Abstract
We have investigated how the abnormal head posture and motility in spasmodic torticollis interferes with ecological movements such as combined eye-to-foot whole-body reorientations to visual targets. Eight mildly affected patients and 10 controls voluntarily rotated eyes and body in response to illuminated targets of eccentricities up to ±180°. The experimental protocol allowed separate evaluation of the effects of target location, visibility and predictability on movement parameters. Patients’ latencies of eye, head, trunk and foot motion were prolonged but showed a normal modification pattern when target location was predictable. Peak head-on-trunk displacement and velocity were reduced both ipsi- and contralaterally with respect to the direction of torticollis. Surprisingly, peak trunk velocity was also reduced, even more than in previously studied patients with Parkinson’s disease. As a consequence, patients made short, hypometric gaze saccades and only exceptionally foveated initially nonvisible targets with a single large gaze shift (4 % of predictable trials as opposed to 30 % in controls). Foveation of distant targets was massively delayed by more than half a second on average. Spontaneous dystonic head movements did not interfere with the execution of voluntary gaze shifts. The results show that neck dystonia does not arise from gaze (head-eye) motor centres but the eye-to-foot turning synergy is seriously compromised. For the first time we identify significant ‘secondary’ complications of torticollis such as trunk bradykinesia and foveation delays, likely to cause additional disability in patients. Eye movements per se are intact and compensate for the reduced head/trunk performance in an adaptive manner.
Keywords: Cervical dystonia, Movement coordination, Turning, Gaze, Torticollis
Introduction
Head posture is abnormal in cervical dystonia due to involuntary neck muscle contractions. Voluntary head movements have been recently reported to be slow in these patients [1–4]. However, the effect of head slowness on motor behaviour involving head turns during gaze reorientations is unknown. Earlier work suggested that imbalance of a so-called ‘versional’ substrate, thought to be located in basal ganglia and midbrain, leads to postural and voluntary head movement abnormalities in spasmodic torticollis (ST) [5]. More recently, the interstitial nucleus of Cajal in the midbrain has been identified as the site implementing integration of head velocity signals in primates and it has been postulated that imbalance in this structure could be one of the mechanisms underlying spasmodic torticollis [6, 7]. This view appeared for the first time in Denny-Brown’s work who thought that torticollis arises from distortion of optokinetic reflexes as a result of damage to the pretectal area [6]. However, while eye movements are compromised after INC lesions or inactivation experiments, eye saccadic function is normal in spasmodic torticollis [7]. Further, abnormalities of eye-head coordination such as reduced head contribution and reduced head velocity in the direction of torticollis have been thought by Maurer and colleagues [8] to be compensatory to the abnormal head posture. However, in their study, combined eye-head movements were elicited upon the presentation of visual stimuli in seated patients. On the other hand, some vestibular abnormalities and abnormal interaction of vestibular signals with high order motor commands have been reported, that cannot solely be explained as being secondary to abnormal head posture [7, 9].
Large gaze shifts in standing (‘pivot turns’) are common in everyday life, requiring combined rotations of the eye, head, trunk and feet [10–12]. This ‘ecological’ movement requires complex interactions between eye-to-foot voluntary motor systems with reflex mechanisms controlling posture and eye movements, such as the vestibulo-ocular reflex (VOR). Latencies and movement patterns during such coordinated target acquisition vary according to the behavioral paradigm and can be modified by predictability and visibility of target appearance. In spatially predictable conditions, normal subjects are able to foveate targets of up to 180° eccentricity with single-step gaze shifts, a behaviour requiring precise eye/head/trunk synergistic movements, high speed head velocity [12] and the suppression of the VOR [13]. Such ‘single-step’ gaze shifts are highly efficient movements that allow acquisition of visual targets of large eccentricities in “one go”, de facto extending the range of gaze saccades to 90–180°—well beyond the normal oculomotor range of 45°.
In this study we ask whether the disruption of head-on-trunk movements in ST patients compromises the execution of large gaze reorientations. By recording multiple body sites we will also investigate the coordination of simultaneous rotating segments and whether cervical dystonia is a disorder of orienting movements as previously hypothesized [6–8]. Finally, as in our paradigm patients also move towards initially non-visible targets (i.e., ≥90°), we can compare visually versus non-visually guided movements. On the basis of previous findings in patients with basal ganglia disorders [14–17], namely degraded motor performance in the absence of visual input, movements to initially nonvisible targets would be expected to be worse, but this has not been explored in ST.
Materials and methods
Patients and control subjects
Eight patients (five males, age 58.3 ± 8 years, mean ± SD) with idiopathic ST were compared with 10 control subjects from a previous investigation (Anastasopoulos et al. [14]; 52 ± 2.6 years; seven males; Table 1). Neck dystonia was assessed with a large protractor using the severity scale of Tsui et al. [19] while patients were sitting at rest. In particular, head turn in the horizontal (transverse) plane of the body, tilt in the frontal plane, and anterior-posterior deviations in the sagittal plane were evaluated separately by referencing the chin-nasion line to the anterior median line of the thorax and by measuring the angle of the Reid’s base line relative to the horizontal plane. For each of these planes the head deviation was quantified as absent (0), mild (grade 1; <15°), moderate (grade 2; 15–30°) or extreme (grade 3; >30°). Patients were selected to be on average mildly affected and have almost exclusively abnormal head deviation in the horizontal plane (total score across planes, 3.1 ± 2.4, duration of head-on-trunk deviation, i.e., intermittent/constant, taken into account). None had tremor or shoulder elevation. Disease duration ranged between 2 and 10 years. All patients were treated with regular botulinum toxin injections in the neck muscles. In order to minimize possible effects of botulinum toxin on muscle function, the measurements were performed after a minimum interval of 3 months following the last injection, immediately prior to the new treatment. One patient was taking clonazepam at the time of measurements, for at least a year without any change in dosage. No subject wore spectacles and selection was careful to guarantee good physical condition. All subjects were right hand/leg dominant (Waterloo Footedness Questionnaire-Revised [18]). All subjects provided informed consent as approved by the IC Riverside Ethics Committee.
