of erythroleukemia cells. Cancer Res. 58, 5057–5060 [PubMed] [Google Scholar]
- 6. Faenza I., Matteucci A., Bavelloni A., Marmiroli S., Martelli A. M., Gilmour R. S., Suh P. G., Manzoli L., Cocco L. (2002) Nuclear PLCbeta(1) acts as a negative regulator of p45/NF-E2 expression levels in Friend erythroleukemia cells. Biochim. Biophys. Acta 1589, 305–310 [DOI] [PubMed] [Google Scholar]
- 7. Fiume R., Faenza I., Matteucci A., Astolfi A., Vitale M., Martelli A. M., Cocco L. (2005) Nuclear phospholipase C beta1 (PLCbeta1) affects CD24 expression in murine erythroleukemia cells. J. Biol. Chem. 280, 24221–24226 [DOI] [PubMed] [Google Scholar]
- 8. Follo M. Y., Mongiorgi S., Clissa C., Paolini S., Martinelli G., Martelli A. M., Fioravanti G., Manzoli L., Finelli C., Cocco L. (2012) Activation of nuclear inositide signalling pathways during erythropoietin therapy in low-risk MDS patients. Leukemia 26, 2474–2482 [DOI] [PubMed] [Google Scholar]
- 9. Ramazzotti G., Faenza I., Gaboardi G. C., Piazzi M., Bavelloni A., Fiume R., Manzoli L., Martelli A. M., Cocco L. (2008) Catalytic activity of nuclear PLC-beta(1) is required for its signalling function during C2C12 differentiation. Cell Signal. 20, 2013–2021 [DOI] [PubMed] [Google Scholar]
- 10. Faenza I., Bavelloni A., Fiume R., Lattanzi G., Maraldi N. M., Gilmour R. S., Martelli A. M., Suh P. G., Billi A. M., Cocco L. (2003) Up-regulation of nuclear PLCbeta1 in myogenic differentiation. J. Cell. Physiol. 195, 446–452 [DOI] [PubMed] [Google Scholar]
- 11. O'Carroll S. J., Mitchell M. D., Faenza I., Cocco L., Gilmour R. S. (2009) Nuclear PLCbeta1 is required for 3T3-L1 adipocyte differentiation and regulates expression of the cyclin D3-cdk4 complex. Cell Signal. 21, 926–935 [DOI] [PubMed] [Google Scholar]
- 12. Faenza I., Ramazzotti G., Bavelloni A., Fiume R., Gaboardi G. C., Follo M. Y., Gilmour R. S., Martelli A. M., Ravid K., Cocco L. (2007) Inositide-dependent phospholipase C signaling mimics insulin in skeletal muscle differentiation by affecting specific regions of the cyclin D3 promoter. Endocrinology 148, 1108–1117 [DOI] [PubMed] [Google Scholar]
- 13. Somervaille T. C., Cleary M. L. (2006) Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell 10, 257–268 [DOI] [PubMed] [Google Scholar]
- 14. Blalock W. L., Bavelloni A., Piazzi M., Tagliavini F., Faenza I., Martelli A. M., Follo M. Y., Cocco L. (2011) Multiple forms of PKR present in the nuclei of acute leukemia cells represent an active kinase that is responsive to stress. Leukemia 25, 236–245 [DOI] [PubMed] [Google Scholar]
- 15. Keller A., Nesvizhskii A. I., Kolker E., Aebersold R. (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 74, 5383–5392 [DOI] [PubMed] [Google Scholar]
- 16. Nesvizhskii A. I., Keller A., Kolker E., Aebersold R. (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 75, 4646–4658 [DOI] [PubMed] [Google Scholar]
- 17. Shteynberg D., Deutsch E. W., Lam H., Eng J. K., Sun Z., Tasman N., Mendoza L., Moritz R. L., Aebersold R., Nesvizhskii A. I. (2011) iProphet: multi-level integrative analysis of shotgun proteomic data improves peptide and protein identification rates and error estimates. Mol. Cell. Proteomics 10, M111.007690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bavelloni A., Faenza I., Cioffi G., Piazzi M., Parisi D., Matic I., Maraldi N. M., Cocco L. (2006) Proteomic-based analysis of nuclear signaling: PLCbeta1 affects the expression of the splicing factor SRp20 in Friend erythroleukemia cells. Proteomics 6, 5725–5734 [DOI] [PubMed] [Google Scholar]