Table 1.
Clinical details of eight patients with spasmodic torticollis
| Patient | Age (years) | Disease duration (years) | Severity score | Head turn at rest (°) |
|---|---|---|---|---|
| 1 | 60 | 7 | 4 | −17 |
| 2 | 55 | 2 | 2 | −10 |
| 3 | 72 | 10 | 1 | −10 |
| 4 | 61 | 5 | 1 | +12 |
| 5 | 60 | 4 | 4 | −20 |
| 6 | 57 | 3 | 8 | +35 |
| 7 | 42 | 4 | 4 | +10 |
| 8 | 54 | 4 | 1 | +10 |
Severity score established by Tsui et al. [19]. Positive values of head turn indicate deviation of the chin to the right
Protocol and data acquisition
Participants stood in the centre of a circular array (radius 1.2 m) of 8 LEDs, placed at 45° intervals at eye level, in darkness. At the beginning of each trial, subjects, standing with legs comfortably separated, in their common, everyday footwear, fixated and aligned their head, body and feet with the central LED (Fig. 1 left schematic). After a delay of 10 s the central target was extinguished, thus indicating that an eccentric LED in one of seven locations (±45°, 90°, 135° and 180°) had been lit (Fig. 1, top, second schematic from the left). At this point, the subject had to align his whole body with the new target (outbound trials). After an interval of 15 s the eccentric LED was extinguished (Fig. 1, fourth schematic from the left) thus cueing subjects to return to the initial, centrally-positioned LED (inbound or ‘return’ trials). This protocol guaranteed that the starting position of the eyes of control subjects before a trial was near primary gaze, thus eliminating changes in orbital eye position as confound. In patients, however, eyes, head and trunk were not always perfectly aligned before trial begin due to the abnormal head posture (cf. “Results”).
Fig. 1.
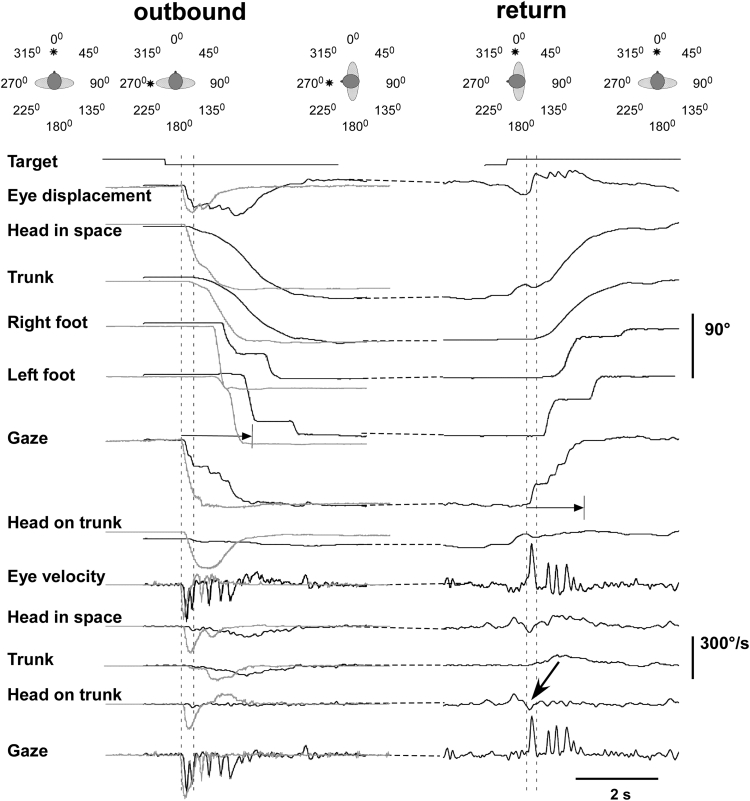
Representative examples of combined movements to a target of 90° offset. The panels above show a cartoon with the successive target presentations and head/trunk positions adopted before and after outbound and inbound turns. Left panels show recordings of a leftward outbound (non predictable) turn in a patient (black traces) and a control subject (gray traces) for comparison. Right hand records show traces of the patient’s rightward (inbound) turn to the central target. Displacement signals are displayed at the top and velocity signals at the bottom. The primary gaze shift in the patient (between dotted vertical markers) fell short of the target and more than 50 % of the visual angle in the patient was covered by the sum of fast nystagmic phases and head motion in space. In contrast, the primary gaze shift executed by the control covered approximately 80 % of the target eccentricity. Acquisition time is defined between the onset of the primary gaze shift and target fixation (for the patient indicated by the length of horizontal arrows). The oblique bold arrow shows an involuntary head-on-trunk dystonic movement which does not interfere with the execution of the inbound voluntary turn
It should be noted that trials to 45° targets are visually driven. For 90° or larger trials the target is initially not visible and patients had no hint as to its location during outbound trials or in which direction they should turn. In inbound or ‘return’ trials however, they knew where the centrally positioned target was. Thus, the protocol allowed separate evaluation of the effects of visual input and spatial prediction on subjects’ performance. Note also that in approximately 50 % of trials to non-visible outbound targets all subjects turned first in the wrong direction (as they were unaware of the target location) and, after realizing their mistake, moved abruptly back in the opposite direction. “Wrong direction” turns are not considered further as starting position cannot be controlled for.
Subjects randomly performed four trials to each LED location. Turns were accomplished at natural, freely chosen speeds rather than forcing subjects to execute time-optimal movements. Our previous studies showed normal intra- and intersubject strategy variability and we wanted to see how ST interfered with this.