- 19. Piazzi M., Bavelloni A., Faenza I., Blalock W., Urbani A., D'Aguanno S., Fiume R., Ramazzotti G., Maraldi N. M., Cocco L. (2010) eEF1A phosphorylation in the nucleus of insulin-stimulated C2C12 myoblasts: Ser(5)(3) is a novel substrate for protein kinase C betaI. Mol. Cell. Proteomics 9, 2719–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szklarczyk D., Franceschini A., Kuhn M., Simonovic M., Roth A., Minguez P., Doerks T., Stark M., Muller J., Bork P., Jensen L. J., von Mering C. (2011) The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 39, D561–D568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. UniProt Consortium (2012) Reorganizing the protein space at the Universal Protein Resource (UniProt). Nucleic Acids Res. 40, D71–D75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hornbeck P. V., Kornhauser J. M., Tkachev S., Zhang B., Skrzypek E., Murray B., Latham V., Sullivan M. (2012) PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res. 40, D261–D270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peng Y., Li X., Wu M., Yang J., Liu M., Zhang W., Xiang B., Wang X., Li G., Shen S. (2012) New prognosis biomarkers identified by dynamic proteomic analysis of colorectal cancer. Mol. Biosyst. 8, 3077–3088 [DOI] [PubMed] [Google Scholar]
- 24. Ritterson Lew C., Tolan D. R. (2012) Targeting of several glycolytic enzymes using RNA interference reveals aldolase affects cancer cell proliferation through a non-glycolytic mechanism. J. Biol. Chem. 287, 42554–42563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kowalski W., Nocon D., Gamian A., Kolodziej J., Rakus D. (2012) Association of C-terminal region of phosphoglycerate mutase with glycolytic complex regulates energy production in cancer cells. J. Cell. Physiol. 227, 2613–2621 [DOI] [PubMed] [Google Scholar]
- 26. Suhane S., Berel D., Ramanujan V. K. (2011) Biomarker signatures of mitochondrial NDUFS3 in invasive breast carcinoma. Biochem. Biophys. Res. Commun. 412, 590–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang G., Chen Y., Lu H., Cao X. (2007) Coupling mitochondrial respiratory chain to cell death: an essential role of mitochondrial complex I in the interferon-beta and retinoic acid-induced cancer cell death. Cell Death Differ. 14, 327–337 [DOI] [PubMed] [Google Scholar]
- 28. Schildgen V., Wulfert M., Gattermann N. (2011) Impaired mitochondrial gene transcription in myelodysplastic syndromes and acute myeloid leukemia with myelodysplasia-related changes. Exp. Hematol. 39, 666–675 e661 [DOI] [PubMed] [Google Scholar]
- 29. Armean I. M., Lilley K. S., Trotter M. W. (2013) Popular computational methods to assess multiprotein complexes derived from label-free affinity purification and mass spectrometry (AP-MS) experiments. Mol. Cell. Proteomics 12, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaake R. M., Wang X., Huang L. (2010) Profiling of protein interaction networks of protein complexes using affinity purification and quantitative mass spectrometry. Mol. Cell. Proteomics 9, 1650–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oeffinger M. (2012) Two steps forward–one step back: advances in affinity purification mass spectrometry of macromolecular complexes. Proteomics 12, 1591–1608 [DOI] [PubMed] [Google Scholar]