Head-in-space, upper trunk, and feet horizontal movements were recorded using a Polhemus Fastrak motion analysis system. Horizontal eye-in-head rotations were recorded using bi-temporal DC electro-oculography (flat response 0–90 Hz). On-off target signals, EOG and body markers were sampled at 240 Hz and stored for off-line analysis. Gaze (eye-in-space) was obtained by adding EOG and head signals. In turn, subtracting trunk from head signals yields head-on-trunk movement. Further details are given in previous reports [11, 12].
Statistics
As the distribution of patients’ data was not always normal, conservative nonparametric tests were used to compare group variables unless otherwise stated. Accordingly, larger numbers of patients have to be recruited in future studies in order to confirm the present findings. Consequently, data are given in text and figures as medians and interquartile range (Q75–Q25),
Results
Patterns of voluntary reorientations
Details of the movement pattern in controls have been given and illustrated extensively in earlier publications (Figs. 1, 2, 3, [12]; Figs. 1, 2, 3, [19]; Figs. 1, 2, [20]). Latency and kinematics depended on target location and predictability but patterns were similar during outbound (unpredictable) and return (predictable) conditions. Here we concentrate on patients’ findings which, in summary were: patients had prolonged movement initiation times and slow velocity of head and trunk rotations; eye velocity was normal. Single-step gaze shifts (see the “Introduction”) were less frequent and target acquisition was therefore prolonged in patients. Visual input did not improve head motor performance. Details are given below.
Fig. 2.
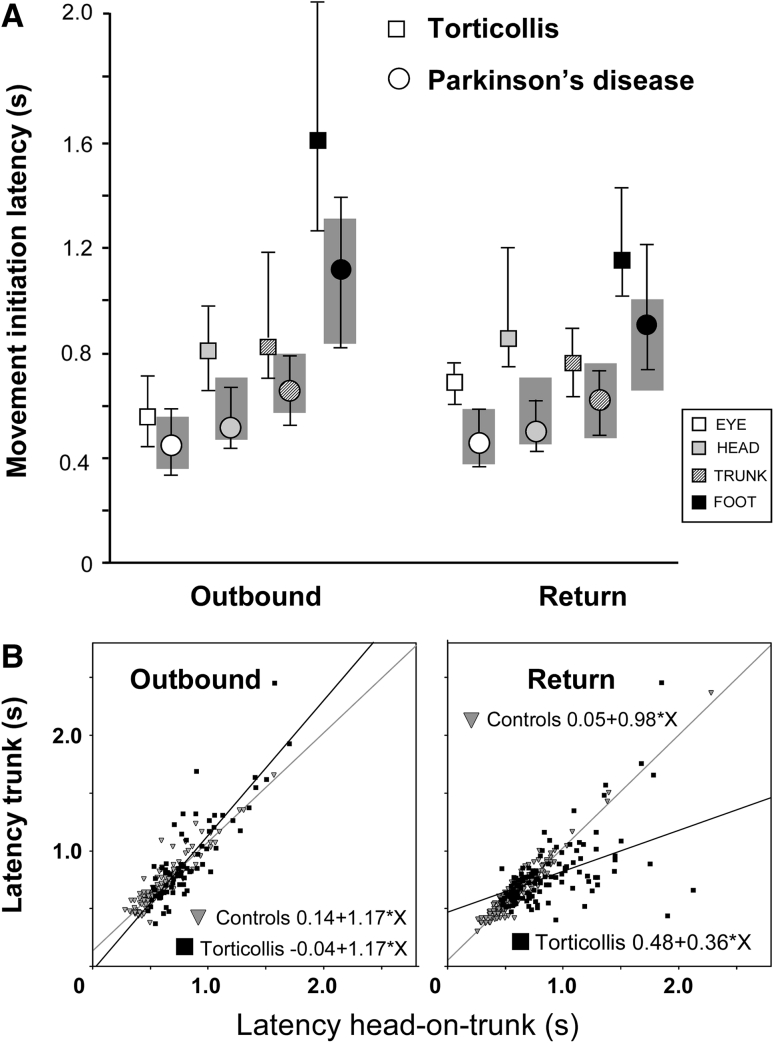
Movement initiation times (latencies). a Normal values are given by the shaded areas (75–25 interquartile range) while torticollis patients median and interquartiles ranges are represented by quadrangles and error bars respectively (for comparison data from Parkinson’s disease patients taken from Anastasopoulos et al. [22] are represented as circles). Note that during inbound (return) trials eye and head latencies in the torticollis patients are further delayed with respect to normal than during outbound trials. In contrast, trunk and foot latencies tend to normalise during inbound trials. b Plots of latency of head-on-trunk versus the latency of trunk movement initiation (straight lines best linear regression fits). The relationship is linear in control subjects (gray triangles) both for outbound and inbound trials. However, note that the head-trunk coupling is loose in torticollis patients (filled quadrangles) particularly during predictable (inbound) trials as reflected in the low R 2 value. Here, in many instances the trunk preceded the head
Fig. 3.
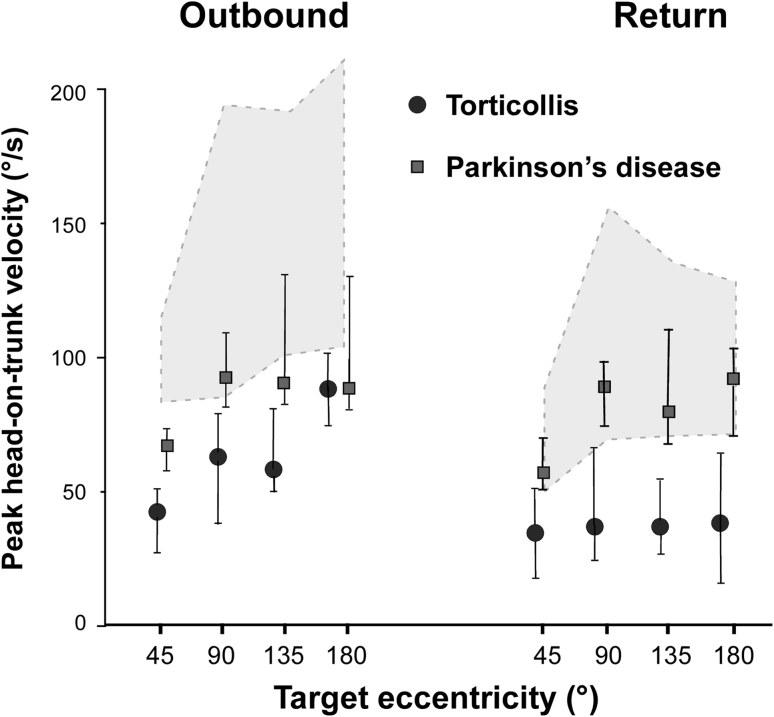
Peak head-on-trunk velocity as a function of target eccentricity. Filled circles represent median values and error bars upper and lower quartiles in torticollis patients; shaded area represents interquartile range in controls. For comparison data from parkinsonian patients investigated with the same methodology were added (filled quadrangles)
The pathological head deviation present whilst standing at the beginning of the trials amounted to 3.4 ± 14.8º on average (11.3 ± 9.2 when normalized with respect to the torticollis direction; head-on-trunk deviation in controls: −1.2 ± 3.8). Figure 1 exemplifies the prevailing movement pattern. Here (left records), the patient reorients to a spatially nonpredictable (outbound) target 90° to the left. The eye moved first with saccadic velocity quickly rising to 260°/s, while head velocity was still very low. The initial gaze shift (primary or main gaze saccade) terminated as the eye approached an eccentric position of 23° in the orbit. This was followed by a compensatory eye rotation (i.e., in the opposite direction of head rotation) towards the primary orbital position. Thereafter, gaze continued to shift towards the target by the sum of head-in-space displacement and repetitive fast eye movements, presumably quick-phases of vestibular nystagmus [12, 21, 22]. These were interspersed with oppositely directed slow-phases of similar amplitude; note that the velocity of the latter approximately equals head-in-space velocity, such that gaze remains thereby stationary (seventhh trace from above). Comparable movement patterns were observed in control subjects (superimposed trajectories from a representative control subject in gray in Fig. 1), but peak head-on-trunk and trunk velocity were clearly reduced in patients (Figs. 3, 4, 5). Note that the control subject continued to rotate the head on the trunk until the target was visible so that peak head-on-trunk deviation was attained around the time of target acquisition. Note also that head-on-trunk in the patient before movement initiation was shifted approximately 11° to the left and that eye, head and trunk were not perfectly aligned with the central target. Occasionally, the patient displays involuntary, short lasting head-on-trunk movements (arrow, right records). The later part of this movement is directed leftward (in the opposite direction of the required voluntary movement) and clearly does not interfere with the execution of the eye/head/trunk combined voluntary movement to the right. In the many occasions where these dystonic intrusions were observed the gaze transfer pattern was never disturbed.
Fig. 4.
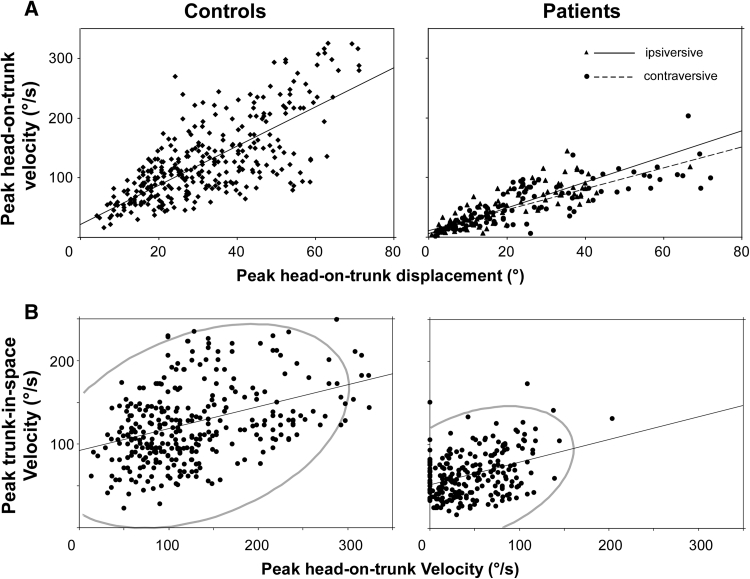
Peak head-on-trunk velocity as a function of head-on-trunk displacement amplitude and peak trunk-in-space velocity. a Patient data separately for ipsiversive and contraversive turns with respect to the direction of torticollis. b Plots of patient (right diagram) and control data (left). The area covered by the density ellipse including 95 % of data points is 2.81 times smaller in patients
Fig. 5.