- 32. Dunham W. H., Mullin M., Gingras A. C. (2012) Affinity-purification coupled to mass spectrometry: basic principles and strategies. Proteomics 12, 1576–1590 [DOI] [PubMed] [Google Scholar]
- 33. Ramazzotti G., Faenza I., Fiume R., Matteucci A., Piazzi M., Follo M. Y., Cocco L. (2011) The physiology and pathology of inositide signaling in the nucleus. J. Cell. Physiol. 226, 14–20 [DOI] [PubMed] [Google Scholar]
- 34. Follo M. Y., Marmiroli S., Faenza I., Fiume R., Ramazzotti G., Martelli A. M., Gobbi P., McCubrey J. A., Finelli C., Manzoli F. A., Cocco L. (2013) Nuclear phospholipase C beta1 signaling, epigenetics and treatments in MDS. Adv. Biol. Regul. 53, 2–7 [DOI] [PubMed] [Google Scholar]
- 35. Suh P. G., Park J. I., Manzoli L., Cocco L., Peak J. C., Katan M., Fukami K., Kataoka T., Yun S., Ryu S. H. (2008) Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB Rep. 41, 415–434 [DOI] [PubMed] [Google Scholar]
- 36. Moroianu J. (1999) Nuclear import and export pathways. J. Cell. Biochem. 33 Suppl 32, 76–83 [DOI] [PubMed] [Google Scholar]
- 37. Wagstaff K. M., Jans D. A. (2009) Importins and beyond: non-conventional nuclear transport mechanisms. Traffic 10, 1188–1198 [DOI] [PubMed] [Google Scholar]
- 38. Yagisawa H. (2006) Nucleocytoplasmic shuttling of phospholipase C-delta1: a link to Ca2+. J. Cell. Biochem. 97, 233–243 [DOI] [PubMed] [Google Scholar]
- 39. Aisiku O., Dowal L., Scarlata S. (2011) Protein kinase C phosphorylation of PLCbeta1 regulates its cellular localization. Arch. Biochem. Biophys. 509, 186–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Makishima H., Visconte V., Sakaguchi H., Jankowska A. M., Abu Kar S., Jerez A., Przychodzen B., Bupathi M., Guinta K., Afable M. G., Sekeres M. A., Padgett R. A., Tiu R. V., Maciejewski J. P. (2012) Mutations in the spliceosome machinery, a novel and ubiquitous pathway in leukemogenesis. Blood 119, 3203–3210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bejar R., Stevenson K. E., Caughey B. A., Abdel-Wahab O., Steensma D. P., Galili N., Raza A., Kantarjian H., Levine R. L., Neuberg D., Garcia-Manero G., Ebert B. L. (2012) Validation of a prognostic model and the impact of mutations in patients with lower-risk myelodysplastic syndromes. J. Clin. Oncol. 30, 3376–3382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thol F., Kade S., Schlarmann C., Loffeld P., Morgan M., Krauter J., Wlodarski M. W., Kolking B., Wichmann M., Gorlich K., Gohring G., Bug G., Ottmann O., Niemeyer C. M., Hofmann W. K., Schlegelberger B., Ganser A., Heuser M. (2012) Frequency and prognostic impact of mutations in SRSF2, U2AF1, and ZRSR2 in patients with myelodysplastic syndromes. Blood 119, 3578–3584 [DOI] [PubMed] [Google Scholar]
- 43. Lasho T. L., Jimma T., Finke C. M., Patnaik M., Hanson C. A., Ketterling R. P., Pardanani A., Tefferi A. (2012) SRSF2 mutations in primary myelofibrosis: significant clustering with IDH mutations and independent association with inferior overall and leukemia-free survival. Blood 120, 4168–4171 [DOI] [PubMed] [Google Scholar]
- 44. Matsuda K., Ishida F., Ito T., Nakazawa H., Miura S., Taira C., Sueki A., Kobayashi Y., Honda T. (2012) Spliceosome-related gene mutations in myelodysplastic syndrome can be used as stable markers for monitoring minimal residual disease during follow-up. Leuk. Res. 36, 1393–1397 [DOI] [PubMed] [Google Scholar]