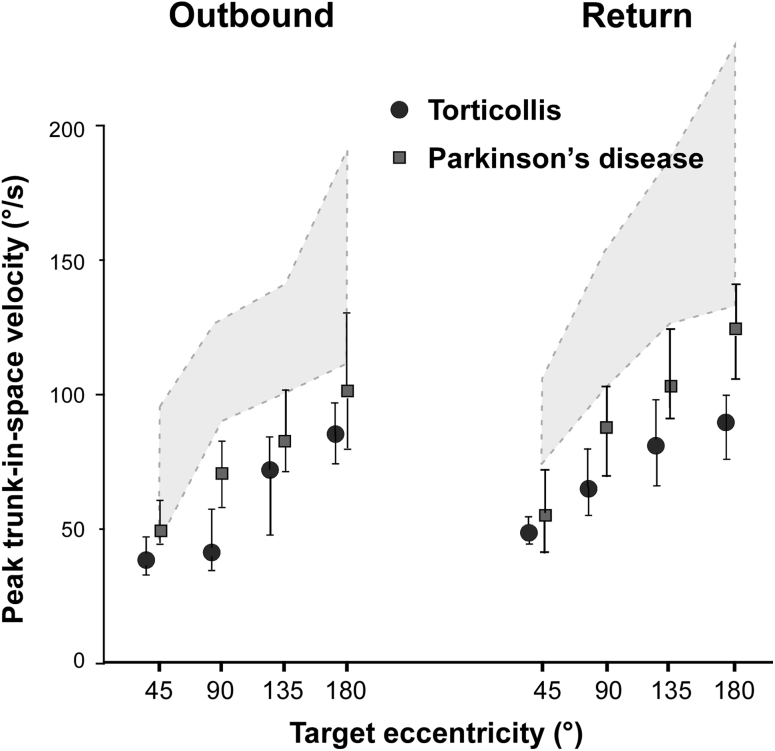
Peak trunk velocity as a function of target eccentricity. Filled circles represent median values and error bars upper and lower quartiles in patients; shaded area represents interquartile range in controls. For comparison data from parkinsonian patients investigated with the same methodology were added (filled quadrangles). Note that the trunk moved slower in torticollis than in mildly to moderately affected Parkinson’s disease patients
Importantly, in 31 % of the return, inbound trials (and occasionally in outbound trials, e.g. see Fig. 1, left “Gaze” trace), control subjects covered >85 % of the displacement to the central target with a single-step large gaze saccade—that is, the gaze displacement was not interrupted by oppositely directed slow phases. This had the net effect of considerably reducing target acquisition time (e.g. up to 1 s, compare in Fig. 1, left, the two superimposed “Gaze” traces). Patients produced significantly less single-step gaze saccades (4 % of return trials, chi-square test p < 0.0001).
Movement initiation (latencies)
Movement onset of body segments was on average prolonged in patients (Fig. 2). In outbound trials statistical significance was reached only for head and foot latency (p = 0.03 and 0.01 respectively; Mann–Whitney U test). All segments started to move with significant delays in patients as compared with controls during return trials (eye, p = 0.01; head, p = 0.002; trunk, p = 0.01; foot, p = 0.006; Mann–Whitney U test). Both groups modified latencies in return trials for each body part, reducing differences between segment latencies (Fig. 2). This en bloc strategy during return, predictable trials (as opposed to a top-down, eye-to-foot progression of latencies during outbound unpredictable trials) was described previously [12].
Head and trunk latencies were strongly linearly related in controls both during inbound and outbound trials (Pearsons’s r = 0.98 and 0.93 respectively, p < 0.0001, Fig. 2b). The relationship was less tight in ST patients (r = 0.55 and 0.86, p < 0.001). In turn this may indicate that delays in trunk motion onset in torticollis patients may be due to the slow and difficult onset of head movements Patients’ intersegmental latency variability was greatest in return, predictive trials. On these occasions head often lagged trunk movement, confirming the patients’ commitment to the task despite the reduced ability to control the head (see also Fig. 2a).
Visualization of the targets (45°) significantly shortened latencies of the eye-to-foot turning synergy in controls [12]. If only the outbound trials are taken into account (i.e., thus removing the effect of prediction), comparisons of head latency to visual targets (45°) vs. head latency to initially non-visual targets (90–135–180°) show no effect of vision in patients (p = 0.007 in controls, paired Wilcoxon test). Similar results were obtained for trunk latencies. In contrast to the head and trunk, eye and foot latencies were significantly shortened in trials to visual targets (in both cases p = 0.01, paired Wilcoxon test). This means that visual input did not improve head and trunk motor performance. Of note, there were no differences in movement latencies between ipsiversive and contraversive turns with respect to the direction of torticollis.
Metrics of the initial (primary) gaze shift
The initial gaze shift amplitude in patients was significantly reduced for return trials to 90°, 135° and 180° targets (p = 0.01, 0.03 and 0.05 respectively; Mann–Whitney U test) while the accuracy of their gaze shifts to visual targets of 45° was normal. This means that during large gaze shifts, the primary gaze shift in patients was only a small fraction of the total gaze shift (median values: 0.80, 0.38, 0.27 and 017 for targets at 45°, 90°, 135° and 180° eccentricity respectively; controls: 0.84, 0.77, 0.59, 0.43 respectively). The remaining visual angle to the target was covered by combined nystagmic quick-phases and head-in-space displacement similarly in both groups (Fig. 1).
Body segmental velocity
Eye: Peak eye velocity was a saturating function of eye saccade amplitude (‘main sequence’ [23]. There was no Group difference.
Head: Peak head-on-trunk velocity was significantly reduced in patients, both inbound and outbound (patients: 27 (55–21) and 47 (65–30) respectively vs. 98 (132–70) and 115 (170–81) /s in controls, Fig. 3; p < 0.05, Mann–Whitney U test, separately for trials to 45°, 90°, 135° and 180° targets). This speed reduction was more pronounced (by approximately 20 %) in turns directed ipsilaterally to the torticollis. Still, the relationship between peak head-on-trunk displacement vs. velocity had similar slopes in both directions, indicating that ipsiversive head movements were slower because they were smaller, Fig. 4a. Peak head-on-trunk velocity was significantly reduced in patients even in trials with visible targets (Mann–Whitney U test, p < 0.009; Fig. 3); i.e., the normal gaze accuracy in these 45° trials was due to the fact that patients compensated for the reduced head-on-trunk contribution with larger eye saccades.
Noticeably, in the few (n = 5) single-step trials carried out by patients, peak head-on-trunk velocity (and consequently head-in-space velocity) was relatively high, reaching 119°/s on average (controls 156°/s, compare Fig. 3). In contrast, parkinsonian patients in a previous investigation with the same protocol [20] were able to execute long duration single-step gaze shifts even if the velocity of head and trunk was considerably low as compared to controls.
Trunk: Peak trunk velocity was significantly reduced in patients for all but outbound trials to 180° (p < 0.001, Mann–Whitney U test; Fig. 5) Trunk velocity correlated significantly with head-on-trunk velocity in normal subjects (Pearson rho 0.36; p = 0.0001) and patients (Pearson rho 0.45; p = 0.0001). Note that density ellipses of 95 % of data points (computed from bivariate normal distribution fit to these variables) cover a 2.81 times smaller area in patients than controls (Fig. 4b). Thus, for a particular head-on-trunk velocity in patients, only low trunk velocity values (and consequently peak head-in-space velocity) can be expected. In contrast, for a particular head-on-trunk velocity in normal controls peak trunk velocity can vary considerably. This indicates a more flexible control of trunk motion in normal subjects.
Foot: Peak foot stepping rotational velocity was normal.
Task performance
Since subjects performed a goal-oriented visual task, performance was ultimately defined as target acquisition time (defined in Fig. 1). This was overall significantly prolonged in patients. Separate nonparametric tests show significant differences for trials ≥90° (p < 0.05; Mann–Whitney U test); that is, only for target amplitude of 45° were the patients not significantly different to the control subjects. Patients needed on average 480, 685 and 800 ms more than controls to acquire targets of 90°, 135° and 180° eccentricity respectively [control values 641 (837–485), 1,010 (1,300–754) and 1,263 (1,683–958) ms respectively]. The acquisition time in patients was thus, surprisingly, even longer than in a group of parkinsonian patients investigated previously [20].
Total score across planes of the Tsui scale correlated significantly with acquisition time for targets at >90° eccentricity (p < 0.001). The prolongation of target acquisition time in patients was due to: (a) the less frequent single-step gaze displacement patterns, (b) slower head-in-space velocity.
Discussion
Reorientation of gaze in cervical dystonia has been reported when the head [7, 8] or the trunk is stationary [8]. By incorporating the lower body segments in the task, we were able to examine a more natural task. The paradigm used also allowed us to investigate the effects of vision and target location/prediction on initiation and multi-segmental coordination. In particular, whether the abnormal head posture and motility in patients disrupt eye, head and trunk coordination during large gaze shifts. We now report that, indeed, such coordination in patients is defective, because single-step gaze shifts are rarely executed. These have been replaced by less efficient nystagmic-based mode of gaze transfers [12]. The abnormally prolonged foveation time in patients documents for the first time how severely impaired these patients are in terms of gaze control and turning behavior. The abnormal head control compromises saccadic function in situations where eye movements become an integral part of a more widespread motor synergy, rather than a somewhat artificial ‘head-clamped’ ocular experiment; e.g., head-fixed saccadic latencies are normal in ST [7].
Head movement control in the context of gaze transfers
As previously reported [8], initiation of head movement was significantly delayed in our patient group. The initiation of head movement in ST patients was delayed for all amplitudes of target eccentricity. We found that visualization of the target (i.e., at 45°) did not restore this deficit. Patients did not normalize head latencies during return (predictive) trials either but were able to reduce foot latencies in return trials, just as control subjects did. The effect of these modifications in both groups was that the various body segments began to move more en bloc in return trials. We previously interpreted this as an attempt to coordinate the various body segments such that single-step gaze shifts can be generated [12, 19, 20]. Thus it can be concluded that the neck disorder does not interfere with higher order movement control of other body segments, such as prediction-led reorganization of movement patterns.
Head-on-trunk displacement and velocity were significantly reduced in patients, as expected. Although the amplitude-velocity relationship remained linear as in control subjects, its slope was less steep. Of note, both ipsiversive and contraversive head movements are slower and last longer in patients [2–4, 8], but the mechanism of this bilateral slowing remains be elucidated.
On several occasions, involuntary, short-lasting head-on-trunk movements occurred synchronously or immediately before the eye-to-foot synergy to the target in three out of eight patients. This is exemplified in Fig. 1 (bold arrow), where it is important to note that the involuntary movement ‘intrusion’ does not disrupt, delay, or interrupt the goal-directed combined movement. More importantly, the dystonic movement does not resemble a synergistic eye-head voluntary gaze shift as previously (i.e., by Denny-Brown and Hassler) and more recently hypothesized [6]. Hence, our findings contradict previous hypotheses that the involuntary, dystonic head movement in torticollis is due to pathological activation of gaze or orienting movement mechanisms. This conclusion is also supported by current and previous findings in ST patients [7, 8] suggesting sparing of visually-controlled oculomotor function, an essential component of gaze control.
Trunk bradykinesia
Voluntary trunk rotations have not been previously examined in ST. Trunk movement was significantly slow in patients which may appear at first sight surprising. However, impairment of movements beyond the neck region has been reported in spasmodic torticollis; for example, impairment of fast voluntary arm extension movements and reduction of arm swing [24, 25], although such findings are likely to represent direct caudal extension of the underlying dystonic process.
Trunk slowness during turning, both standing and in bed, is a well recognized feature of akinetic-rigid syndromes [20, 26–30]. Strikingly, the reduction of trunk velocity in our patients was even more pronounced than that observed in a group of mild to moderately affected parkinsonian patients ([20]; Fig. 5 current manuscript). Superficially, this finding may also indicate segmental spread of motor dysfunction by the underlying disease process. However, such trunk ‘bradykinesia’ may be associated with (or be secondary to) the reduction of head-on-trunk velocity, as these parameters correlate in both patient and control groups (Fig. 4b) and head-on-trunk and trunk movement are functionally tightly coupled [19]. Viewed in this way, the trunk bradykinesia in ST can be termed ‘secondary’ (to the slow head movement) as opposed to the ‘primary’ bradykinesia in Parkinsonism. The tighter than normal association between head-on-trunk velocity and trunk velocity observed in torticollis patients (Fig. 4b) supports this view.