- 45. Cazzola M., Rossi M., Malcovati L. (2013) Biologic and clinical significance of somatic mutations of SF3B1 in myeloid and lymphoid neoplasms. Blood 121, 260–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lamond A. I., Spector D. L. (2003) Nuclear speckles: a model for nuclear organelles. Nat. Rev. Mol. Cell. Biol. 4, 605–612 [DOI] [PubMed] [Google Scholar]
- 47. Ramazzotti G., Faenza I., Follo M. Y., Fiume R., Piazzi M., Giardino R., Fini M., Cocco L. (2011) Nuclear phospholipase C in biological control and cancer. Crit. Rev. Eukaryot. Gene Expr. 21, 291–301 [DOI] [PubMed] [Google Scholar]
- 48. Follo M. Y., Marmiroli S., Faenza I., Fiume R., Ramazzotti G., Martelli A. M., Gobbi P., McCubrey J. A., Finelli C., Manzoli F. A., Cocco L. (2013) Nuclear phospholipase C beta1 signaling, epigenetics and treatments in MDS. Adv. Biol. Regul. 53, 2–7 [DOI] [PubMed] [Google Scholar]
- 49. Morey L., Brenner C., Fazi F., Villa R., Gutierrez A., Buschbeck M., Nervi C., Minucci S., Fuks F., Di Croce L. (2008) MBD3, a component of the NuRD complex, facilitates chromatin alteration and deposition of epigenetic marks. Mol. Cell. Biol. 28, 5912–5923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Czyzyk-Krzeska M. F., Bendixen A. C. (1999) Identification of the poly(C) binding protein in the complex associated with the 3′ untranslated region of erythropoietin messenger RNA. Blood 93, 2111–2120 [PubMed] [Google Scholar]
- 51. Waggoner S. A., Johannes G. J., Liebhaber S. A. (2009) Depletion of the poly(C)-binding proteins alphaCP1 and alphaCP2 from K562 cells leads to p53-independent induction of cyclin-dependent kinase inhibitor (CDKN1A) and G1 arrest. J. Biol. Chem. 284, 9039–9049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee Y. H., Kim S. Y., Kim J. R., Yoh K. T., Baek S. H., Kim M. J., Ryu S. H., Suh P. G., Kim J. H. (2000) Overexpression of phospholipase Cbeta-1 protects NIH3T3 cells from oxidative stress-induced cell death. Life Sci. 67, 827–837 [DOI] [PubMed] [Google Scholar]
- 53. Shen S. M., Yu Y., Wu Y. L., Cheng J. K., Wang L. S., Chen G. Q. (2010) Downregulation of ANP32B, a novel substrate of caspase-3, enhances caspase-3 activation and apoptosis induction in myeloid leukemic cells. Carcinogenesis 31, 419–426 [DOI] [PubMed] [Google Scholar]
- 54. Hsieh M. M., Hegde V., Kelley M. R., Deutsch W. A. (2001) Activation of APE/Ref-1 redox activity is mediated by reactive oxygen species and PKC phosphorylation. Nucleic Acids Res. 29, 3116–3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vascotto C., Fantini D., Romanello M., Cesaratto L., Deganuto M., Leonardi A., Radicella J. P., Kelley M. R., D'Ambrosio C., Scaloni A., Quadrifoglio F., Tell G. (2009) APE1/Ref-1 interacts with NPM1 within nucleoli and plays a role in the rRNA quality control process. Mol. Cell. Biol. 29, 1834–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nagano-Ito M., Ichikawa S. (2012) Biological effects of mammalian translationally controlled tumor protein (TCTP) on cell death, proliferation, and tumorigenesis. Biochem. Res. Int. 2012, 204960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li Z., Qi C. F., Shin D. M., Zingone A., Newbery H. J., Kovalchuk A. L., Abbott C. M., Morse H. C., 3rd (2010) Eef1a2 promotes cell growth, inhibits apoptosis and activates JAK/STAT and AKT signaling in mouse plasmacytomas. PLoS One 5, e10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.