Fragmentation of gaze shifts and the execution of coordinated movements in ST
Similarly to latencies, visual input did not improve head velocity to 45° targets, which remained abnormally slow (Fig. 3). This finding is in contrast to that observed in patients with Parkinson’s disease who are more dependent on visual information for the execution of oculomotor and somatomotor tasks [14, 29, 31, 32]. Still, gaze accuracy and acquisition time to 45° targets remained normal in patients because larger eye movements managed to adaptively compensate for the reduced range of head motion [8].
When reorienting movements are towards initially nonvisible, remembered targets (i.e., return to 0° from ≥90°), normal subjects frequently replace the nystagmic-based (i.e., multiple stepped) target-acquisition pattern by single-step gaze shifts. Earlier work has shown that higher head-in-space velocity and precise control of individual segment timing determine the release of the single-step gaze pattern [12, 20]. In addition, both vestibular neuronal activity and the VOR have been shown to be cancelled during large active gaze shifts, presumably by signals generated within cerebellar structures [33]. This CNS-controlled synergistic motor behaviour was only rarely observed in ST patients, whereas parkinsonian patients studied previously [20] executed more frequently long duration, single-step gaze shifts despite occasionally very low head and trunk velocity. Inadequate central cancellation of the VOR, when large gaze shifts are to be implemented may thus be an additional unfavorable factor impeding the release of single-step gaze pattern in ST. The disruption of this motor synergy, together with the finding that the visual input cannot restore head motor performance may thus represent evidence for the recently discussed cerebellar dysfunction in ST [34].
In conclusion, the coordination of large gaze reorientations in the horizontal plane is significantly impaired in cervical dystonia leading to gross prolongations in target acquisition times. This finding and the secondary trunk bradykinesia observed likely contribute to clinical disability in patients. Whether targeted therapy, drug based or rehabilitation, can have an impact on this specific dysfunction in ST patients is not known. The different configuration of the dystonic neck movements, and the fact that they do not disrupt voluntary gaze shifts, indicates that the abnormal motor command in ST does not originate in head-eye gaze centers. Eye movements per se are normal and in fact compensate for the reduce head motion in this disease.
Acknowledgments
The authors wish to thank Julie Apostolou for technical assistance. Financial support from MRC (UK) and ELKE, University of Athens.
Conflicts of interest
There are not any conflicts of interest from any of the authors that relate to the research covered in the article.
Ethics approval
Subjects provided informed consent as approved by the IC Riverside Ethics Committee in accordance with the WMA Declaration of Helsinki.
References
- 1.Zangemeister WH, Stark L (1982) Understanding dynamics and control of normal and abnormal head movements by use of a parameterized head movement model. TRANS IEEE Biomed Soc 82 CH1720 3:468–473
- 2.Boccagni C, Carpaneto J, Micera S, et al. Motion analysis in cervical dystonia. Neurol Sci. 2008;29:375–381. doi: 10.1007/s10072-008-1033-z. [DOI] [PubMed] [Google Scholar]
- 3.Gregori B, Agostino R, Bologna M, et al. Fast voluntary neck movements in patients with cervical dystonia: A kinematic study before and after therapy with botulinum toxin type A. Clin Neurophysiol. 2008;119:273–280. doi: 10.1016/j.clinph.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 4.De Beyl DZ, Salvia P. Neck movement speed in cervical dystonia. Mov Disord. 2009;26:2267–2271. doi: 10.1002/mds.22830. [DOI] [PubMed] [Google Scholar]
- 5.Hassler R, Dieckmann G (1970) Die stereotaktische Behandlung des Torticollis aufgrund tierexperimenteller Erfahrungen über die richtungsbetimmten Bewegungen. (Stereotaxic treatment of torticollis according to animal experiment experiences about direction determined movement). Nervenarzt 41:437–87 [PubMed]
- 6.Klier E, Wang H, Constantin A, et al. Midbrain control of three-dimensional head orientation. Science. 2002;295:1314–1316. doi: 10.1126/science.1067300. [DOI] [PubMed] [Google Scholar]
- 7.Farshadmanesh F, Klier EM, Chang P, et al. Three-dimensional eye-head coordination after injection of muscimol into the interstitial nucleus of Cajal. J Neurophysiol. 2007;97:2322–2338. doi: 10.1152/jn.00752.2006. [DOI] [PubMed] [Google Scholar]
- 8.Gilman S, Vilensky J, Morecraft RW, et al. Denny-Brown’s views on the pathophysiology of dystonia. J Neurol Sci. 1999;167:142–147. doi: 10.1016/S0022-510X(99)00149-5. [DOI] [PubMed] [Google Scholar]
- 9.Stell R, Bronstein AM, Gresty M, et al. Saccadic function in spasmodic torticollis. J Neurol Nurosurg Psychiatry. 1990;53:496–501. doi: 10.1136/jnnp.53.6.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maurer C, Mergner T, Luecking CH, et al. Adaptive changes of saccadic eye head coordination resulting from altered head posture in torticollis spasmodicus. Brain. 2001;124:413–426. doi: 10.1093/brain/124.2.413. [DOI] [PubMed] [Google Scholar]
- 11.Münchau A, Corna S, Gresty MA, et al. Abnormal interaction between vestibular and voluntary head control in patients with spasmodic torticollis. Brain. 2001;124:47–59. doi: 10.1093/brain/124.1.47. [DOI] [PubMed] [Google Scholar]
- 12.Land M. The coordination of rotations of the eyes, head and trunk in saccadic turns produced in natural situations. Exp Brain Res. 2004;159:151–160. doi: 10.1007/s00221-004-1951-9. [DOI] [PubMed] [Google Scholar]
- 13.Hollands M, Ziavra N, Bronstein A. A new paradigm to investigate the roles of head and eye movements in the coordination of whole-body movements. Exp Brain Res. 2004;154:161–166. doi: 10.1007/s00221-003-1718-8. [DOI] [PubMed] [Google Scholar]
- 14.Anastasopoulos D, Ziavra N, Hollands M, et al. Gaze displacement and inter-segmental coordination during large whole body voluntary rotations. Exp Brain Res. 2009;217:336–346. doi: 10.1007/s00221-008-1627-y. [DOI] [PubMed] [Google Scholar]
- 15.Cullen KE, Roy JE. Signal processing in the vestibular system during active versus passive head movements. J Neurophysiol. 2004;91:1919–1933. doi: 10.1152/jn.00988.2003. [DOI] [PubMed] [Google Scholar]
- 16.Flowers KA. Visual ‘closed-loop’ and ‘open-loop’ characteristics of voluntary movement in patients with Parkinsonism and intention tremor. Brain. 1976;99:269–310. doi: 10.1093/brain/99.2.269. [DOI] [PubMed] [Google Scholar]
- 17.Currá A, Berardelli A, Agostino R, et al. Movement cueing and motor execution in patients with dystonia: a kinematic study. Mov Disord. 2000;15:103–112. doi: 10.1002/1531-8257(200001)15:1<103::AID-MDS1016>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa K, Inui T, Sugio T. Separating brain regions involved in internally guided and visual feedback control of moving effectors: an event-related fMRI study. NeuroImage. 2006;32:1760–1770. doi: 10.1016/j.neuroimage.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Tsui JK, Eisen A, Stoessl A, et al. Double-blind study of botulinum toxin in spasmodic torticollis. Lancet. 1986;8501:246. doi: 10.1016/s0140-6736(86)92070-2. [DOI] [PubMed] [Google Scholar]
- 20.Elias LJ, Bryden MP, Bulman-Fleming MB. Footedness is a better predictor than is handedness of emotional lateralisation. Neuropsychologia. 1998;36:37–43. doi: 10.1016/S0028-3932(97)00107-3. [DOI] [PubMed] [Google Scholar]
- 21.Sklavos S, Anastasopoulos D, Bronstein A. Kinematic redundancy and variance of eye, head and trunk displacements in earth the horizontal plane during large gaze reorientations in humans. Exp Brain Res. 2010;202:879–890. doi: 10.1007/s00221-010-2192-8. [DOI] [PubMed] [Google Scholar]
- 22.Anastasopoulos D, Ziavra N, Savvidou E, et al. Altered eye-to-foot coordination in standing parkinsonian patients during large gaze and whole body re-orientations. Mov Disord. 2011;26:2201–2211. doi: 10.1002/mds.23798. [DOI] [PubMed] [Google Scholar]
- 23.Mishkin S, Mellvil Jones G. Predominant direction of gaze during slow head rotation. Aerospace Med. 1966;37:897–901. [PubMed] [Google Scholar]
- 24.Barnes G. Vestibulo-ocular function during co-ordinated head and eye movements to acquire visual targets. J Physiol. 1979;287:127–147. doi: 10.1113/jphysiol.1979.sp012650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becker W. Metrics. In: Wurtz RH, Goldberg ME, editors. The neurobiology of saccadic eye movements. Amsterdam: Elsevier; 1989. pp. 13–67. [PubMed] [Google Scholar]
- 26.Carboncini MC, Manzoni D, Strambi S, et al. Impaired agonists recruitment during voluntary arm movements in patients affected by spasmodic torticollis. Arch Ital Biol. 2004;142:113–124. [PubMed] [Google Scholar]
- 27.Kägi MDG, Schwingenschuh P, Bhatia K. Arm swing is reduced in idiopathic cervical dystonia. Mov Disord. 2008;23:1784–1787. doi: 10.1002/mds.22216. [DOI] [PubMed] [Google Scholar]
- 28.Steiger MJ, Thompson PD, Marsden CD. Disordered axial movement in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1996;61:645–648. doi: 10.1136/jnnp.61.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verheyden G, Willems A-M, Ooms L, et al. Validity of the trunk impairment scale as a measure of trunk performance in people with Parkinson’s disease. Arch Phys Med Rehabil. 2007;88:1303–1308. doi: 10.1016/j.apmr.2007.06.772. [DOI] [PubMed] [Google Scholar]
- 30.Carpenter MG, Bloem BR. A new twist on turning movements in Parkinson’s disease patients. Mov Disord. 2011;26:21511–21513. doi: 10.1002/mds.23980. [DOI] [PubMed] [Google Scholar]
- 31.Kennard C, Lueck CJ. Oculomotor abnormalities in diseases of the basal ganglia (Review) Rev Neurol (Paris) 1989;145:587–595. [PubMed] [Google Scholar]
- 32.Nakamura T, Branstein AM, Lueck C, et al. Vestibular, cervical and visual remembered saccades in Parkinson’s disease. Brain. 1994;117:1423–1432. doi: 10.1093/brain/117.6.1423. [DOI] [PubMed] [Google Scholar]
- 33.Cullen KE. The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 2012;35:185–196. doi: 10.1016/j.tins.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadnicka A, Hoffland BS, Bhatia KP, et al. The cerebellum in dystonia-Help or hindrance? Clin Neurophysiol. 2012;123:65–70. doi: 10.1016/j.clinph.2011.04.027. [DOI] [PubMed] [Google Scholar]